Wastewater Treatment Plant: Modelling and Validation of an Activated Sludge Process
Abstract
1. Introduction
2. Method
2.1. System Layout
2.2. Calibration and Validation Procedure
3. Results and Discussion
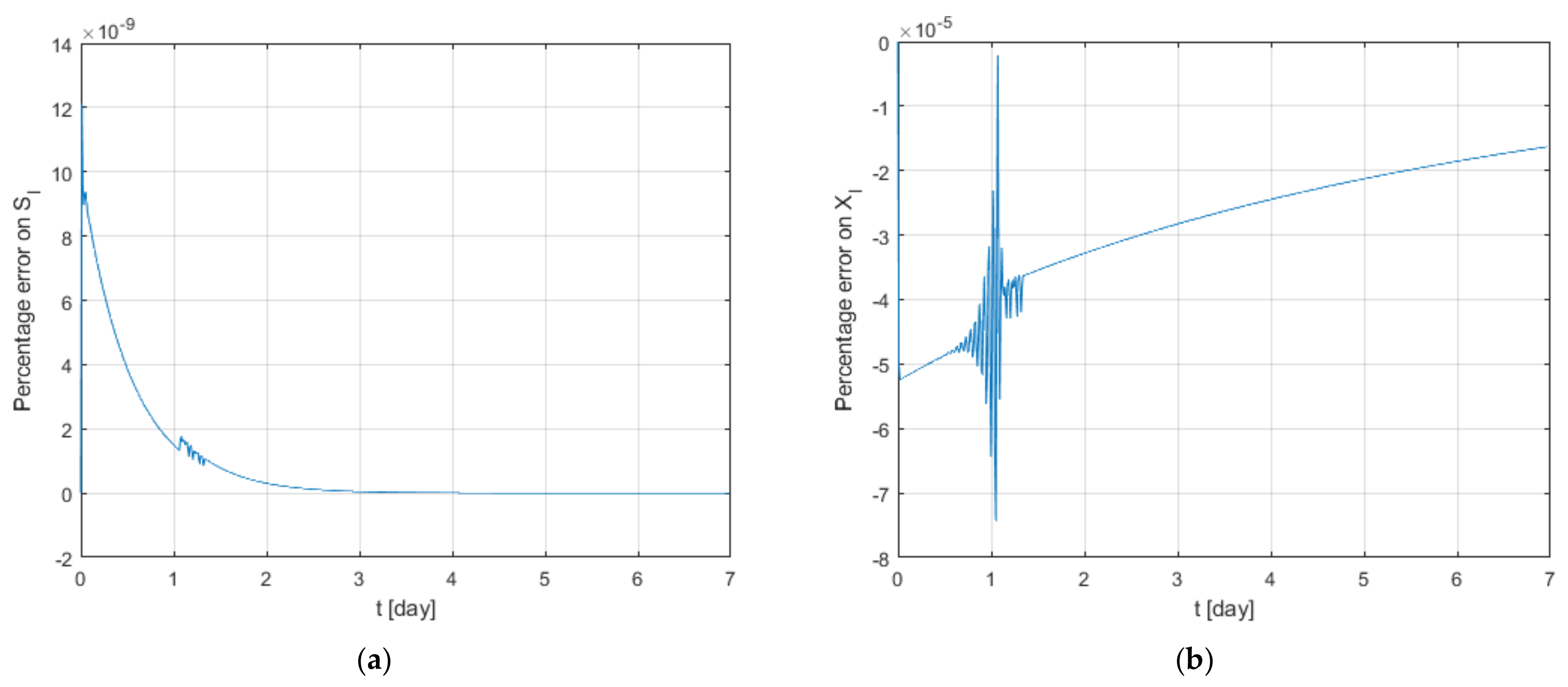
3.1. Sensitivity Analysis
3.2. Energy Analysis
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
Appendix A
Appendix A.1. System Model
| Parameter | Description | Unit | |
|---|---|---|---|
| 1 | SI | Soluble inert organic matter: consists of organic compounds that do not take part in the wastewater treatment processes | mgCODl−3 |
| 2 | SS | Readily biodegradable substrate: simple organic carbon compounds that provide energy to heterotrophic bacteria | mgCODl−3 |
| 3 | XI | Particulate inert organic matter: undissolved organic particles such as soil organic matter or other particulates isolated by sieving or filtration | mgCODl−3 |
| 4 | XS | Slowly biodegradable substrate: consists of relatively complex compounds that must be hydrolysed to simple compounds by extracellular enzymes in order to be assimilated by microorganisms | mgCODl−3 |
| 5 | XBH | Active heterotrophic biomass: microorganisms using organic carbon for the formation of new biomass | mgCODl−3 |
| 6 | XBA | Active autotrophic biomass: microorganisms using carbon from carbon dioxide for the formation of new biomass | mgCODl−3 |
| 7 | XP | Particulate products arising from biomass decay: products which are inert to further biological attack after the decay of the biomass | mgCODl−3 |
| 8 | SO | Oxygen: the oxygen concentration for the biological process | mgl−3 |
| 9 | SNO | Nitrate and nitrite nitrogen: products obtained by autotrophic bacteria in aerobic condition and removed by heterotrophic bacteria in anoxic condition | mgNl−3 |
| 10 | SNH | NH4++ NH3 nitrogen: the soluble ammonia nitrogen, assumed to be the sum of the ionized (ammonium, NH4+) and un-ionized form (ammonia, NH3) | mgNl−3 |
| 11 | SND | Soluble biodegradable organic nitrogen: products formed by the hydrolysis of particular organic nitrogen by ammonification | mgNl−3 |
| 12 | XND | Particulate biodegradable organic nitrogen: products generated from decay of autotrophic and heterotrophic bacteria | mgNl−3 |
| 13 | Salk | Alkalinity | mol m−3 |
| Process, j | Component, i | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | Process Rate | |
| SI | SS | XI | XS | XBH | XBA | XP | SO | SNO | SNH | SND | XND | Salk | ρj | |
| 1. Aerobic growth of heterotrophs | 1 | |||||||||||||
| 2. Anoxic growth of heterotrophs | 1 | |||||||||||||
| 3. Aerobic growth of autotrophs | 1 | |||||||||||||
| 4. Decay of heterotrophs | 1-fP | −1 | fP | bHXB,H | ||||||||||
| 5. Decay of autotrophs | −1 | baXB,A | ||||||||||||
| 6. Ammonification of soluble nitrogen | 1 | −1 | kaSNDXB,H | |||||||||||
| 7. Hydrolysis of entrapped organics | 1 | −1 | ||||||||||||
| 8. Hydrolysis of entrappedorganic nitrogen | 1 | −1 | ρ7(XND/XS) | |||||||||||
- H = the maximum heterotrophic growth rate, equal to 4.0 (day−1).
- KS = the saturation (heterotrophic growth), equal to 10.0 (g COD m−3).
- KO,H = the half saturation (heterotrophic oxygen), equal to 0.2 (g O2 m−3).
- KNO = the half saturation (nitrate), equal to 0.5 (g NO3-Nm−3).
- bH = the heterotrophic decay rate, equal to 0.3 (day−1).
- ηg = the anoxic growth rate correction factor, equal to 0.8 (-).
- ηh = the anoxic hydrolysis rate correction factor, equal to 0.8 (-).
- kh = the maximum specific hydrolysis rate, equal to 3.0 (g, slowly biodegradable COD (g XBH COD day)−1).
- KX = the half saturation (hydrolysis), equal to 0.1 (g, slowly biodegradable COD (g XBH COD)−1).
- A = the maximum autotrophic growth rate, equal to 0.5 (day−1).
- KNH = the half saturation (auto: growth), equal to 1.0 (g NH3-Nm−3).
- KOA = the half saturation (auto: oxygen), equal to 0.4 (g O2 m−3).
- bA = the autotrophic decay rate, equal to 0.05 (day−1).
- ka = the ammonification rate, equal to 0.05 (m3 COD (g day)−1).
Appendix A.2. Aeration Energy Demand Evaluation
References
- Salgot, M.; Folch, M. Wastewater treatment and water reuse. Curr. Opin. Environ. Sci. Health 2018, 2, 64–74. [Google Scholar] [CrossRef]
- Filipe, J.; Bessa, R.J.; Reis, M.; Alves, R.; Póvoa, P. Data-driven predictive energy optimization in a wastewater pumping station. Appl. Energy 2019, 252, 113423. [Google Scholar] [CrossRef]
- Longo, S.; Mirkod’Antoni, B.; Bongards, M.; Chaparro, A.; Cronrath, A.; Fatone, F.; Lema, J.M.; Mauricio-Iglesias, M.; Soares, A.; Hospido, A. Monitoring and diagnosis of energy consumption in wastewater treatment plants. A state of the art and proposals for improvement. Appl. Energy 2016, 179, 1251–1268. [Google Scholar] [CrossRef]
- Zhang, Z.; Kusiak, A.; Zeng, Y.; Wei, X. Modeling and optimization of a wastewater pumping system with data-mining methods. Appl. Energy 2016, 164, 303–311. [Google Scholar] [CrossRef]
- Guven, H.; Dereli, R.K.; Ozgun, H.; Ersahin, M.E.; Ozturk, I. Towards sustainable and energy efficient municipal wastewater treatment by up-concentration of organics. Prog. Energy Combust. Sci. 2019, 70, 145–168. [Google Scholar] [CrossRef]
- Shen, Y.; Linville, J.L.; Urgun-Demirtas, M.; Mintz, M.M.; Snyder, S.W. An overview of biogas production and utilization at full-scale wastewater treatment plants (WWTPs) in the United States: Challenges and opportunities towards energy-neutral WWTPs. Renew. Sustain. Energy Rev. 2015, 50, 346–362. [Google Scholar] [CrossRef]
- Wang, J.; You, S.; Zong, Y.; Træholt, C.; Dong, Z.Y.; Zhou, Y. Flexibility of combined heat and power plants: A review of technologies and operation strategies. Appl. Energy 2019, 252, 113445. [Google Scholar] [CrossRef]
- Salman, C.A.; Naqvi, M.; Thorin, E.; Yan, J. Impact of retrofitting existing combined heat and power plant with polygeneration of biomethane: A comparative techno-economic analysis of integrating different gasifiers. Energy Convers. Manag. 2017, 152, 250–265. [Google Scholar] [CrossRef]
- Picardo, A.; Soltero, V.M.; Peralta, M.E.; Chacartegui, R. District heating based on biogas from wastewater treatment plant. Energy 2019, 180, 649–664. [Google Scholar] [CrossRef]
- Calise, F.; Cappiello, F.L.; Dentice d’Accadia, M.; Infante, A.; Vicidomini, M. Modeling of the Anaerobic Digestion of Organic Wastes: Integration of Heat Transfer and Biochemical Aspects. Energies 2020, 13, 2702. [Google Scholar] [CrossRef]
- Di Fraia, S.; Macaluso, A.; Massarotti, N.; Vanoli, L. Energy, exergy and economic analysis of a novel geothermal energy system for wastewater and sludge treatment. Energy Convers. Manag. 2019, 195, 533–547. [Google Scholar] [CrossRef]
- Nelson, M.I.; Sidhu, H.S.; Watt, S.; Hai, F.I. Performance analysis of the activated sludge model (number 1). Food Bioprod. Process. 2019, 116, 41–53. [Google Scholar] [CrossRef]
- Henze, M.; Grady, C.P.L., Jr.; Gujer, W.; Marais, G.V.R.; Matsuo, T. A general model for single-sludge wastewater treatment systems. Water Res. 1987, 21, 505–515. [Google Scholar] [CrossRef]
- Hauduc, H.; Rieger, L.; Oehmen, A.; van Loosdrecht, M.C.M.; Comeau, Y.; Héduit, A.; Vanrolleghem, P.A.; Gillot, S. Critical review of activated sludge modeling: State of process knowledge, modeling concepts, and limitations. Biotechnol. Bioeng. 2013, 110, 24–46. [Google Scholar] [CrossRef] [PubMed]
- Moretti, C.J.; Jahan, K.; Schmit, K.H.; Debik, E.; Mahendraker, V. Activated Sludge and Other Aerobic Suspended Culture Processes. Water 2011, 3, 806–818. [Google Scholar] [CrossRef]
- Available online: https://pdfs.semanticscholar.org/b076/822899ab977bd49e271e51ae6fa8ec9ad951.pdf (accessed on 30 July 2020).
- Available online: https://op.europa.eu/en/publication-detail/-/publication/8448ef88-37dd-4d1a-823f-3143e7902429 (accessed on 30 July 2020).
- The European Co-Operation in the Field of Scient. ific and Technical Research, Action 624: Optimal Management of Wastewater SystemsCOST Action 624 Website. Available online: http://www.ensic.inpl-nancy.fr/COSTWWTP (accessed on 30 July 2020).
- Asadi, A.; Yang, K.; Mejabi, B. Wastewater treatment aeration process optimization: A data mining approach. J. Environ. Manag. 2017, 203, 630–639. [Google Scholar] [CrossRef] [PubMed]
- Sheridan, C.; Petersen, J.; Rohwer, J. Technical note on modifying the Arrhenius equation to compensate for temperature changes for reactions within biological systems. Water SA 2012, 38, 149–152. [Google Scholar] [CrossRef]
- Henze, M.; Gujer, W.; Mino, T.; van Loosdrecht, M.C.M. Activated Sludge Models ASM1, ASM2, ASM2d and ASM3; IWA Publishing: London, UK, 2000. [Google Scholar]
- Takacs, I.; Patry, G.G.; Nolasco, D. A dynamic model of the clarification-thickening process. Wat. Res. 1991, 25, 1263–1271. [Google Scholar] [CrossRef]
- Alex, J.; Benedetti, L.; Copp, J.; Gernaey, K.V.; Jeppsson, U.; Nopens, I.; Pons, M.N.; Rieger, L.; Rosen, C.; Steyer, J.P.; et al. Benchmark Simulation Model no 1 (BSM1). Department of Industrial Electrical Engineering and Automation, Lund University. Available online: https://www.iea.lth.se/publications/Reports/LTH-IEA-7229.pdf (accessed on 30 July 2020).
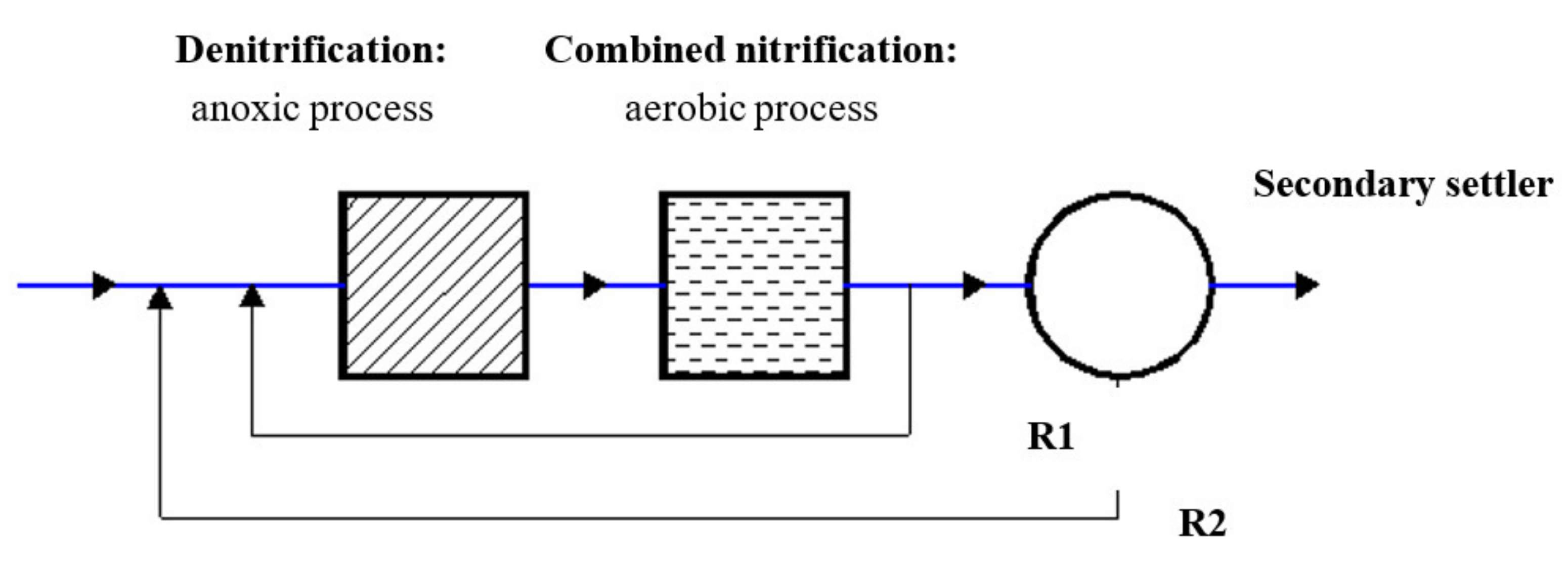










| Concentration of | Inflow | Anoxic Tank 1 Initial | Anoxic Tank 2 Initial | Aeration Tank 1 Initial | Aeration Tank 2 Initial | Aeration Tank 3 Initial |
|---|---|---|---|---|---|---|
| (gm−3) | ||||||
| Readily biodegradable substrate | 69.500 | 3.732 | 2.061 | 1.454 | 1.226 | 1.067 |
| Soluble inert organic | 30.000 | 30.000 | 30.000 | 30.000 | 30.000 | 30.000 |
| Particulate inert organic matter | 51.200 | 814.301 | 813.912 | 813.393 | 812.874 | 812.354 |
| Slowly biodegradable substrate | 202.320 | 83.073 | 81.401 | 68.023 | 57.215 | 49.092 |
| Active heterotrophic biomass | 28.170 | 2085.021 | 2083.728 | 2088.379 | 2091.048 | 2091.868 |
| Active autotrophic biomass | 0 | 69.621 | 69.535 | 69.839 | 70.152 | 70.382 |
| Particulate products arising from biomass decay | 0 | 227.672 | 228.044 | 228.540 | 229.037 | 229.534 |
| Dissolved oxygen | 0 | 0.012 | 0.000 | 2.938 | 3.752 | 1.076 |
| Nitrate and nitrite nitrogen | 0 | 1.649 | 0.543 | 2.177 | 3.881 | 5.089 |
| Ammonia nitrogen | 31.560 | 17.672 | 18.135 | 16.549 | 15.029 | 13.901 |
| Soluble biodegradable organic nitrogen | 6.950 | 1.319 | 0.859 | 0.914 | 0.881 | 0.808 |
| Particulate biodegradable organic nitrogen | 10.590 | 5.234 | 5.235 | 4.485 | 3.872 | 3.410 |
| Concentration of | Results Obtained | Biowin [4] | Simba [19] | Stoat [5] |
|---|---|---|---|---|
| (gm−3) | ||||
| Readily biodegradable substrate | 0.890 | 0.890 | 0.889 | 0.900 |
| Soluble inert organic | 30.000 | 30.000 | 30.000 | 30.000 |
| Particulate inert organic matter | 4.392 | 4.270 | 4.392 | 6.100 |
| Slowly biodegradable substrate | 0.188 | 0.210 | 0.188 | 0.140 |
| Active heterotrophic biomass | 9.782 | 9.510 | 9.782 | 6.600 |
| Active autotrophic biomass | 0.573 | 0.560 | 0.573 | 0.400 |
| Particulate products arising from biomass decay | 1.728 | 1.680 | 1.728 | - |
| Dissolved oxygen | 0.491 | 0.500 | 0.491 | 0.500 |
| Nitrate and nitrite nitrogen | 10.415 | 10.450 | 10.415 | 10.400 |
| Ammonia nitrogen | 1.733 | 1.740 | 1.733 | 1.700 |
| Soluble biodegradable organic nitrogen | 0.688 | 0.690 | 0.688 | 0.700 |
| Particulate biodegradable organic nitrogen | 0.013 | 0.010 | 0.013 | 0.000 |
| TSS Concentrations Along the Settler Height | Results Obtained | Biowin [4] | Simba [19] | Stoat [5] |
|---|---|---|---|---|
| (gSSm−3) | ||||
| Layer 1 (effluent source) | 12.497 | 12.170 | 12.500 | 12.500 |
| Layer 2 | 18.113 | n/a | 18.110 | 18.100 |
| Layer 3 | 29.540 | n/a | 29.540 | 29.500 |
| Layer 4 | 68.978 | n/a | 68.980 | 68.900 |
| Layer 5 | 356.075 | n/a | 356.070 | 356.100 |
| Layer 6 | 356.075 | n/a | 356.070 | 356.100 |
| Layer 7 | 356.075 | n/a | 356.070 | 356.100 |
| Layer 8 | 356.075 | n/a | 356.070 | 356.100 |
| Layer 9 | 356.075 | n/a | 356.070 | 356.100 |
| Layer 10 (activated sludge source) | 6393.984 | 6406.030 | 6393.980 | 6394.100 |
| Volume Tank 1, 2 (m3) | Volume Tank 3, 4, 5 (m3) | kLa (day−1) | EQI (kg pollutions d−1) | Electric Demand (kW) | Primary Energy Consumption (MWh/y) | ΔPE (%) | CO2 (tCO2/y) | ΔCO2 (%) |
|---|---|---|---|---|---|---|---|---|
| 1000 | 1333 | 240 | 5508 | 185 | 3520 | - | 782 | - |
| 1000 | 1666 | 144 | 5109 | 161 | 3061 | 13.0 | 680 | 13.0 |
| 1250 | 1666 | 144 | 4941 | 163 | 3105 | 11.8 | 690 | 11.8 |
| 1250 | 2000 | 120 | 4546 | 165 | 3145 | 10.7 | 699 | 10.6 |
| 1500 | 2000 | 108 | 4968 | 163 | 3103 | 11.8 | 689 | 11.9 |
| 2000 | 2666 | 84 | 4129 | 175 | 3339 | 5.1 | 742 | 5.1 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Calise, F.; Eicker, U.; Schumacher, J.; Vicidomini, M. Wastewater Treatment Plant: Modelling and Validation of an Activated Sludge Process. Energies 2020, 13, 3925. https://doi.org/10.3390/en13153925
Calise F, Eicker U, Schumacher J, Vicidomini M. Wastewater Treatment Plant: Modelling and Validation of an Activated Sludge Process. Energies. 2020; 13(15):3925. https://doi.org/10.3390/en13153925
Chicago/Turabian StyleCalise, Francesco, Ursula Eicker, Juergen Schumacher, and Maria Vicidomini. 2020. "Wastewater Treatment Plant: Modelling and Validation of an Activated Sludge Process" Energies 13, no. 15: 3925. https://doi.org/10.3390/en13153925
APA StyleCalise, F., Eicker, U., Schumacher, J., & Vicidomini, M. (2020). Wastewater Treatment Plant: Modelling and Validation of an Activated Sludge Process. Energies, 13(15), 3925. https://doi.org/10.3390/en13153925








