Structural, Textural, and Catalytic Properties of Ni-CexZr1−xO2 Catalysts for Methane Dry Reforming Prepared by Continuous Synthesis in Supercritical Isopropanol
Abstract
1. Introduction
2. Materials and Methods
3. Results
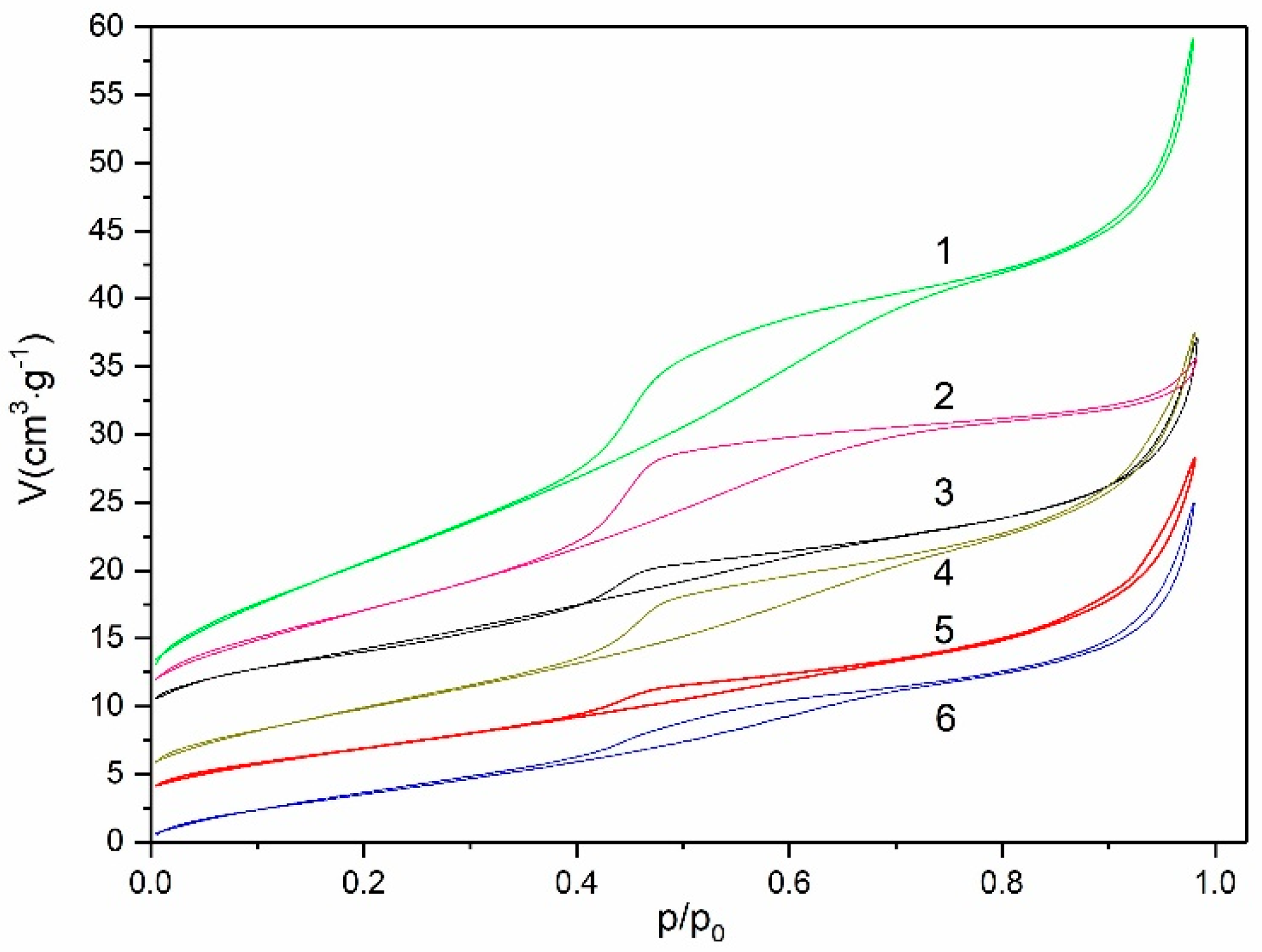
3.1. Textural Properties of Supports and Catalysts
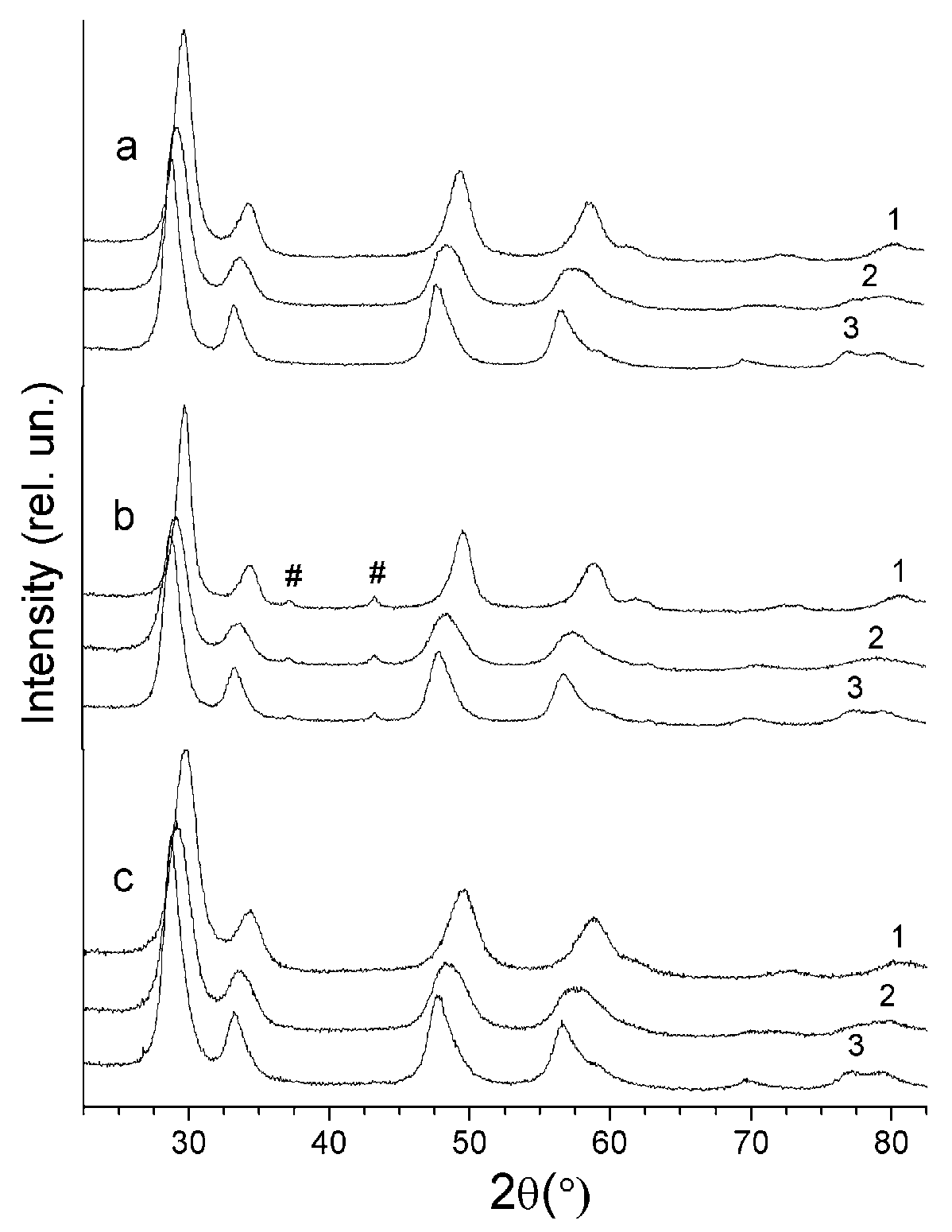
3.2. Structural Properties of Supports and Catalysts
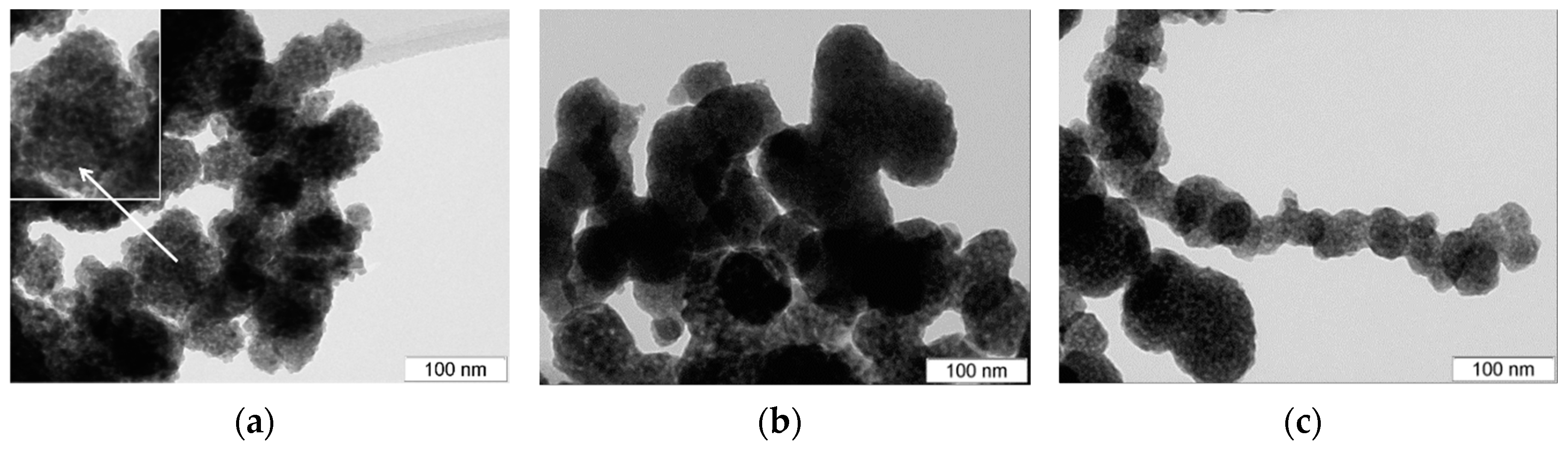
3.3. TEM Analysis
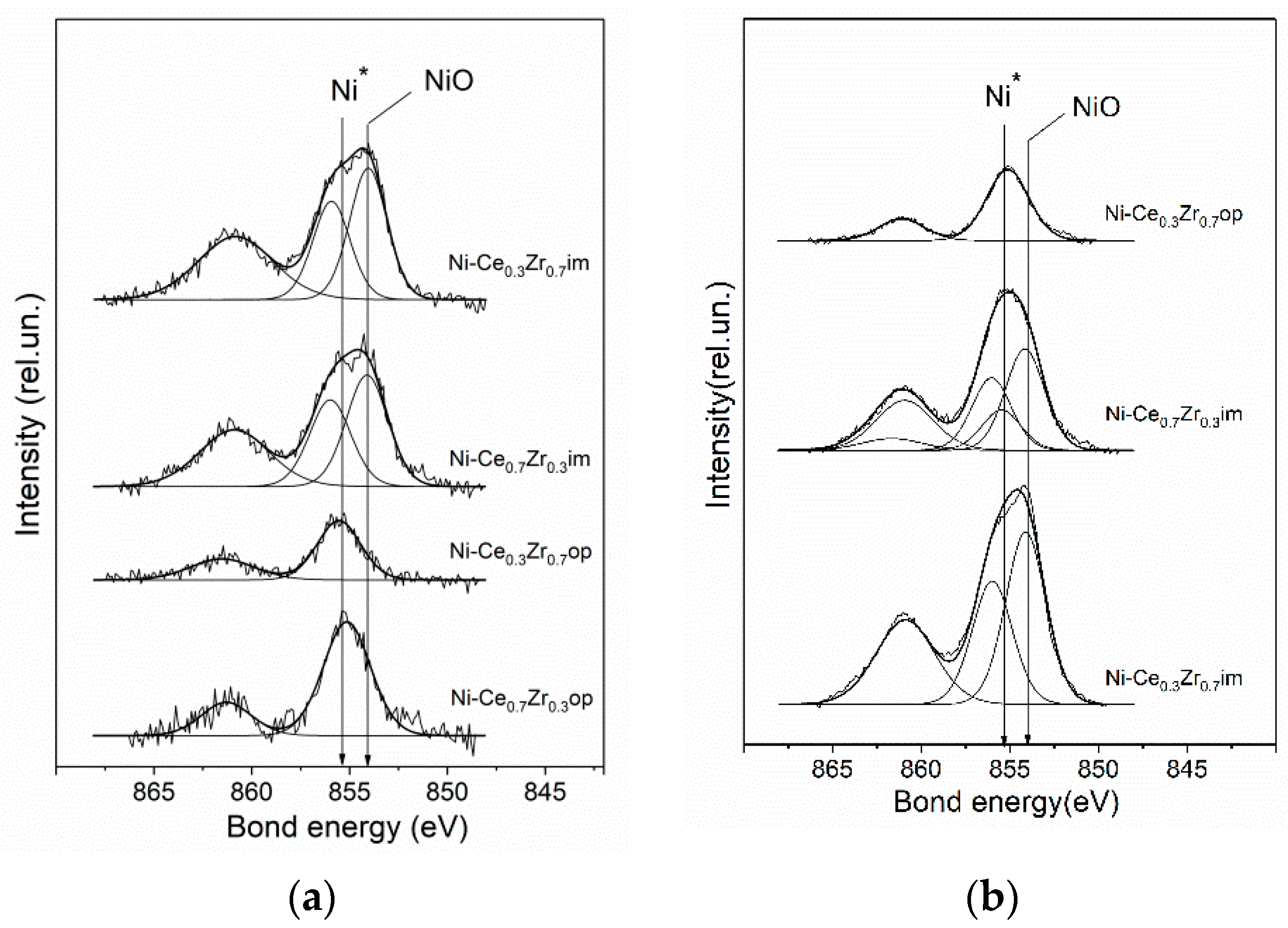
3.4. XPS Analysis
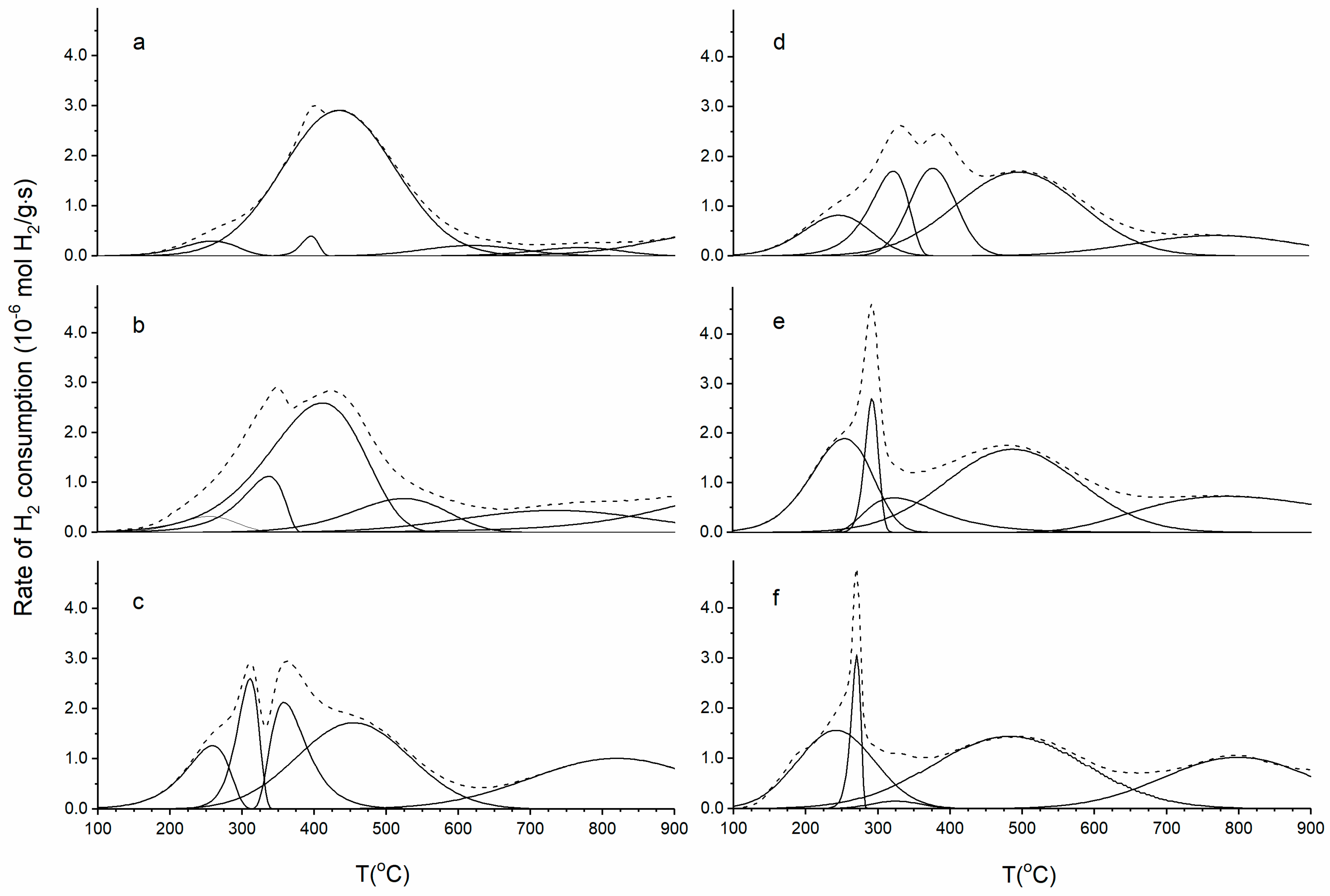
3.5. H2-TPR Analysis
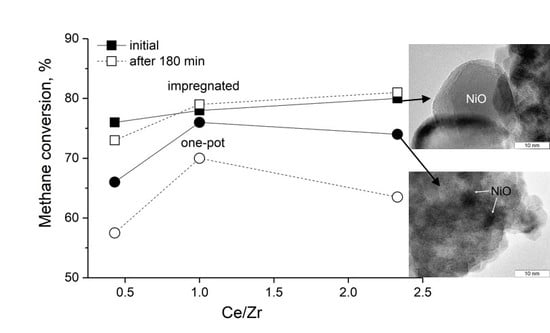
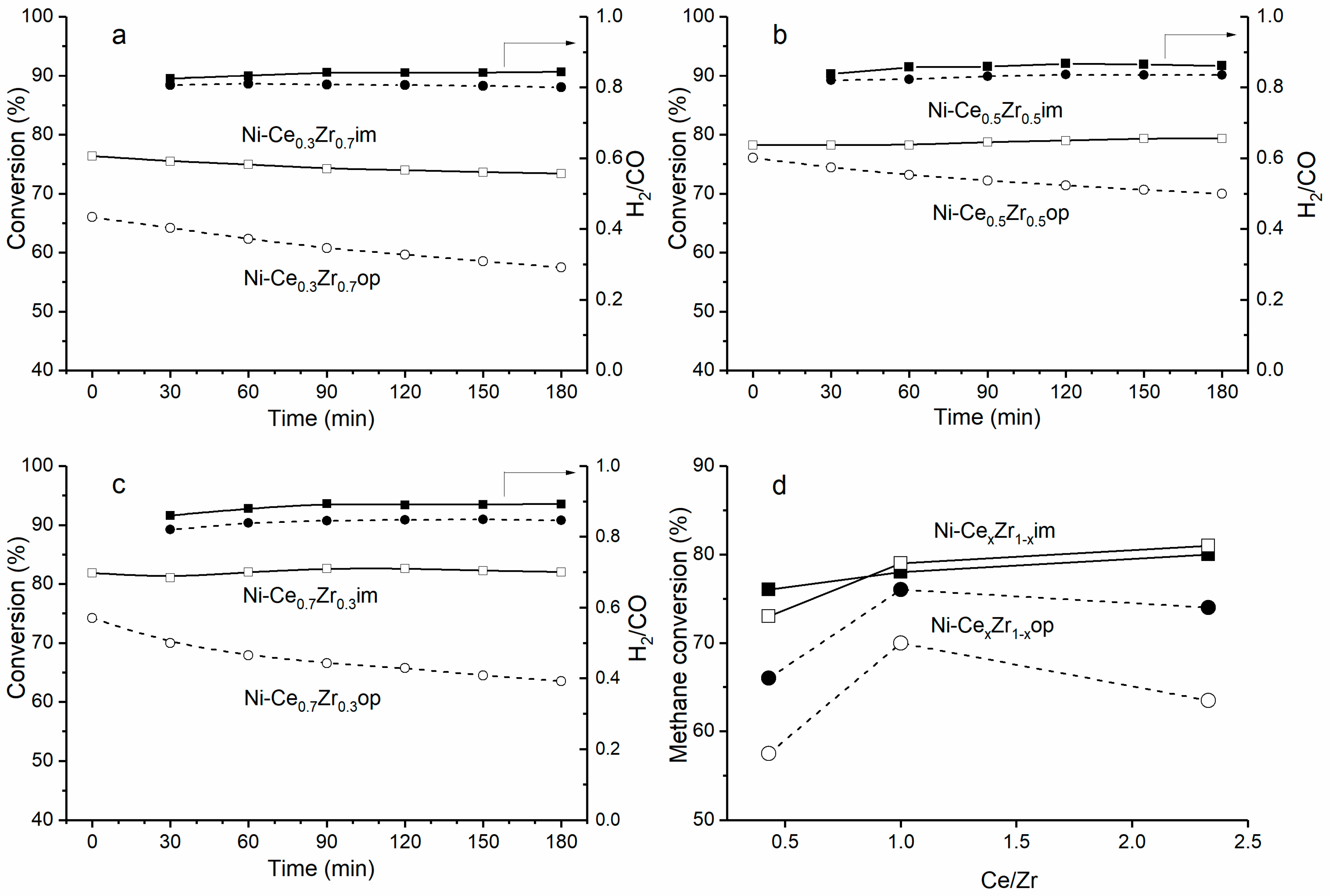
3.6. Catalytic Properties
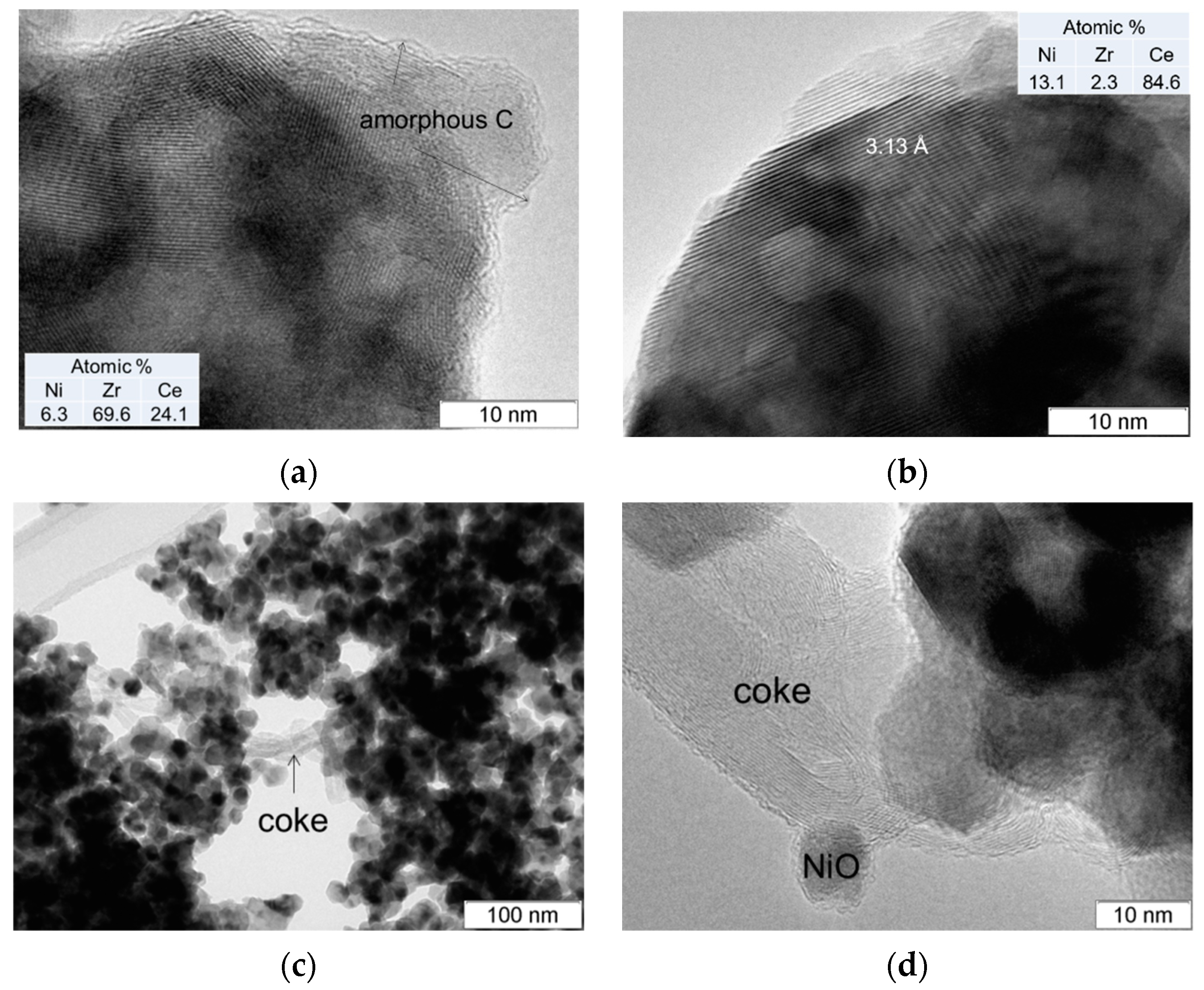
3.7. TEM Analysis of Used Catalysts
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kumar, N.; Shojaee, M.; Spivey, J. Catalytic bi-reforming of methane: From greenhouse gases to syngas. Curr. Opin. Chem. Eng. 2015, 9, 8–15. [Google Scholar] [CrossRef]
- Jang, W.-J.; Shim, J.-O.; Kim, H.-M.; Yoo, S.-Y.; Roh, H.-S. A review on dry reforming of methane in aspect of catalytic properties. Catal. Today 2019, 324, 15–26. [Google Scholar] [CrossRef]
- Montoya, J.A. Methane reforming with CO2 over Ni/ZrO2–CeO2 catalysts prepared by sol–gel. Catal. Today 2000, 63, 71–85. [Google Scholar] [CrossRef]
- Chen, J.; Wu, Q.; Zhang, J. Effect of preparation methods on structure and performance of Ni/Ce0.75Zr0.25O2 catalysts for CH4–CO2 reforming. Fuel 2008, 87, 2901–2907. [Google Scholar] [CrossRef]
- Kambolis, A.; Matralis, H.; Trovarelli, A.; Papadopoulou, C. Ni/CeO2-ZrO2 catalysts for the dry reforming of methane. Appl. Catal. A Gen. 2010, 377, 16–26. [Google Scholar] [CrossRef]
- Romero-Nunez, A.; Diaz, G. High oxygen storage capacity and enhanced catalytic performance of NiO/NixCe1−xO2−δ nanorods: Synergy between Ni-doping and 1D morphology. RSC Adv. 2015, 5, 54571–54579. [Google Scholar] [CrossRef]
- Wolfbeisser, A.; Sophiphun, O.; Bernardi, J.; Wittayakun, J.; Föttinger, K.; Rupprechter, G. Methane dry reforming over ceria-zirconia supported Ni catalysts. Catal. Today 2016, 277, 234–245. [Google Scholar] [CrossRef]
- Roh, H.-S.; Potdar, H.; Jun, K.-W.; Kim, J.-W.; Oh, Y.-S. Carbon dioxide reforming of methane over Ni incorporated into Ce–ZrO2 catalysts. Appl. Catal. A Gen. 2004, 276, 231–239. [Google Scholar] [CrossRef]
- Kumar, P.; Sun, Y.; Idem, R.O. Nickel-Based Ceria, Zirconia, and Ceria–Zirconia Catalytic Systems for Low-Temperature Carbon Dioxide Reforming of Methane. Energy Fuels 2007, 21, 3113–3123. [Google Scholar] [CrossRef]
- Makri, M.; Vasiliades, M.A.; Petallidou, K.; Efstathiou, A.M. Effect of support composition on the origin and reactivity of carbon formed during dry reforming of methane over 5wt% Ni/Ce1−xMxO2−δ (M=Zr4+, Pr3+) catalysts. Catal. Today 2016, 259, 150–164. [Google Scholar] [CrossRef]
- Zonetti, P.C.; Letichevsky, S.; Gaspar, A.B.; Sousa-Aguiar, E.F.; Appel, L.G. The NixCe0.75Zr0.25−xO2 solid solution and the RWGS. Appl. Catal. A Gen. 2014, 475, 48–54. [Google Scholar] [CrossRef]
- Bacani, R.; Toscani, L.M.; Martins, T.S.; Fantini, M.C.A.; Lamas, D.G.; Larrondo, S.A. Synthesis and characterization of mesoporous NiO2/ZrO2-CeO2 catalysts for total methane conversion. Ceram. Int. 2017, 43, 7851–7860. [Google Scholar] [CrossRef]
- Barrio, L.; Kubacka, A.; Zhou, G.; Estrella, M.; Martínez-Arias, A.; Hanson, J.C.; Fernández-García, M.; Rodriguez, J.A. Unusual Physical and Chemical Properties of Ni in Ce1−xNixO2−y oxides: Structural Characterization and Catalytic Activity for the Water Gas Shift Reaction. J. Phys. Chem. C 2010, 114, 12689–12697. [Google Scholar] [CrossRef]
- Adachi, G.-Y.; Masui, T. Synthesis and modification of ceria-based materials. In Catalysis by Ceria and Related Materials; Trovarelli, A., Ed.; Imperial College Press: London, UK, 2002; pp. 51–83. [Google Scholar]
- Masui, T.; Fujiwara, K.; Peng, Y.; Sakata, T.; Machida, K.-I.; Mori, H.; Adachi, G.-Y. Characterization and catalytic properties of CeO2–ZrO2 ultrafine particles prepared by the microemulsion method. J. Alloy. Compd. 1998, 269, 116–122. [Google Scholar] [CrossRef]
- Trovarelli, A.; Zamar, F.; Llorca, J.; De Leitenburg, C.; Dolcetti, G.; Kiss, J.T. Nanophase Fluorite-Structured CeO2–ZrO2 Catalysts Prepared by High-Energy Mechanical Milling. J. Catal. 1997, 169, 490–502. [Google Scholar] [CrossRef]
- Aymonier, C.; Philippot, G.; Erriguible, A.; Marre, S. Playing with chemistry in supercritical solvents and the associated technologies for advanced materials by design. J. Supercrit. Fluids 2018, 134, 184–196. [Google Scholar] [CrossRef]
- Darr, J.; Zhang, J.; Makwana, N.M.; Weng, X. Continuous Hydrothermal Synthesis of Inorganic Nanoparticles: Applications and Future Directions. Chem. Rev. 2017, 117, 11125–11238. [Google Scholar] [CrossRef]
- Adschiri, T.; Hakuta, Y.; Arai, K. Hydrothermal Synthesis of Metal Oxide Fine Particles at Supercritical Conditions. Ind. Eng. Chem. Res. 2000, 39, 4901–4907. [Google Scholar] [CrossRef]
- Cabañas, A.; Darr, J.A.; Lester, E.; Poliakoff, M. Continuous hydrothermal synthesis of inorganic materials in a near-critical water flow reactor; the one-step synthesis of nano-particulate Ce1−xZrxO2 (x = 0–1) solid solutions. J. Mater. Chem. 2001, 11, 561–568. [Google Scholar] [CrossRef]
- Kim, J.-R.; Myeong, W.-J.; Ihm, S.-K. Characteristics in oxygen storage capacity of ceria–zirconia mixed oxides prepared by continuous hydrothermal synthesis in supercritical water. Appl. Catal. B Environ. 2007, 71, 57–63. [Google Scholar] [CrossRef]
- Kim, J.-R.; Myeong, W.-J.; Ihm, S.-K. Characteristics of CeO2–ZrO2 mixed oxide prepared by continuous hydrothermal synthesis in supercritical water as support of Rh catalyst for catalytic reduction of NO by CO. J. Catal. 2009, 263, 123–133. [Google Scholar] [CrossRef]
- Zhang, J.; Ohara, S.; Umetsu, M.; Naka, T.; Hatakeyama, Y.; Adschiri, T. Colloidal Ceria Nanocrystals: A Tailor-Made Crystal Morphology in Supercritical Water. Adv. Mater. 2007, 19, 203–206. [Google Scholar] [CrossRef]
- Kim, J.; Park, Y.-S.; Veriansyah, B.; Kim, J.-D.; Lee, Y.-W. Continuous Synthesis of Surface-Modified Metal Oxide Nanoparticles Using Supercritical Methanol for Highly Stabilized Nanofluids. Chem. Mater. 2008, 20, 6301–6303. [Google Scholar] [CrossRef]
- Veriansyah, B.; Park, H.; Kim, J.-D.; Min, B.K.; Shin, Y.H.; Lee, Y.-W.; Kim, J. Characterization of surface-modified ceria oxide nanoparticles synthesized continuously in supercritical methanol. J. Supercrit. Fluids 2009, 50, 283–291. [Google Scholar] [CrossRef]
- Wang, P.; Kobiro, K. Synthetic versatility of nanoparticles: A new, rapid, one-pot, single-step synthetic approach to spherical mesoporous (metal) oxide nanoparticles using supercritical alcohols. Pure Appl. Chem. 2014, 86, 785–800. [Google Scholar] [CrossRef]
- Slostowski, C.; Marre, S.; Babot, O.; Toupance, T.; Aymonier, C. Near- and Supercritical Alcohols as Solvents and Surface Modifiers for the Continuous Synthesis of Cerium Oxide Nanoparticles. Langmuir 2012, 28, 16656–16663. [Google Scholar] [CrossRef] [PubMed]
- Slostowski, C.; Marre, S.; Babot, O.; Toupance, T.; Aymonier, C. Effect of Thermal Treatment on the Textural Properties of CeO2Powders Synthesized in Near- and Supercritical Alcohols. ChemPhysChem 2015, 16, 3493–3499. [Google Scholar] [CrossRef]
- Smirnova, M.Y.; Pavlova, S.N.; Krieger, T.A.; Bespalko, Y.N.; Anikeev, V.I.; Chesalov, Y.A.; Kaichev, V.V.; Mezentseva, N.V.; Sadykov, V.A. The synthesis of Ce1–xZrxO2 oxides in supercritical alcohols and catalysts for carbon dioxide reforming of methane on their basis. Russ. J. Phys. Chem. B 2017, 11, 1312–1321. [Google Scholar] [CrossRef]
- Smirnova, M.Y.; Bobin, A.S.; Pavlova, S.N.; Ishchenko, A.V.; Selivanova, A.; Kaichev, V.; Cherepanova, S.V.; Krieger, T.A.; Arapova, M.V.; Roger, A.-C.; et al. Methane dry reforming over Ni catalysts supported on Ce–Zr oxides prepared by a route involving supercritical fluids. Open Chem. 2017, 15, 412–425. [Google Scholar] [CrossRef]
- Anikeev, V.I. Hydrothermal synthesis of metal oxide nano- and microparticles in supercritical water. Russ. J. Phys. Chem. A 2011, 85, 377–382. [Google Scholar] [CrossRef]
- Lamas, D.G.; Lascalea, G.E.; Juárez, R.E.; Djurado, E.; Pérez, L.; De Reca, N.E.W. Metastable forms of the tetragonal phase in compositionally homogeneous, nanocrystalline zirconia–ceria powders synthesised by gel-combustion. J. Mater. Chem. 2003, 13, 904–910. [Google Scholar] [CrossRef]
- Varez, A.; Garcia-Gonzalez, E.; Jolly, J.; Sanz, J. Structural characterization of Ce1−xZrxO2 (0≤ x≤ 1) samples prepared at 1650 °C by solid state reaction: A combined TEM and XRD study. J. Eur. Ceram. Soc. 2007, 27, 3677–3682. [Google Scholar] [CrossRef]
- Scofield, J. Hartree-Slater subshell photoionization cross-sections at 1254 and 1487 eV. J. Electron Spectrosc. Relat. Phenom. 1976, 8, 129–137. [Google Scholar] [CrossRef]
- Sing, K.S.; Williams, R.T. Physisorption Hysteresis Loops and the Characterization of Nanoporous Materials. Adsorpt. Sci. Technol. 2004, 22, 773–782. [Google Scholar] [CrossRef]
- Lamas, D.; Fuentes, R.O.; Fábregas, I.O.; De Rapp, M.E.F.; Lascalea, G.E.; Casanova, J.R.; De Reca, N.E.W.; Craievich, A.F. Synchrotron X-ray diffraction study of the tetragonal–cubic phase boundary of nanocrystalline ZrO 2 –CeO 2 synthesized by a gel-combustion process. J. Appl. Crystallogr. 2005, 38, 867–873. [Google Scholar] [CrossRef]
- Yashima, M.; Arashi, H.; Kakihana, M.; Yoshimura, M. Raman Scattering Study of Cubic-Tetragonal Phase Transition in Zr1−xCexO2 Solid Solution. J. Am. Ceram. Soc. 1994, 77, 1067–1071. [Google Scholar] [CrossRef]
- Kim, D.-J.; Jung, H.-J.; Yang, I.-S. Raman Spectroscopy of Tetragonal Zirconia Solid Solutions. J. Am. Ceram. Soc. 1993, 76, 2106–2108. [Google Scholar] [CrossRef]
- Mahammadunnisa, S.; Manoj Kumar Reddy, P.; Lingaiah, N.; Subrahmanyam, C. NiO/Ce1−xNixO2−δ as an alternative to noble metal catalysts for CO oxidation. Catal. Sci. Technol. 2013, 3, 730–736. [Google Scholar] [CrossRef]
- Koubaissy, B.; Pietraszek, A.; Roger, A.-C.; Kiennemann, A. CO2 reforming of methane over Ce-Zr-Ni-Me mixed catalysts. Catal. Today 2010, 157, 436–439. [Google Scholar] [CrossRef]
- Alders, D.; Voogt, F.C.; Hibma, T.; Sawatzky, G.A. Nonlocal screening effects in 2 p x-ray photoemission spectroscopy of NiO (100). Phys. Rev. B 1996, 54, 7716–7719. [Google Scholar] [CrossRef]
- Carley, A.; Jackson, S.; O’Shea, J.; Roberts, M. The formation and characterisation of Ni3+—an X-ray photoelectron spectroscopic investigation of potassium-doped Ni(110)–O. Surf. Sci. 1999, 440, L868–L874. [Google Scholar] [CrossRef]
- Kosova, N.V.; Devyatkina, E.; Kaichev, V. Mixed layered Ni–Mn–Co hydroxides: Crystal structure, electronic state of ions, and thermal decomposition. J. Power Sources 2007, 174, 735–740. [Google Scholar] [CrossRef]
- Kaichev, V.; Teschner, D.; Saraev, A.A.; Kosolobov, S.; Gladky, A.; Prosvirin, I.P.; Rudina, N.; Ayupov, A.; Blume, R.; Havecker, M.; et al. Evolution of self-sustained kinetic oscillations in the catalytic oxidation of propane over a nickel foil. J. Catal. 2016, 334, 23–33. [Google Scholar] [CrossRef]
- Daza, C.; Gamba, O.A.; Hernández, Y.; Centeno, M.Á.; Mondragón, F.; Moreno, S.; Molina, R. High-Stable Mesoporous Ni-Ce/Clay Catalysts for Syngas Production. Catal. Lett. 2011, 141, 1037–1046. [Google Scholar] [CrossRef]
- Wrobel, G.; Sohier, M.; D’Huysser, A.; Bonnelle, J.; Marcq, J. Hydrogenation catalysts based on nickel and rare earth oxides. Appl. Catal. A Gen. 1993, 101, 73–93. [Google Scholar] [CrossRef]
- Bernal, S.; Calvino, J.J.; Cauqui, M.A.; Gatica, J.; Cartes, C.L.; Pérez-Omil, J.A.; Pintado, J. Some contributions of electron microscopy to the characterisation of the strong metal–support interaction effect. Catal. Today 2003, 77, 385–406. [Google Scholar] [CrossRef]
- Hosokawa, S.; Imamura, S.; Iwamoto, S.; Inoue, M. Synthesis of CeO2–ZrO2 solid solution by glycothermal method and its oxygen release capacity. J. Eur. Ceram. Soc. 2011, 31, 2463–2470. [Google Scholar] [CrossRef]
- Pojanavaraphan, C.; Luengnaruemitchai, A.; Gulari, E. Effect of catalyst preparation on Au/Ce1−xZrxO2 and Au–Cu/Ce1−xZrxO2 for steam reforming of methanol. Int. J. Hydrogen Energy 2013, 38, 1348–1362. [Google Scholar] [CrossRef]
- Zhang, G.; Zhao, Z.; Xu, J.; Zheng, J.; Liu, J.; Jiang, G.; Duan, A.; He, H. Comparative study on the preparation, characterization and catalytic performances of 3DOM Ce-based materials for the combustion of diesel soot. Appl. Catal. B Environ. 2011, 107, 302–315. [Google Scholar] [CrossRef]
- Wei, Y.; Liu, J.; Zhao, Z.; Duan, A.; Jiang, G. The catalysts of three-dimensionally ordered macroporous Ce1−xZrxO2-supported gold nanoparticles for soot combustion: The metal–support interaction. J. Catal. 2012, 287, 13–29. [Google Scholar] [CrossRef]
- Tang, C.; Li, J.; Yao, X.; Sun, J.; Cao, Y.; Zhang, L.; Gao, F.; Deng, Y.; Dong, L. Mesoporous NiO–CeO2 catalysts for CO oxidation: Nickel content effect and mechanism aspect. Appl. Catal. A Gen. 2015, 494, 77–86. [Google Scholar] [CrossRef]
- Pino, L.; Vita, A.; Cipitì, F.; Laganà, M.; Recupero, V. Catalytic Performance of Ce1−xNixO2 Catalysts for Propane Oxidative Steam Reforming. Catal. Lett. 2007, 122, 121–130. [Google Scholar] [CrossRef]
- Zhang, T.; Amiridis, M.D. Hydrogen production via the direct cracking of methane over silica-supported nickel catalysts. Appl. Catal. A Gen. 1998, 167, 161–172. [Google Scholar] [CrossRef]
- San-José-Alonso, D.; Juan-Juan, J.; Illán-Gómez, M.; Román-Martínez, M. Ni, Co and bimetallic Ni–Co catalysts for the dry reforming of methane. Appl. Catal. A Gen. 2009, 371, 54–59. [Google Scholar] [CrossRef]
- Villacampa, J.I.; Royo, C.; Romeo, E.; Montoya, J.A.; Del Angel, P.; Monzón, A. Catalytic decomposition of methane over Ni-Al2O3 coprecipitated catalysts: Reaction and regeneration studies. Appl. Catal. A Gen. 2003, 252, 363–383. [Google Scholar] [CrossRef]
- Bradford, M.C.J.; Vannice, M.A. CO2 Reforming of CH4. Catal. Rev. 1999, 41, 1–42. [Google Scholar] [CrossRef]
- Matte, L.P.; Kilian, A.S.; Luza, L.; Alves, M.D.C.; Morais, J.; Baptista, D.L.; Dupont, J.; Bernardi, F. Influence of the CeO2 Support on the Reduction Properties of Cu/CeO2 and Ni/CeO2 Nanoparticles. J. Phys. Chem. C 2015, 119, 26459–26470. [Google Scholar] [CrossRef]
- Caballero, A.; Holgado, J.P.; Gonzalez-Delacruz, V.M.; Habas, S.; Herranz, T.; Salmeron, M. In situ spectroscopic detection ofSMSI effect in a Ni/CeO2 system: Hydrogen-induced burial and dig out of metallic nickel. Chem. Commun. 2010, 46, 1097–1099. [Google Scholar] [CrossRef]


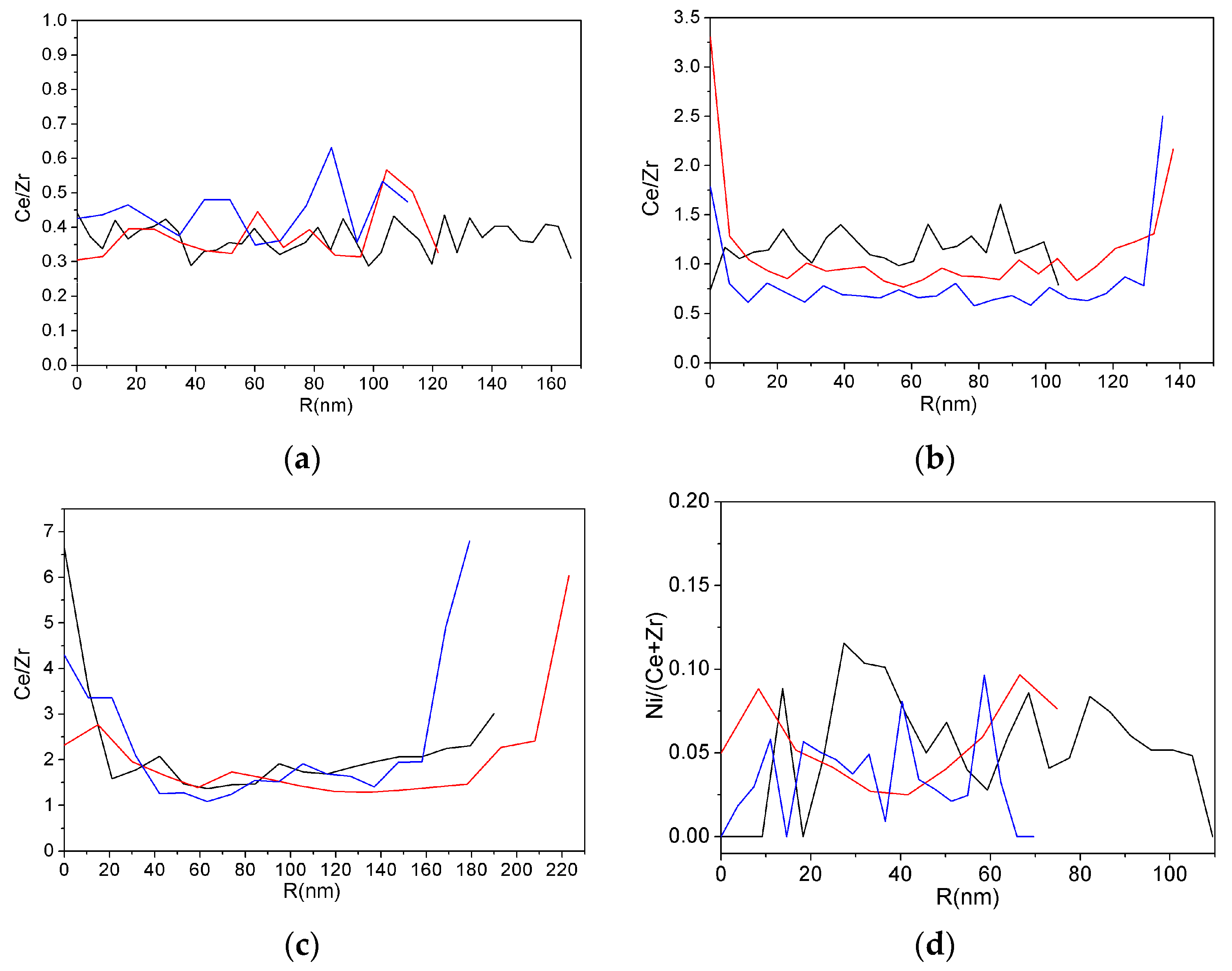
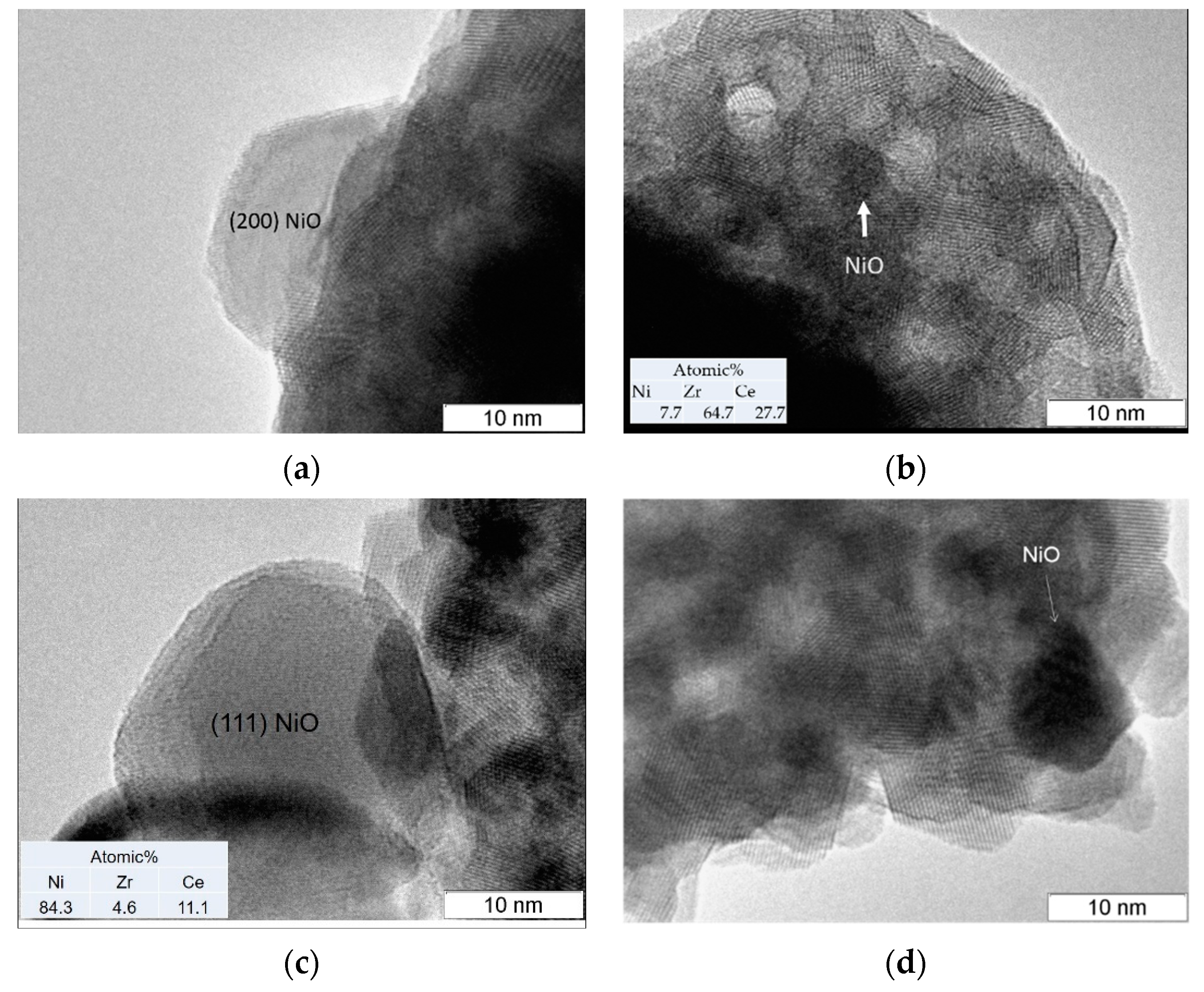
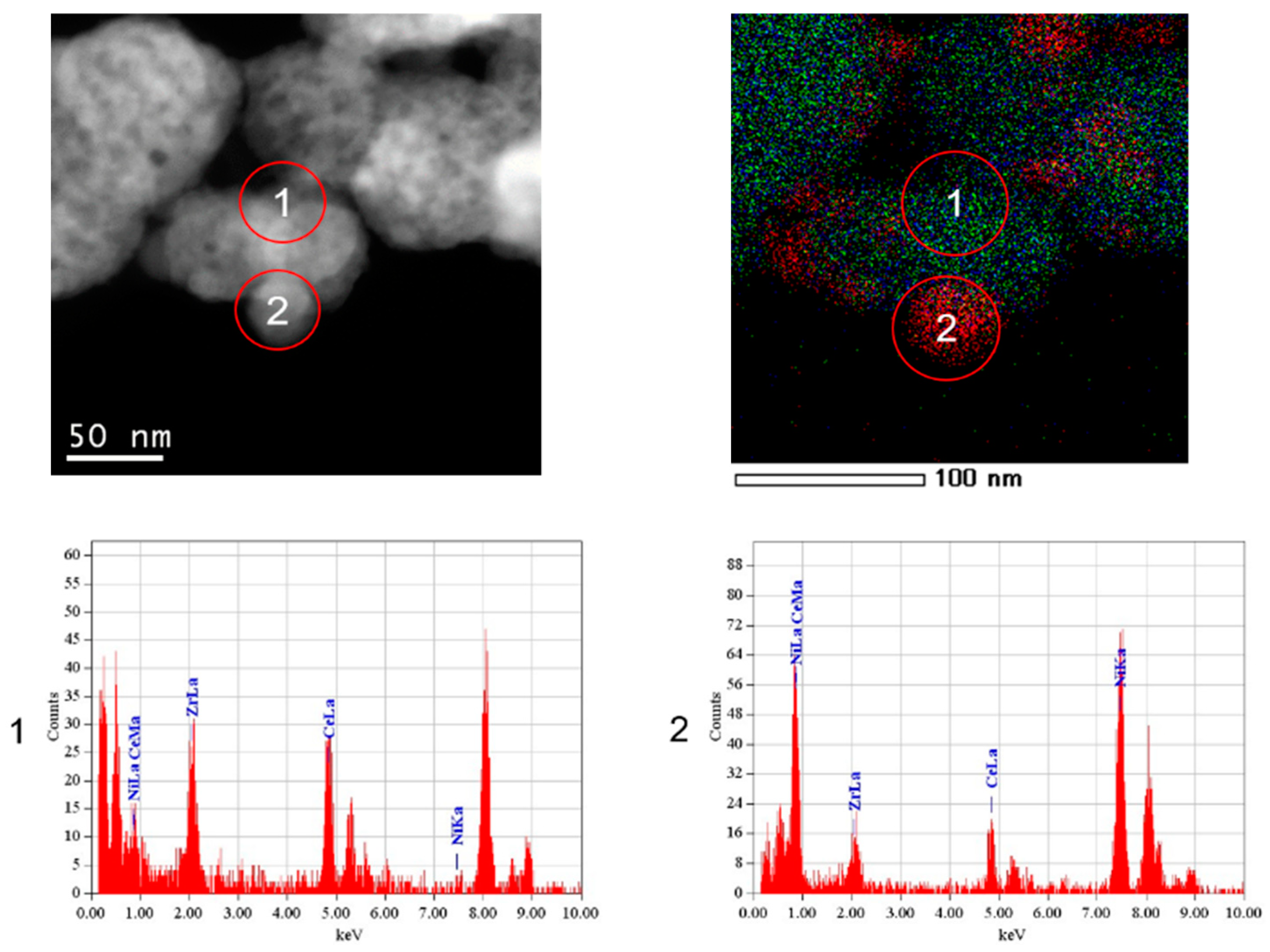
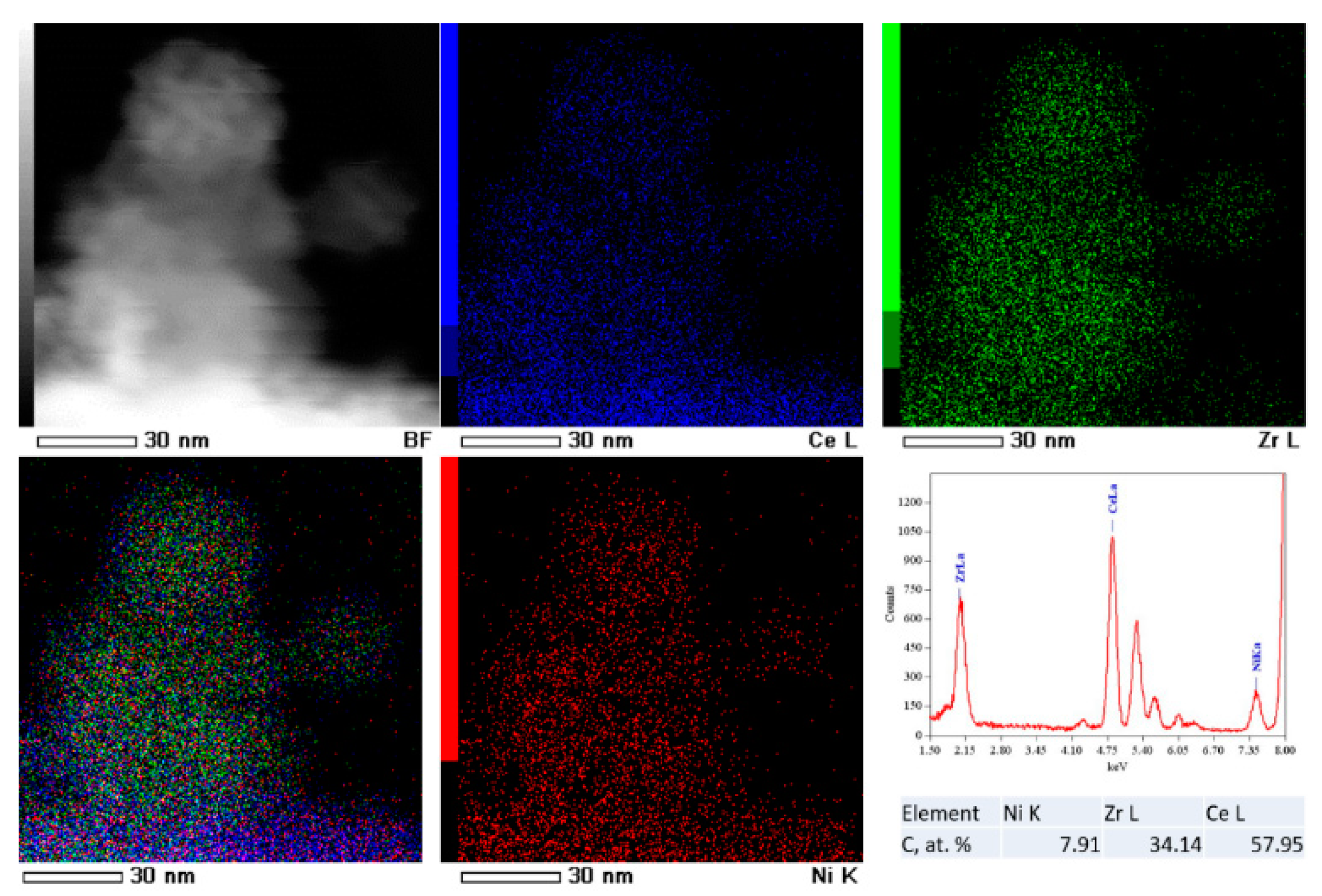

| Sample | SSA (m2∙g−1) | Vpore (cm3∙g−1) | Vmicropore (cm3∙g−1) | D 1 (nm) |
|---|---|---|---|---|
| Ce0.3Zr0.7 | 32 | 0.052 | 0.0013 | 9.9 |
| Ce0.5Zr0.5 | 42 | 0.047 | 0.0002 | 5.6 |
| Ce0.7Zr0.3 | 66 | 0.090 | 0.0033 | 7.5 |
| Ni-Ce0.3Zr0.7im | 20 | n.d. | n.d. | n.d. |
| Ni-Ce0.5Zr0.5im | 23 | n.d. | n.d. | n.d. |
| Ni-Ce0.7Zr0.3im | 50 | n.d. | n.d. | n.d. |
| Ni-Ce0.3Zr0.7op | 29 | 0.045 | 0 | 9.8 |
| Ni-Ce0.5Zr0.5op | 25 | 0.045 | 0.0011 | 16.9 |
| Ni-Ce0.7Zr0.3op | 36 | 0.060 | 0.0018 | 8.9 |
| Sample | a (Å) | c (Å) | c/a’ 1 | V | D (nm) | N(Ce) 2 | W 3 (%) |
|---|---|---|---|---|---|---|---|
| Ce0.3Zr0.7 | 3.686(7) | 5.291(1) | 1.015 | 71.9 | 14 | 0.34 | 100 |
| Ce0.5Zr0.5 | 3.754(1) | 5.335(2) | 1.005 | 75.19 | 14 | 0.59 | 93 |
| 3.674(3) | 5.27(1) | 1.014 | 71.16 | 10 | 0.28 | 4 | |
| 5.402(3) | - | - | 157.7 | 11 | 0.94 | 3 | |
| Ce0.7Zr0.3 | 3.780(1) | 5.372(2) | 1.005 | 76.75 | 14 | 0.71 | 86 |
| 5.41(1) | - | - | 158.3 | 14 | 0.97 | 14 | |
| Ni-Ce0.3Zr0.7op | 3.677(1) | 5.277(1) | 1.015 | 71.3 | 10 | 0.29 | 100 |
| Ni-Ce0.5Zr0.5op | 3.748(1) | 5.326(1) | 1.005 | 74.8 | 14 | 0.56 | 98 |
| 5.405(3) | - | - | 157.9 | 11 | 0.95 | 2 | |
| Ni-Ce0.7Zr0.3op | 3.78(2) | 5.36(7) | 1.003 | 76.6 | 14 | 0.70 | 86 |
| 5.410(1) | - | - | 158.3 | 13 | 0.97 | 14 |
| Catalyst | Ce/Zr | [Ni]/([Ce]+[Zr]) | C(NiO)/C(Ni*) |
|---|---|---|---|
| Ni-Ce0.3Zr0.7im | 0.33 | 0.19 | 100/0 |
| Ni-Ce0.3Zr0.7im reduced | 0.37 | 0.21 | 100/0 |
| Ni-Ce0.3Zr0.7op | 0.22 | 0.06 | 0/100 |
| Ni-Ce0.3Zr0.7op reduced | 0.27 | 0.05 | 0/100 |
| Ni-Ce0.7Zr0.3im | 2.7 | 0.19 | 100/0 |
| Ni-Ce0.7Zr0.3im reduced | 1.9 | 0.15 | 80/20 |
| Ni-Ce0.7Zr0.3op | 3.4 | 0.10 | 0/100 |
| Sample | H2 Consumption (mmol∙g−1) | ||||
|---|---|---|---|---|---|
| 100–300 °C | 300–700 °C | 100–700 °C | 100–900 °C | CeO2-ZrO21 | |
| Ce0.3Zr0.7 | 0 | 0.93 | 0.93 | 1.07 | |
| Ce0.5Zr0.5 | 0 | 0.94 | 0.94 | 1.40 | |
| Ce0.7Zr0.3 | 0 | 0.70 | 0.70 | 1.40 | |
| Ni-Ce0.3Zr0.7im | 0.08 | 1.59 | 1.67 | 1.83 | 0.98 |
| Ni-Ce0.5Zr0.5im | 0.08 | 1.46 | 1.75 | 2.04 | 1.19 |
| Ni-Ce0.7Zr0.3im | 0.25 | 1.41 | 1.66 | 2.22 | 1.37 |
| Ni-Ce0.3Zr0.7op | 0.24 | 1.59 | 1.83 | 2.09 | 1.24 |
| Ni-Ce0.5Zr0.5op | 0.68 | 1.23 | 1.9 | 2.38 | 1.53 |
| Ni-Ce0.7Zr0.3op | 0.64 | 0.97 | 1.7 | 2.17 | 1.32 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pavlova, S.; Smirnova, M.; Bobin, A.; Cherepanova, S.; Kaichev, V.; Ishchenko, A.; Selivanova, A.; Rogov, V.; Roger, A.-C.; Sadykov, V. Structural, Textural, and Catalytic Properties of Ni-CexZr1−xO2 Catalysts for Methane Dry Reforming Prepared by Continuous Synthesis in Supercritical Isopropanol. Energies 2020, 13, 3728. https://doi.org/10.3390/en13143728
Pavlova S, Smirnova M, Bobin A, Cherepanova S, Kaichev V, Ishchenko A, Selivanova A, Rogov V, Roger A-C, Sadykov V. Structural, Textural, and Catalytic Properties of Ni-CexZr1−xO2 Catalysts for Methane Dry Reforming Prepared by Continuous Synthesis in Supercritical Isopropanol. Energies. 2020; 13(14):3728. https://doi.org/10.3390/en13143728
Chicago/Turabian StylePavlova, Svetlana, Marina Smirnova, Aleksei Bobin, Svetlana Cherepanova, Vasily Kaichev, Arcady Ishchenko, Aleksandra Selivanova, Vladimir Rogov, Anne-Cécile Roger, and Vladislav Sadykov. 2020. "Structural, Textural, and Catalytic Properties of Ni-CexZr1−xO2 Catalysts for Methane Dry Reforming Prepared by Continuous Synthesis in Supercritical Isopropanol" Energies 13, no. 14: 3728. https://doi.org/10.3390/en13143728
APA StylePavlova, S., Smirnova, M., Bobin, A., Cherepanova, S., Kaichev, V., Ishchenko, A., Selivanova, A., Rogov, V., Roger, A.-C., & Sadykov, V. (2020). Structural, Textural, and Catalytic Properties of Ni-CexZr1−xO2 Catalysts for Methane Dry Reforming Prepared by Continuous Synthesis in Supercritical Isopropanol. Energies, 13(14), 3728. https://doi.org/10.3390/en13143728