Production of Hydrogen-Rich Gas by Oxidative Steam Reforming of Dimethoxymethane over CuO-CeO2/γ-Al2O3 Catalyst
Abstract
1. Introduction
2. Materials and Methods
3. Results
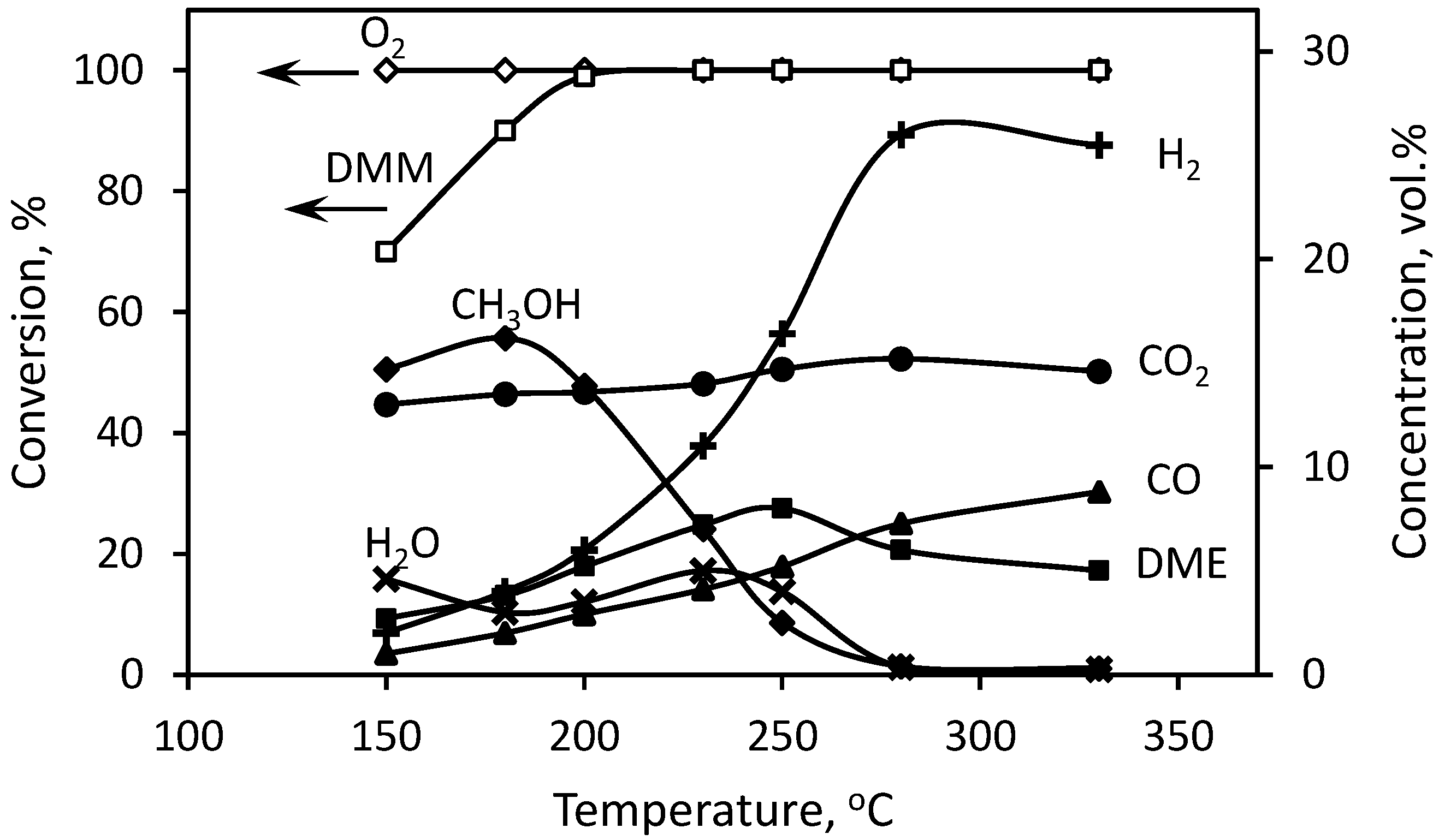
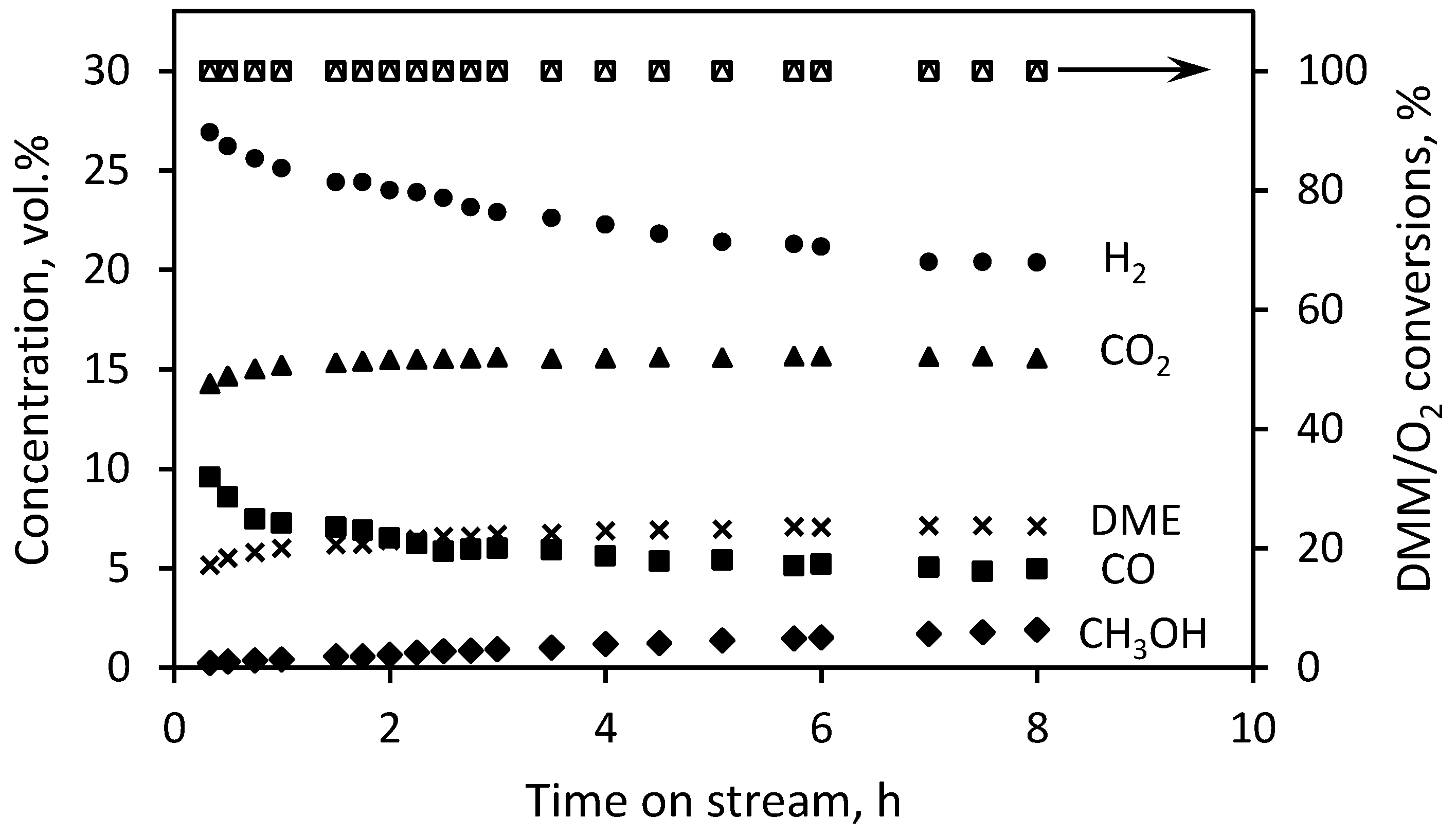
3.1. DMM Partial Oxidation
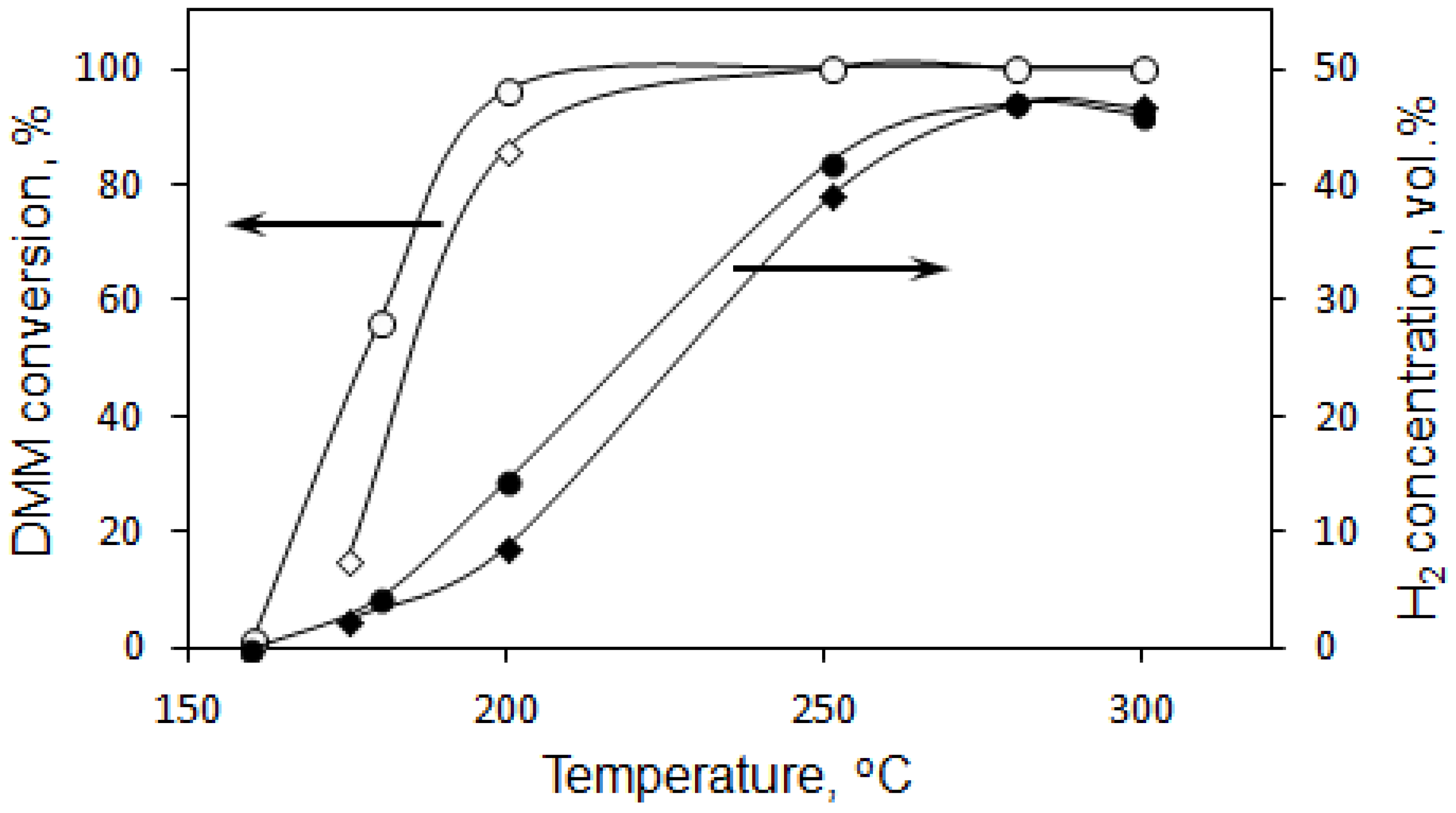
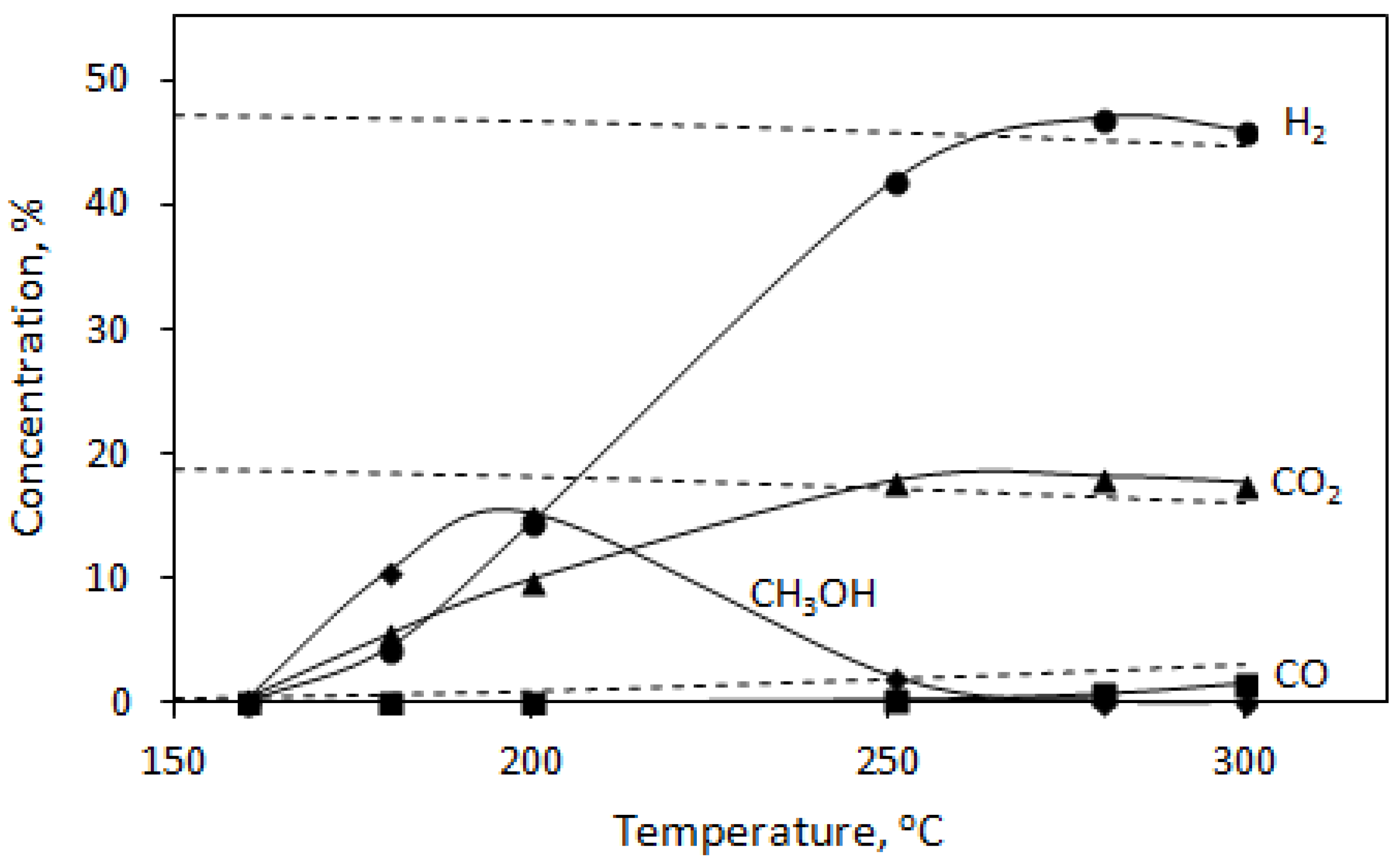
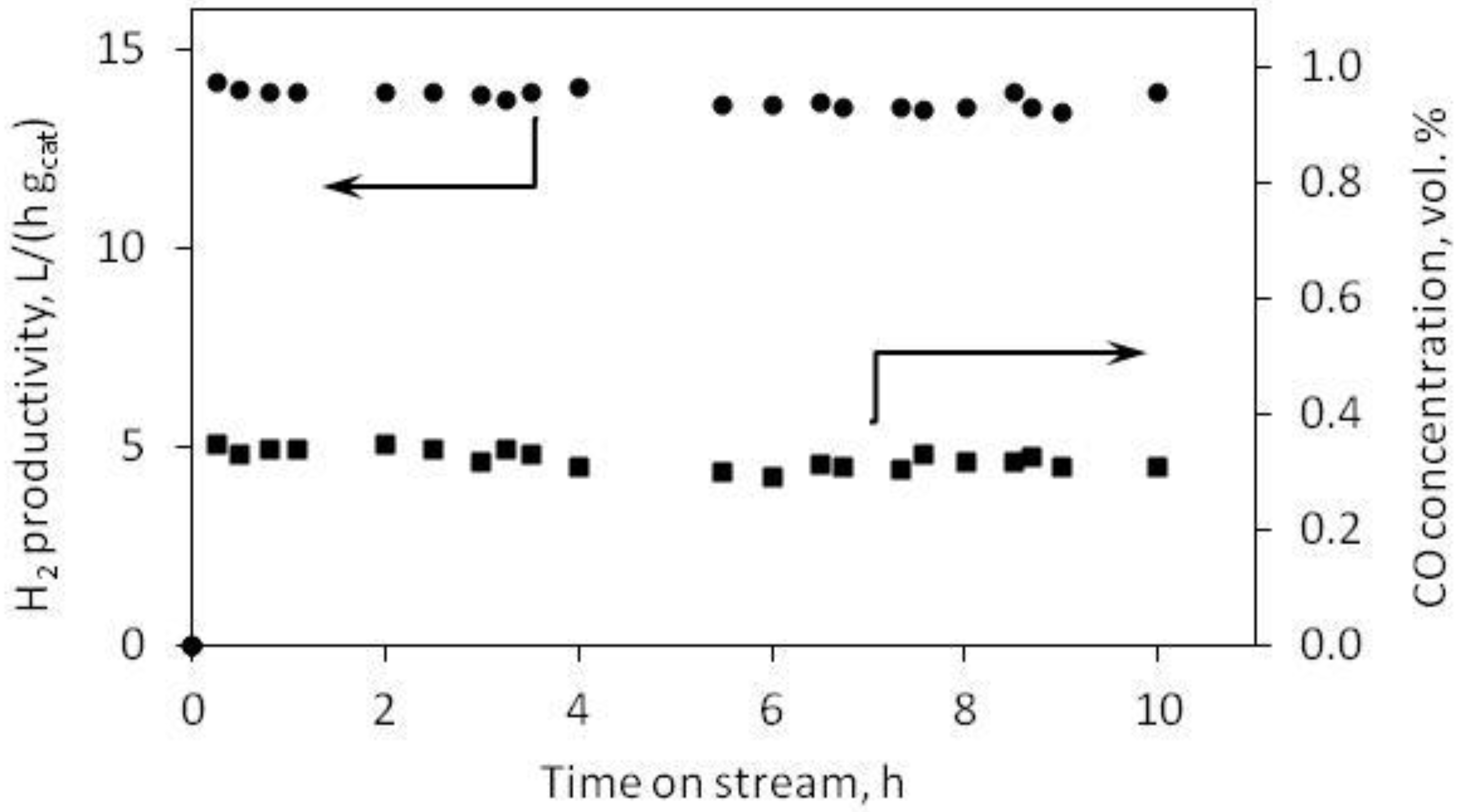
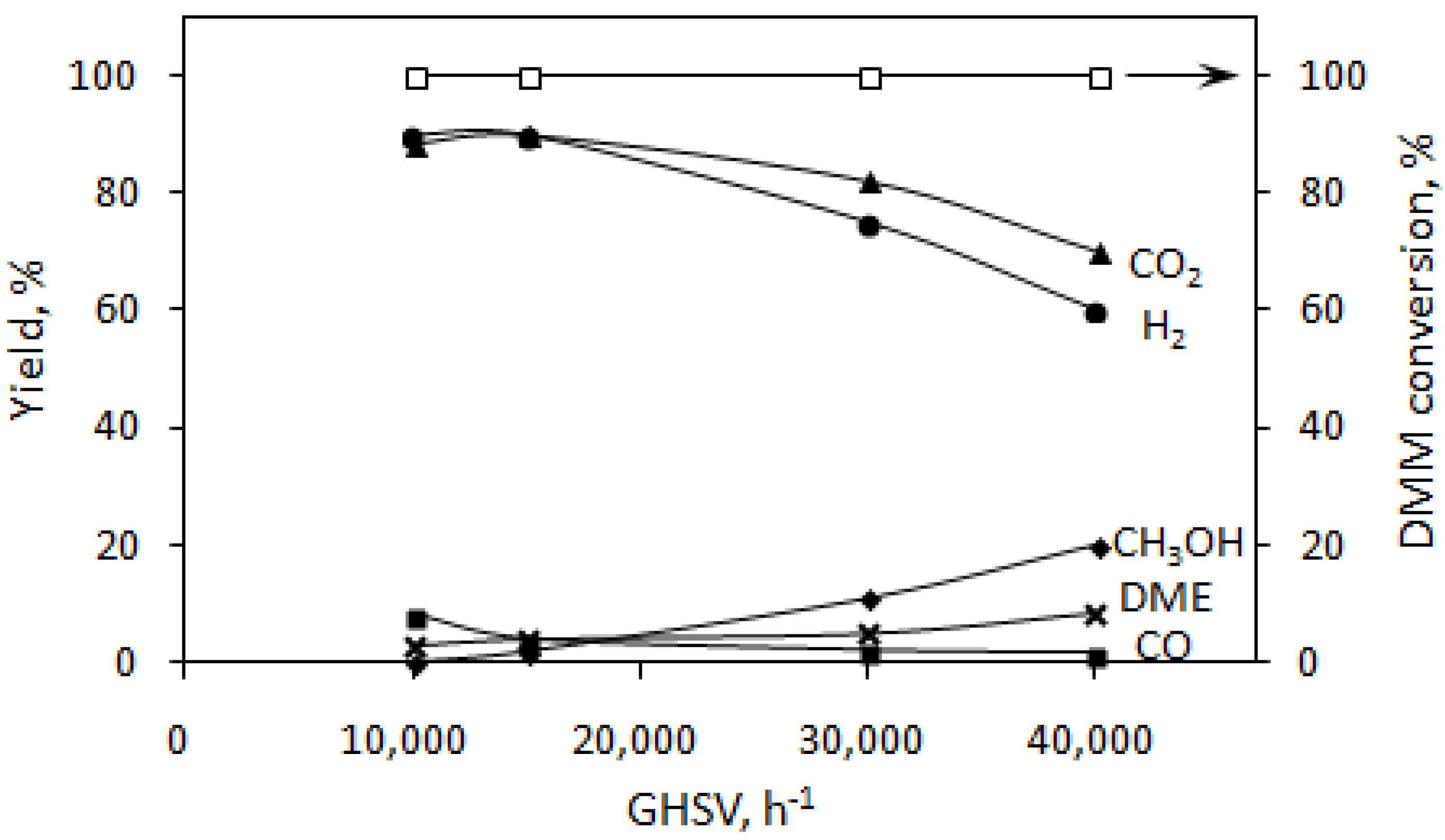
3.2. DMM Oxidative Steam Reforming
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Felseghi, R.-A.; Carcadea, E.; Raboaca, M.S.; Trufin, C.N.; Filote, C. Hydrogen fuel cell technology for the sustainable future of stationary applications. Energies 2019, 12, 4593. [Google Scholar] [CrossRef]
- Simon Araya, S.; Liso, V.; Cui, X.; Li, N.; Zhu, J.; Sahlin, S.L.; Jensen, S.H.; Nielsen, M.P.; Kær, S.K. A review of the methanol economy: The fuel cell route. Energies 2020, 13, 596. [Google Scholar] [CrossRef]
- Navarro, R.M.; Pena, M.A.; Fierro, J.L.G. Hydrogen Production Reactions from Carbon Feedstocks: Fossil Fuels and Biomass. Chem. Rev. 2007, 107, 3952–3991. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Li, X.; Gong, J. Catalytic Reforming of Oxygenates: State of the Art and Future Prospects. Chem. Rev. 2016, 116, 11529–11653. [Google Scholar] [CrossRef]
- Zyryanova, M.M.; Badmaev, S.D.; Belyaev, V.D.; Amosov, Y.I.; Snytnikov, P.V.; Kirillov, V.A.; Sobyanin, V.A. Catalytic reforming of hydrocarbon feedstocks into fuel for power generation units. Catal. Ind. 2013, 5, 312–317. [Google Scholar] [CrossRef]
- Sun, Q.; Auroux, A.; Shen, J. Surface acidity of niobium phosphate and steam reforming of dimethoxymethane over CuZnO/Al2O3–NbP complex catalysts. J. Catal. 2006, 244, 1–9. [Google Scholar] [CrossRef]
- Fu, Y.; Shen, J. Production of hydrogen by catalytic reforming of dimethoxymethane over bifunctional catalysts. J. Catal. 2007, 248, 101–110. [Google Scholar] [CrossRef]
- Badmaev, S.D.; Pechenkin, A.A.; Belyaev, V.D.; Ven’yaminov, S.A.; Snytnikov, P.V.; Sobyanin, V.A.; Parmon, V.N. Steam reforming of dimethoxymethane to hydrogen-rich gas for fuel cell feeding application. Dokl. Phys. Chem. 2013, 452, 251–253. [Google Scholar] [CrossRef]
- Pechenkin, A.A.; Badmaev, S.D.; Belyaev, V.D.; Sobyanin, V.A. Performance of bifunctional CuO–CeO2/γ-Al2O3 catalyst in dimethoxymethane steam reforming to hydrogen-rich gas for fuel cell feeding. Appl. Catal. B Environ. 2015, 166, 535–543. [Google Scholar] [CrossRef]
- Badmaev, S.D.; Pechenkin, A.A.; Belyaev, V.D.; Sobyanin, V.A. Hydrogen production by steam reforming of dimethoxymethane over bifunctional CuO-ZnO/γ-Al2O3 catalyst. Int. J. Hydrog. Energy 2015, 40, 14052–14057. [Google Scholar] [CrossRef]
- Pechenkin, A.A.; Badmaev, S.D.; Belyaev, V.D.; Sobyanin, V.A. Steam reforming of dimethoxymethane, methanol and dimethyl ether on CuO–ZnO/γ-Al2O3 catalyst. Kinet. Catal. 2017, 58, 577–584. [Google Scholar] [CrossRef]
- Badmaev, S.D.; Sobyanin, V.A. Steam reforming of dimethoxymethane to hydrogen-rich gas over bifunctional CuO-ZnO/η-Al2O3 catalyst-coated FeCrAl wire mesh. Catal. Today 2020, 348, 9–14. [Google Scholar] [CrossRef]
- Pechenkin, A.A.; Badmaev, S.D.; Belyaev, V.D.; Sobyanin, V.A. Production of hydrogen-rich gas by formic acid decomposition over CuO-CeO2/γ-Al2O3 catalyst. Energies 2019, 12, 3577. [Google Scholar] [CrossRef]
- Thattarathody, R.; Katheria, S.; Sheintuch, M. Methylal Steam Reforming with Pt/Al2O3, Ni/Al2O3, and Mixed Cu/ZnO/Al2O3 Catalysts. Ind. Eng. Chem. Res. 2019, 58, 21382–21391. [Google Scholar] [CrossRef]
- Thattarathody, R.; Sheintuch, M. Product composition and kinetics of methylal decomposition on alumina-supported Pt, Ni, and Rh catalysts. Ind. Eng. Chem. Res. 2019, 58, 11902–11909. [Google Scholar] [CrossRef]
- Badmaev, S.D.; Akhmetov, N.O.; Sobyanin, V.A. Partial oxidation of dimethoxymethane to syngas over supported noble metal catalysts. Top. Catal. 2020, 63, 196–202. [Google Scholar] [CrossRef]
- Kaichev, V.V.; Popova, G.Y.; Chesalov, Y.A.; Saraev, A.A.; Zemlyanov, D.Y.; Beloshapkin, S.A.; Knop-Gericke, A.; Schlögl, R.; Andrushkevich, T.V. Selective oxidation of methanol to form dimethoxymethane and methyl formate over a monolayer V2O5/TiO2 catalyst. J. Catal. 2014, 311, 59–70. [Google Scholar] [CrossRef]
- Sturt, N.R.M.; Terra, J.C.S.; Lara Sangiorge, D.; Oliveira, L.C.A.; Moura, F.C.C. Performance of niobium catalysts in a one-pot system for selective methanol conversion to dimethoxymethane under mild conditions. Fuel 2020, 262, 116417–116428. [Google Scholar] [CrossRef]
- Sun, R.; Delidovich, I.; Palkovits, R. Dimethoxymethane as a Cleaner Synthetic Fuel: Synthetic Methods, Catalysts, and Reaction Mechanism. ACS Catal. 2019, 9, 1298–1318. [Google Scholar] [CrossRef]
- Velu, S.; Suzuki, K.; Okazaki, M.; Kapoor, M.P.; Osaki, T.; Ohashi, F. Oxidative Steam Reforming of Methanol over CuZnAl(Zr)-Oxide Catalysts for the Selective Production of Hydrogen for Fuel Cells: Catalyst Characterization and Performance Evaluation. J. Catal. 2000, 194, 373–384. [Google Scholar] [CrossRef]
- Velu, S.; Suzuki, K. Selective Production of Hydrogen for Fuel Cells via Oxidative Steam Reforming of Methanol over CuZnAl Oxide Catalysts: Effect of Substitution of Zirconium and Cerium on the Catalytic Performance. Top. Catal. 2003, 22, 235–244. [Google Scholar] [CrossRef]
- Jiménez-González, C.; Gil-Calvo, M.; de Rivas, B.; González-Velasco, J.R.; Gutiérrez-Ortiz, J.I.; López-FonsecaInd, R. Oxidative Steam Reforming and Steam Reforming of Methane, Isooctane, and N-Tetradecane over an Alumina Supported Spinel-Derived Nickel Catalyst. Ind. Eng. Chem. Res. 2016, 55, 3920–3929. [Google Scholar] [CrossRef]
- Chi, H.; Andolina, C.M.; Li, J.; Curnan, M.T.; Saidi, W.A.; Zhou, G.; Yang, J.C.; Veser, G. Dependence of H2 and CO2 selectivity on Cu oxidation state during partial oxidation of methanol on Cu/ZnO. Appl. Catal. 2018, 556, 64–72. [Google Scholar] [CrossRef]
- Badmaev, S.D.; Akhmetov, N.O.; Pechenkin, A.A.; Sobyanin, V.A.; Parmon, V.N. Low-temperature partial oxidation of dimethyl ether to hydrogen-rich gas over CuO–CeO2/γ-Al2O3 catalysts for fuel cell supply. Dokl. Phys. Chem. 2019, 487, 95–98. [Google Scholar] [CrossRef]
- Badmaev, S.D.; Akhmetov, N.O.; Belyaev, V.D.; Kulikov, A.V.; Pechenkin, A.A.; Potemkin, D.I.; Konishcheva, M.V.; Rogozhnikov, V.N.; Snytnikov, P.V.; Sobyanin, V.A. Syngas production via partial oxidation of dimethyl ether over Rh/Ce0.75Zr0.25O2 catalyst and its application for SOFC feeding. Int. J. Hydrog. Energy 2020. [Google Scholar] [CrossRef]
- Takezawa, N.; Iwasa, N. Steam reforming and dehydrogenation of methanol: Difference in the catalytic functions of copper and group VIII metals. Catal. Today 1997, 36, 45–56. [Google Scholar] [CrossRef]
- Murcia-Mascaros, S.; Navarro, R.M.; Gomez-Sainero, L.; Constantino, U.; Nocchetti, M.; Fierro, J.L.G. Oxidative methanol reforming reactions on CuZnAl catalysts derived from hydrotalcite-like precursors. J. Catal. 2001, 198, 338–347. [Google Scholar] [CrossRef]
- Carrazan, S.R.G.; Wojcieszak, R.; Blanco, R.M.; Mateos-Pedrero, C.; Ruiz, P. Modulation of the selectivity in partial oxidation of methanol over CuZnAl catalysts by adding CO2 and/or H2 into the reaction feed. Appl. Catal. B Environ. 2015, 168, 14–24. [Google Scholar] [CrossRef]
- Sun, Z.; Zhang, X.; Li, H.; Liu, T.; Sang, S.; Chen, S.; Duan, L.; Zeng, L.; Xiang, W.; Gong, J. Chemical looping oxidative steam reforming of methanol: A new pathway for auto-thermal conversion. Appl. Catal. B Environ. 2020, 269, 118758. [Google Scholar] [CrossRef]
- Zhou, X.; Meng, M.; Sun, Z.; Li, Q.; Jiang, Z. Prominent enhancement of Mn or Co addition on the performance of Cu-Ce-O catalyst used for H2 production via dimethyl ether steam reforming. Chem. Eng. J. 2011, 174, 400–407. [Google Scholar] [CrossRef]
- Chandan, A.; Hattenberger, M.; El-kharouf, A.; Du, S.; Dhir, A.; Self, V.; Pollet, B.G.; Ingram, A.; Bujalski, W. High temperature (HT) polymer electrolyte membrane fuel cells (PEMFC)–A review. J. Power Sources 2013, 231, 264–278. [Google Scholar] [CrossRef]
- Garbis, P.; Kern, C.; Jess, A. Kinetics and reactor design aspects of selective methanation of CO over a Ru/γ-Al2O3 catalyst in CO2/H2 rich gases. Energies 2019, 12, 469. [Google Scholar] [CrossRef]
| Inlet Reaction Mixture DMM:O2:H2O:N2, vol.% | W(H2), L h−1 gcat−1 | Yield, % | ||||
|---|---|---|---|---|---|---|
| CO2 | CO | CH3OCH3 | CH3OH | |||
| 1 | 10 : 0 : 45 : 45 | 12.4 | 82 | 1.4 | 4 | 12.6 |
| 2 | 10 : 2.5 : 40 : 47.5 | 14 | 87.7 | 1.8 | 3.2 | 7.3 |
| 3 | 10 : 5 : 25 : 60 | 10 | 70.6 | 3.3 | 16.6 | 9.5 |
| 4 | 10 : 10 : 0 : 80 | 5 | 43 | 21 | 34 | 1 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Badmaev, S.; Sobyanin, V. Production of Hydrogen-Rich Gas by Oxidative Steam Reforming of Dimethoxymethane over CuO-CeO2/γ-Al2O3 Catalyst. Energies 2020, 13, 3684. https://doi.org/10.3390/en13143684
Badmaev S, Sobyanin V. Production of Hydrogen-Rich Gas by Oxidative Steam Reforming of Dimethoxymethane over CuO-CeO2/γ-Al2O3 Catalyst. Energies. 2020; 13(14):3684. https://doi.org/10.3390/en13143684
Chicago/Turabian StyleBadmaev, Sukhe, and Vladimir Sobyanin. 2020. "Production of Hydrogen-Rich Gas by Oxidative Steam Reforming of Dimethoxymethane over CuO-CeO2/γ-Al2O3 Catalyst" Energies 13, no. 14: 3684. https://doi.org/10.3390/en13143684
APA StyleBadmaev, S., & Sobyanin, V. (2020). Production of Hydrogen-Rich Gas by Oxidative Steam Reforming of Dimethoxymethane over CuO-CeO2/γ-Al2O3 Catalyst. Energies, 13(14), 3684. https://doi.org/10.3390/en13143684




