Steam Explosion Pretreatment of Beechwood. Part 1: Comparison of the Enzymatic Hydrolysis of Washed Solids and Whole Pretreatment Slurry at Different Solid Loadings
Abstract
1. Introduction
2. Materials and Methods
2.1. Biomass Feedstock
2.2. Steam Explosion Pretreatment
2.3. Xylan Mass Balance
2.4. Enzymatic Hydrolysis
2.4.1. Enzymatic Hydrolysis of Washed Solids
2.4.2. Whole Slurry Enzymatic Hydrolysis
2.5. Analytical Methods
3. Results and Discussion
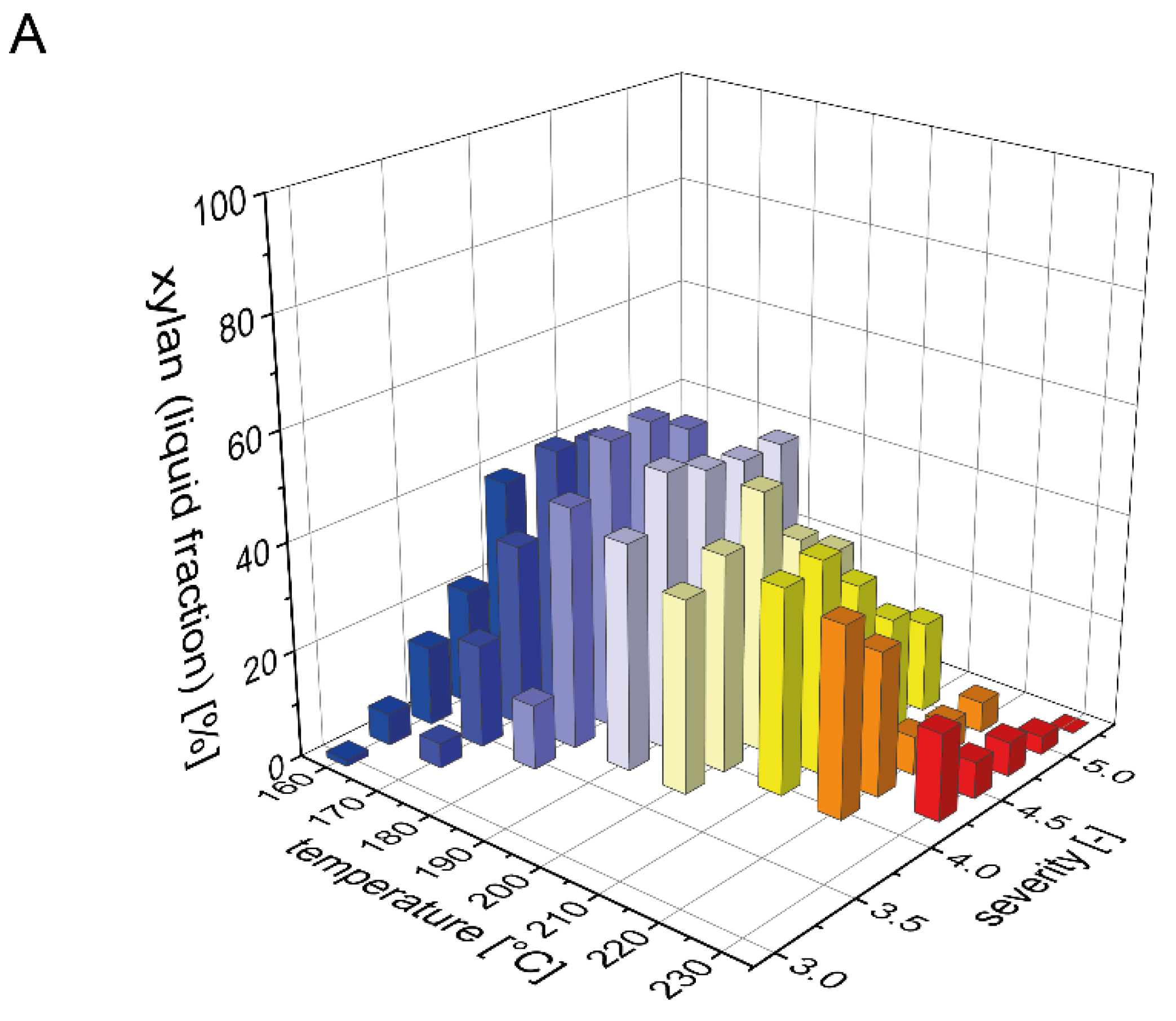
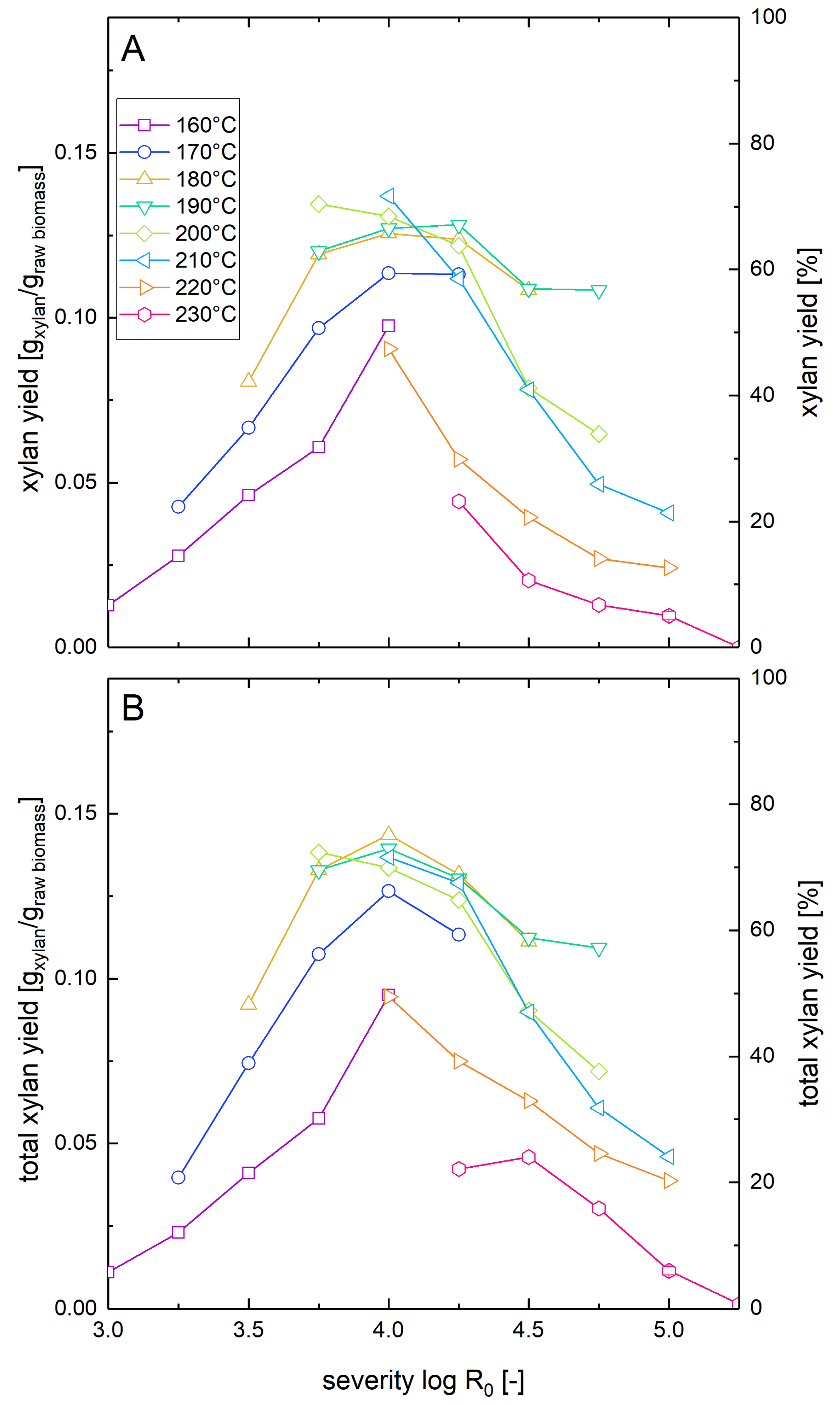
3.1. Xylan Mass Balance after Pretreatment
3.2. Xylan Recovery after Steam Explosion Pretreatment and Enzymatic Hydrolysis at Low Solid Loading
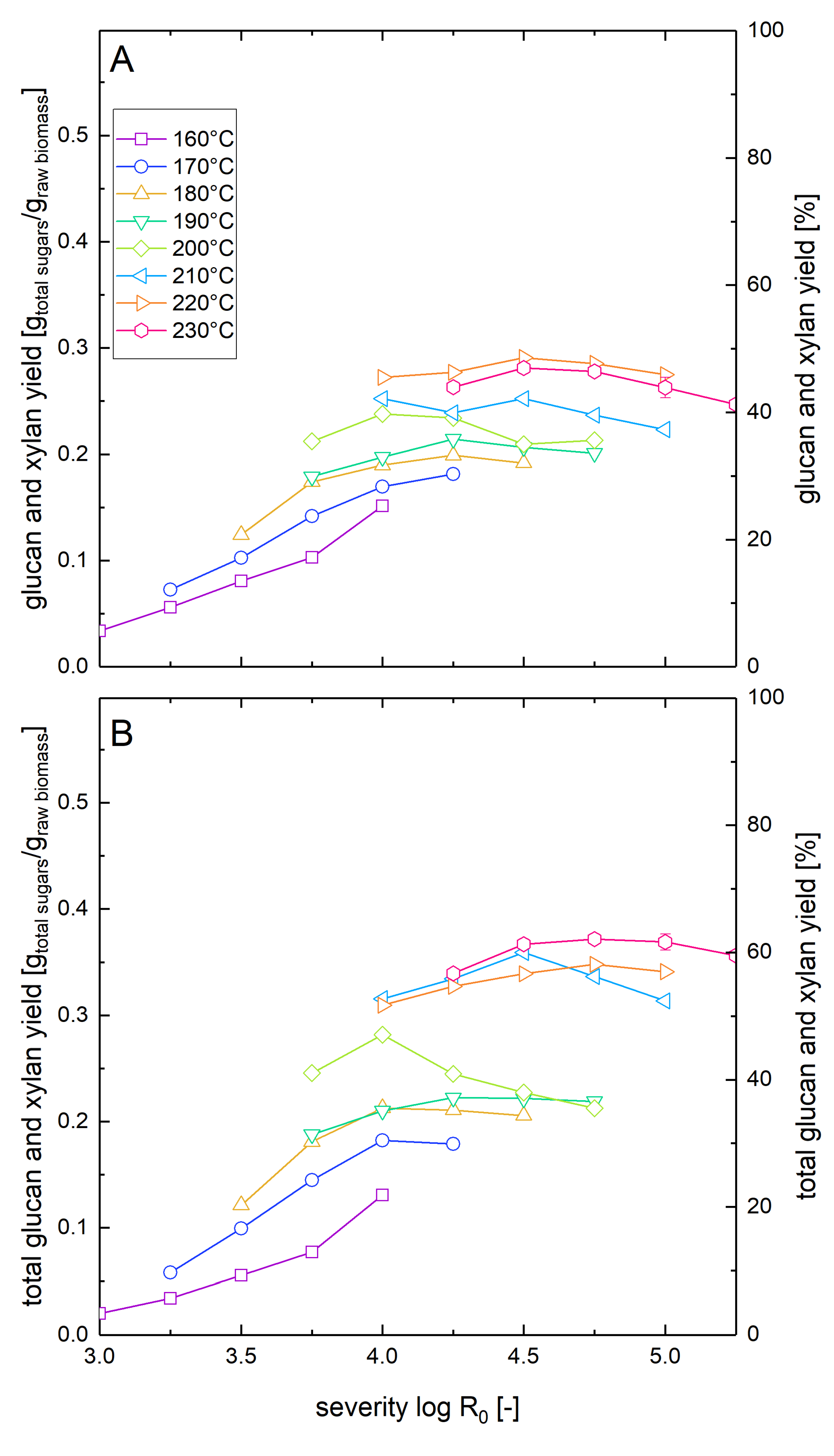
3.3. Glucose Yields after Pretreatment and Enzymatic Hydrolysis at Low Solid Loading
3.4. Total Sugar Yields after Steam Explosion Pretreatment and Enzymatic Hydrolysis at Low Solid Loading
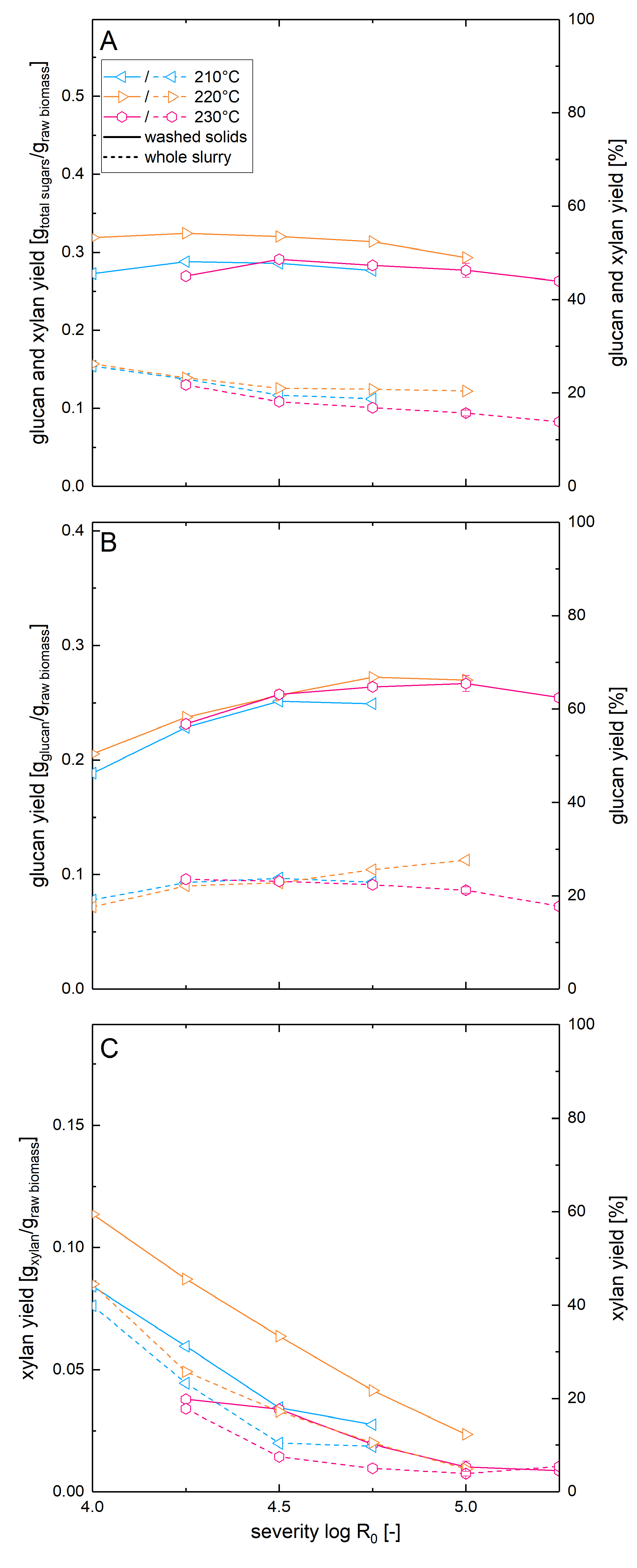
3.5. Optimization of Steam Explosion Pretreatment Conditions for High Solids Enzymatic Hydrolysis
4. Summary and Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lynd, L.R.; Liang, X.; Biddy, M.J.; Allee, A.; Cai, H.; Foust, T.; Himmel, M.E.; Laser, M.S.; Wang, M.; Wyman, C.E. Cellulosic ethanol: Status and innovation. Curr. Opin. Biotechnol. 2017, 45, 202–211. [Google Scholar] [CrossRef]
- Lynd, L.R.; Laser, M.S.; Bransby, D.; Dale, B.E.; Davison, B.; Hamilton, R.; Himmel, M.; Keller, M.; McMillan, J.D.; Sheehan, J.; et al. How biotech can transform biofuels. Nat. Biotechnol. 2008, 26, 169–172. [Google Scholar] [CrossRef] [PubMed]
- Limayem, A.; Ricke, S.C. Lignocellulosic biomass for bioethanol production: Current perspectives, potential issues and future prospects. Prog. Energy Combust. Sci. 2012, 38, 449–467. [Google Scholar] [CrossRef]
- Cioldi, F.; Keller, M.; Rösler, E.; Speich, S.; Brändli, U.-B.; Vidondo, B.; Abegg, M.; Meile, R.; Huber, M.; Traub, B.; et al. Schweizerisches Landesforstinventar—Ergebnistabelle Nr. 177425; Eidgenössische Forschungsanstalt WSL: Birmensdorf, Switzerland, 2014. [Google Scholar]
- Himmel, M.E.; Ding, S.-Y.; Johnson, D.K.; Adney, W.S.; Nimlos, M.R.; Brady, J.W.; Foust, T.D. Biomass recalcitrance: Engineering plants and enzymes for biofuels production. Science 2007, 315, 804–807. [Google Scholar] [CrossRef]
- Lynd, L.R.; Wyman, C.E.; Gerngross, T.U. Biocommodity engineering. Biotechnol. Prog. 1999, 15, 777–793. [Google Scholar] [CrossRef] [PubMed]
- Mosier, N.; Wyman, C.E.; Dale, B.; Elander, R.; Lee, Y.Y.; Holtzapple, M.; Ladisch, M. Features of promising technologies for pretreatment of lignocellulosic biomass. Bioresour. Technol. 2005, 96, 673–686. [Google Scholar] [CrossRef] [PubMed]
- Wyman, C.E.; Dale, B.E.; Balan, V.; Elander, R.T.; Holtzapple, M.T.; Ramirez, R.S.; Ladisch, M.R.; Mosier, N.S.; Lee, Y.Y.; Gupta, R.; et al. Comparative performance of leading pretreatment technologies for biological conversion of corn stover, poplar wood, and switchgrass to sugars. In Aqueous Pretreatment of Plant Biomass for Biological and Chemical Conversion to Fuels and Chemicals; Wyman, C.E., Ed.; John Wiley & Sons Inc.: Chichester, West Sussex, UK, 2013; pp. 239–259. ISBN 9780470975831. [Google Scholar]
- Wyman, C.E.; Balan, V.; Dale, B.E.; Elander, R.T.; Falls, M.; Hames, B.; Holtzapple, M.T.; Ladisch, M.R.; Lee, Y.Y.; Mosier, N.; et al. Comparative data on effects of leading pretreatments and enzyme loadings and formulations on sugar yields from different switchgrass sources. Bioresour. Technol. 2011, 102, 11052–11062. [Google Scholar] [CrossRef] [PubMed]
- Hendriks, A.T.W.M.; Zeeman, G. Pretreatments to enhance the digestibility of lignocellulosic biomass. Bioresour. Technol. 2009, 100, 10–18. [Google Scholar] [CrossRef]
- Yang, B.; Wyman, C.E. Pretreatment: The key to unlocking low-cost cellulosic ethanol. Biofuels Bioprod. Bioref. 2008, 2, 26–40. [Google Scholar] [CrossRef]
- Holtzapple, M.T.; Humphrey, A.E.; Taylor, J.D. Energy requirements for the size reduction of poplar and aspen wood. Biotechnol. Bioeng. 1989, 33, 207–210. [Google Scholar] [CrossRef]
- Vidal, B.C.; Dien, B.S.; Ting, K.C.; Singh, V. Influence of feedstock particle size on lignocellulose conversion—A review. Appl. Biochem. Biotechnol. 2011, 164, 1405–1421. [Google Scholar] [CrossRef] [PubMed]
- Cara, C.; Ruiz, E.; Ballesteros, M.; Manzanares, P.; Negro, M.J.; Castro, E. Production of fuel ethanol from steam-explosion pretreated olive tree pruning. Fuel 2008, 87, 692–700. [Google Scholar] [CrossRef]
- Romaní, A.; Garrote, G.; Ballesteros, I.; Ballesteros, M. Second generation bioethanol from steam exploded Eucalyptus globulus wood. Fuel 2013, 111, 66–74. [Google Scholar] [CrossRef]
- Park, J.Y.; Kang, M.; Kim, J.S.; Lee, J.P.; Choi, W.I.; Lee, J.S. Enhancement of enzymatic digestibility of Eucalyptus grandis pretreated by NaOH catalyzed steam explosion. Bioresour. Technol. 2012, 123, 707–712. [Google Scholar] [CrossRef] [PubMed]
- Cantarella, M.; Cantarella, L.; Gallifuoco, A.; Spera, A.; Alfani, F. Effect of inhibitors released during steam-explosion treatment of poplar wood on subsequent enzymatic hydrolysis and SSF. Biotechnol. Prog. 2004, 20, 200–206. [Google Scholar] [CrossRef]
- Sipos, B.; Réczey, J.; Somorai, Z.; Kádár, Z.; Dienes, D.; Réczey, K. Sweet sorghum as feedstock for ethanol production: Enzymatic hydrolysis of steam-pretreated bagasse. Appl. Biochem. Biotechnol. 2009, 153, 151–162. [Google Scholar] [CrossRef]
- Petersen, M.Ø.; Larsen, J.; Thomsen, M.H. Optimization of hydrothermal pretreatment of wheat straw for production of bioethanol at low water consumption without addition of chemicals. Biomass Bioenerg. 2009, 33, 834–840. [Google Scholar] [CrossRef]
- Alvira, P.; Negro, M.J.; Ballesteros, I.; González, A.; Ballesteros, M. Steam explosion for wheat straw pretreatment for sugars production. Bioethanol 2016, 2, 66–75. [Google Scholar] [CrossRef]
- Galbe, M.; Wallberg, O. Pretreatment for biorefineries: A review of common methods for efficient utilisation of lignocellulosic materials. Biotechnol. Biofuels 2019, 12, 294. [Google Scholar] [CrossRef]
- Galbe, M.; Zacchi, G. Pretreatment of lignocellulosic materials for efficient bioethanol production. Adv. Biochem. Eng. Biotechnol. 2007, 108, 41–65. [Google Scholar] [CrossRef]
- Chen, H.; Qiu, W. Key technologies for bioethanol production from lignocellulose. Biotechnol. Adv. 2010, 28, 556–562. [Google Scholar] [CrossRef]
- Asada, C.; Sasaki, C.; Hirano, T.; Nakamura, Y. Chemical characteristics and enzymatic saccharification of lignocellulosic biomass treated using high-temperature saturated steam: Comparison of softwood and hardwood. Bioresour. Technol. 2015, 182, 245–250. [Google Scholar] [CrossRef]
- Pazitny, A.; Russ, A.; Bohacek, S.; Stankovska, M.; Ihnat, V.; Suty, S. Various lignocellulosic raw materials pretreatment processes utilizable for increasing holocellulose accessibility for hydrolytic enzymes part II. Effect of steam explosion temperature on beech enzymatic hydrolysis. Wood Res. 2019, 64, 437–447. [Google Scholar]
- Ramos, L.P. The chemistry involved in the steam treatment of lignocellulosic materials. Química Nova 2003, 26, 863–871. [Google Scholar] [CrossRef]
- Yang, B.; Wyman, C.E. Effect of xylan and lignin removal by batch and flowthrough pretreatment on the enzymatic digestibility of corn stover cellulose. Biotechnol. Bioeng. 2004, 86, 88–95. [Google Scholar] [CrossRef] [PubMed]
- Lloyd, T.A.; Wyman, C.E. Combined sugar yields for dilute sulfuric acid pretreatment of corn stover followed by enzymatic hydrolysis of the remaining solids. Bioresour. Technol. 2005, 96, 1967–1977. [Google Scholar] [CrossRef]
- Overend, R.P.; Chornet, E.; Gascoigne, J.a. Fractionation of lignocellulosics by steam-aqueous pretreatments [and discussion]. Philos. Trans. R. Soc. A 1987, 321, 523–536. [Google Scholar] [CrossRef]
- Negro, M.J.; Manzanares, P.; Ballesteros, I.; Oliva, J.M.; Cabañas, A.; Ballesteros, M. Hydrothermal Pretreatment Conditions to Enhance Ethanol Production from Poplar Biomass. Appl. Biochem. Biotechnol. 2003, 105, 87–100. [Google Scholar] [CrossRef]
- Ballesteros, I.; Oliva, J.M.; Navarro, A.A.; González, A.; Carrasco, J.; Ballesteros, M. Effect of chip size on steam explosion pretreatment of softwood. Appl. Biochem. Biotechnol. 2000, 84–86, 97–110. [Google Scholar] [CrossRef]
- Liu, Z.-H.; Qin, L.; Pang, F.; Jin, M.-J.; Li, B.-Z.; Kang, Y.; Dale, B.E.; Yuan, Y.-J. Effects of biomass particle size on steam explosion pretreatment performance for improving the enzyme digestibility of corn stover. Ind. Crops Prod. 2013, 44, 176–184. [Google Scholar] [CrossRef]
- Wyman, C.E.; Dale, B.E.; Elander, R.T.; Holtzapple, M.; Ladisch, M.R.; Lee, Y.Y. Comparative sugar recovery data from laboratory scale application of leading pretreatment technologies to corn stover. Bioresour. Technol. 2005, 96, 2026–2032. [Google Scholar] [CrossRef]
- Wyman, C.E.; Dale, B.E.; Elander, R.T.; Holtzapple, M.; Ladisch, M.R.; Lee, Y.Y.; Mitchinson, C.; Saddler, J.N. Comparative sugar recovery and fermentation data following pretreatment of poplar wood by leading technologies. Biotechnol. Prog. 2009, 25, 333–339. [Google Scholar] [CrossRef]
- Garlock, R.J.; Balan, V.; Dale, B.E.; Pallapolu, V.R.; Lee, Y.Y.; Kim, Y.; Mosier, N.S.; Ladisch, M.R.; Holtzapple, M.T.; Falls, M.; et al. Comparative material balances around pretreatment technologies for the conversion of switchgrass to soluble sugars. Bioresour. Technol. 2011, 102, 11063–11071. [Google Scholar] [CrossRef] [PubMed]
- Vane, L.M. Separation technologies for the recovery and dehydration of alcohols from fermentation broths. Biofuels Bioprod. Bioref. 2008, 2, 553–588. [Google Scholar] [CrossRef]
- McMillan, J.D.; Jennings, E.W.; Mohagheghi, A.; Zuccarello, M. Comparative performance of precommercial cellulases hydrolyzing pretreated corn stover. Biotechnol. Biofuels 2011, 4, 29. [Google Scholar] [CrossRef] [PubMed]
- Geddes, C.C.; Nieves, I.U.; Ingram, L.O. Advances in ethanol production. Curr. Opin. Biotechnol. 2011, 22, 312–319. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Pielhop, T.; Amgarten, J.; von Rohr, P.R.; Studer, M.H. Steam explosion pretreatment of softwood: The effect of the explosive decompression on enzymatic digestibility. Biotechnol. Biofuels 2016, 9, 152. [Google Scholar] [CrossRef] [PubMed]
- Schütt, F.; Puls, J.; Saake, B. Optimization of steam pretreatment conditions for enzymatic hydrolysis of poplar wood. Holzforschung 2011, 65. [Google Scholar] [CrossRef]
- Nitsos, C.K.; Matis, K.A.; Triantafyllidis, K.S. Optimization of hydrothermal pretreatment of lignocellulosic biomass in the bioethanol production process. ChemSusChem 2013, 6, 110–122. [Google Scholar] [CrossRef]
- Sassner, P.; Galbe, M.; Zacchi, G. Steam pretreatment of Salix with and without SO2 impregnation for production of bioethanol. Appl. Biochem. Biotechnol. 2005, 121, 1101–1117. [Google Scholar] [CrossRef]
- Romaní, A.; Garrote, G.; Alonso, J.L.; Parajó, J.C. Bioethanol production from hydrothermally pretreated Eucalyptus globulus wood. Bioresour. Technol. 2010, 101, 8706–8712. [Google Scholar] [CrossRef] [PubMed]
- Ghose, T.K. Measurement of cellulase activities. Pure Appl. Chem. 1987, 59, 257–268. [Google Scholar] [CrossRef]
- Muzamal, M.; Arnling Bååth, J.; Olsson, L.; Rasmuson, A.S. Contribution of structural modification to enhanced enzymatic hydrolysis and 3-D structural analysis of steam-exploded wood using X-ray tomography. BioResources 2016, 11. [Google Scholar] [CrossRef]
- Kim, Y.; Kreke, T.; Mosier, N.S.; Ladisch, M.R. Severity factor coefficients for subcritical liquid hot water pretreatment of hardwood chips. Biotechnol. Bioeng. 2014, 111, 254–263. [Google Scholar] [CrossRef] [PubMed]
- Kristensen, J.B.; Felby, C.; Jorgensen, H. Yield-determining factors in high-solids enzymatic hydrolysis of lignocellulose. Biotechnol. Biofuels 2009, 2, 11. [Google Scholar] [CrossRef] [PubMed]
- Modenbach, A.A.; Nokes, S.E. Enzymatic hydrolysis of biomass at high-solids loadings—A review. Biomass Bioenerg. 2013, 56, 526–544. [Google Scholar] [CrossRef]
- Kumar, R.; Wyman, C.E. An improved method to directly estimate cellulase adsorption on biomass solids. Enzyme Microb. Technol. 2008, 42, 426–433. [Google Scholar] [CrossRef]
- Martins, L.H.d.S.; Rabelo, S.C.; da Costa, A.C. Effects of the pretreatment method on high solids enzymatic hydrolysis and ethanol fermentation of the cellulosic fraction of sugarcane bagasse. Bioresour. Technol. 2015, 191, 312–321. [Google Scholar] [CrossRef]
- Cara, C.; Moya, M.; Ballesteros, I.; Negro, M.J.; González, A.; Ruiz, E. Influence of solid loading on enzymatic hydrolysis of steam exploded or liquid hot water pretreated olive tree biomass. Process. Biochem. 2007, 42, 1003–1009. [Google Scholar] [CrossRef]


| Temperature [°C] | Pretreatment Severity log R0 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 3 | 3.25 | 3.5 | 3.75 | 4 | 4.25 | 4.5 | 4.75 | 5 | 5.25 | |
| 160 | 17.1 | 30.4 | 54.1 | 96.2 | 171.2 | - | - | - | - | - |
| 170 | - | 15.5 | 27.5 | 48.9 | 86.9 | 154.5 | - | - | - | - |
| 180 | - | - | 13.9 | 24.8 | 44.1 | 78.4 | 139.5 | - | - | - |
| 190 | - | - | - | 12.6 | 22.4 | 39.8 | 70.8 | 125.9 | - | - |
| 200 | - | - | - | 6.4 | 11.4 | 20.2 | 35.9 | 63.9 | - | - |
| 210 | - | - | - | - | 5.8 | 10.3 | 18.2 | 32.4 | 57.7 | - |
| 220 | - | - | - | - | 2.9 | 5.2 | 9.3 | 16.5 | 29.3 | - |
| 230 | - | - | - | - | - | 2.6 | 4.7 | 8.4 | 14.9 | 26.4 |
| Whole Slurry Enzymatic Hydrolysis | Combined Washed Solids Enzymatic Hydrolysis and Dilute Acid Hydrolysis of Prehydrolyzate | |||
|---|---|---|---|---|
| Sugar, Glucan Concentration in Enzymatic Hydrolysis | Yield (%) | Optimal Pretreatment Conditions (log R0, T [°C]) | Yield (%) | Optimal Pretreatment Conditions (log R0, T [°C]) |
| Glucan, 1% | 65 | 4.75, 230 | 88 ± 2 | 5.0, 230 |
| Xylan, 1% | 72 | 4.0, 210 | 75 | 4.0, 180 |
| Total sugars, 1% | 49 | 4.5, 220 | 62 | 4.75, 230 |
| Glucan, 5% | 28 | 5.0, 220 | 67 | 4.75, 220 |
| Xylan, 5% | 44 | 4.0, 220 | 59 | 4.0, 220 |
| Total sugars, 5% | 26 | 4.0, 220 | 54 | 4.25, 220 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Balan, R.; Antczak, A.; Brethauer, S.; Zielenkiewicz, T.; Studer, M.H. Steam Explosion Pretreatment of Beechwood. Part 1: Comparison of the Enzymatic Hydrolysis of Washed Solids and Whole Pretreatment Slurry at Different Solid Loadings. Energies 2020, 13, 3653. https://doi.org/10.3390/en13143653
Balan R, Antczak A, Brethauer S, Zielenkiewicz T, Studer MH. Steam Explosion Pretreatment of Beechwood. Part 1: Comparison of the Enzymatic Hydrolysis of Washed Solids and Whole Pretreatment Slurry at Different Solid Loadings. Energies. 2020; 13(14):3653. https://doi.org/10.3390/en13143653
Chicago/Turabian StyleBalan, Robert, Andrzej Antczak, Simone Brethauer, Tomasz Zielenkiewicz, and Michael H. Studer. 2020. "Steam Explosion Pretreatment of Beechwood. Part 1: Comparison of the Enzymatic Hydrolysis of Washed Solids and Whole Pretreatment Slurry at Different Solid Loadings" Energies 13, no. 14: 3653. https://doi.org/10.3390/en13143653
APA StyleBalan, R., Antczak, A., Brethauer, S., Zielenkiewicz, T., & Studer, M. H. (2020). Steam Explosion Pretreatment of Beechwood. Part 1: Comparison of the Enzymatic Hydrolysis of Washed Solids and Whole Pretreatment Slurry at Different Solid Loadings. Energies, 13(14), 3653. https://doi.org/10.3390/en13143653





