Pretreatment of Animal Manure Biomass to Improve Biogas Production: A Review
Abstract
1. Introduction
2. Hydrolysis in Anaerobic Digestion of Animal Waste
3. Pretreatments and Techniques to Improve the Digestion of Animal Manure
3.1. Physical Pretreatments
3.1.1. Mechanical Pretreatment
3.1.2. Heat Pretreatment
3.2. Physicochemical Pretreatments
3.2.1. Steam Explosion
3.2.2. Plasma
3.2.3. CO2 Explosion
3.2.4. Ammonia Fiber Expansion (AFEX)
3.3. Chemical Pretreatments
3.3.1. Alkaline Hydrolysis
3.3.2. Acid Hydrolysis
3.3.3. Organosolv
3.3.4. Wet Oxidation
3.3.5. Alkaline Peroxide
3.4. Biological Thermal Pretreatments
4. Application of Pretreatments to Livestock Waste
4.1. Pretreatments Applied to Cow Manure
4.2. Pretreatments Applied to Pig Manure
4.3. Pretreatments Applied to Poultry Manure
5. Summary of the Effects of Pretreatment on Animal Manure
5.1. Comparison of the Main Pretreatments
5.2. Effect of Pretreatments on Cow, Pig and Poultry Manure
6. Perspectives and Challenges of Animal Manure Pretreatments
7. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Nasir, I.; Ghazi, T.; Omar, R. Production of biogas from solid organic wastes through anaerobic digestion: A review. Appl. Microbiol. Biotechnol. 2012, 95, 321–329. [Google Scholar] [CrossRef] [PubMed]
- Tallou, A.; Haouas, A.; Jamali, M.; Atif, K.; Amir, S.; Aziz, F. Review on Cow Manure as Renewable Energy. In BT—Smart Village Technology: Concepts and Developments; Patnaik, S., Sen, S., Mahmoud, M.S., Eds.; Springer International Publishing: Cham, Switzerland, 2020; Volume 17, pp. 341–352. [Google Scholar]
- Nielsen, H.; Mladenovska, Z.; Westermann, P.; Ahring, B. Comparison of two-stage thermophilic (68 °C/55 °C) anaerobic digestion with one-stage thermophilic (55 °C) digestion of cattle manure. Biotechnol. Bioeng. 2004, 86, 291–300. [Google Scholar] [CrossRef] [PubMed]
- Tsapekos, P.; Kougias, P.G.; Frison, A.; Raga, R.; Angelidaki, I. Improving methane production from digested manure biofibers by mechanical and thermal alkaline pretreatment. Bioresour. Technol. 2016, 216, 545–552. [Google Scholar] [CrossRef] [PubMed]
- Issah, A.; Kabera, T.; Kemausuor, F. Biogas optimisation processes and effluent quality: A review. Biomass Bioenerg. 2020, 133, 105449. [Google Scholar] [CrossRef]
- Nasir, I.; Ghazi, T.; Omar, R. Anaerobic digestion technology in livestock manure treatment for biogas production: A review. Eng. Life Sci. 2012, 12, 258–269. [Google Scholar] [CrossRef]
- Chen, S.; Liao, W.; Liu, C.; Wen, Z.; Kincaid, R.; Harrison, J.; Elliott, D.; Brown, M.; Solana, A.; Stevens, D. Value-Added Chemicals from Animal Manure; Pacific Northwest National Laboratory: Richland, WA, USA; Environmental Molecular: Washington, WA, USA, 2003. [Google Scholar]
- McKendry, P. Energy production from biomass: Overview of biomass. Bioresour. Technol. 2002, 83, 55–63. [Google Scholar] [CrossRef]
- Seppälä, M.; Paavola, T.; Lehtomäki, A.; Pakarinen, O.; Rintala, J. Biogas from energy crops—Optimal pre-treatments and storage, co-digestion and energy balance in boreal conditions. Water Sci. Technol. 2008, 58, 1857–1863. [Google Scholar] [CrossRef]
- Carlsson, M.; Lagerkvist, A.; Morgan-Sagastume, F. The effects of substrate pre-treatment on anaerobic digestion systems: A review. Waste Manag. 2012, 32, 1634–1650. [Google Scholar] [CrossRef]
- Nasir, I.; Mohd, T.I. Pretreatment of lignocellulosic biomass from animal manure as a means of enhancing biogas production. Eng. Life Sci. 2015, 15, 733–742. [Google Scholar]
- Taherzadeh, M.; Karimi, K. Pretreatment of Lignocellulosic Wastes to Improve Ethanol and Biogas Production: A Review. Int. J. Mol. Sci. 2008, 9, 1621–1651. [Google Scholar] [CrossRef]
- Kaparaju, P.; Serrano, M.; Thomsen, A.; Kongjan, P.; Angelidaki, I. Bioethanol, biohydrogen and biogas production from wheat straw in a biorefinery concept. Bioresour. Technol. 2009, 100, 2562–2568. [Google Scholar] [CrossRef] [PubMed]
- Baratieri, M.; Baggio, P.; Fiori, L.; Grigiante, M. Biomass as an energy source: Thermodynamic constraints on the performance of the conversion process. Bioresour. Technol. 2008, 99, 7063–7073. [Google Scholar] [CrossRef] [PubMed]
- Vavilin, V.; Rytov, S.; Lokshina, L. A description of hydrolysis kinetics in anaerobic degradation of particulate organic matter. Bioresour. Technol. 1996, 56, 229–237. [Google Scholar] [CrossRef]
- Millati, R.; Wikandari, R.; Ariyanto, T.; Putri, R.; Taherzadeh, M. Pretreatment technologies for anaerobic digestion of lignocelluloses and toxic feedstocks. Bioresour. Technol. 2020, 304, 122998. [Google Scholar] [CrossRef]
- Yang, B.; Wyman, C.E. Pretreatment: The key to unlocking low-cost cellulosic ethanol. Biofuel. Bioprod. Biorefin. 2008, 2, 26–40. [Google Scholar] [CrossRef]
- Pavlostathis, S.G.; Giraldo-Gomez, E. Kinetics of anaerobic treatment: A critical review. Crit. Rev. Environ. Control 1991, 21, 411–490. [Google Scholar] [CrossRef]
- Li, Y.; Jin, Y.; Li, J.; Li, H.; Yu, Z.; Nie, Y. Effects of thermal pretreatment on degradation kinetics of organics during kitchen waste anaerobic digestion. Energy 2017, 118, 377–386. [Google Scholar] [CrossRef]
- Atelge, M.; Atabani, A.; Banu, J.; Krisa, D.; Kaya, M.; Eskicioglu, C.; Kumar, G.; Lee, C.; Yildiz, Y.; Unalan, S. A critical review of pretreatment technologies to enhance anaerobic digestion and energy recovery. Fuel 2020, 270, 117494. [Google Scholar] [CrossRef]
- Abraham, A.; Mathew, A.; Park, H.; Choi, O.; Sindhu, R.; Parameswaran, B.; Pandey, A.; Park, J.; Sang, B. Pretreatment strategies for enhanced biogas production from lignocellulosic biomass. Bioresour. Technol. 2020, 301, 122725. [Google Scholar] [CrossRef]
- Zhu, S. Use of ionic liquids for the efficient utilization of lignocellulosic materials. J. Chem. Technol. Biotechnol. 2008, 83, 777–779. [Google Scholar] [CrossRef]
- Kianmehr, P.; Parker, W.; Seto, P. An evaluation of protocols for characterization of ozone impacts on WAS properties and digestibility. Bioresour. Technol. 2010, 101, 8565–8572. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Liu, R.; Sun, C. Comparison of anaerobic digestion characteristics and kinetics of four livestock manures with different substrate concentrations. Bioresour. Technol. 2015, 198, 133–140. [Google Scholar] [CrossRef] [PubMed]
- Córdoba, V.; Fernadez, M.; Santalla, E. Influencia del inóculo en la digestión anaeróbica de purín de cerdo. Acta XXXVII Reun. Trab. ASADES 2014, 2, 06.29–06.38. [Google Scholar]
- Zhang, C.; Li, J.; Liu, C.; Liu, X.; Wang, J.; Li, S.; Fan, G.; Zhang, L. Alkaline pretreatment for enhancement of biogas production from banana stem and swine manure by anaerobic codigestion. Bioresour. Technol. 2013, 149, 353–358. [Google Scholar] [CrossRef] [PubMed]
- Molinuevo, B.; Mahdy, A.; Ballesteros, M.; González, C. From piggery wastewater nutrients to biogas: Microalgae biomass revalorization through anaerobic digestion. Renew. Energy 2016, 96, 1103–1110. [Google Scholar] [CrossRef]
- Shen, F.; Zhong, B.; Wang, Y.; Xia, X.; Zhai, Z.; Zhang, Q. Cellulolytic Microflora Pretreatment Increases the Efficiency of Anaerobic Co-digestion of Rice Straw and Pig Manure. Bioenergy Res. 2019, 12, 703–713. [Google Scholar] [CrossRef]
- Shen, J.; Zhao, C.; Liu, Y.; Zhang, R.; Liu, G.; Chen, C. Biogas production from anaerobic co-digestion of durian shell with chicken, dairy, and pig manures. Energy Convers. Manag. 2019, 198, 110535. [Google Scholar] [CrossRef]
- Li, R.; Tan, W.; Zhao, X.; Dang, Q.; Song, Q.; Xi, B.; Zhang, X. Evaluation on the Methane Production Potential of Wood Waste Pretreated with NaOH and Co-Digested with Pig Manure. Catalysts 2019, 9, 539. [Google Scholar] [CrossRef]
- Li, R.; Chen, S.; Li, X.; Saifullah Lar, J.; He, Y.; Zhu, B. Anaerobic Codigestion of Kitchen Waste with Cattle Manure for Biogas Production. Energy Fuels 2009, 23, 2225–2228. [Google Scholar] [CrossRef]
- Zhao, Y.; Sun, F.; Yu, J.; Cai, Y.; Luo, X.; Cui, Z.; Hu, Y.; Wang, X. Co-digestion of oat straw and cow manure during anaerobic digestion: Stimulative and inhibitory effects on fermentation. Bioresour. Technol. 2018, 269, 143–152. [Google Scholar] [CrossRef]
- Wei, Y.; Yuan, H.; Wachemo, A.; Li, X. Impacts of Modification of Corn Stover on the Synergistic Effect and Microbial Community Structure of Co-Digestion with Chicken Manure. Energy Fuels 2020, 34, 401–411. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, R.; Chen, C.; Liu, G.; He, Y.; Liu, X. Biogas production from co-digestion of corn stover and chicken manure under anaerobic wet, hemi-solid, and solid-state conditions. Bioresour. Technol. 2013, 149, 406–412. [Google Scholar] [CrossRef] [PubMed]
- Molinuevo, B.; Gómez, X.; Morán, A.; García, M. Anaerobic co-digestion of livestock and vegetable processing wastes: Fibre degradation and digestate stability. Waste Manag. 2013, 33, 1332–1338. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.; Møller, H.; Saha, C.; Alam, M.; Wahid, R.; Feng, L. Anaerobic co-digestion of poultry droppings and briquetted wheat straw at mesophilic and thermophilic conditions: Influence of alkali pretreatment. Renew. Energy 2018, 128, 241–249. [Google Scholar] [CrossRef]
- Wadhwa, M.; Bakshi, M. Utilization of fruit and vegetable wastes as livestock feed and as substrates for generation of other value-added products. Rap Publ. 2013, 4, 1–67. [Google Scholar]
- Vertès, F.; Hatch, D.; Velthof, G.; Taube, F.; Laurent, F.; Loiseau, P.; Recous, S. Short-term and cumulative effects of grassland cultivation on nitrogen and carbon cycling in ley-arable rotations. In The Permanent and Temporary Grassland: Plant, Environment and Economy. Proceedings of the 14th Symposium of the European Grassland Federation, Ghent, Belgium, 3–5 September 2007; Belgian Society for Grassland and Forage Crops: Merelbeke, Belgium, 2007; pp. 227–246. [Google Scholar]
- Paudel, S.; Banjara, S.; Choi, O.; Park, K.; Kim, Y.; Lee, J. Pretreatment of agricultural biomass for anaerobic digestion: Current state and challenges. Bioresour. Technol. 2017, 245, 1194–1205. [Google Scholar] [CrossRef] [PubMed]
- Himmel, M.; Ding, S.; Johnson, D.; Adney, W.; Nimlos, M.; Brady, J.; Foust, T. Biomass Recalcitrance: Engineering Plants and Enzymes for Biofuels Production. Science 2007, 315, 804–807. [Google Scholar] [CrossRef]
- Li, Y.; Park, S.; Zhu, J. Solid-state anaerobic digestion for methane production from organic waste. Renew. Sustain. Energy Rev. 2011, 15, 821–826. [Google Scholar] [CrossRef]
- Kumar, P.; Barrett, D.; Delwiche, M.; Stroeve, P. Methods for Pretreatment of Lignocellulosic Biomass for Efficient Hydrolysis and Biofuel Production. Ind. Eng. Chem. Res. 2009, 48, 3713–3729. [Google Scholar] [CrossRef]
- Kabel, M.; Bos, G.; Zeevalking, J.; Voragen, A.; Schols, H. Effect of pretreatment severity on xylan solubility and enzymatic breakdown of the remaining cellulose from wheat straw. Bioresour. Technol. 2007, 98, 2034–2042. [Google Scholar] [CrossRef]
- Isikgor, F.; Becer, R. Lignocellulosic Biomass: A Sustainable Platform for Production of Bio-Based Chemicals and Polymers. Polym. Chem. 2015, 6, 4497–4559. [Google Scholar] [CrossRef]
- Nhuchhen, D.; Basu, P.; Acharya, B. A Comprehensive Review on Biomass Torrefaction. Int. J. Renew. Energy Biofuels 2014, 2014, 1–56. [Google Scholar] [CrossRef]
- Amin, F.; Khalid, H.; Zhang, H.; Rahman, S.; Zhang, R.; Liu, G.; Chen, C. Pretreatment methods of lignocellulosic biomass for anaerobic digestion. AMB Express 2017, 7, 72. [Google Scholar] [CrossRef] [PubMed]
- Aftab, M. Different pretreatment methods of lignocellulosic biomass for use in biofuel production. In Biomass for Bioenergy-Recent Trends and Future Challenges; Iqbal, I., Ed.; IntechOpen: Rijeka, Croatia, 2019; Chapter 2. [Google Scholar]
- Salmén, L. Viscoelastic properties ofin situ lignin under water-saturated conditions. J. Mater. Sci. 1984, 19, 3090–3096. [Google Scholar] [CrossRef]
- Kumakura, M.; Kaetsu, I. Effect of radiation pretreatment of bagasse on enzymatic and acid hydrolysis. Biomass 1983, 3, 199–208. [Google Scholar] [CrossRef]
- Grabber, J. How Do Lignin Composition, Structure, and Cross-Linking Affect Degradability? A Review of Cell Wall Model Studies. Crop Sci. 2005, 45, 820–831. [Google Scholar] [CrossRef]
- Sun, Y.; Cheng, J. Hydrolysis of lignocellulosic materials for ethanol production: A review. Bioresour. Technol. 2002, 83, 1–11. [Google Scholar] [CrossRef]
- Batista, D.; Montes de Oca, G.; Vega, J.R.; Rojas, M.; Corrales, J.; Murillo, L. Pretreatment methods of lignocellulosic wastes into value-added products: Recent advances and possibilities. Biomass Convers. Biorefin. 2020, 1–18. [Google Scholar] [CrossRef]
- Agbor, V.; Cicek, N.; Sparling, R.; Berlin, A.; Levin, D. Biomass pretreatment: Fundamentals toward application. Biotechnol. Adv. 2011, 29, 675–685. [Google Scholar] [CrossRef]
- Ravindran, R.; Jaiswal, A. A comprehensive review on pre-treatment strategy for lignocellulosic food industry waste: Challenges and opportunities. Bioresour. Technol. 2016, 199, 92–102. [Google Scholar] [CrossRef]
- Kim, K.; Hong, J. Supercritical CO2 pretreatment of lignocellulose enhances enzymatic cellulose hydrolysis. Bioresour. Technol. 2001, 77, 139–144. [Google Scholar] [CrossRef]
- Zheng, Y.; Lin, H.; Wen, J.; Cao, N.; Yu, X.; Tsao, G. Supercritical carbon dioxide explosion as a pretreatment for cellulose hydrolysis. Biotechnol. Lett. 1995, 17, 845–850. [Google Scholar] [CrossRef]
- Bonner, I.; Thompson, D.; Plummer, M.; Dee, M.; Tumuluru, J.; Pace, D.; Teymouri, F.; Campbell, T.; Bals, B. Impact of ammonia fiber expansion (AFEX) pretreatment on energy consumption during drying, grinding, and pelletization of corn stover. Dry. Technol. 2016, 34, 1319–1329. [Google Scholar] [CrossRef]
- Wen, Z.; Liao, W.; Chen, S. Hydrolysis of animal manure lignocellulosics for reducing sugar production. Bioresour. Technol. 2004, 91, 31–39. [Google Scholar] [CrossRef]
- Janker, I.; Sieber, V.; Faulstich, M.; Schieder, D. Solubilization of hemicellulose and lignin from wheat straw through microwave-assisted alkali treatment. Ind. Crops Prod. 2012, 39, 198–203. [Google Scholar] [CrossRef]
- Li, C.; Knierim, B.; Manisseri, C.; Arora, R.; Scheller, H.; Auer, M.; Vogel, K.; Simmons, B.A.; Singh, S. Comparison of dilute acid and ionic liquid pretreatment of switchgrass: Biomass recalcitrance, delignification and enzymatic saccharification. Bioresour. Technol. 2010, 101, 4900–4906. [Google Scholar] [CrossRef]
- López González, L.M.; Pereda Reyes, I.; Escobar Román, R.; Pedraza Garciga, J.; Romero Romero, O. Efecto de la aplicación de métodos de pre-tratamientos en el proceso de digestión anaerobia de la biomasa lignocelulósica. Tecnol. Química 2018, 38, 324–334. [Google Scholar]
- Zhao, X.; Cheng, K.; Liu, D. Organosolv pretreatment of lignocellulosic biomass for enzymatic hydrolysis. Appl. Microbiol. Biotechnol. 2009, 82, 815–827. [Google Scholar] [CrossRef]
- Park, N.; Kim, H.; Koo, B.; Yeo, H.; Choi, I. Organosolv pretreatment with various catalysts for enhancing enzymatic hydrolysis of pitch pine (Pinus rigida). Bioresour. Technol. 2010, 101, 7046–7053. [Google Scholar] [CrossRef]
- Palonen, H.; Thomsen, A.; Tenkanen, M.; Schmidt, A.; Viikari, L. Evaluation of wet oxidation pretreatment for enzymatic hydrolysis of softwood. Appl. Biochem. Biotechnol. 2004, 117, 1–17. [Google Scholar] [CrossRef]
- Schmidt, A.; Thomsen, A. Optimization of wet oxidation pretreatment of wheat straw. Bioresour. Technol. 1998, 64, 139–151. [Google Scholar] [CrossRef]
- Chum, H.; Douglas, L.; Feinberg, D.; Schroeder, H. Evaluation of Pretreatments of Biomass for Enzymatic Hydrolysis of Cellulose. [Organosolv Process, Wet Oxidation, and Steam Explosion of Wood Chips]; Solar Energy Research Institute: Golden, CO, USA; Colorado State University: Fort Collins, CO, USA, 1985. [Google Scholar]
- Varga, E.; Schmidt, A.; Réczey, K.; Thomsen, A. Pretreatment of corn stover using wet oxidation to enhance enzymatic digestibility. Appl. Biochem. Biotechnol. 2003, 104, 37–50. [Google Scholar] [CrossRef]
- McGinnis, G.; Wilson, W.; Prince, S.; Chen, C. Conversion of biomass into chemicals with high-temperature wet oxidation. Ind. Eng. Chem. Prod. Res. Dev. 1983, 22, 633–636. [Google Scholar] [CrossRef]
- Forero, S.; Soto, A.; Martínez, J.; Ayala, O. Evaluación del desempeño del pretratamiento con peróxido de hidrógeno sobre bagazo de caña de azúcar para remoción de lignina. ITECKNE Innovación Investig. Ing. 2019, 16, 21–28. [Google Scholar]
- Saritha, M.; Arora, A. Lata Biological pretreatment of lignocellulosic substrates for enhanced delignification and enzymatic digestibility. Indian J. Microbiol. 2012, 52, 122–130. [Google Scholar] [CrossRef]
- Zheng, Y.; Zhao, J.; Xu, F.; Li, Y. Pretreatment of lignocellulosic biomass for enhanced biogas production. Prog. Energy Combust. Sci. 2014, 42, 35–53. [Google Scholar] [CrossRef]
- Isroi, I.; Ria, M.; Syamsiah, S.; Niklasson, C.; Cahyanto, M.; Lundquist, K.; Taherzadeh, M. Biological pretreatment of lignocelluloses with white-rot fungi and its applications: A review. Bioresources 2011, 6, 5224–5259. [Google Scholar]
- Howard, R.; Abotsi, E.; Van Rensburg, E.; Howard, S. Lignocellulose biotechnology: Issues of bioconversion and enzyme production. Afr. J. Biotechnol. 2003, 2, 602–619. [Google Scholar] [CrossRef]
- Tapia, R.; Avila, J.; Domínguez, J.; Valero, D.; Olguin, E.; Pérez, D.; Alzate, L. Biological pretreatment of mexican caribbean macroalgae consortiums using Bm-2 strain (Trametes hirsuta) and its enzymatic broth to improve biomethane potential. Energies 2018, 11, 494. [Google Scholar] [CrossRef]
- Jeremic, D.; Goacher, R.; Yan, R.; Karunakaran, C.; Master, E. Direct and up-close views of plant cell walls show a leading role for lignin-modifying enzymes on ensuing xylanases. Biotechnol. Biofuels 2014, 7, 496. [Google Scholar] [CrossRef]
- Zhang, Q.; He, J.; Tian, M.; Mao, Z.; Tang, L.; Zhang, J.; Zhang, H. Enhancement of methane production from cassava residues by biological pretreatment using a constructed microbial consortium. Bioresour. Technol. 2011, 102, 8899–8906. [Google Scholar] [CrossRef] [PubMed]
- Haruta, S.; Cui, Z.; Huang, Z.; Li, M.; Ishii, M.; Igarashi, Y. Construction of a stable microbial community with high cellulose-degradation ability. Appl. Microbiol. Biotechnol. 2002, 59, 529–534. [Google Scholar] [PubMed]
- Lin, Y.; Wang, D.; Wu, S.; Wang, C. Alkali pretreatment enhances biogas production in the anaerobic digestion of pulp and paper sludge. J. Hazard. Mater. 2009, 170, 366–373. [Google Scholar] [CrossRef] [PubMed]
- Romano, R.; Zhang, R.; Teter, S.; McGarvey, J. The effect of enzyme addition on anaerobic digestion of Jose Tall Wheat Grass. Bioresour. Technol. 2009, 100, 4564–4571. [Google Scholar] [CrossRef] [PubMed]
- Davison, B. Twenty-Sixth Symposium on Biotechnology for Fuels and Chemicals; Springer Science & Business Media: New Jersey, NJ, USA, 2005; Volume 121, ISBN 1588296970. [Google Scholar]
- Hartmann, H.; Angelidaki, I.; Ahring, B. Increase of anaerobic degradation of particulate organic matter in full-scale biogas plants by mechanical maceration. Water Sci. Technol. 2000, 41, 145–153. [Google Scholar] [CrossRef]
- Angelidaki, I.; Ahring, B. Methods for increasing the biogas potential from the recalcitrant organic matter contained in manure. Water Sci. Technol. 2000, 41, 189–194. [Google Scholar] [CrossRef]
- McVoitte, W.; Clark, O. The effects of temperature and duration of thermal pretreatment on the solid-state anaerobic digestion of dairy cow manure. Heliyon 2019, 5, e02140. [Google Scholar] [CrossRef]
- Qiao, W.; Yan, X.; Ye, J.; Sun, Y.; Wang, W.; Zhang, Z. Evaluation of biogas production from different biomass wastes with/without hydrothermal pretreatment. Renew. Energy 2011, 36, 3313–3318. [Google Scholar] [CrossRef]
- Coarita, H.; Amaya, D.; Teixeira Franco, R.; Buffière, P.; Bayard, R. Methods for the Evaluation of Industrial Mechanical Pretreatments before Anaerobic Digesters. Molecules 2020, 25, 860. [Google Scholar] [CrossRef] [PubMed]
- Seyedy, H.; Karimi, K.; Zilouei, H.; Salehian, P.; Jeihanipour, A. Effects of lime pretreatment on biogas production from dry dairy cattle manure. Minerva Biotecnol. 2011, 23, 77. [Google Scholar]
- Ramos, J.; Gómez, D.; Regueiro, L.; Baeza, A.; Hansen, F. Alkaline and oxidative pretreatments for the anaerobic digestion of cow manure and maize straw: Factors influencing the process and preliminary economic viability of an industrial application. Bioresour. Technol. 2017, 241, 10–20. [Google Scholar] [CrossRef]
- Ferreira, L.; Souza, T.; Polanco, F.; Pérez, S. Thermal steam explosion pretreatment to enhance anaerobic biodegradability of the solid fraction of pig manure. Bioresour. Technol. 2014, 152, 393–398. [Google Scholar] [CrossRef]
- Sambusiti, C.; Monlau, F.; Ficara, E.; Carrère, H.; Malpei, F. A comparison of different pre-treatments to increase methane production from two agricultural substrates. Appl. Energy 2013, 104, 62–70. [Google Scholar] [CrossRef]
- Thomas, H.; Seira, J.; Escudié, R.; Carrère, H. Lime Pretreatment of Miscanthus: Impact on BMP and Batch Dry Co-Digestion with Cattle Manure. Molecules 2018, 23, 1608. [Google Scholar] [CrossRef]
- Palamae, S.; Palachum, W.; Chisti, Y.; Choorit, W. Retention of hemicellulose during delignification of oil palm empty fruit bunch (EFB) fiber with peracetic acid and alkaline peroxide. Biomass Bioenerg. 2014, 66, 240–248. [Google Scholar] [CrossRef]
- Yin, D.; Jing, Q.; AlDajani, W.; Duncan, S.; Tschirner, U.; Schilling, J.; Kazlauskas, R.J. Improved pretreatment of lignocellulosic biomass using enzymatically generated peracetic acid. Bioresour. Technol. 2011, 102, 5183–5192. [Google Scholar] [CrossRef] [PubMed]
- Rafique, R.; Poulsen, T.; Nizami, A.; Asam, Z.; Murphy, J.; Kiely, G. Effect of thermal, chemical and thermo-chemical pre-treatments to enhance methane production. Energy 2010, 35, 4556–4561. [Google Scholar] [CrossRef]
- Liu, M.; Ni, H.; Yang, L.; Chen, G.; Yan, X.; Leng, X.; Liu, P.; Li, X. Pretreatment of swine manure containing β-lactam antibiotics with whole-cell biocatalyst to improve biogas production. J. Clean. Prod. 2019, 240, 118070. [Google Scholar] [CrossRef]
- González, C.; León, C.; García, P. Different pretreatments for increasing the anaerobic biodegradability in swine manure. Bioresour. Technol. 2008, 99, 8710–8714. [Google Scholar] [CrossRef]
- Gómez, X.; Meredith, W.; Fernández, C.; Sánchez, M.; Díez, R.; Garzón, J.; Snape, C. Evaluating the effect of biochar addition on the anaerobic digestion of swine manure: Application of Py-GC/MS. Environ. Sci. Pollut. Res. 2018, 25, 25600–25611. [Google Scholar] [CrossRef]
- Carrère, H.; Sialve, B.; Bernet, N. Improving pig manure conversion into biogas by thermal and thermo-chemical pretreatments. Bioresour. Technol. 2009, 100, 3690–3694. [Google Scholar] [CrossRef] [PubMed]
- Feng, L.; Casas, M.; Ottosen, L.; Møller, H.; Bester, K. Removal of antibiotics during the anaerobic digestion of pig manure. Sci. Total Environ. 2017, 603–604, 219–225. [Google Scholar] [CrossRef] [PubMed]
- Abouelenien, F.; Namba, Y.; Nishio, N.; Nakashimada, Y. Dry Co-Digestion of Poultry Manure with Agriculture Wastes. Appl. Biochem. Biotechnol. 2016, 178, 932–946. [Google Scholar] [CrossRef] [PubMed]
- Costa, J.; Barbosa, S.; Alves, M.; Sousa, D. Thermochemical pre- and biological co-treatments to improve hydrolysis and methane production from poultry litter. Bioresour. Technol. 2012, 111, 141–147. [Google Scholar] [CrossRef]
- Zahan, Z.; Othman, M. Effect of pre-treatment on sequential anaerobic co-digestion of chicken litter with agricultural and food wastes under semi-solid conditions and comparison with wet anaerobic digestion. Bioresour. Technol. 2019, 281, 286–295. [Google Scholar] [CrossRef]
- Cai, C.; Lou, B.; Zheng, X. Keratinase production and keratin degradation by a mutant strain of Bacillus subtilis. J. Zhejiang Univ. Sci. B 2008, 9, 60–67. [Google Scholar] [CrossRef]
- Schumacher, B.; Wedwitschka, H.; Weinrich, S.; Mühlenberg, J.; Gallegos, D.; Oehmichen, K.; Liebetrau, J. The influence of pressure swing conditioning pre-treatment of chicken manure on nitrogen content and methane yield. Renew. Energy 2019, 143, 1554–1565. [Google Scholar] [CrossRef]
- Yin, D.; Qiao, W.; Negri, C.; Adani, F.; Fan, R.; Dong, R. Enhancing hyper-thermophilic hydrolysis pre-treatment of chicken manure for biogas production by in-situ gas phase ammonia stripping. Bioresour. Technol. 2019, 287, 121470. [Google Scholar] [CrossRef]
- Raju, C.; Sutaryo, S.; Ward, A.J.; Møller, H. Effects of high-temperature isochoric pre-treatment on the methane yields of cattle, pig and chicken manure. Environ. Technol. 2013, 34, 239–244. [Google Scholar] [CrossRef]
- Patinvoh, R.; Feuk, E.; Lundin, M.; Sárvári, I.; Taherzadeh, M. Biological Pretreatment of Chicken Feather and Biogas Production from Total Broth. Appl. Biochem. Biotechnol. 2016, 180, 1401–1415. [Google Scholar] [CrossRef]
- Fuchs, W.; Wang, X.; Gabauer, W.; Ortner, M.; Li, Z. Tackling ammonia inhibition for efficient biogas production from chicken manure: Status and technical trends in Europe and China. Renew. Sustain. Energy Rev. 2018, 97, 186–199. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, R.; Liu, X.; Chen, C.; Xiao, X.; Feng, L.; He, Y.; Liu, G. Evaluating Methane Production from Anaerobic Mono- and Co-digestion of Kitchen Waste, Corn Stover, and Chicken Manure. Energy Fuels 2013, 27, 2085–2091. [Google Scholar] [CrossRef]
- Sarker, S.; Lamb, J.; Hjelme, D.; Lien, K. A review of the role of critical parameters in the design and operation of biogas production plants. Appl. Sci. 2019, 9, 1915. [Google Scholar] [CrossRef]
- Velázquez, B.; Meneses, O.; Gaibor, J.; Niño, Z. Review of mathematical models for the anaerobic digestion process. In Anaerobic Digestion; Banu, J.R., Ed.; IntechOpen: London, UK, 2018. [Google Scholar]
- Hren, R.; Petrovič, A.; Čuček, L.; Simonič, M. Determination of Various Parameters during Thermal and Biological Pretreatment of Waste Materials. Energies 2020, 13, 2262. [Google Scholar] [CrossRef]
- Montgomery, L.; Bochmann, G. Pretreatment of Feedstock for Enhanced Biogas Production; IEA Bioenergy: Dublin, Ireland, 2014; pp. 1–20. [Google Scholar]
- Khan, M.; Ahring, B. Lignin degradation under anaerobic digestion: Influence of lignin modifications. A review. Biomass Bioenerg. 2019, 128, 105325. [Google Scholar] [CrossRef]
- Cesaro, A.; Belgiorno, V. Pretreatment methods to improve anaerobic biodegradability of organic municipal solid waste fractions. Chem. Eng. J. 2014, 240, 24–37. [Google Scholar] [CrossRef]
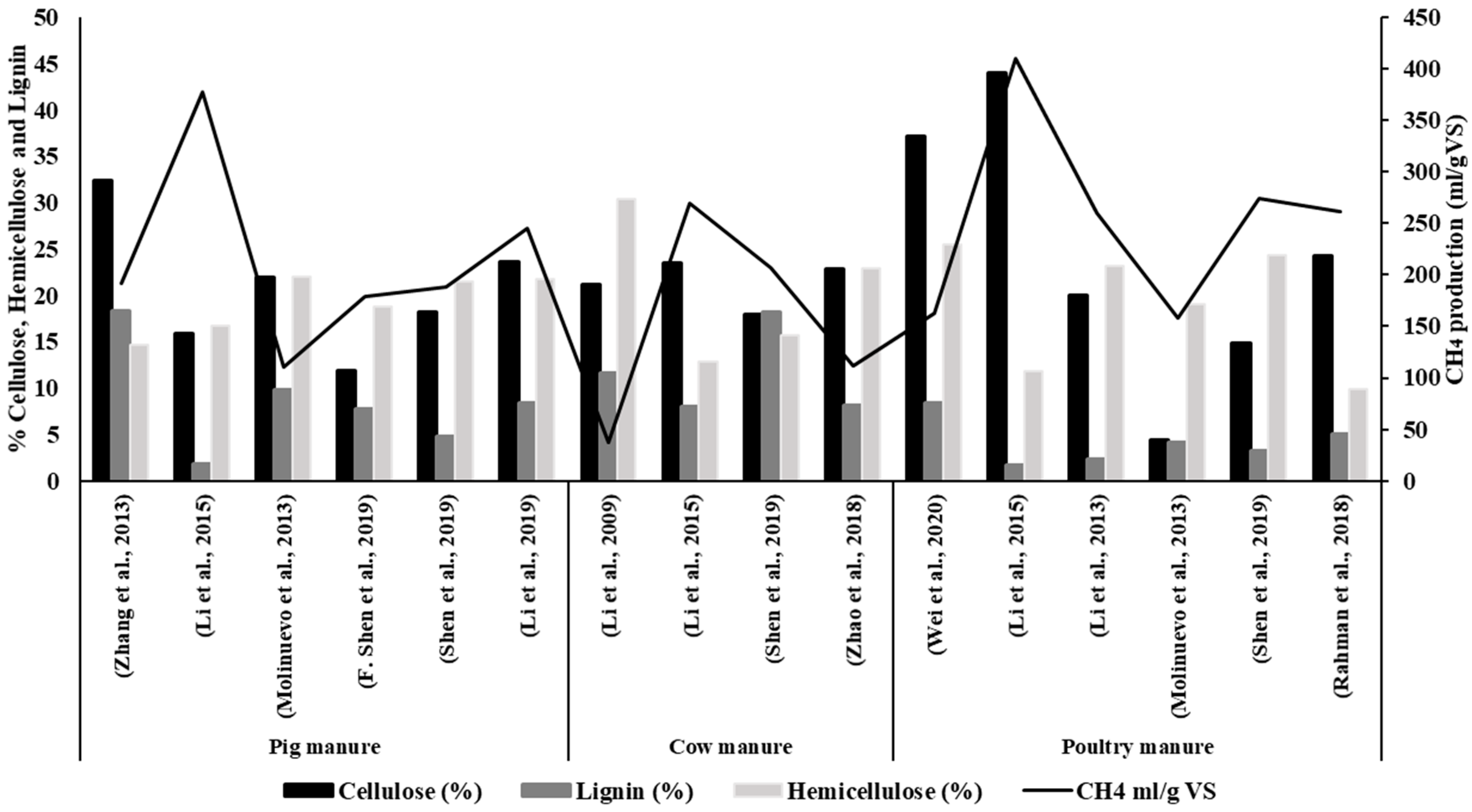


| Feedstock | Cellulose (%) | Lignin (%) | Hemicellulose (%) | CH4 mL/g VS | Inoculum | References |
|---|---|---|---|---|---|---|
| Pig manure | 32.4 | 18.4 | 14.6 | 191.4 | a | [26] |
| Pig manure | 15.9 | 1.8 | 16.7 | 377.0 | b | [24] |
| Pig manure | 22.0 | 9.8 | 22.0 | 111.0 | b | [27] |
| Pig manure | 11.9 | 7.7 | 18.8 | 178.7 | b | [28] |
| Pig manure | 18.2 | 4.8 | 21.5 | 187.7 | b | [29] |
| Pig manure | 23.6 | 8.4 | 21.7 | 245.1 | b | [30] |
| Cow manure | 21.2 | 11.6 | 30.4 | 37.5 | c | [31] |
| Cow manure | 23.5 | 8.0 | 12.8 | 270.0 | b | [24] |
| Cow manure | 17.9 | 18.2 | 15.7 | 206.9 | b | [29] |
| Cow manure | 22.9 | 8.1 | 22.9 | 112.1 | d | [32] |
| Poultry manure | 37.2 | 8.4 | 25.5 | 163.2 | a | [33] |
| Poultry manure | 44.0 | 1.7 | 11.8 | 410.0 | a | [24] |
| Poultry manure | 20.0 | 2.3 | 23.2 | 260.8 | a | [34] |
| Poultry manure | 4.4 | 4.2 | 19 | 158.0 | a | [35] |
| Poultry manure | 14.9 | 3.3 | 24.3 | 273.9 | a | [29] |
| Poultry manure | 24.3 | 5.1 | 9.9 | 261.7 | e | [36] |
| Pretreatments | Effects on Lignocellulosic Structure | References | ||
|---|---|---|---|---|
| Cellulose | Hemicellulose | Lignin | ||
| Physical | ||||
| Milling | Reduces crystallinity Decreases the degree of polymerization | [42,47] | ||
| Extrusion | [11] | |||
| Microwave irradiation | Increases substrate availability for enzymes | [42,49] | ||
| Physicochemicals | ||||
| Steam explosion | Greater solubilization | Solubilization Alteration of the structure | [53] | |
| Plasma | Degrades it into glucose | [52,54] | ||
| CO2 explosion | Break the structures | Break the structures | [56] | |
| Liquid hot water (LHW) | Increased solubilization and depolymerization | |||
| Ammonia Fiber Expansion (AFEX) | Degradation in oligomeric sugars Deacetylation | [47] | ||
| Chemical | ||||
| Alkaline hydrolysis | Solubilization of hemicellulose | Decompose, alter and breakdown of lignin | [58,59] | |
| Acid hydrolysis | Solubilization of hemicellulose | Decompose, alter and breakdown of lignin | [60] | |
| Organosolv process | Solubilization of hemicellulose | Lignin solubilization | [63] | |
| Wet oxidation | Lignin solubilization Altered lignin structure | [12,66] | ||
| Alkaline peroxide | [69] | |||
| Biological | ||||
| Pretreatment with microbial consortia, fungi and enzymes | Degrade cellulose | Degrades hemicellulose | Degrades lignin | [46,75] |
| Pretreatment | Process | Inoculum | Initials Condition | CH4 (mL/g VS) | Methane Enhancement (%) | References |
|---|---|---|---|---|---|---|
| Biological | Incubation (7 days, 70 °C with B4 bacteria to degrade hemicellulose) | Digested manure from a thermophilic laboratory reactor | Vr = 0.117 L; TRH = 40–60 d; T = 55 °C | 300.0 | 30 | [82] |
| Physiochemical | 125 °C, 37.5 min and 24 h | Digestate from a wastewater plant | TS = 16.12%; VS = 13.64%; pH= 7.85; C/N = 16.1; Vr = 2 L; TRH = 40 d | 450.0 | 35 | [83] |
| Physiochemical | Boiler 1l (170 °C at 1 h) | - | TS = 34.66%; VS= 19.52%; pH= 8.57; Vr = 0.250 L; TRH = d; T = 37 °C | 130.2 | −7 | [84] |
| Physiochemical | 68 °C (36, 108 and 168 h) | Digested sludge from cattle manure of a laboratory scale digester | Vr = 116 L; TRH=70 d; T = 68–55 °C | 260.0 | 56 | [3] |
| Physical | Maceration with a blender <0.35 mm and pressurizing the manure to 100 atm | Digested manure from a thermophilic laboratory reactor | Vr = 0.117; TRH = 40–60d; T = 55 °C | 276.0 | 20 | [82] |
| Physical | Mobile hammer mills. Sieving | Sludge from an anaerobic digester from a WWTP | TS = 19.6%; VS = 17.32%; pH = 8.23; Vr = 1 L; TRH = 39 d; T = 35 °C | 316.3 | 15 | [85] |
| Physical | Combination of three plates: aluminum, sandpaper and stainless steel | Sludge from an anaerobic digester from a WWTP | TS = 223.59 g/kg; VS = 191.87 g/kg; pH = 8.32; Vr = 0.164 L, TRH = 30 d; T = 53 °C | 168.0 | - | [4] |
| Chemical | Ca(OH)2, 60 °C, 12 and pH of 12 | Sludge from an anaerobic digester from a WWTP | Vr = 0.118 L; TRH = 45 d; T = 37 °C | 225.0 | 76 | [86] |
| Chemical | Calcium oxide (CaO) | Sludge from an anaerobic digester from a WWTP; sludge from an agroindustrial cow manure digester | TS = 9.84%; VS = 8.34%; pH = 7.15; Vr = 1.6 L; T = 38 °C | 168.2 | 26 | [87] |
| Chemical | Peracetic Acid (C2H4O3) | Sludge from an anaerobic digester from a WWTP; sludge from an agroindustrial cow manure digester | TS = 9.84%; VS = 8.34%; pH = 7.15; Vr = 1.6 L; TRH = 43 d T = 38 °C | 182.4 | 39 | [87] |
| Chemical and Physiochemical | NaOH 6% p/p TS 121 °C, 20 min | Sludge from a WWTP anaerobic digester | TS = 223.59 g/kg; VS = 191.87 g/kg; pH = 8.32; Vr = 0.164 L; T = 53 °C | 168.0 | 155 | [4] |
| Pretreatment | Process | Feedstock | Inoculum | Initials Condition | CH4 (mL/g VS) | Methane Enhancement (%) | References |
|---|---|---|---|---|---|---|---|
| Physiochemical | 170 °C at 1 h | Pig manure | - | TS = 28.14%; VS = 22.26%; pH = 6.91; Vr = 0.250 L; TRH = 43 d; T = 37 °C | 290.8 | 14.6 | [84] |
| Physiochemical | Thermal steam explosion (170 °C and 30 min) | Pig manure | Sludge from a WWTP anaerobic digester | TS = 46.6 g/kg; VS = 36.8 g/kg; C/N = 8.5; Vr = 0.300 L; T = 35.1 °C | 329 | 206.9 | [88] |
| Physiochemical | (100 °C) 1 h | Dehydrated pig manure | Sludge from an anaerobic digester from a WWTP | TS = 46.6 g/kg; VS = 36.8 g/kg; C/N = 8.5; Vr = 0.300 L; TRH = 29 d; T = 35.1 °C | 237.5 | 28 | [93] |
| Chemical | Ca (OH)2 al 5%, 2 h and neutralization of pH with HCl | Dehydrated pig manure | Sludge from an anaerobic digester from a WWTP | TS = 46.6 g/kg; VS = 36.8 g/kg; C/N =8.5; Vr = 0.300 L; TRH = 29 d; T = 35.1 °C | 204.74 | 12 | [93] |
| Chemical | 6% NaOH (p/p) | Pig manure | Anaerobic sludge from a beer plant | TS = 84.5%; VS = 67.76%; Vr = 0.500 L; T = 35 °C | 232.4 | 21.4 | [29] |
| Chemical | Ca(OH)2,1 h (70 °C) | Dehydrated pig manure | Sludge from an anaerobic digester from a WWTP | TS = 46.6 g/kg; VS = 36.8 g/kg; C/N = 8.5; Vr = 0.300 L; TRH = 29 d; T = 35.1 °C | 345 | 72 | [93] |
| Biological | Microbial community cell biocatalyst to accelerate degradation of antibiotics | Pig manure | - | TS = 28.14 %; VS = 22.26 %; pH = 6.91; Vr = 0.420 L; TRH = 7 d | 98.7 | 93.2 | [94] |
| Physical | Liquid and solid matrix separation using a 0.25mm pore size screen | Pig waste slurry | Sludge from an anaerobic digester from a WWTP | TS = 11.4%; VS = 9.34%; Vr = 1 L; TRH = 30 d; T=32 °C | 251 mL/g DQO | −2.33 | [95] |
| Physiochemical | Power at 600 W. The temperature increased with a ramp of 10 °C/min until reaching 80 °C and was maintained for 15 min supplemented with C | Pig manure | Sludge from an anaerobic digester from a WWTP | TS = 23.1g/l; VS = 15.2g/L; pH = 6.9 C/N = 10.9; Vr = 0.250 L; TRH = 30 d; T = 35 °C | 433.2 | 39 | [96] |
| Pretreatment | Process | Feedstock | Inoculum | Initials Condition | CH4 (mL/g VS) | Methane Enhancement (%) | References |
|---|---|---|---|---|---|---|---|
| Chemical | 5% de NaOH 90 min 120 °C + 3% de H2SO4 90 min 120 °C | Chicken litter | Sludge from an anaerobic digester from a WWTP | TS = 77.2%; VS = 39.1%; pH = 8.15; C/N = 13.02; Vr = 1 L; T = 37 °C | 481.5 | 50 | [101] |
| Chemical | Ca(OH)2 at 90 °C y 1.27 bar pressure | Chicken litter and chicken feathers | Anaerobic sludge from a wastewater treatment plant | TRH = 80 d; T = 37 °C | 137 | - | [100] |
| Biological | Clostridium cellulolyticum, Clostridium saccharolyticum and Clostridium thermocellum as bioaccumulation strains | Poultry manure | Sludge from an anaerobic digester from a WWTP | TS = 77%; VS = 70%; Vr = 0.05 L; T =37 °C | 102 | 15% | [100] |
| Biological | 2–8 days at total solid concentrations of 5–20% by Bacillus sp. C4 | chicken feathers | Sludge from an anaerobic digester from a WWTP | TS = 92.05 %; VS = 89.78%; C/N = 3.66; Vr = 0.056 L; TRH = 55 d; T = 37 °C | 430 | 292 | [102] |
| Thermal | Pressure in a stirred tank 150 °C/5 min and 4.8 bar | Poultry manure | Digestate from a biogas plant from cattle manure and corn silage | TS = 52.73 %; VS = 37.25%; Vr = 0.05 L; T = 39 °C | 288 | 14.4 | [103] |
| Physiochemical | (70 °C) from chicken manure under 3-day HRT | Poultry manure | Sludge from an anaerobic chicken manure reactor | Reactor CSTR; Vr = 16 L; TRH = 120 d; T = 55 °C | 518 | 54.6 | [104] |
| Physiochemical | High pressure and temperature reactor (T = 200 °C, 15 min) | Poultry manure | Anaerobic sludge from anaerobic digester from cow, corn and grass manure | Vr = 0.500 L; TRH = 90 d; T = 35 °C | 340 | −7.86 | [105] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Orlando, M.-Q.; Borja, V.-M. Pretreatment of Animal Manure Biomass to Improve Biogas Production: A Review. Energies 2020, 13, 3573. https://doi.org/10.3390/en13143573
Orlando M-Q, Borja V-M. Pretreatment of Animal Manure Biomass to Improve Biogas Production: A Review. Energies. 2020; 13(14):3573. https://doi.org/10.3390/en13143573
Chicago/Turabian StyleOrlando, Meneses-Quelal, and Velázquez-Martí Borja. 2020. "Pretreatment of Animal Manure Biomass to Improve Biogas Production: A Review" Energies 13, no. 14: 3573. https://doi.org/10.3390/en13143573
APA StyleOrlando, M.-Q., & Borja, V.-M. (2020). Pretreatment of Animal Manure Biomass to Improve Biogas Production: A Review. Energies, 13(14), 3573. https://doi.org/10.3390/en13143573






