Assessment of Green Methanol Production Potential and Related Economic and Environmental Benefits: The Case of China
Abstract
1. Introduction
2. Literature Review
2.1. Benefits of Methanol Based Fuels
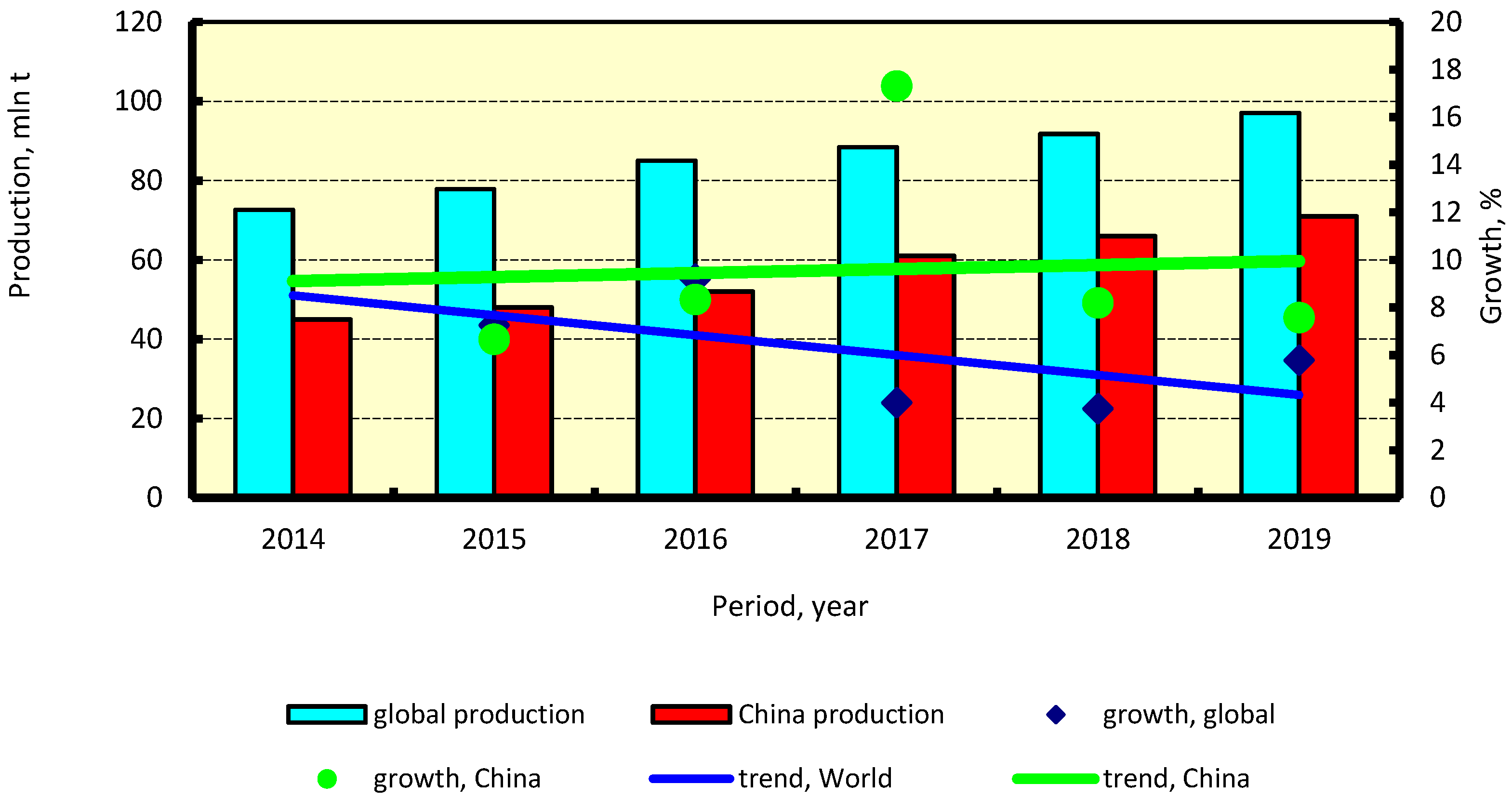
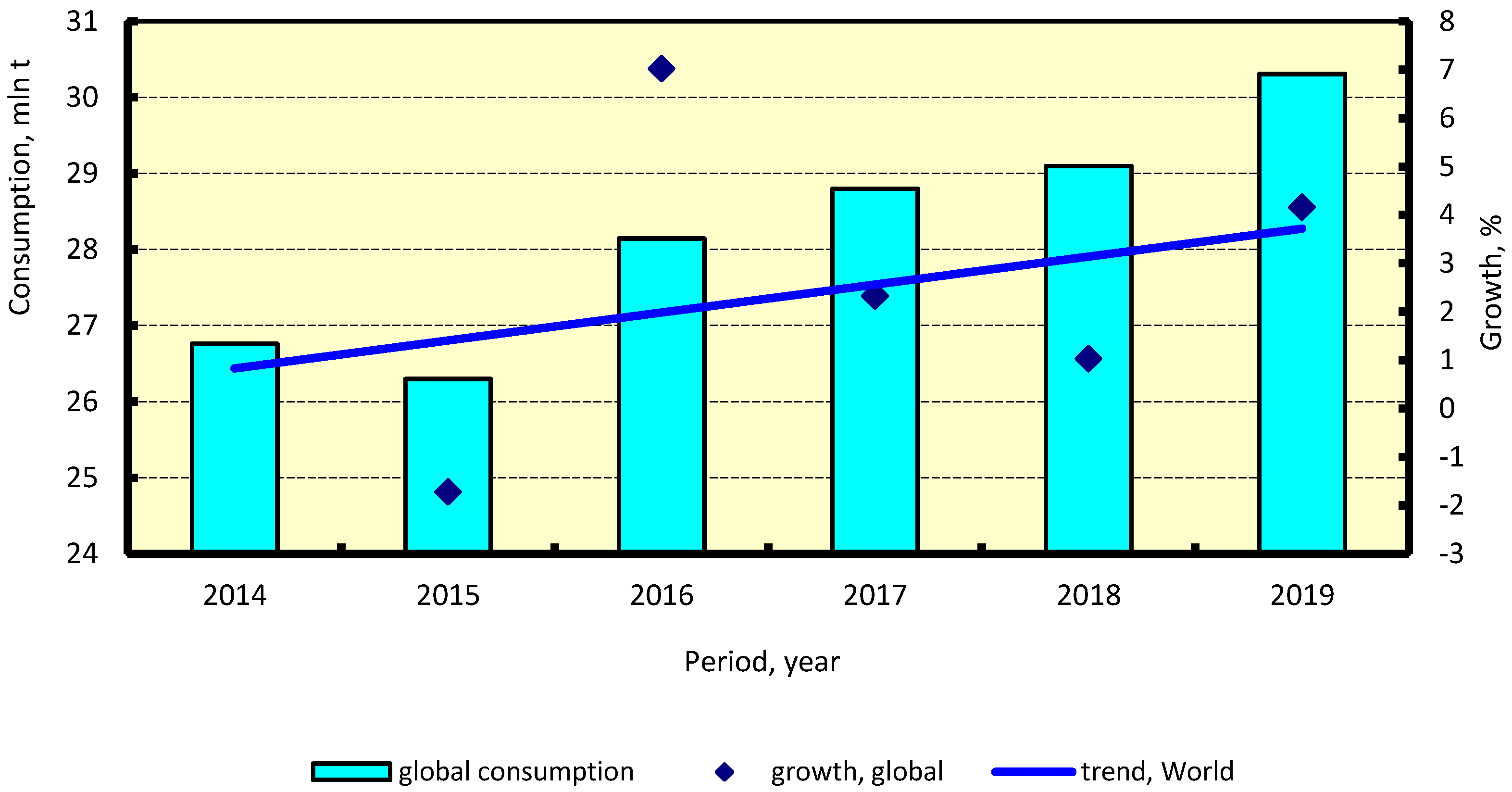
2.2. Methanol Market Forecasts
2.3. Feedstock and Methanol Production
3. Materials and Methods
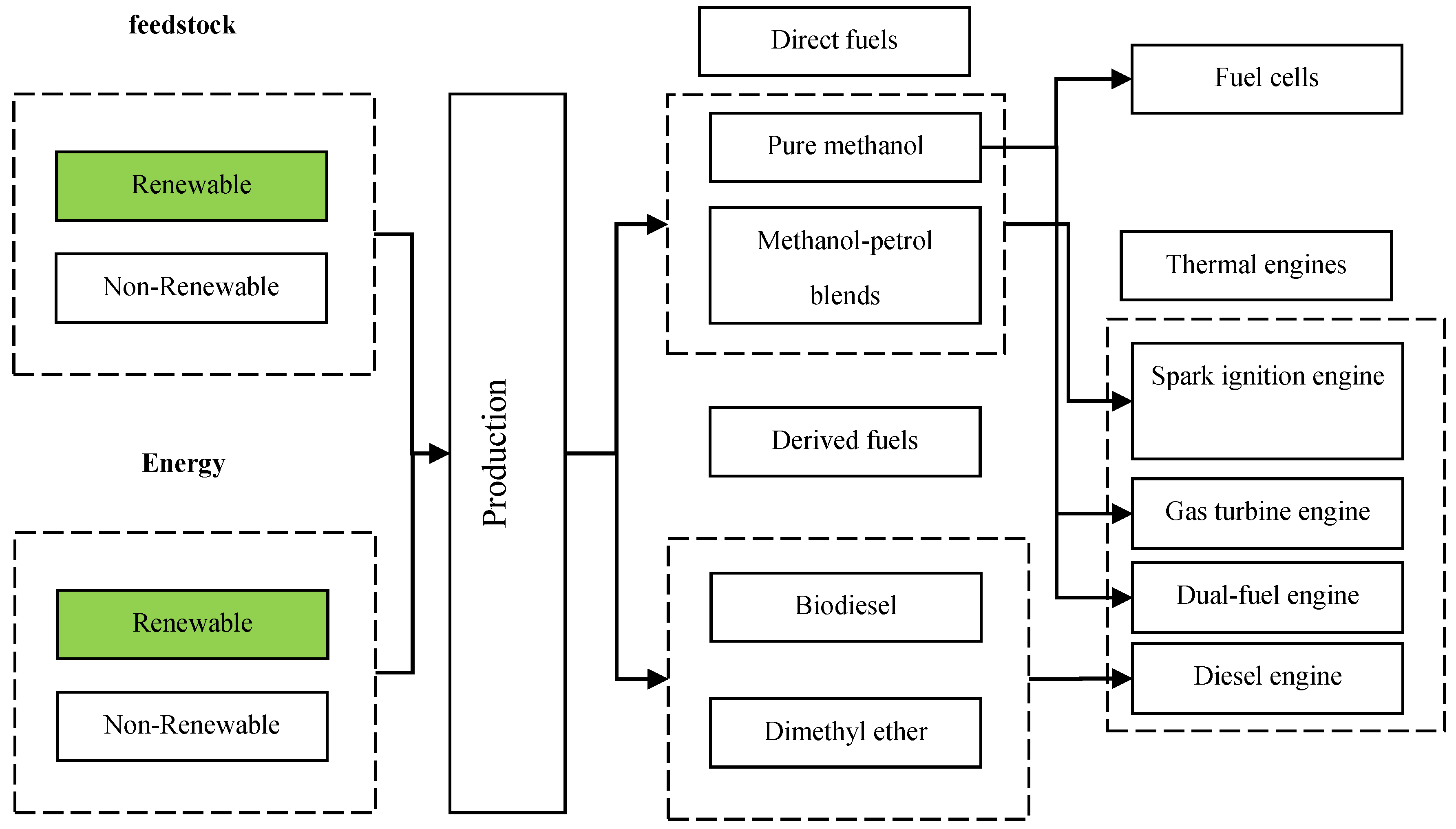
3.1. Fuels
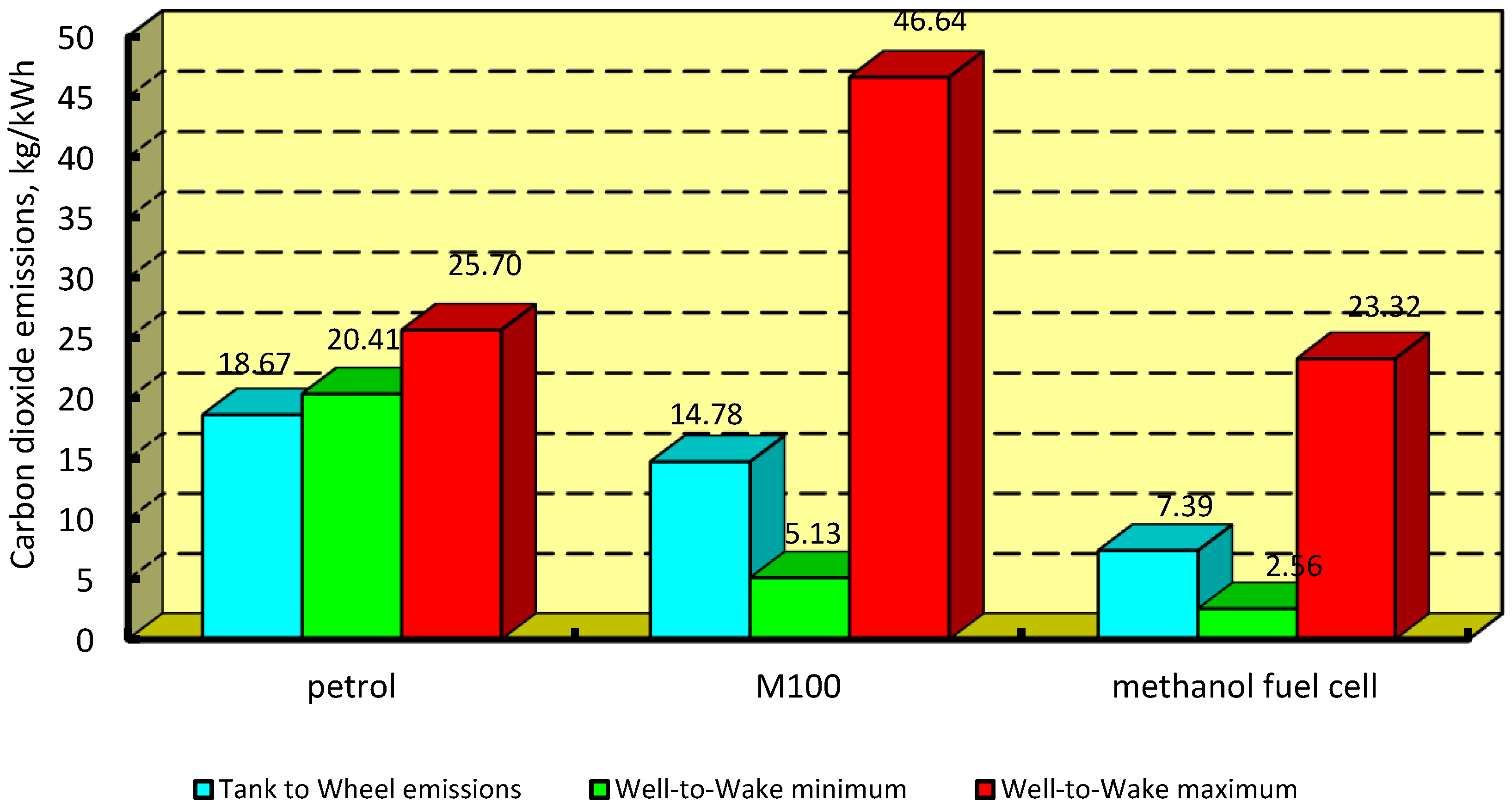
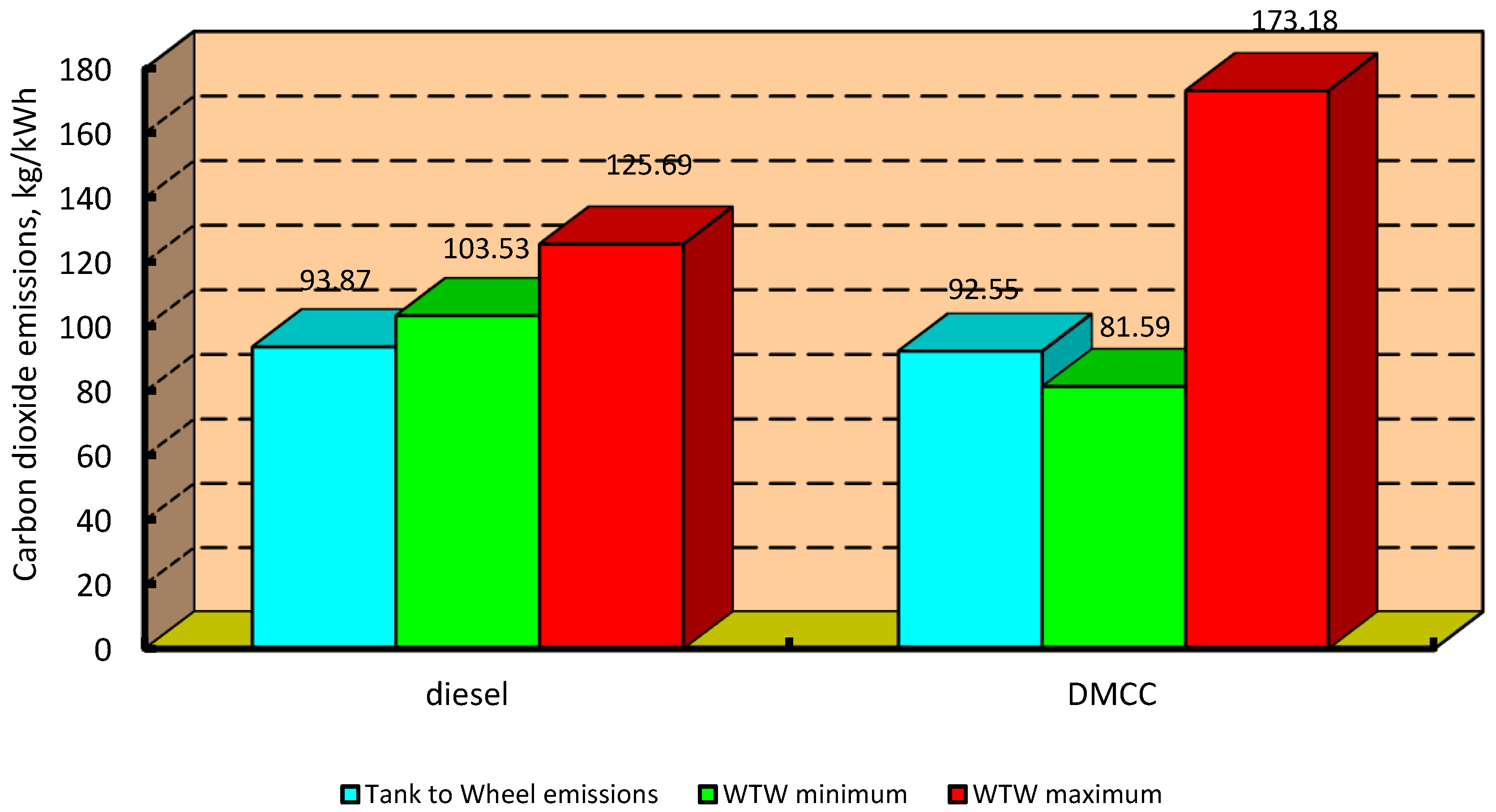
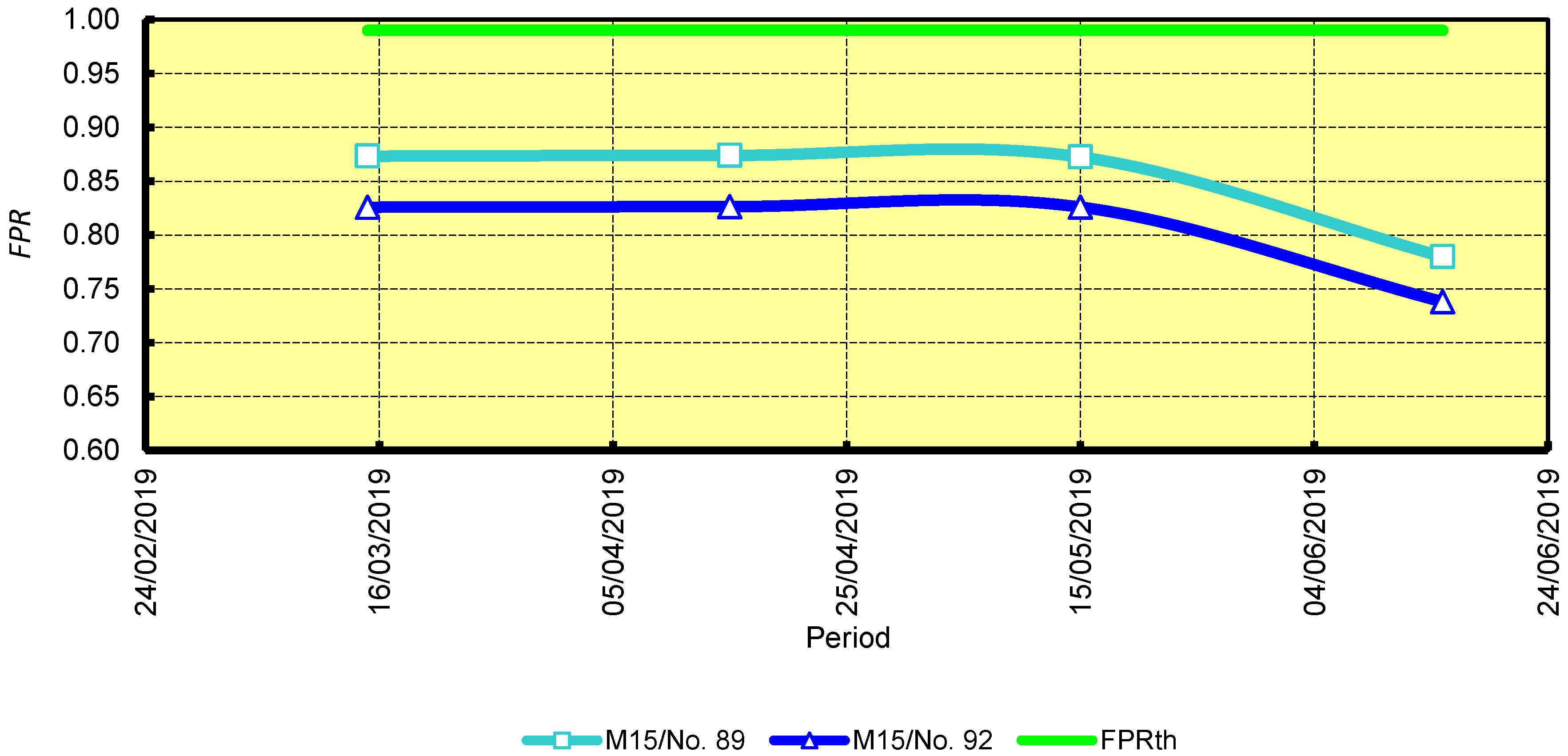
3.2. Carbon Dioxide Emissions Indicator
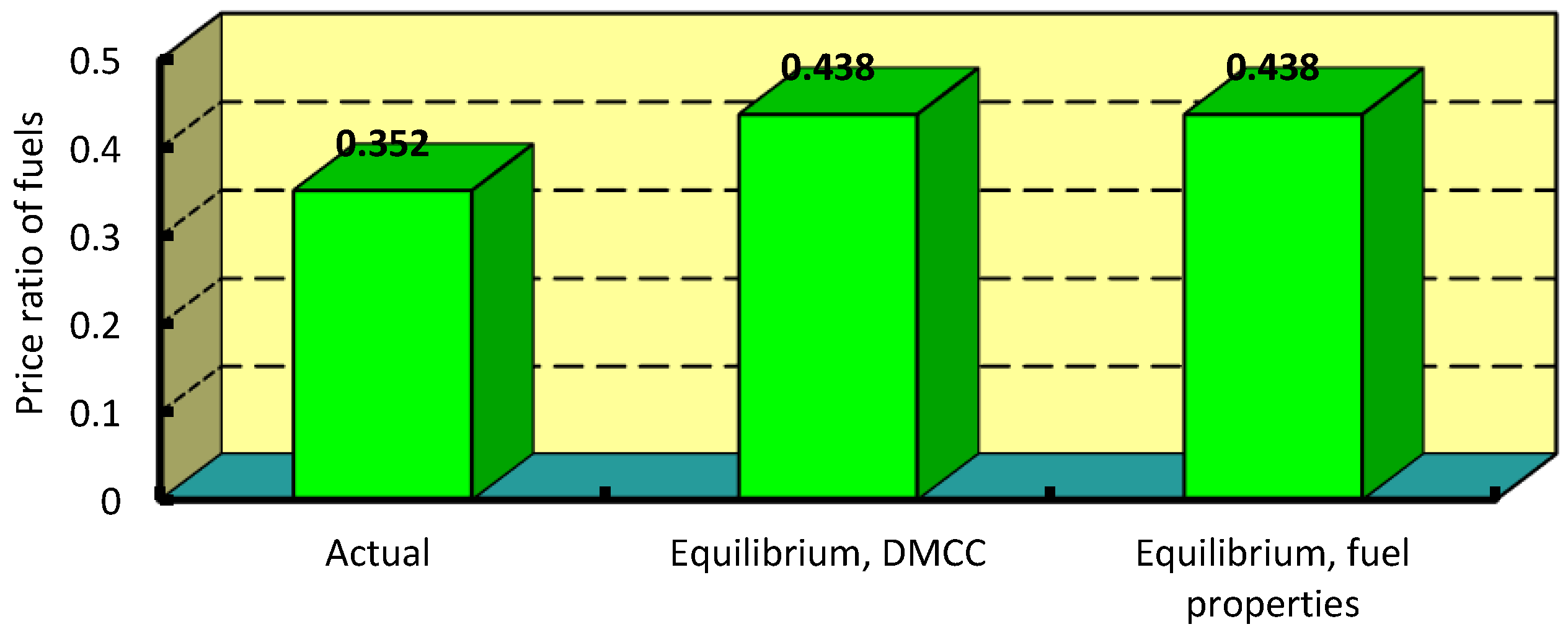
3.3. Efficiency Indicators and Economic Assessment
- Engine efficiency;
- Specific fuel consumption;
- Fuel economy.
3.4. Information Base
4. Results
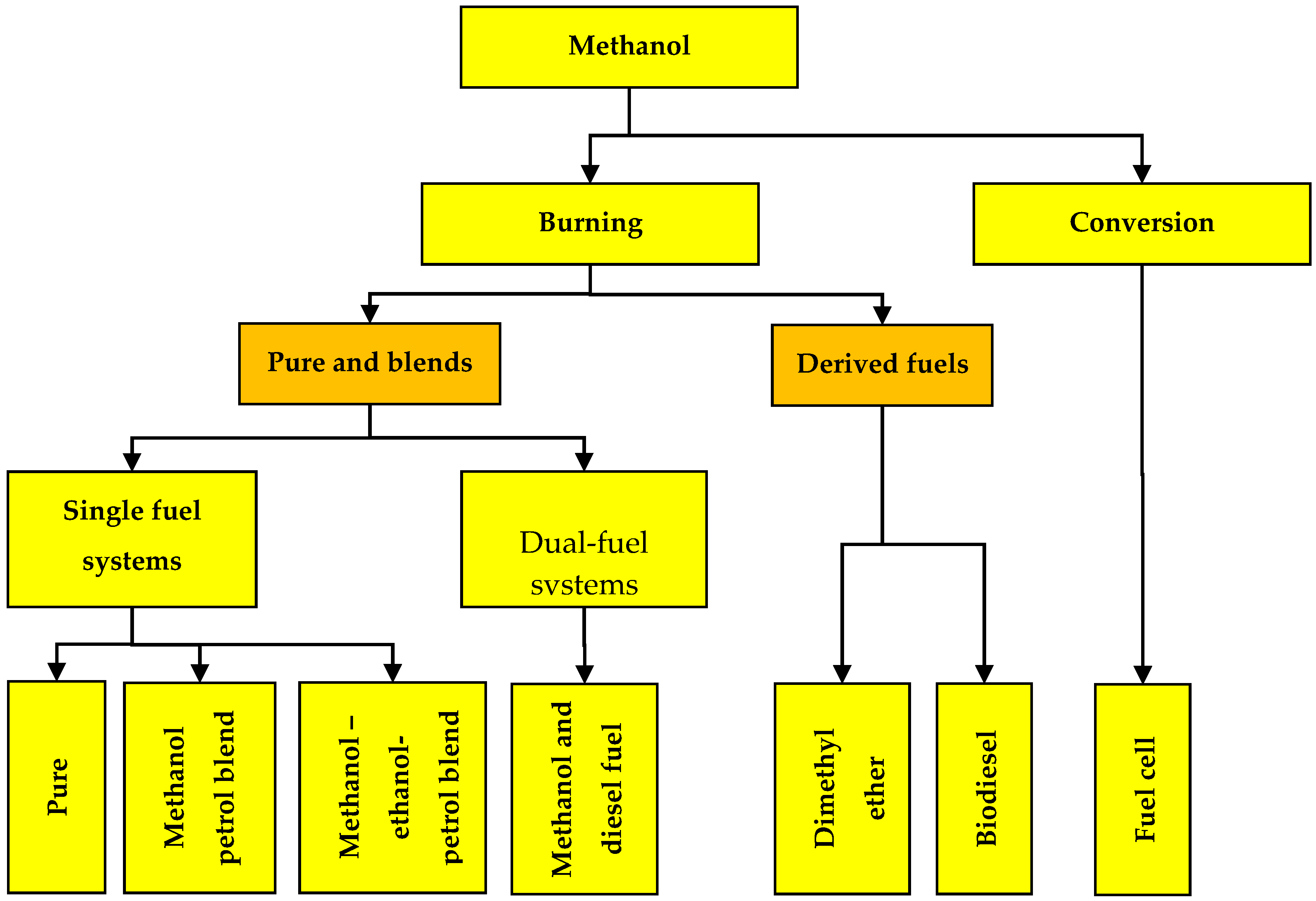
4.1. Pathway for Methanol Utilization
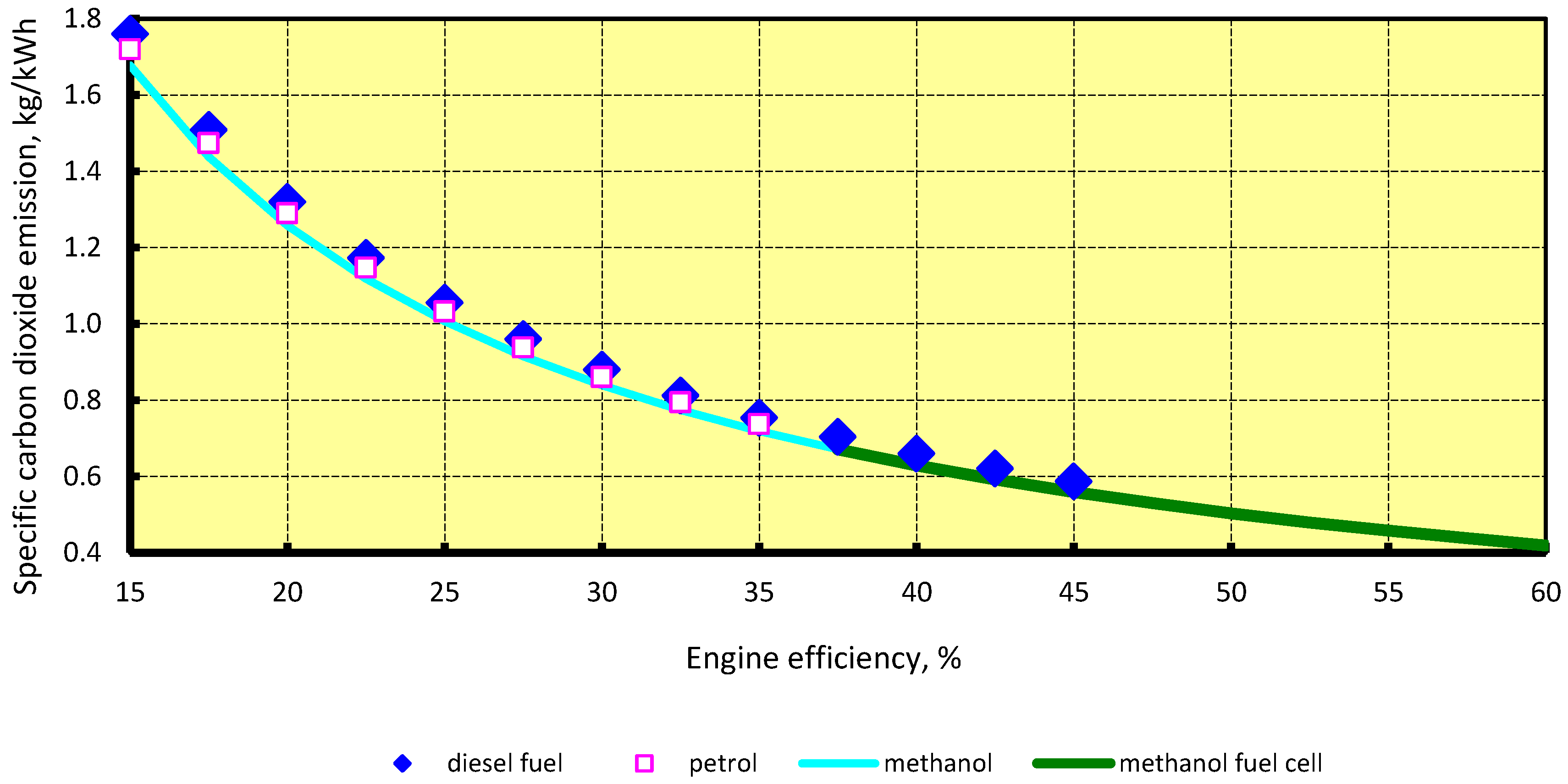
4.2. Carbon Dioxide Emissions
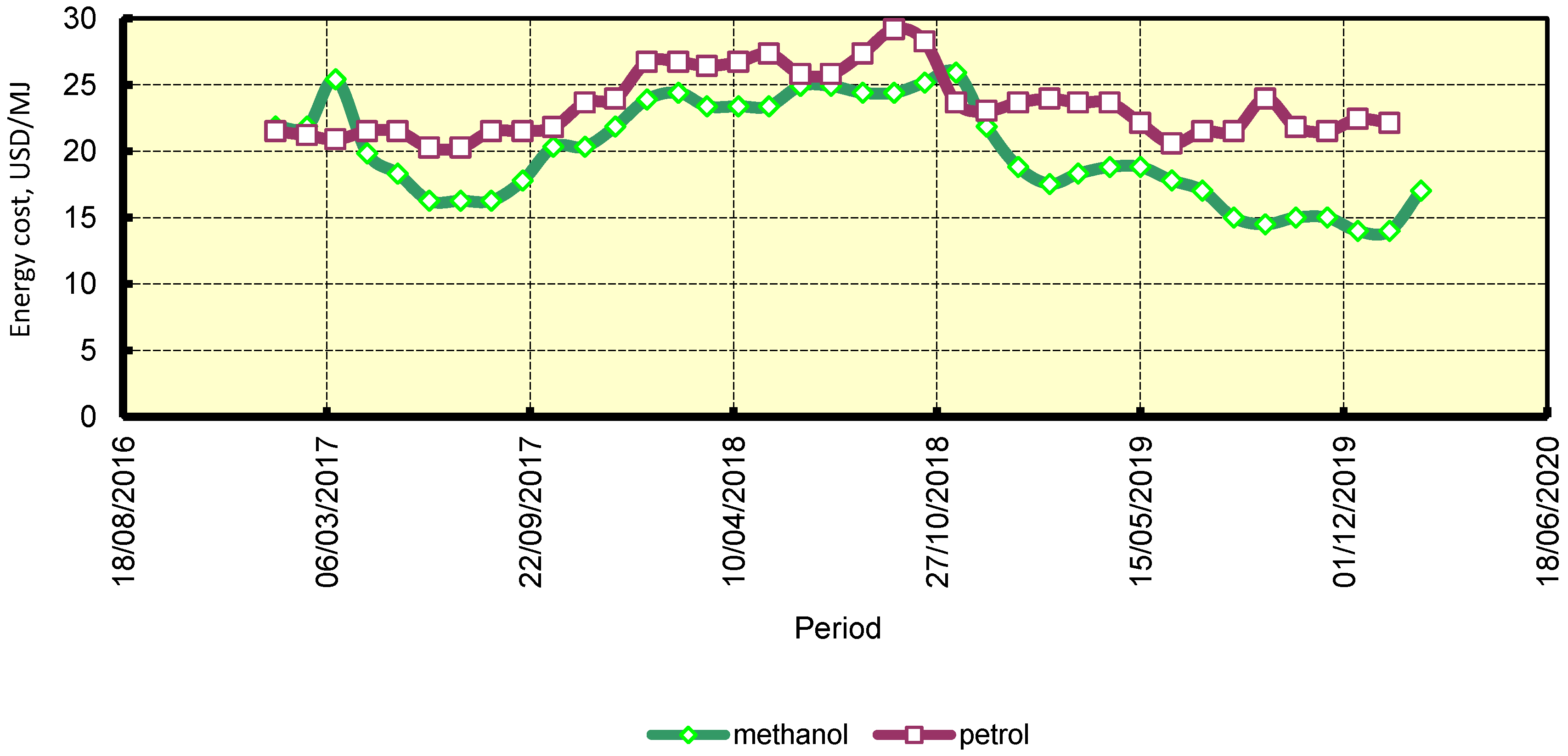
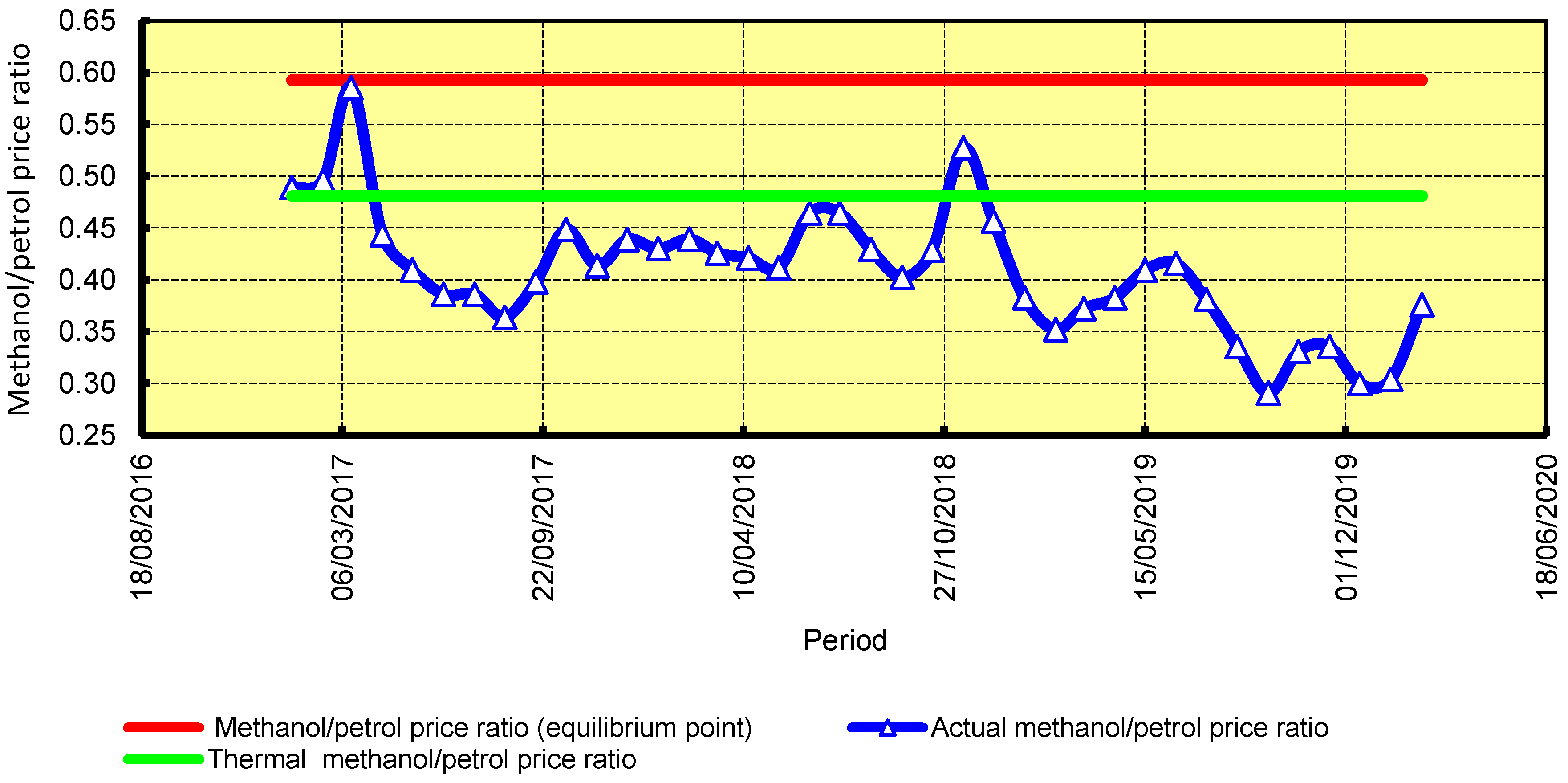
4.3. Economic Assessment
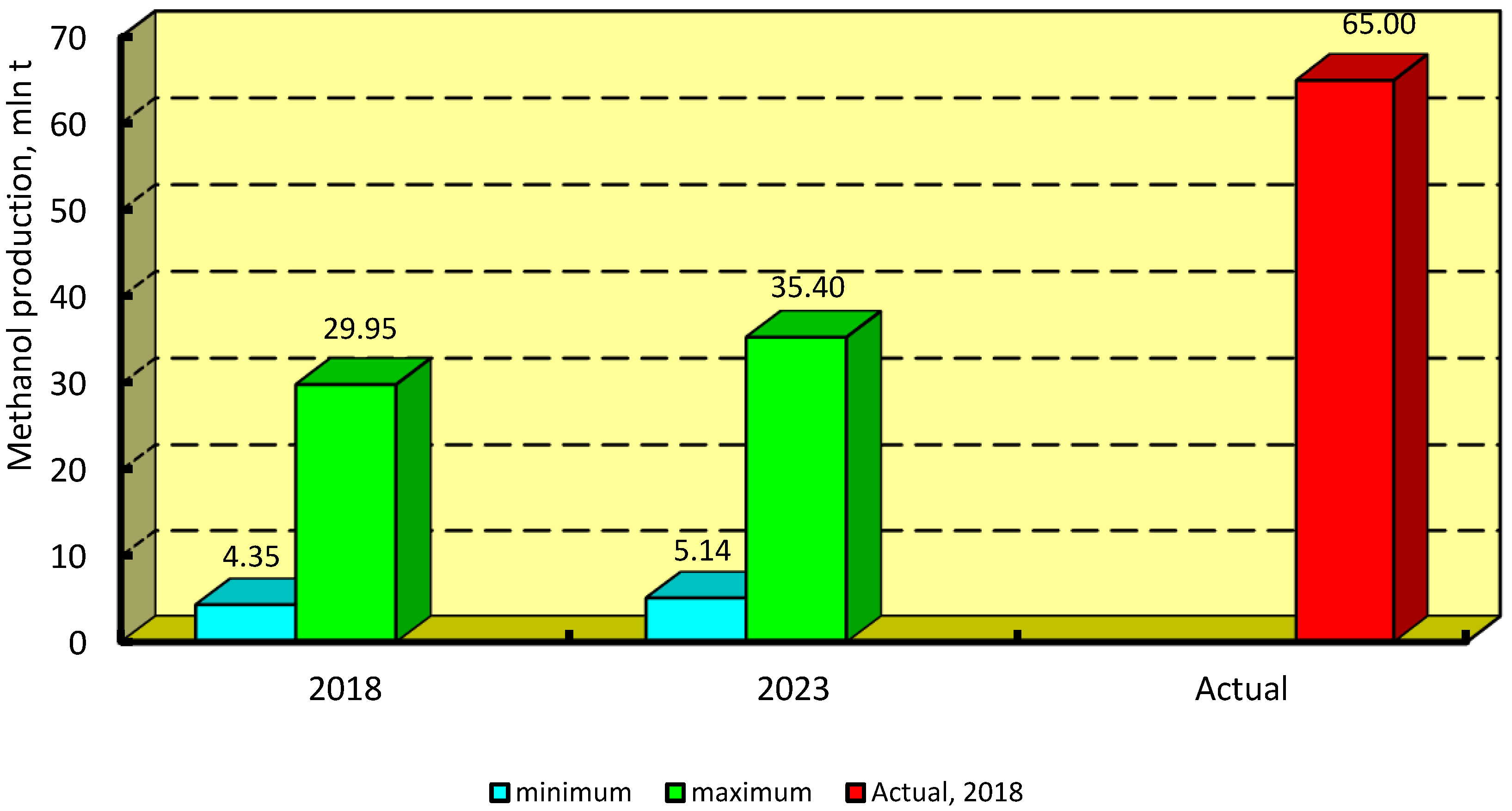
4.4. Methanol Production
4.4.1. Green Methanol
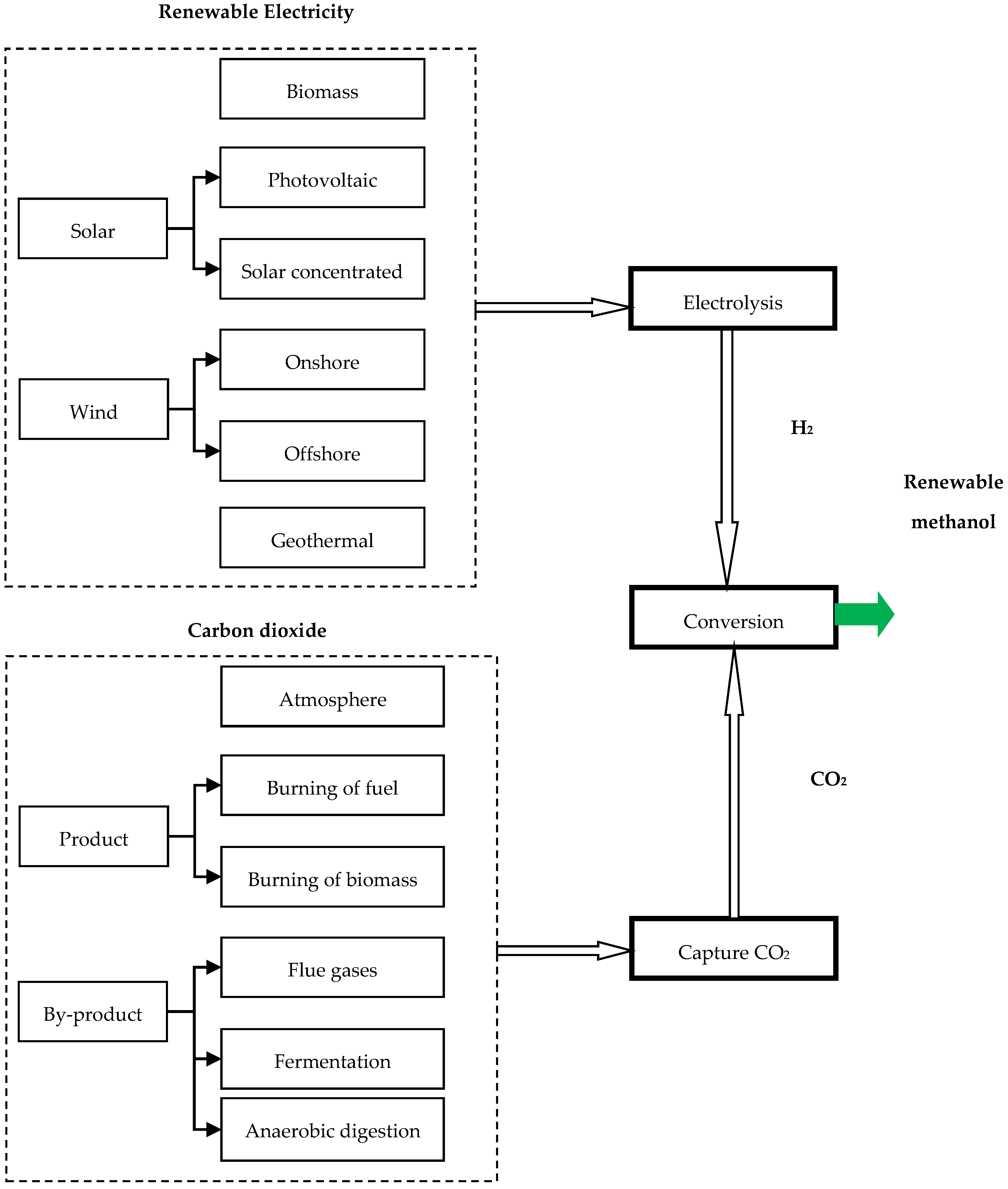
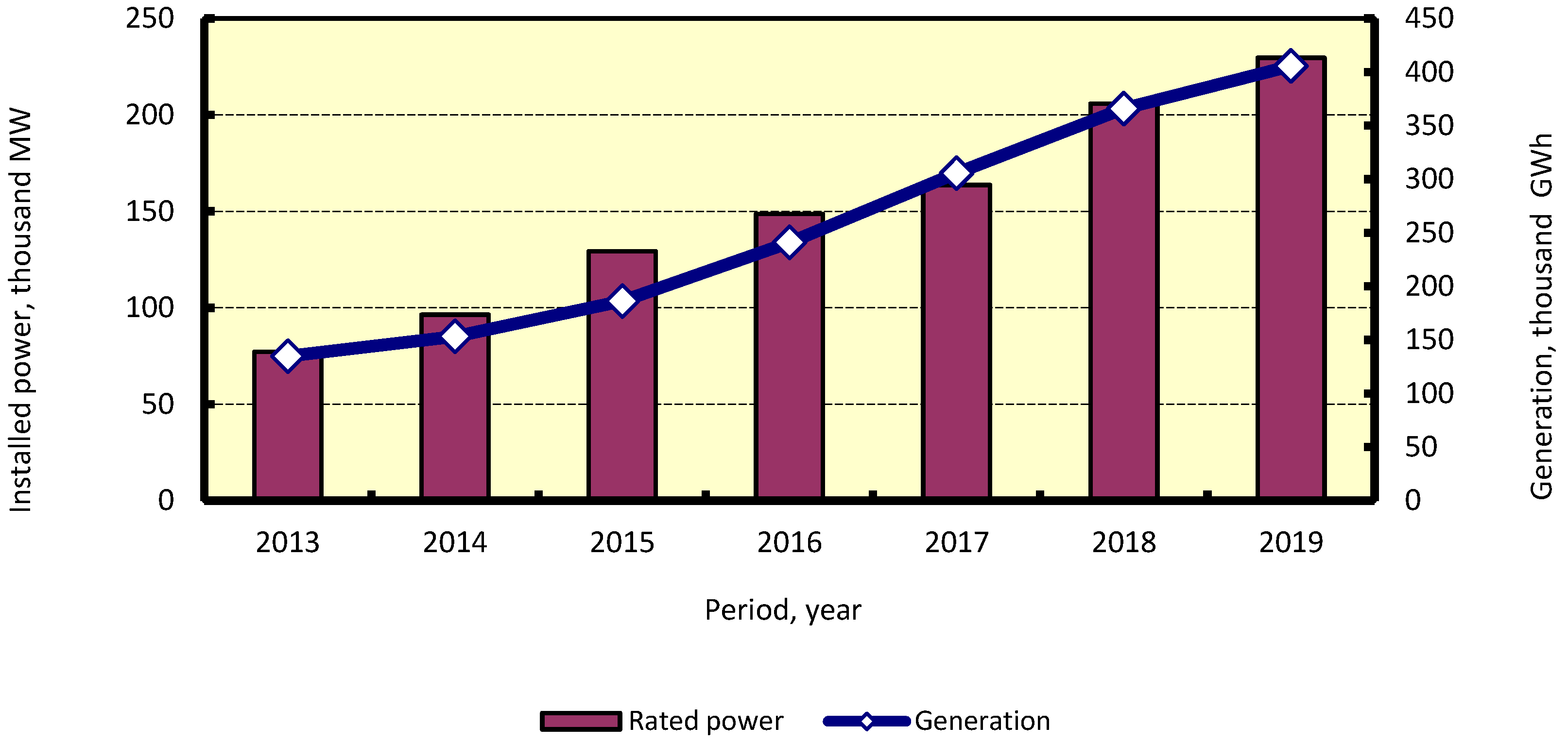
4.4.2. Renewable Methanol (Carbon Dioxide and Renewable Electricity)
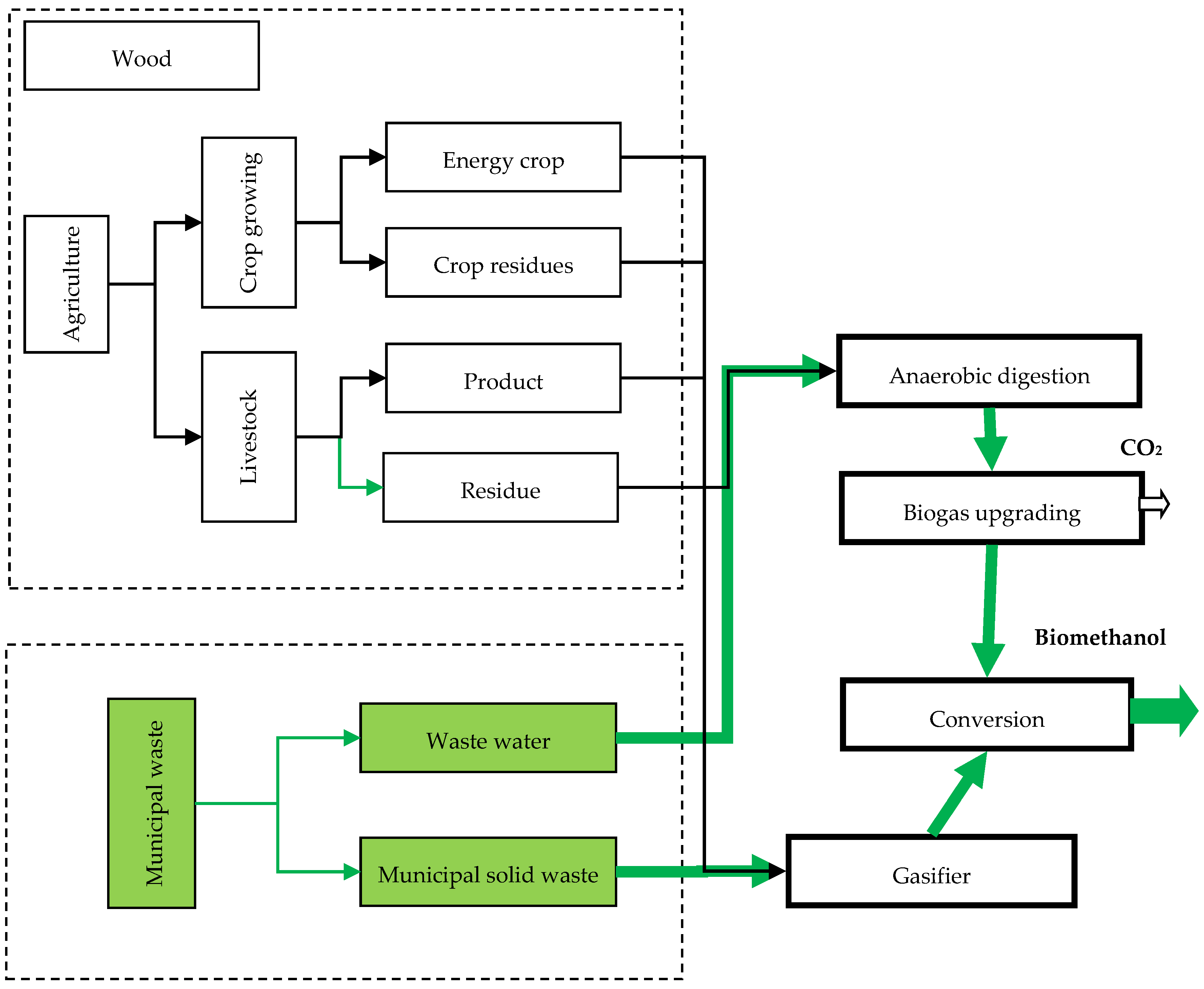
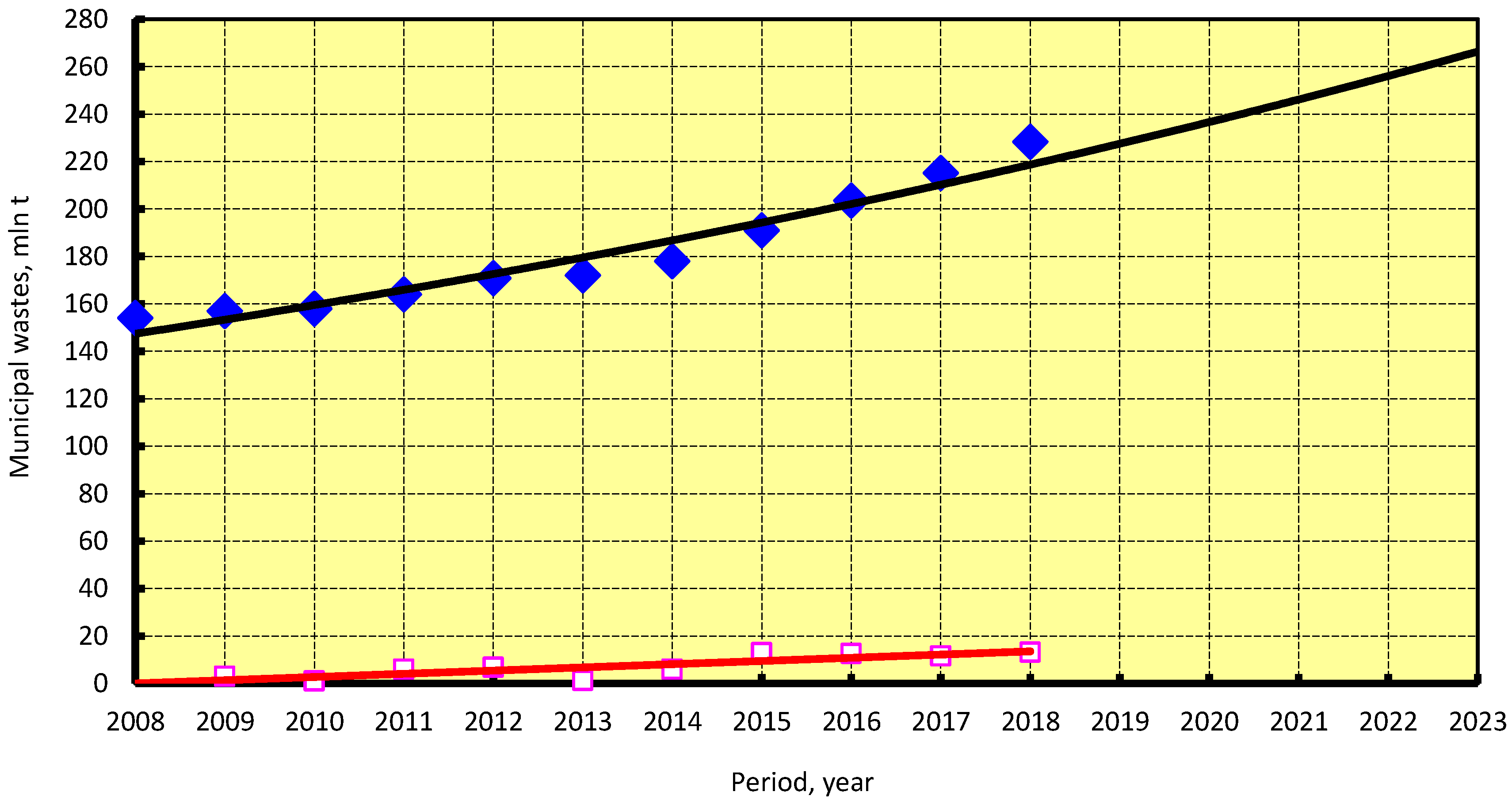
4.4.3. Biomethanol
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- International Energy Agency. Transport, Energy and CO2: Moving Toward Sustainability; OECD/IEA: Paris, France, 2009; Available online: https://www.iea.org/news/transport-energy-and-co2-moving-toward-sustainability (accessed on 11 April 2020).
- IEA. Global Energy & CO2 Status Report 2019. Available online: https://www.iea.org/reports/global-energy-co2-status-report-2019/emissions (accessed on 20 April 2020).
- IPCC—Intergovernmental Panel on Climate Change. IPCC Fourth Assessment Report; UN IPCC: Geneva, Switzerland, 2007; Available online: https://www.ipcc.ch/assessment-report/ar4/ (accessed on 11 April 2020).
- Yuan, X.; Liu, X.; Zuo, J. The development of new energy vehicles for a sustainable future: A review. Renew. Sustain. Energy Rev. 2015, 42, 298–305. [Google Scholar] [CrossRef]
- Zhang, X.; Liang, Y.; Yu, E.; Rao, R.; Xie, J. Review of electric vehicle policies in China: Content summary and effect analysis. Renew. Sustain. Energy Rev. 2017, 70, 698–714. [Google Scholar] [CrossRef]
- Hao, H.; Liu, Z.; Zhao, F.; Ren, J.; Chang, S.; Rong, K.; Du, J. Biofuel for vehicle use in China: Current status, future potential and policy implications. Renew. Sustain. Energy Rev. 2018, 82, 645–653. [Google Scholar] [CrossRef]
- Li, C.; Negnevitsky, M.; Wang, X. Review of methanol vehicle policies in China: Current status and future implications. Energy Procedia 2019, 160, 324–331. [Google Scholar] [CrossRef]
- National Bureau of Statistics. China Energy Statistics Yearbook 2019; China Statistics Press: Beijing, China. Available online: http://www.stats.gov.cn/tjsj/ndsj/2019/indexeh.htm (accessed on 11 April 2020).
- Keil, F.J. Methanol-to-Hydrocarbons: Process Technology. Microporous Mesoporous Mater. 1999, 29, 49–66. [Google Scholar] [CrossRef]
- Catizzone, E.; Cirelli, Z.; Aloise, A.; Lanzafame, P.; Migliori, M.; Giordano, G. Methanol conversion over ZSM-12, ZSM-22 and EU-1 zeolites: From DME to hydrocarbons production. Catal. Today 2018, 304, 39–50. [Google Scholar] [CrossRef]
- Zhao, K. A Brief Review of China’s Methanol Vehicle Pilot and Policy. Available online: https://www.methanol.org/wp-content/uploads/2019/03/A-Brief-Review-of-Chinas-Methanol-Vehicle-Pilot-and-Policy-20-March-2019.pdf (accessed on 12 February 2020).
- Chan, T. Methanol Fuel Blending In China. In Proceedings of the Enmore 14th International Me OH Week, Nanjing, China, 9–10 July 2019; Available online: https://www.methanol.org/wp-content/uploads/2019/07/Methanol-Fuel-Blending-in-China.pdf (accessed on 29 March 2020).
- Methanol Institute. Methanol Price and Supply/Demand. Available online: https://www.methanol.org/methanol-price-supply-demand/ (accessed on 15 April 2020).
- Methanol Production in the United States from 1990 to 2018. Available online: https://www.statista.com/statistics/974802/us-methanol-production-volume/ (accessed on 30 May 2020).
- Methanol Production in Russia in 2018 Increased by 7%. Available online: http://rcc.ru/article/proizvodstvo-metanola-v-rossii-v-2018-g-vyroslo-na-7–69007 (accessed on 30 May 2020).
- Methanex Investor Presentation. March 2019. Available online: https://www.methanex.com/sites/default/files/investor/MEOH-Presentation-2019–03–04.pdf (accessed on 30 May 2020).
- Olah, G.A.; Goeppert, A.; Prakash, G.K.S. Beyond Oil and Gas: The Methanol Economy; Wiley-VCH: Hoboken, NJ, USA, 2006; Available online: https://onlinelibrary.wiley.com/doi/book/10.1002/9783527627806 (accessed on 11 April 2020).
- Olah, G.A. Towards Oil Independence Through Renewable Methanol Chemistry. Angew. Chem. Int. Ed. 2013, 52, 104–107. [Google Scholar] [CrossRef]
- Liao, H.; Long, Y.; Ming, T.; Mardani, A.; Xu, J. Low carbon supplier selection using a hesitant fuzzy linguistic span method integrating the analytic network. Transform. Bus. Econ. 2019, 18, 67–88. [Google Scholar]
- Tian, Z.; Zhen, X.; Wang, Y.; Liu, D.; Li, X. Comparative study on combustion and emission characteristics of methanol, ethanol and butanol fuel in TISI engine. Fuel 2020, 259, 116199. [Google Scholar] [CrossRef]
- Wang, X.; Ge, Y.; Liu, L.; Peng, Z.; Hao, L.; Yin, H.; Ding, Y.; Wang, J. Evaluation on toxic reduction and fuel economy of a gasoline direct injection- (GDI-) powered passenger car fueled with methanol–gasoline blends with various substitution ratios. Appl. Energy 2015, 157, 134–143. [Google Scholar] [CrossRef]
- Sun, H.; Wang, W.; Koo, K.-P. The practical implementation of methanol as a clean and efficient alternative fuel for automotive vehicles. Int. J. Engine Res. 2019, 20, 350–358. [Google Scholar] [CrossRef]
- Huang, P.; Ju, H.; Tan, S.; Wang, H.; Zhao, T. The Future of Methanol Fuel an Analysis on the Feasibility of Methanol as an Alternative Fuel. 4 December 2015. Team 1. Available online: http://franke.uchicago.edu/bigproblems/BPRO29000–2015/Team01-TheFutureofMethanolFuel.pdf (accessed on 16 January 2020).
- Duraisamy, G.; Rangasamy, M.; Govindan, N. A comparative study on methanol/diesel and methanol/PODE dual fuel RCCI combustion in an automotive diesel engine. Renew. Energy 2020, 145, 542–556. [Google Scholar] [CrossRef]
- Jia, Z.; Denbratt, I. Experimental investigation into the combustion characteristics of a methanol-Diesel heavy duty engine operated in RCCI mode. Fuel 2018, 226, 745–753. [Google Scholar] [CrossRef]
- Prasad, B.S.N.; Pandey, J.K.; Kumar, G.N. Impact of changing compression ratio on engine characteristics of an SI engine fueled with equi-volume blend of methanol and gasoline. Energy 2020, 191, 116605. [Google Scholar] [CrossRef]
- Bromberg, L.; Cheng, W.K. Methanol as an Alternative Transportation Fuel in the US: Options for Sustainable and/or Energy-Secure Transportation. Massachusetts Institute of Technology. Final Report. 2010. Available online: https://afdc.energy.gov/files/pdfs/mit_methanol_white_paper.pdf (accessed on 30 May 2020).
- Infoholic Research LLP. Methanol Market—Global Drivers, Restraints, Opportunities, Trends Forecast up to 2023; Infoholic Research LLP: Karnataka, India, 2019. [Google Scholar]
- IMARC Group. Methanol Market: Global Industry Trends, Share, Size, Growth, Opportunity and Forecast 2019–2024; IMARC Group: Noida, India, 2019; ID: 4763079. [Google Scholar]
- Grand View Research. Methanol Market Size, Share Trends Analysis Report by Application (Formaldehyde, Acetic Acid, MTBE, DME, Fuel Blending, MTO, Biodiesel), by Region, and Segment Forecasts, 2019–2025; Grand View Research: San Francisco, CA, USA, 2019; ID: GVR-4-68038-016-3. [Google Scholar]
- Prismane Consulting. Global Methanol Market Study 2014–2025; Prismane Consulting: Maharashtra, India, 2018. [Google Scholar]
- Mordor Intelligence. Methanol Market—Growth, Trends, and Forecast (2020–2025); Mordor Intelligence: Hyderabad, India, 2020; ID: 4514928. [Google Scholar]
- Polaris Market Research. Methanol Market Share, Size, Trends Industry Analysis Report, by Feedstock (Natural Gas, Coal), by Derivatives (Formaldehyde, Acetic Acid, Gasoline, MTO, Gasoline, MMA), by End-Use (Automotive, Construction, Electronics, Paints Coatings, Pharmaceuticals), by Regions: Segment Forecast, 2019—2026; Polaris Market Research: Maharashtra, India, 2019; ID: PM1543. [Google Scholar]
- Triton Market Research. Global Methanol Market 2019–2027; Triton Market Research, July 2019. ID: 4791833. Available online: https://www.tritonmarketresearch.com/reports/methanol-market (accessed on 16 June 2020).
- Inkwood Research. Global Methanol Market Forecast 2019–2027; Inkwood Research: Westford, MA, USA, 2019; ID: INKR15970824. [Google Scholar]
- Statista. Production Capacity of Methanol Worldwide in 2018 and 2030 (in Million Metric Tons). Available online: https://www.statista.com/statistics/1065891/global-methanol-production-capacity/ (accessed on 5 January 2020).
- Joshi, A. 160,000 Jobs can be Created by Manufacturing Petrochems Using Green Methanol: CEEW. ETEnergyWorld. 17 October 2019. Available online: https://energy.economictimes.indiatimes.com/news/oil-and-gas/160000-jobs-can-be-created-by-manufacturing-petrochems-using-green-methanol-ceew/71423985 (accessed on 12 December 2019).
- Bassani, A.; Bozzano, G.; Pirola, C.; Ranzi, E.; Pierucci, S.; Manenti, F. Low Impact Methanol Production from Sulfur Rich Coal Gasification. Energy Procedia 2017, 105, 4519–4524. [Google Scholar] [CrossRef]
- Wang, X. Sustainable Clean Coal Technology with Power and Methanol Production. Chem. Biomol. Eng. Theses Diss. Stud. Res. 2017, 30. [Google Scholar]
- Liu, Z. Economic analysis of methanol production from coal/biomass upgrading. Energy Sources Part. B Econ. Plan. Policy 2018, 13, 66–71. [Google Scholar] [CrossRef]
- Blumberg, T.; Tsatsaronis, G.; Morosuk, T. On the economics of methanol production from natural gas. Fuel 2019, 256, 115824. [Google Scholar] [CrossRef]
- Yang, S.; Chen, Q.; Liu, Z.; Wang, Y.; Tang, Z.; Sun, Y. Performance analysis of the wind energy integrated with a natural-gas-to-methanol process. Energy Convers. Manag. 2018, 173, 735–742. [Google Scholar] [CrossRef]
- Yang, S.; Li, B.; Zheng, J.; Kankala, R.K. Biomass-to-Methanol by dual-stage entrained flow gasification: Design and techno-economic analysis based on system modeling. J. Clean. Prod. 2018, 205, 364–374. [Google Scholar] [CrossRef]
- Liu, Z.; Peng, W.; Motahari-Nezhad, M.; Shahraki, S.; Beheshti, M. Circulating fluidized bed gasification of biomass for flexible end-use of syngas: A micro and nano scale study for production of bio-methanol. J. Clean. Prod. 2016, 129, 249–255. [Google Scholar] [CrossRef]
- Roode-Gutzmer, Q.I.; Kaiser, D.; Bertau, M. Renewable Methanol Synthesis. ChemBioEng Rev. 2019, 6, 209–236. [Google Scholar] [CrossRef]
- Borgogna, A.; Salladini, A.; Spadacini, L.; Pitrelli, A.; Annesini, M.C.; Iaquaniello, G. Methanol production from Refuse Derived Fuel: Influence of feedstock composition on process yield through gasification analysis. J. Clean. Prod. 2019, 235, 1080–1089. [Google Scholar] [CrossRef]
- Borgwardt, R.H. Transportation fuel from cellulosic biomass: A comparative assessment of ethanol and methanol options. Proc. Inst. Mech. Eng. Part A J. Power Energy 1999, 213, 399–407. [Google Scholar] [CrossRef]
- Andres Soria, J.; McDonald, A.G. Liquefaction of Softwoods and Hardwoods in Supercritical Methanol: A Novel Approach to Bio-Oil Production. In Biomass Conversion; Baskar, C., Baskar, S., Dhillon, R., Eds.; Springer: Berlin/Heidelberg, Germany, 2012. [Google Scholar] [CrossRef]
- Franca, R.G.; Souza, P.A.; Lima, E.R.; Costa, A.L. An extended techno-economic analysis of the utilization of glycerol as an alternative feedstock for methanol production. Clean Technol. Environ. Policy 2017, 19, 1855–1865. [Google Scholar] [CrossRef]
- Zhang, C.; Jun, K.W.; Gao, R.; Kwak, G.; Park, H.G. Efficient Way of Carbon Dioxide Utilization in a Gas-to-Methanol Process: From Fundamental Research to Industrial Demonstration. Top. Catal. 2018, 61, 1794–1809. [Google Scholar] [CrossRef]
- Hank, C.; Gelpke, S.; Schnabl, A.; White, R.J.; Full, J.; Wiebe, N.; Smolinka, T.; Schaadt, A.; Henning, H.-M.; Hebling, C. Economics carbon dioxide avoidance cost of methanol production based on renewable hydrogen and recycled carbon dioxide—Power-to-methanol. Sustain. Energy Fuels 2018, 2, 1244–1261. [Google Scholar] [CrossRef]
- Bergins, C.; Fox, E.L.; Tran, K.C.; Wuebben, P. Commercialization of Low Carbon Methanol. ATZextra Worldw. 2016, 21, 22–25. [Google Scholar] [CrossRef]
- Ishaq, H.; Dincer, I. Evaluation of a wind energy based system for co-generation of hydrogen and methanol production. Int. J. Hydrogen Energy 2020. [Google Scholar] [CrossRef]
- Perathoner, S.; Centi, G. CO2 Recycling: A Key Strategy to Introduce Green Energy in the Chemical Production Chain. Chem. Sus. Chem. 2014, 7, 1274–1282; [Google Scholar] [CrossRef]
- Bonura, G.; Cannilla, C.; Frusteri, L.; Catizzone, E.; Todaro, S.; Migliori, M.; Giordano, G.; Frusteri, F. Interaction effects between CuO-ZnO-ZrO2 methanol phase and zeolite surface affecting stability of hybrid systems during one-step CO2 hydrogenation to DME. Catal. Today 2020, 345, 175–182. [Google Scholar] [CrossRef]
- Catizzone, E.; Bonura, G.; Migliori, M.; Frusteri, F.; Giordano, G. CO2 Recycling to Dimethyl Ether: State-of-the-Art and Perspectives. Molecules 2018, 23, 31. [Google Scholar] [CrossRef]
- Dolan, G. Fuel Application for Methanol. In Proceedings of the HIS World Methanol Conference, Berlin, Germany, 5 October 2019; Available online: https://www.methanol.org/wp-content/uploads/2019/10/Fuel-Applications-for-Methanol-IHS-2019.pdf (accessed on 12 February 2020).
- Fayyazbakhsh, A.; Pirouzfar, V. Comprehensive overview on diesel additives to reduce emissions, enhance fuel properties and improve engine performance. Renew. Sustain. Energy Rev. 2017, 74, 891–901. [Google Scholar] [CrossRef]
- Yusri, I.M.; Mamat, R.; Najafi, G.; Razman, A.; Awad, O.I.; Azmi, W.H.; Ishak, W.F.W.; Shaiful, A.I.M. Alcohol based automotive fuels from first four alcohol family in compression and spark ignition engine: A review on engine performance and exhaust emissions. Renew. Sustain. Energy Rev. 2017, 77, 169–181. [Google Scholar] [CrossRef]
- Kumar, M.S.; Nataraj, G.; Arulselvan, S. A comprehensive assessment on the effect of high octane fuels induction on engine’s combustion behaviour of a Mahua oil based dual fuel engine. Fuel 2017, 199, 176–184. [Google Scholar] [CrossRef]
- Yates, A.; Bell, A.; Swarts, A. Insights relating to the autoignition characteristics of alcohol fuels. Fuel 2010, 89, 83–93. [Google Scholar] [CrossRef]
- Goncharuk, A.G.; Havrysh, V.I.; Nitsenko, V.S. National features for alternative motor fuels market. Int. J. Energy Technol. Pol. 2018, 14, 226–249. [Google Scholar] [CrossRef]
- Cherednichenko, O.; Havrysh, V.; Shebanin, V.; Kalinichenko, A.; Mentel, G.; Nakonieczny, J. Local Green Power Supply Plants Based on Alcohol Regenerative Gas Turbines: Economic and Environmental Aspects. Energies 2020, 13, 2156. [Google Scholar] [CrossRef]
- Zhao, K. Methanol Fuel Blending in China. Trinidad and Tobago Methanol Forum. 24 January 2019. Available online: https://www.methanol.org/wp-content/uploads/2019/02/6.-Kai-Zhao-Methanol-Fuel-Blending-in-China.pdf (accessed on 14 March 2020).
- Avenues for Collaboration. Recommendations for US—China Transportation Fuel Cooperation. United State Energy Security Council. 2015. Available online: http://www.iags.org/USChinaFC.pdf (accessed on 3 March 2020).
- Winther, K. Methanol as Motor Fuel. IEA-AMF Annex 56. Danish Technological Institute. 2019. Available online: http://danskbiomethanol.dk/Papers/Report%20DK.pdf (accessed on 29 March 2020).
- Dolan, G. Overview of Global Methanol Fuel Blending, Trinidad and Tobago Methanol Fuel Blending Forum. 24 January 2019. Available online: https://www.methanol.org/wp-content/uploads/2019/02/4.-Greg-Dolan-Overview-of-Global-Methanol-Fuel-Blending.pdf (accessed on 13 February 2020).
- Gumpert Presents Serial-Ready Methanol Fuel Cell. Available online: https://www.electrive.com/2019/11/08/gumpert-presents-serial-ready-methanol-fuel-cell/ (accessed on 29 March 2020).
- Yao, C.; Cheung, C.S.; Cheng, C.; Wang, Y.; Chan, T.L.; Lee, S.C. Effect of Diesel/methanol compound combustion on Diesel engine combustion and emissions. Energy Convers. Manag. 2008, 49, 1696–1704. [Google Scholar] [CrossRef]
- Methanol for Power Generation: A White Paper. ADI Analytics. Energy Insight and Consulting. September 2017. Available online: https://adi-analytics.com/wp-content/uploads/2017/09/ADI-MTP-White-Paper-Sep-2017-vf.pdf (accessed on 17 March 2020).
- China Gasoline Prices. Available online: https://tradingeconomics.com/china/gasoline-prices (accessed on 14 March 2020).
- Methanex Posts Regional Contract Methanol Prices for North America, Europe and Asia. Available online: https://www.methanex.com/our-business/pricing (accessed on 10 February 2020).
- Balussou, D. An Analysis of Current and Future Electricity Production from Biogas in Germany; Karlsruhe Institute of Technology: Karlsruhe, Germany, 2018; Available online: https://publikationen.bibliothek.kit.edu/1000084909 (accessed on 12 February 2020).
- Kalinichenko, A.; Havrysh, V.; Atamanyuk, I. The Acceptable Alternative Vehicle Fuel Price. Energies 2019, 12, 3889. [Google Scholar] [CrossRef]
- Shacman SX3317DR456HM Methanol/Diesel Dual Fuel Dump Truck Manufactured by Shaanxi Automobile Group Co.; Ltd. Available online: http://shaanxi.su/en/auto/sx/shacman-sx3317dr456hm.html (accessed on 20 March 2020).
- Sinotruk Howo ZZ3257N3847D1M Methanol/Diesel Dual Fuel Dump Truck Manufactured by Sinotruk Jinan Truck Co.; Ltd. Available online: http://howo.biz/howo/zz/sinotruk-howo-zz3257n3847d1m.html (accessed on 20 March 2020).
- Global Petrol Prices. Available online: https://www.globalpetrolprices.com/diesel_prices/ (accessed on 20 March 2020).
- Broeren, M. Production of Bio-Methanol: Technology Brief. IRENA. 2013. Available online: https://irena.org/publications/2013/Jan/Production-of-Bio-methanol (accessed on 30 May 2020).
- Giuliano, A.; Catizzone, E.; Barisano, D.; Nanna, F.; Villone, A.; De Bari, I.; Cornacchia, G.; Braccio, G. Towards Methanol Economy: A Techno-environmental Assessment for a Bio-methanol OFMSW/Biomass/Carbon Capture-based Integrated Plant. Int. J. Heat Technol. 2019, 37, 665–674. [Google Scholar] [CrossRef]
- Giuliano, A.; Catizzone, E.; Freda, C.; Cornacchia, G. Valorization of OFMSW Digestate-Derived Syngas toward Methanol, Hydrogen, or Electricity: Process Simulation and Carbon Footprint Calculation. Processes 2020, 8, 526. [Google Scholar] [CrossRef]
- Hobson, C.; Márquez, C. Renewable Methanol Report. Methanol Institute. December 2018. Available online: https://www.methanol.org/wp-content/uploads/2019/01/MethanolReport.pdf (accessed on 3 March 2020).
- Law, K.; Rosenfeld, J.; Jackson, M. Methanol as a Renewable Energy Resource; White Paper; TIAX LLCL: Cupertino, CA, USA, 2013; Available online: https://mafiadoc.com/queue/white-paper-methanol-as-a-renewable-energy-resource_59f9e63b1723dd8aab15caac.html (accessed on 29 March 2020).
- Su, L.W.; Li, X.R.; Sun, Z.Y. The consumption, production and transportation of methanol in China: A review. Energy Policy 2013, 63, 130–138. [Google Scholar] [CrossRef]
- Boulamanti, A.; Moya, J.A. Production costs of the chemical industry in the EU and other countries: Ammonia, methanol and light olefins. Renew. Sustain. Energy Rev. 2017, 68, 1205–1212. [Google Scholar] [CrossRef]
- Bozzano, G.; Manenti, F. Efficient methanol synthesis: Perspectives, technologies and optimization strategies. Prog. Energy Combust. Sci. 2016, 56, 71–105. [Google Scholar] [CrossRef]
- Deforestation Statistics for China. Available online: https://rainforests.mongabay.com/deforestation/archive/China.htm (accessed on 8 April 2020).
- Havrysh, V.; Kalinichenko, A.; Minkova, O.; Lyashenko, S. Agricultural feedstock for solid and liquid biofuel production in Ukraine: Cluster analysis. Procedia Environ. Sci. Eng. Manag. 2019, 6, 649–658. Available online: http://procedia-esem.eu/pdf/issues/2019/no4/73_Havrysh_19.pdf (accessed on 18 April 2020).
- Jiang, Y.; Havrysh, V.; Klymchuk, O.; Nitsenko, V.; Balezentis, T.; Streimikiene, D. Utilization of Crop Residue for Power Generation: The Case of Ukraine. Sustainability 2019, 11, 7004. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, L.; Van herle, J.; Maréchal, F.; Desideri, U. Techno-Economic Optimization of CO2-to-Methanol with Solid-Oxide Electrolyzer. Energies 2019, 12, 3742. [Google Scholar] [CrossRef]
- Netherlands Environmental Assessment Agency. Trends in Global CO2 Emissions: 2016 Report. Available online: https://edgar.jrc.ec.europa.eu/news_docs/jrc-2016-trends-in-global-co2-emissions-2016-report-103425.pdf (accessed on 29 March 2020).
- Asif, M.; Gao, X.; Lv, H.; Xi, X.; Dong, P. Catalytic hydrogenation of CO2 from 600 MW supercritical coal power plant to produce methanol: A techno-economic analysis. Int. J. Hydrogen Energy 2018, 43, 2726–2741. [Google Scholar] [CrossRef]
- Rubin, E.S.; Davison, J.E.; Herzog, H.J. The cost of CO2 capture and storage. Int. J. Greenh. Gas. Control. 2015, 40, 378–400. [Google Scholar] [CrossRef]
- Anicic, B.; Trop, P.; Goricanec, D. Comparison between two methods of methanol production from carbon dioxide. Energy 2014, 77, 279–289. [Google Scholar] [CrossRef]
- IRENA. Renewable Power Generation Costs in 2018; International Renewable Energy Agency: Abu Dhabi, UAE, 2019; Available online: https://www.irena.org/-/media/Files/IRENA/Agency/Publication/2019/May/IRENA_Renewable-Power-Generations-Costs-in-2018.pdf (accessed on 3 January 2020).
- GWEC. Global Wind Report 2019. 2019. Available online: https://gwec.net/global-wind-report-2019/ (accessed on 8 April 2020).
- Qinghe Wastewater Treatment Plant. Available online: https://www.water-technology.net/projects/qinghewatertreatment/ (accessed on 8 April 2020).
- Lu, J.-Y.; Wang, X.-M.; Liu, H.-Q.; Yu, H.-Q.; Li, W.-W. Optimizing operation of municipal wastewater treatment plants in China: The remaining barriers and future implications. Environ. Int. 2019, 129, 273–278. [Google Scholar] [CrossRef]
- Li, H.; Feng, K. Life cycle assessment of the environmental impacts and energy efficiency of an integration of sludge anaerobic digestion and pyrolysis. J. Clean. Prod. 2018, 195, 476–485. [Google Scholar] [CrossRef]
- Cano, R.; Perez-Elvira, S.I.; Fdz-Polanco, F. Energy feasibility study of sludge pretreatments: A review. Appl. Energy 2015, 149, 176–185. [Google Scholar] [CrossRef]
- Halaby, A.; Ghoneim, W.; Helal, A. Sensitivity analysis and comparative studies for energy sustainability in sewage treatment. Sustain. Energy Technol. Assess. 2017, 19, 42–50. [Google Scholar] [CrossRef]
- Yang, G.; Zhang, G.; Wang, H. Current state of sludge production, management, treatment and disposal in China. Water Res. 2015, 78, 60–73. [Google Scholar] [CrossRef] [PubMed]
- Kiselev, A.; Magaril, E.; Magaril, R.; Panepinto, D.; Ravina, M.; Zanetti, M.C. Towards Circular Economy: Evaluation of Sewage Sludge Biogas Solutions. Resources 2019, 8, 91. [Google Scholar] [CrossRef]
- Qu, J.; Wang, H.; Wang, K.; Yu, G.; Ke, B.; Yu, H.Q.; Ren, H.; Zheng, X.; Li, J.; Li, W.W.; et al. Municipal wastewater treatment in China: Development history and future perspectives. Front. Environ. Sci. Eng. 2019, 13, 88. [Google Scholar] [CrossRef]
- Tyagi, K.V.; Fdez-Güelfo, L.A.; Zhou, Y.; Álvarez-Gallego, C.J.; Romero Garcia, L.I.; Nga, W.J. Anaerobic co-digestion of organic fraction of municipal solid waste (OFMSW): Progress and challenges. Renew. Sustain. Energy Rev. 2018, 93, 380–399. [Google Scholar] [CrossRef]
- Zhang, Q.H.; Yang, W.N.; Ngo, H.H.; Guo, W.S.; Jin, P.K.; Dzakpasu, M.; Yang, S.J.; Wang, Q.; Wang, X.C.; Ao, D. Current status of urban wastewater treatment plants in China. Environ. Int. 2016, 92–93, 11–22. [Google Scholar] [CrossRef]
- Von Friesen, M.P.; Odhner, P.; Arnell, M.; Svensson, M.; Ivarsson, P.; Jannasch, A.-K.; Boss, A.; Hjornhede, A. More Efficient Use of Biogas at Waste Water Treatment Plants. Report 2018:476. Available online: https://energiforskmedia.blob.core.windows.net/media/24303/more-efficient-use-of-biogas-at-waste-water-treatment-plants-energiforskrapport-2018–476.pdf (accessed on 8 April 2020).
- Iaquaniello, G.; Centi, G.; Salladini, A.; Palo, E.; Perathoner, S.; Spadaccini, L. Waste-to-methanol: Process and economics assessment. Bioresour. Technol. 2017, 243, 611–619. [Google Scholar] [CrossRef]
- The Rise, Rise, Rise of Bio-Methanol for Fuels and Chemical Markets. Available online: http://www.biofuelsdigest.com/bdigest/2018/01/24/the-rise-rise-rise-of-bio-methanol-for-fuels-and-chemical-markets/ (accessed on 29 March 2020).
- National Bureau of Statistics of China. Collection, Transport and Disposal of Consumption Waste in Cities. 2018. Available online: http://data.stats.gov.cn/easyquery.htm?cn=C01 (accessed on 29 March 2020).
- Ji, L.; Lu, S.; Yang, J.; Du, C.; Chen, Z.; Buekens, A.; Yan, J. Municipal solid waste incineration in China and the issue of acidification: A review. Waste Manag. Res. 2016, 34, 280–297. [Google Scholar] [CrossRef]
- Morandin, M.; Harvey, S. Methanol via Biomass Gasification. Department of Energy and Environment Division of Industrial Energy Systems and Technologies, Chalmers University of Technology, Göteborg, Sweden. 2015. Available online: https://publications.lib.chalmers.se/records/fulltext/218484/local_218484.pdf (accessed on 29 March 2020).

| Properties | Unit | Diesel | Gasoline | Methanol |
|---|---|---|---|---|
| Density | kg/m3 | 840 | 740 | 796 |
| Cetane number | - | >40 | <5 | |
| Octane number | - | - | 95 | - |
| Boiling point | K | 453–643 | 298–488 | 338 |
| Lower heating value | MJ/kg | 42.5 | 44 | 19.67 |
| Stoichiometric air–fuel ratio | - | 14.6 | 14.7 | 6.45 |
| Heat of vaporization | kJ/kg | 243 | 180–350 | 1100 |
| Viscosity | cSt | 4.59 | 0.57 | 0.65 |
| Auto-ignition temperature | K | 503 | 465–743 | 736 |
| Carbon content by mass | % | 85 | 86 | 37.5 |
| Hydrogen content by mass | % | 15 | 14 | 12.5 |
| Oxygen content by mass | % | 0 | 0 | 50 |
| Specific carbon dioxide emission | g/MJ | 73.33 | 73.95 | 68.44 |
| Fuel | WTT Carbon Dioxide Emissions (g CO2-eq/kg) | References | |
|---|---|---|---|
| Minimum | Maximum | ||
| Diesel fuel | 284 | 1020 | [57] |
| Gasoline | 294.8 | 1188 | [57] |
| Natural gas | 909.5 | 1290.9 | [57,58,59,60] |
| Biomethanol (biomass and renewable electricity) | −898 | [61] | |
| Methanol (coal) | 2965 | [61] | |
| Bioethanol | −1493 | −352 | [62] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bazaluk, O.; Havrysh, V.; Nitsenko, V.; Baležentis, T.; Streimikiene, D.; Tarkhanova, E.A. Assessment of Green Methanol Production Potential and Related Economic and Environmental Benefits: The Case of China. Energies 2020, 13, 3113. https://doi.org/10.3390/en13123113
Bazaluk O, Havrysh V, Nitsenko V, Baležentis T, Streimikiene D, Tarkhanova EA. Assessment of Green Methanol Production Potential and Related Economic and Environmental Benefits: The Case of China. Energies. 2020; 13(12):3113. https://doi.org/10.3390/en13123113
Chicago/Turabian StyleBazaluk, Oleg, Valerii Havrysh, Vitalii Nitsenko, Tomas Baležentis, Dalia Streimikiene, and Elena A. Tarkhanova. 2020. "Assessment of Green Methanol Production Potential and Related Economic and Environmental Benefits: The Case of China" Energies 13, no. 12: 3113. https://doi.org/10.3390/en13123113
APA StyleBazaluk, O., Havrysh, V., Nitsenko, V., Baležentis, T., Streimikiene, D., & Tarkhanova, E. A. (2020). Assessment of Green Methanol Production Potential and Related Economic and Environmental Benefits: The Case of China. Energies, 13(12), 3113. https://doi.org/10.3390/en13123113










