1. Introduction
It appears likely that, to limit the atmospheric CO
2 concentration to levels compatible even with the 2 °C warming goal, and particularly the 1.5 °C goal, substantially net negative CO
2 emission balance must be achieved by the second half of the century [
1,
2]. Among a variety of different negative emission technologies (NETs) to remove CO
2 from the atmosphere, bioenergy with carbon capture and storage (BECCS) offers a promising combination of a carbon removal potential estimated at 0.5–5 Gt
CO2/year and removal cost estimated at 100–200 USD/t
CO2 [
3]. The potential for BECCS has significant regional variation; it appears particularly attractive in the Nordic countries where the biomass potential per capita is among the highest in the world.
BECCS can be utilized in the power and heat industry, where CCS technology has been developed with the goal of curbing emissions from fossil fuel use. As the direct emission of CO
2 from biomass combustion is similar to what the plant sequestered from the atmosphere during its growth, BECCS will result in net negative emissions, provided that an appropriate storage option is available for the captured CO
2. The significant energy penalties and equipment costs usually associated with the capture process still hinder the feasibility of BECCS technologies for widespread use, however [
4].
The main CO
2 capture technologies are based on pre-combustion, oxy-combustion, or post-combustion. The large parasitic loads of these processes reduce the net power generation of a power plant considerably [
5]. Chemical looping combustion (CLC), a technology allowing intrinsic separation of CO
2 from the combustion process with a relatively low net efficiency penalty, is one promising concept proposed as a way to alleviate this problem [
6]. The comparatively simple CLC process is also likely to offer lower additional equipment cost than what would be possible with most carbon capture technologies [
7].
Existing CLC installations typically consist of two interconnected fluidized bed reactors: an air reactor (AR) and a fuel reactor (FR). A solid metallic oxide oxygen carrier circulates between the reactors. The concept has been demonstrated successfully for gaseous, liquid, and solid fuels; particularly, natural gas CLC applications have attracted much attention. In the FR, the fuel is oxidized, taking the oxygen from the solid oxygen carrier rather than from molecular oxygen in the air as happens in conventional combustion. After being reduced in the FR, the solid oxygen carrier is recirculated back to the AR, where it is re-oxidized with atmospheric air. The exhaust gas from the FR consists mainly of CO
2 and H
2O. Ideally, the condensation of the water vapor leaves a CO
2 stream ready for sequestration, allowing the capture of CO
2 without any gas separation step, which normally constitutes the main cost of CCS [
8].
With solid fuels, reactions between solid fuel and solid oxygen carrier would not take place at a useful rate [
6]. One solution to this is an intermediate fuel gasification step to form syngas. In in-situ gasification CLC (
iG-CLC), the fuel is fed into the FR, where it is first gasified to CO, CH
4, and various other gaseous products, which then react with the solid oxygen carrier. The reaction rate of the entire process is limited by the gasification step, which can be up to 100 times slower than the combustion step [
9]. Chemical looping with oxygen uncoupling (CLOU) represents a way of avoiding the rate-limiting gasification step, relying instead on the capability of some oxygen carriers to release the oxygen to gas phase at temperature levels useful for combustion (800–1200 °C) [
10]. This way the solid fuel combustion takes place in an oxygen-rich atmosphere facilitating faster char conversion and oxygen carrier reduction, and thereby allowing smaller oxygen carrier inventories and smaller reactor dimensions [
11,
12]. The energy output from a CuO-based CLOU process using sawdust as fuel was recently determined to be 27% greater than that from a Fe
2O
3-based
iG-CLC process [
13], demonstrating the benefits of oxygen carriers with CLOU properties in solid-fuel CLC-based CCS. Although early studies on solid-fuel CLOU mostly focused on fossil coal, biomass has recently attracted increasing attention; in addition to allowing the possibility of negative emission, biomass also has the additional advantage of higher reactivity.
CHP production combines the production of electricity and usable heat in the same plant. In separate production of electricity, much of the fuel energy must be discarded as waste heat, whereas in a CHP plant much or all of this can become a heat energy product. This allows a clear improvement in overall efficiency over separate stand-alone production. The heat is typically used either as steam in industrial processes, or as district heat (DH), the latter usually sent to the customer in the form of 70–120 °C water. Modern biomass-fired CHP plants typically achieve 75–90% overall efficiencies. Biomass-fired CHP production can thus represent a low-carbon, high-efficiency energy system in and of itself.
A CHP plant can also be equipped with a carbon capture process, but, depending on the carbon capture technology used and the temperature levels required for the heat, in a CHP plant, some of the energy lost in the carbon capture process can be recovered to augment heat production, resulting in efficiency and cost benefits. The combination of district heating and CLC-based biomass CCS implementation can be particularly advantageous in this respect: the fuel reactor gas stream has a high concentration of water due to the combination of fuel characteristics and lack of nitrogen and excess oxygen in the stream, while the DH network offers a large, relatively low-temperature heat sink to which the condensing heat can be recovered. In Finland, the potential for biomass-fired CHP CCS is significant; in 2017, 33% of total electricity generation and 65% of district heat supply originated from CHP plants with forest wood the most common source of fuel [
14].
The goal of this work is to evaluate the thermodynamic and technical potential of a next-generation biomass-fired CCS concept in a CHP context. This is performed by combining process simulation of a CuO/Cu
2O-based CLOU process with a flowsheet model of a cogeneration plant producing electricity, district heat, and process steam for industry. The CLOU reactor model is based on earlier work performed at LUT University [
11,
15]. The CHP plant model is implemented using commercial process simulation software. A condensing heat recovery scheme based on the use of multi-stage packed bed scrubbers and heat pumps was designed for heat integration of the CO
2 capture process, DH network, and the CHP plant steam cycle.
The key performance indicators such as power and heat outputs, energy and exergy efficiencies, auxiliary consumption, carbon capture rate, and specific CO2 emissions of the CLOU-integrated CHP plant are quantified and compared with those of a reference plant equipped with a conventional CFB boiler without CO2 capture.
2. Methods
The study focuses on a large-scale biomass-fired CHP plant using Nordic forest wood biomass as fuel, with a lower heating value of LHVd = 19.5 MJ/kg for the dry fraction, 8.53 MJ/kg as fired (50% wet-basis moisture). The plant configuration is based on an actual operating one. The reference case model, equipped with a conventional CFB boiler, was designed to replicate the actual plant as closely as available data allow. This is then used as the basis for the CLOU plant model. The CLOU plant has identical heat production with the reference plant: 110 MW district heat, 40 MW 10 bar(a) high pressure steam, and 90 MW 4.5 bar(a) low pressure steam. The same fuel input is also maintained, at 430 MWLHV, and power generation is allowed vary.
The CHP plant and integration modeling were implemented using IPSEpro process simulation software by SimTech, version 7. IPSEpro is an equation-oriented process simulation tool widely used for power plant simulation; it has been used for modeling chemical looping CCS systems [
16], and the authors have also used it for numerous studies investigating various aspects of biomass-fired CHP plant optimization [
17] and process integration [
18]. The standard module library of IPSEpro includes the majority of typical power plant components, and additional modules can be created with a module development kit.
The CLOU reactors were modeled separately from the main IPSEpro plant model, using a core-annulus type fluidized bed model. The model is based on mass and energy balances, as well as semi-empirical heat transfer, fluid dynamics, and chemical reaction models. The reactor model was developed earlier in MATLAB/Simulink environment, and is described in more detail in [
11,
15]. For the IPSEpro CHP plant model, a CLOU reactor system module was created in the module development kit. The reactor module takes as manual input CLOU reactor simulation results, and gives out exiting gas stream sources matching said results.
2.1. Performance Metrics
The simulation results were used to determine the main plant performance indicators in terms of both carbon capture effectiveness and performance penalty compared to the reference plant. Based on the first law of thermodynamics, the net energy efficiency
ηnet of the plant is
where
Pel,net (MW) is the net electrical output after deducting the auxiliary consumption
Pel,aux from the generator power
Pel,gen,
ΦDH (MW) is the district heat production,
Φsteam (MW) is the steam production,
(kg/s) is the fuel feed rate, and
LHVf (MJ/kg) is the lower heating value of the fuel.
The first law analysis allows a simple comparison between CO
2 capture plants or against a conventional reference plant to assess the efficiency penalty due to carbon capture. The shortcoming of first law analysis is that it fails to differentiate between the values of the electricity and heat, treating both as additive properties with the same relative value. In practice, they are not interchangeable: electricity can be easily converted to heat without significant losses, but the reverse is not true. Moreover, the first law analysis cannot be regarded as generic because the CO
2 option assessment depends strongly on the selection of the process specifications of each unit operation, and fundamental improvements to the unit operations cannot be made without examining their thermodynamic limitations [
19]. A second law analysis (exergy analysis) was thus also conducted.
Exergy measures also the quality of energy. An exergy analysis allows identifying thermodynamic inefficiencies and has been used to evaluate the performances and integration potentials of various energy conversion processes, including CLC [
20] and CLOU [
21]. For a unit, the irreversibilities
I can be calculated through the exergy balance:
where
Exin and
Exout are the total exergy flows into and out of the system, including both the thermomechanical exergy and chemical exergy of the material streams as well as exergy associated with heat transfer. The exergy efficiency of the CHP plant
φ is defined as
where
Exp is the exergy of the products and
Exf is the exergy of the fuel input. The exergy in the exhaust gas stream is unrecoverable and excluded from the product exergy. Instead, the captured CO
2 is a valuable product as a source of negative emissions or raw material for products with higher economic value (e.g., biofuels or plastics). In the results, the exergetic performance of CLOU plant is given for both cases, including and excluding the exergy value of CO
2, and compared to exergetic efficiencies of the reference plants.
The CLOU process performance can be evaluated using the CO
2 capture efficiency
ηCC, defined as the fraction of the fuel carbon captured from the fuel reactor gas stream:
where
is the carbon mass fraction in the char, [C] is the total carbon in the fuel, and
Xchar is the conversion of char in the fuel reactor,
where
is the char flow with the fuel and
is the char flow rate into the air reactor. The specific CO
2 emissions (CO
2 intensity)
SECO2 is the ratio of CO
2 emission to the atmosphere relative to the plant net power and heat generation:
2.2. CLOU Reactors and Modeling
An important factor in a CLC system is the choice of oxygen carrier. There is a limited selection of possible metal oxides having suitable equilibrium partial pressures of oxygen at temperature levels relevant for combustion to facilitate releasing oxygen in the fuel reactor. Of these, copper oxide, CuO/Cu
2O has received interest due to its high reactivity and oxygen transport capacity, and lack of thermodynamic limitations for complete hydrocarbon combustion. For these reasons, CuO/Cu
2O oxygen carrier was considered in this work, as a 50:50 mixture of active CuO/Cu
2O and supporting TiO
2, with physical properties representing Geldart B particles (100-micron average particle size, 4650 kg/m
3 apparent density). In the CLOU regime, the oxygen carrier cycles between the CuO and Cu
2O states,
where ∆
H850 is the reaction enthalpy at 850 °C.
In the fuel reactor, the CuO releases oxygen in an endothermic reaction. The biomass undergoes devolatilization and combustion takes place with this gaseous oxygen, for a net exothermic process in the fuel reactor. The oxygen carrier is then transferred in its reduced form Cu2O to the air reactor, where is re-oxidized back to CuO in an exothermic reaction.
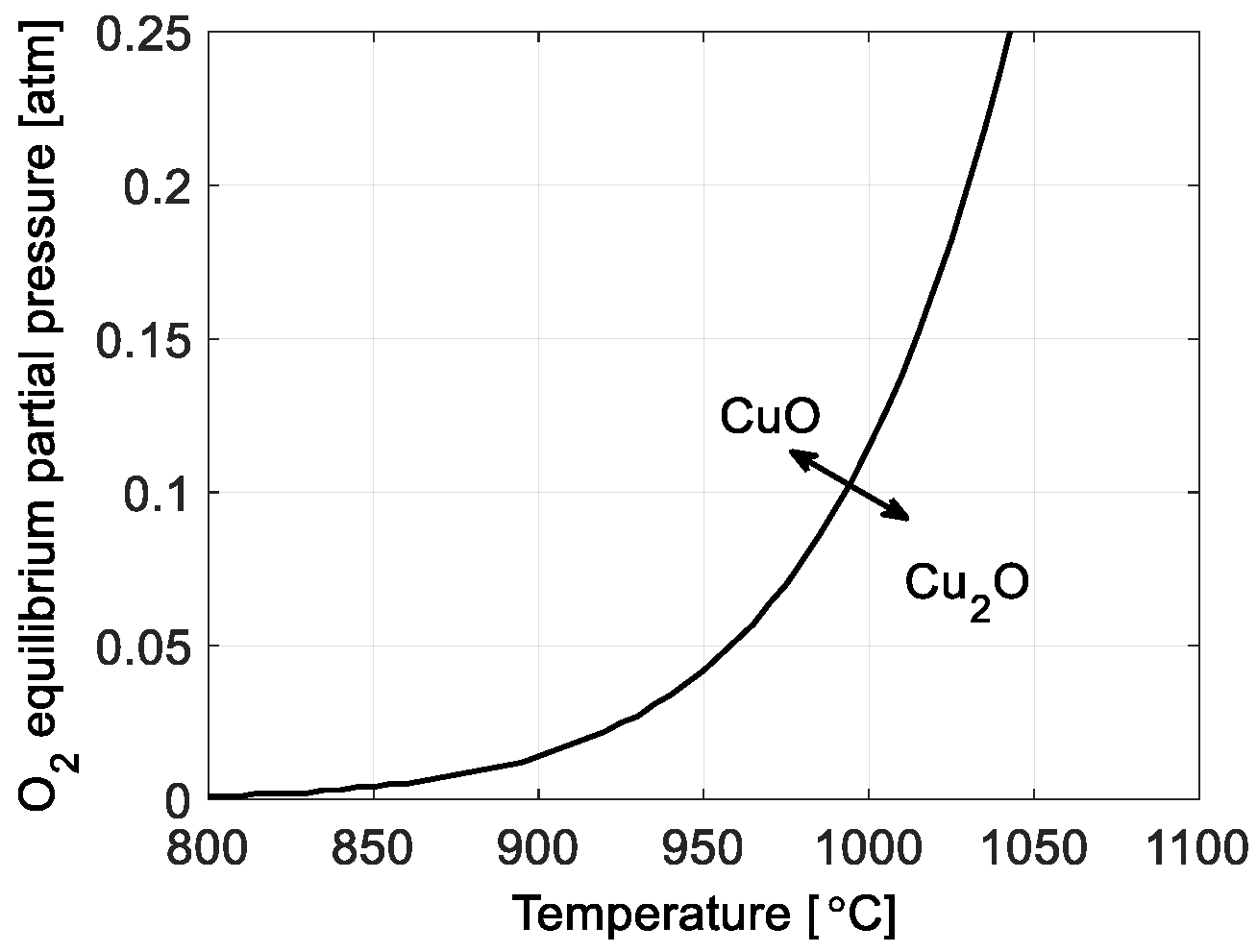
The oxidation and decomposition of the oxygen carrier are both affected by a thermodynamic driving force, which is governed by the difference between the partial pressure of oxygen in a reactor,
pO2, and the equilibrium
pO2. The equilibrium curve for a CuO/Cu
2O system is shown in
Figure 1. A detailed description of the oxygen carrier redox model used in this work is found in [
12]. For both CuO reduction and Cu
2O oxidation, a conversion rate equation can be formulated as
where
f(
pO2,eq.) represents the role of the thermodynamic driving force,
f(
X) accounts for the reaction mechanism, and
f(
T) expresses an Arrhenius-type dependency on temperature. The OC oxidation and reduction kinetics as applied in the air and fuel reactor models are shown in
Table 1.
Reactor performance has a significant impact on plant performance: a fuel conversion reduction of 2% typically reduces the
ηnet of the plant by 1% [
24]. The procedure to determine the reactor characteristics and operating conditions in this study is mostly similar to that presented in [
12]; however, a full optimization of the reactor design was considered premature at this stage for the scope of the work. The main parameters at design-point operation are summarized in
Table 2.
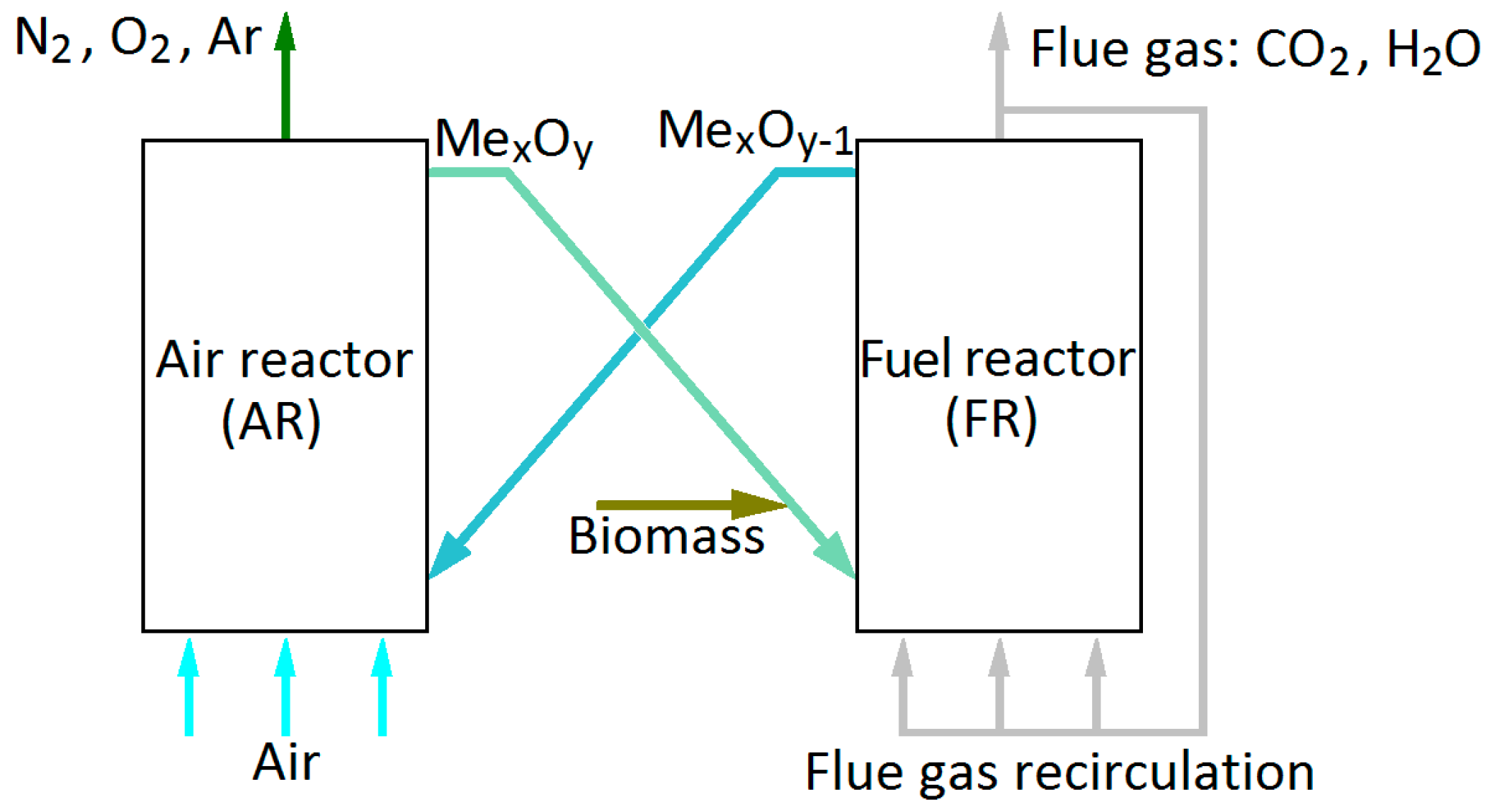
The CLOU reactor system employed in this work is shown in
Figure 2. It consists of an oxidizer (air reactor, AR) and a reducer (fuel reactor, FR), both designed as atmospheric CFBs. Compared to other configurations, this design has many advantages [
25]. Both the AR and the FR are operated in the fast fluidization regime, enabling gas–solids contact throughout the reactor height for maximum fuel conversion. The AR is fluidized with pre-heated air and the FR with partially recirculated flue gas. The FR gas wet basis recirculation ratio is defined as
where
is the recirculation mass flow rate and
is the total exit flow from the fuel reactor. The formation of combustion gases along the reactor height also affects the appropriate choice of the recirculation ratio. A recirculation gas input of 40 kg/s, resulting in
ϕfg = 0.31, was considered. The recirculation gas is cooled to 450 °C or less prior to being returned to the FR. The cross-sectional areas of the reactors were sized so as to obtain desired gas velocities.
The reaction kinetics and the equilibrium
pO2 depend heavily on temperature; the operating temperatures of the reactors are thus important. Temperatures of 900–950 °C have been identified as suitable for CuO-based oxygen carriers [
26]. At typical CLC/CLOU operating conditions, approximately half of the fuel energy input is extracted from the reactors themselves [
27]. The height of the reactors is determined by the heat transfer area required for cooling, as well as ensuring that there is space on the side wall for the particle separator and return systems.
To reduce fan power consumption and the reactor size, the bed material quantity in each reactor should be minimized. The oxygen carrier loading needed for effective combustion depends on the CuO reduction, Cu
2O oxidation and fuel combustion kinetics. In the current case, a total solids loading of 460 kg/MW
th was considered sufficient for >95% fuel conversion [
11,
12]. A carbon stripper to remove unreacted char from the solids stream from FR to AR was considered unnecessary: the reactivity of biomass char particles allows CO
2 capture efficiencies of over 95% [
28].
The ash from the combustion must be purged periodically to avoid accumulation in the reactor system, although some oxygen carrier will be lost in the process. An excessive accumulation of the ash in circulating oxygen carrier can be prevented by a proper cyclone design and by employing other particle separation techniques [
29]. In the current layout, 50% of the ash that accumulates in the solids stream is removed after the air reactor, assuming that the purge stream consists of 99% of ash and 1% of oxygen carrier. With this high selectivity toward ash, the oxygen carrier and ash purge/make-up flows have a negligible effect on the total material and energy balances, as shown in [
12]. At this point, the assumptions regarding the disposal of ash and separation of oxygen carrier particles from the ash are somewhat tentative, and the topic represents a future research need for CLC/CLOU systems.
The behavior and performance of the suggested dual-fluidized bed CLOU system is evaluated using a 1.5D model applicable to chemical looping processes. The models are based on mass and energy balances, using semi-empirical correlations to evaluate the gas–solid fluid dynamics, heat transfer, and chemical reactions [
11,
12]. Data specific to the process (reactor cooling rates as well as flow rates, temperatures, and compositions of the exiting gas flows) can then be obtained and used in the CLOU plant model to determine the plant performance.
2.3. Plant Configurations and Modeling
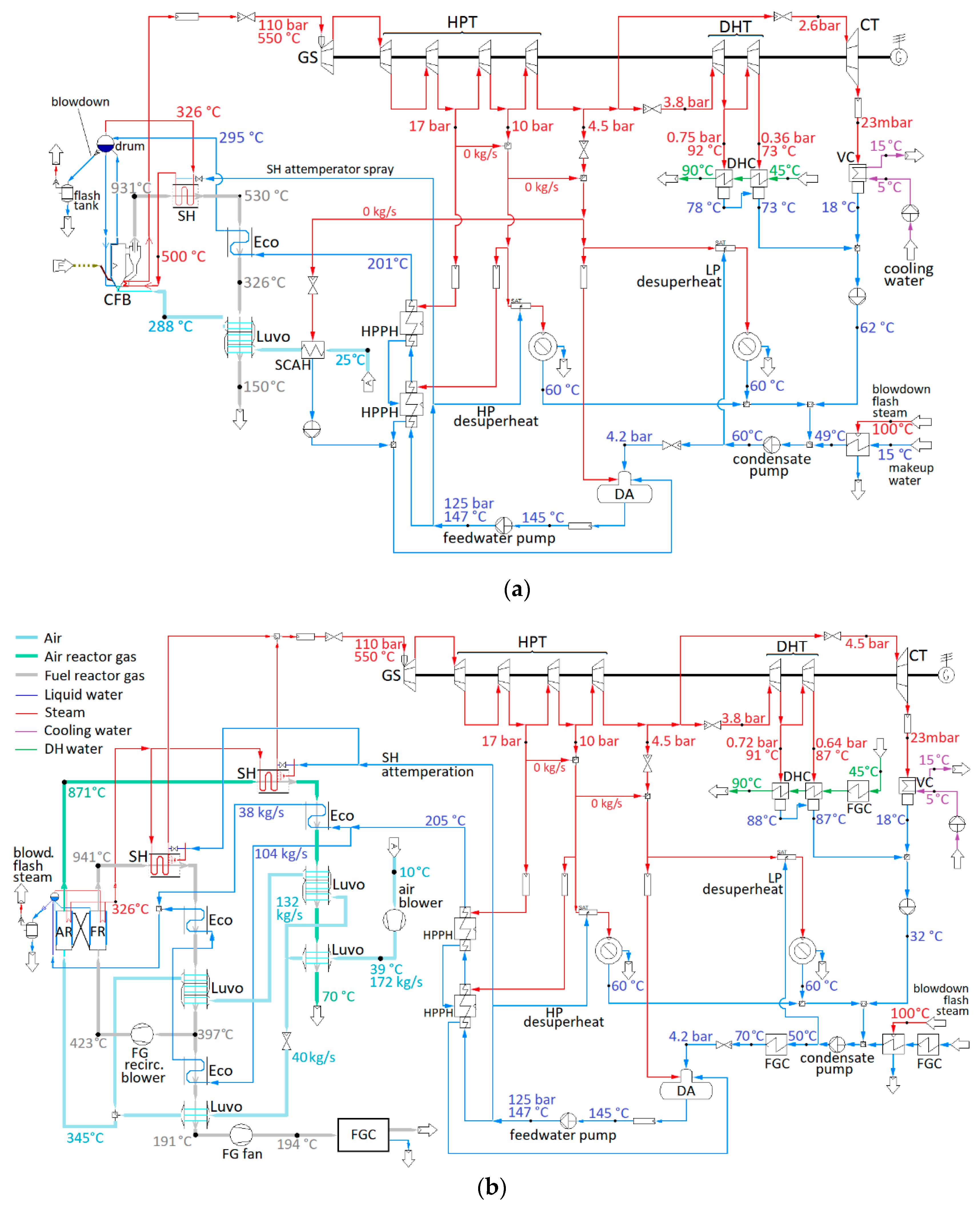
Figure 3a depicts the reference plant and
Figure 3b the CLOU plant. The turbine is divided into three parts on a single shaft. After expansion in the high-pressure (HP) part, equipped with a partial-admission regulating stage, the remaining steam is split between a DH turbine with two DH condensers in series, and a condensing turbine. Both plants are modeled with IPSEpro process simulation software, but, in the CLOU plant, a reactor module takes as manual input the data obtained from MATLAB/Simulink CLOU reactor model.
Both plants take 430 MWLHV fuel input and have steam values of 550 °C and 110 bar at turbine inlet, producing 110 MW of district heat, 40 MW of 10 bar(a) HP steam, and 90 MW 4.5 bar(a) LP steam. Condensate returns from steam consumers at 60 °C; there is a combined total of 8 kg/s condensate loss from HP and LP steam usages. The CLOU plant has the same fuel input and same heat loads as the reference plant.
The principal differences between the reference and the CLOU plant are the more complex back pass heat recovery scheme needed in the CLOU plant, and the opportunity to produce much of the DH by condensing the water vapor from the FR gas flow in the CLOU plant to separate it from the CO
2. This will affect the sizing of the two parallel low-pressure turbines; the condensing one would be relatively larger and DH turbine smaller in the CLOU plant. The details of the condensing heat recovery scheme, omitted for space and clarity in
Figure 3, are described later in
Section 2.3.3.
2.3.1. Steam Cycle
The turbines are modeled similarly as described in [
30]. The turbine module dry isentropic efficiencies at optimum flow rate are estimated to range from 87% (first HP module) to 92% (LP end of condensing and DH turbines). For the regulating stage, the optimum efficiency is 75%. The pressure levels are estimated using the ellipse law; efficiency variation is estimated using correlations based on steam flow rate.
The condensing turbine and DH turbine of the reference plant have swallowing capacities of 21 and 51 kg/s, respectively; as all turbines are on the same shaft and thus constantly turning, a small minimum cooling flow is always required in both turbines. CLOU plant condensing and DH turbine sizing is based on the DH production obtained from the condensing heat recovery scheme.
Both plants have a deaerator operating as an open feed heater at 4.2 bar(a), and also two closed high-pressure heaters using approximately 10 and 16.5 bar(a) steam to heat the boiler feedwater to slightly over 200 °C.
2.3.2. Boiler
The reference plant CFB boiler is modeled with four main modules. The furnace module contains a simple steam generator heat transfer model based on an average furnace temperature and heat transfer coefficient, according to Basu [
31]. All boiler losses except for the stack loss are also determined in the furnace module. The superheater, economizer and air preheater are each represented by a single heat exchanger component. A steam coil air heater (SCAH) is present so that a stack temperature sufficient to prevent acid condensation can always be maintained. One percent of the feedwater flow rate is removed as blowdown from the drum. The blowdown is depressurized, and flash steam is used to heat the makeup water.
In the IPSEpro CLOU plant model, the CFB furnace module is replaced with a CLOU reactor module, which takes the results of the separate CLOU reactor simulation as manual input, and gives out the gas streams of specified composition, state, and flow rate. The steam generator and superheater surfaces located in the air and fuel reactors are considered by specifying the cooling rates, obtained as output of the reactor model, into the IPSEpro plant model’s reactor module.
The back pass heat transfer surfaces are designed so as to obtain the same live steam parameters, broadly similar temperature approach to the drum and combustion air temperatures as in the baseline CHP plant. The fuel reactor requires some flue gas to be recirculated. To permit affordable materials in the recirculation fan, the recirculation temperature can be no greater than 450 °C. Satisfying these requirements required a somewhat more complex interconnected configuration of heat transfer surfaces, as seen in
Figure 3.
Having two separate gas streams, of which one is almost completely devoid of chlorine and water, while the other one must have the water vapor mostly condensed for CO2 purification purposes, yields opportunities for improved heat recovery and thus efficiency. It was assumed that the stack temperature for the gas from the air reactor could be safely reduced to 70 °C without significant corrosion risk while still using inexpensive materials. In the flue gas stream from the fuel reactor, the exit temperature from the last conventional heat transfer surface, the last luvo stage, was increased to 195 °C compared to the 150 °C stack temperature of the baseline CHP plant.
2.3.3. Fuel Reactor Gas Water Removal and Heat Recovery
To purify and compress the CO2 from the fuel reactor gas stream, the water vapor must be first removed by condensation. Compared to fossil coal, the moisture and hydrogen contents of biomass are high, resulting in a high partial pressure of water in the flue gas stream; in this case, 62 kPa partial pressure of the flue gas (44% mass fraction). While the large amount of water condensation increases the size and cost of the equipment, the high partial pressure and thus condensation temperature also allows easier utilization of the latent heat of condensation for improved efficiency. DH water is used as the main heat sink in the flue gas condensers.
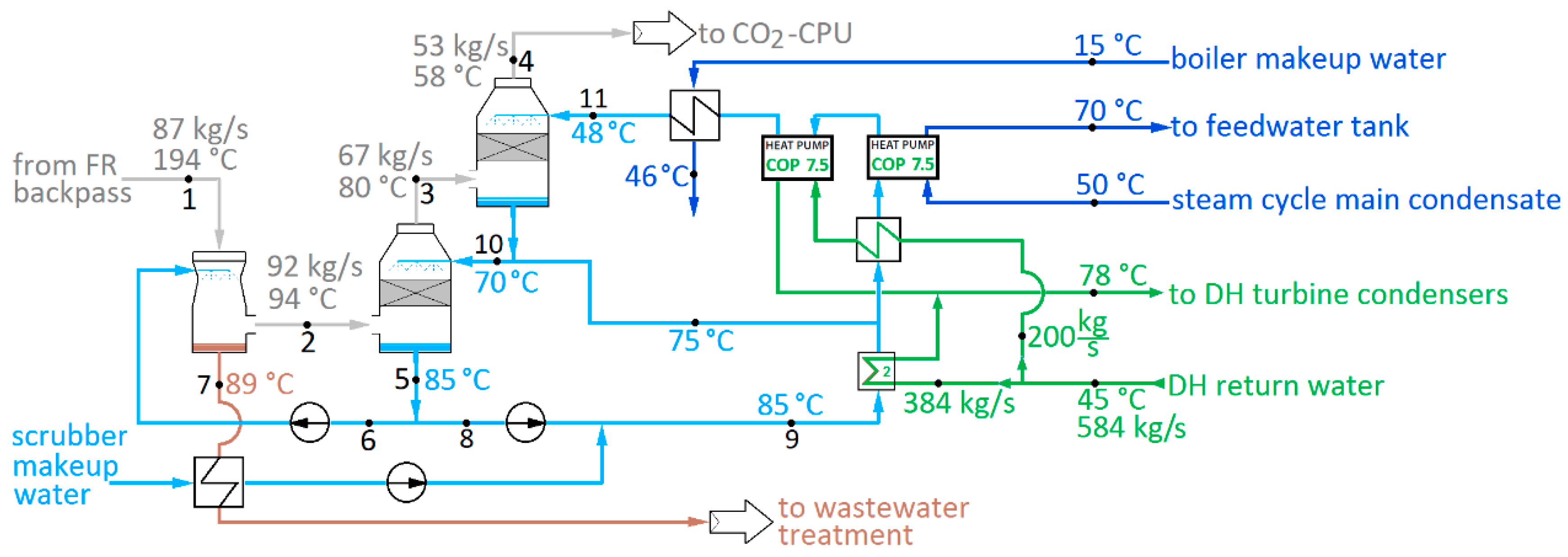
A condensing heat recovery scheme for a boiler can be implemented either as surface condensers or spray towers (scrubbers). In this work, a heat recovery scheme based on spray towers is considered. A typical configuration of such includes two different types of component, each with different functions: a packed-bed heat recovery scrubber (HRS), and a venturi scrubber (VS).
The configuration of a two-stage condensing heat recovery scheme is shown in
Figure 4. The heat recovery takes place in the counter-flow HRS, where cold water is sprayed from above on a flue gas stream flowing up. A packed bed of filler elements provides an increased contact area for water and gas. Moisture in the flue gas condenses on the spray droplets and on the water pellicle formed on the bed filler elements.
The co-flow VS is located before the HRS (
Figure 4). The temperature of the gas entering the VS is far above the dew point, and also too high to permit low-cost light-weight materials for the packed bed to be used in the HRS. By spraying water taken from the bottom of the HRS into the gas stream, evaporation rather than condensation will take place. The gas is cooled, and while the mass flow rate increases, the volumetric flow rate is considerably reduced. Although evaporation transfers heat to the gas rather than from it, the overall efficiency is not reduced: the heat will be recovered through condensation in the HRS. The VS can also serve as the final gas cleaning stage, and pH control can take place by NaOH injection.
The spray water mass flow rate in the VS is typically much less than in the HRS, and dew point temperature is unlikely to be reached. A flue gas exit temperature T2 = Tsat + 5 °C is assumed here, with spray mass flow rate of = 60 kg/s.
The water outlet temperature
T7 from the VS is typically very close to the wet bulb temperature
Twb [
32].
Twb is found by applying the analogy of heat and mass transfer, yielding a well-known equation
where
Ru is the universal gas constant,
T the absolute temperature (K),
hfg the latent heat of evaporation, subscripts g and H
2O refer to mean wet flue gas conditions and water, respectively, and
Le is the Lewis number,
where
DAB is the binary diffusion coefficient between water vapor and wet flue gas (m
2/s) and
kg is the thermal conductivity of wet flue gas (W/mK). Flue gas properties are determined as mass-weighted sums of the properties of its components evaluated at film temperature. With the preceding assumptions and equations, and applying mass and energy balances, the flue gas exit temperature and moisture content are found.
The spray water flow in the HRS is much greater than in the VS. The main mode of operation is condensation; relative moisture of the gas at exit is approximately 100%. The temperature difference ∆
Tg of exiting flue gas flow to spray water can be affected by water flow rate and bed sizing. Optimal flow depends on the cost of packing material and desired flue gas outlet temperature. Detailed component design was ruled beyond the scope of this work; a value of ∆
Tg = 10 °C was considered typical for large-scale equipment and used for both
T4−
T11 and
T3−
T10 (see
Figure 4). The heat exchangers in the scrubbing water cycle are assumed to be plate heat exchangers, dimensioned for 2 °C terminal temperature difference.
It can be seen that, due to the high concentration of water in the fuel reactor gas, further increased by spraying in the first scrubber, heat can be recovered at a comparatively high temperature level for condensing flue gas heat recovery. In terms of power plant steam cycle temperature levels, the temperature levels are still low, however, and there is a limited amount of low-temperature heat sinks available. To obtain more useful heat out of the scrubbing water stream, heat pumps are thus used. A coefficient of performance (COP) of 7.5 was assumed possible for a modern industrial heat pump obtaining a 20 °C temperature lift. With heat pumps, the clear majority of the district heat, approximately 75%, can be produced from the recovered condensation heat, leaving only 25% to be produced in turbine condensers.
2.3.4. Auxiliary Systems
Auxiliary power consumption
Paux in the reference plant was estimated by determining the steam cycle pump power consumptions from the IPSEpro model, and using a published figure of 11.9 MW for all other auxiliary equipment in an operating CHP plant of same type of fuel, boiler capacity and similar configuration and power and heat generation [
33].
The CLOU plant
Paux was assumed to be similar to the reference plant except for the steam cycle pumps, boiler/reactor fans, heat pumps, and the CO
2 compression and purification unit. The higher ∆
p of the CLOU reactor system compared to the conventional CFB boiler of the reference plant requires an air blower with a pressure ratio of slightly over 1.3. In addition to the increased power consumptions, there is a temperature increase of approximately 30 °C at design point over both the air and flue gas recirculation fans. To consider these effects accurately, both the air and flue gas recirculation blowers are considered as components in the CLOU plant model. The power consumption of fuel and ash handling, and other pumps and fans not directly related to the boiler or steam cycle was assumed to remain similar to that of the reference plant, at approximately 2.9 MW according to Ikonen [
33].
3. Results
3.1. Overall Performance
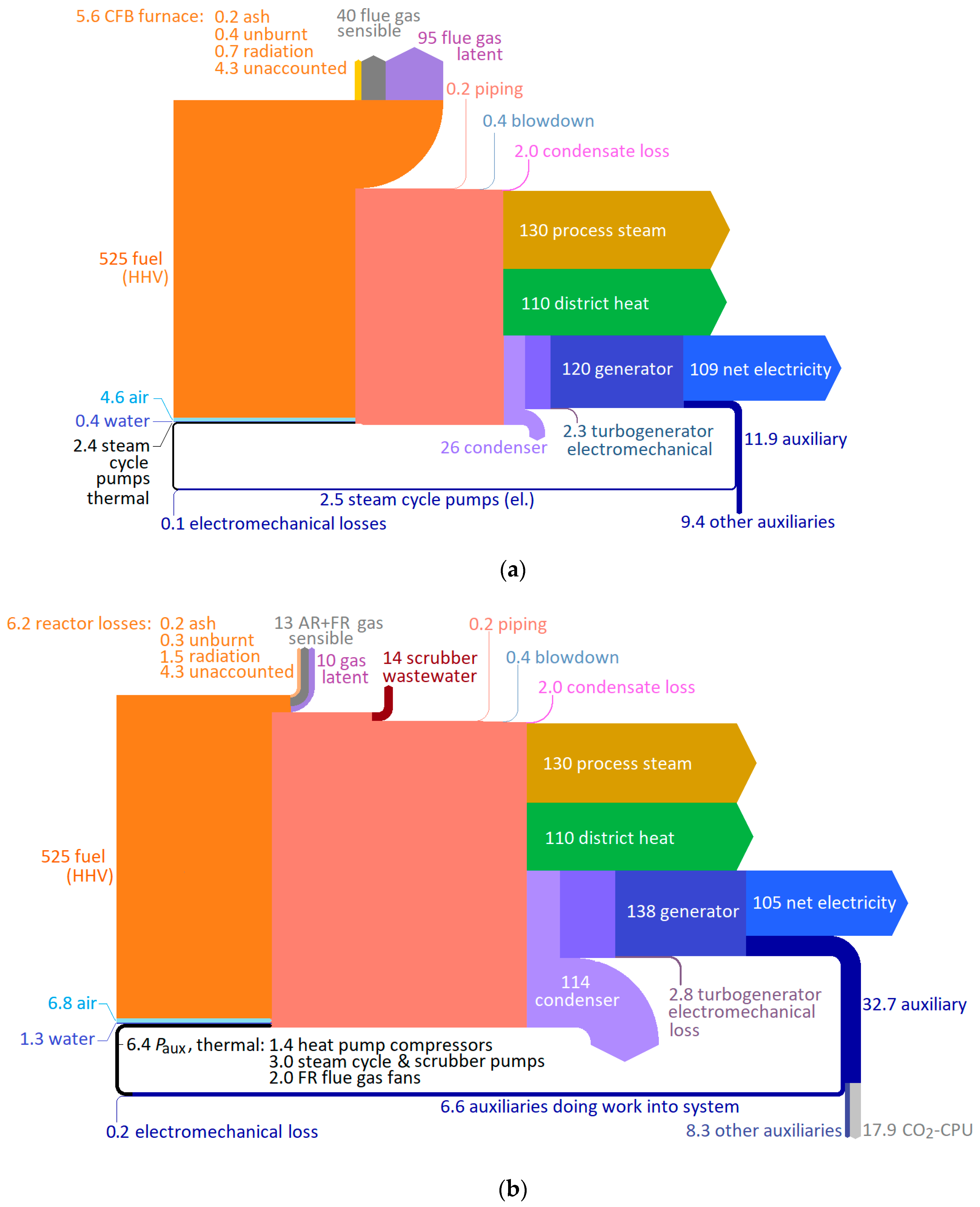
The main operating characteristics of the reference plant and the CLOU-CHP plant are listed in
Table 3. At nominal load, the reference plant with conventional CFB boiler and 150 °C flue gas stack temperature has thermal output of 387 MW, and a net plant efficiency of
ηnet = 81.1%. With the same fuel input, the CLOU system yields a 491 MW thermal output, and
ηnet = 80.3%. Although the CLOU plant has slightly reduced net efficiency, the increase of thermal power is considerable, explained by low-grade heat recovered mainly in the condensation of water from the fuel reactor gas, as well as to a lesser extent the low stack temperature of the air reactor gas flow. The benefit of condensing flue gas heat recovery is much less in conventional boilers, and such equipment is still uncommon in most operational large-scale plants, although recently they have started to appear in some new plants. For comparability such a scheme is also included, labeled “Ref. + FGC” in
Table 3. The FGC-equipped variant of the reference plant uses the same two-stage heat recovery scheme, with the same heat sinks and same heat pump arrangement, temperature lift, and COP as in the CLOU system. At 448 MW of thermal power, the conventional CFB boiler achieves only little over half of the thermal power gain from the FGC compared to the CLOU system.
The second law efficiencies of reference plants were compared by exergy analysis as described in
Section 2.1. By considering the separated CO
2 as a valuable product for later utilization, an exergetic efficiency of 42.0% was obtained for the CLOU plant, exceeding that of the reference plant without flue gas condensation (40.9%) or only slightly less than with the FGC (42.2%). Even if the exergy within the captured CO
2 stream was not considered, the efficiency of 40.2% was close to the figures of the reference plants. The superior performance of the CLOU plant is mainly due to the high-temperature flue gas condensation, which provides a large share of the DH production in the CLOU plant. It is clear from the results that in CCS-equipped CHP plants, a large part of the exergy penalty from CO
2 capture can be recovered by utilizing the heat from flue gas water condensation.
Figure 5 depicts the overall energy balance of both plants. It is evident that the main differences lie in the considerably smaller boiler losses, and greater turbine condenser loss, in the CLOU plant. The latter is a direct consequence of the former: based on the operating strategy of meeting the steam and DH demand, generating much of the district heat in the flue gas condensers leaves much more LP steam to be directed to the condensing turbine. The condensing turbine takes 54 kg/s steam flow in the CLOU-CHP plant compared to 12 kg/s in the reference case. The generator power is also clearly increased, but due to the considerable increase of auxiliary power consumption, net electricity production is still slightly reduced. The significant steam flow increase in the condensing turbine results in only a 19.6 MW increase in generator power at design point, or 16.9% of the 105 MW additional heat recovery. This is a result of the low pressure and temperature of the steam entering the condensing turbine; the live steam flow, with greater capacity for doing work, increases far less at only 5.1 kg/s.
The bulk of the increased auxiliary power consumption is due to the CO
2 compression and purification unit, estimate to have a power consumption of 110 kWh/t
CO2 based on [
34], although the scrubber pumps and heat pumps also contribute to the increase by approximately 3 MW. Steam cycle pump and air and flue gas fan power consumptions are also slightly increased over those of the reference plant due to the increased steam cycle flow rate and increased pressure drop of the twin-CFB reactor system compared to the single CFB boiler of the reference plant. In contrast to the reference plant, more of the auxiliary equipment does work back into the system, however: not only the flue gas recirculation fan, but also the main flue gas fan before the heat recovery scrubbers leaves the majority of their power consumptions, except for the electromechanical losses, into the gas stream and available for use and recovery. Due to the greater pressure drop of the two reactors, the great majority of the pressure increase generated in the fans now takes place in the air fan; while this is not considered as work done into the system due to control volume boundary being set between air entry to the back pass air heaters and the forced draft fan or blower, it does show as increased energy input with the air.
3.2. Boiler and CLOU Reactor Convective Heat Transfer Surface Design
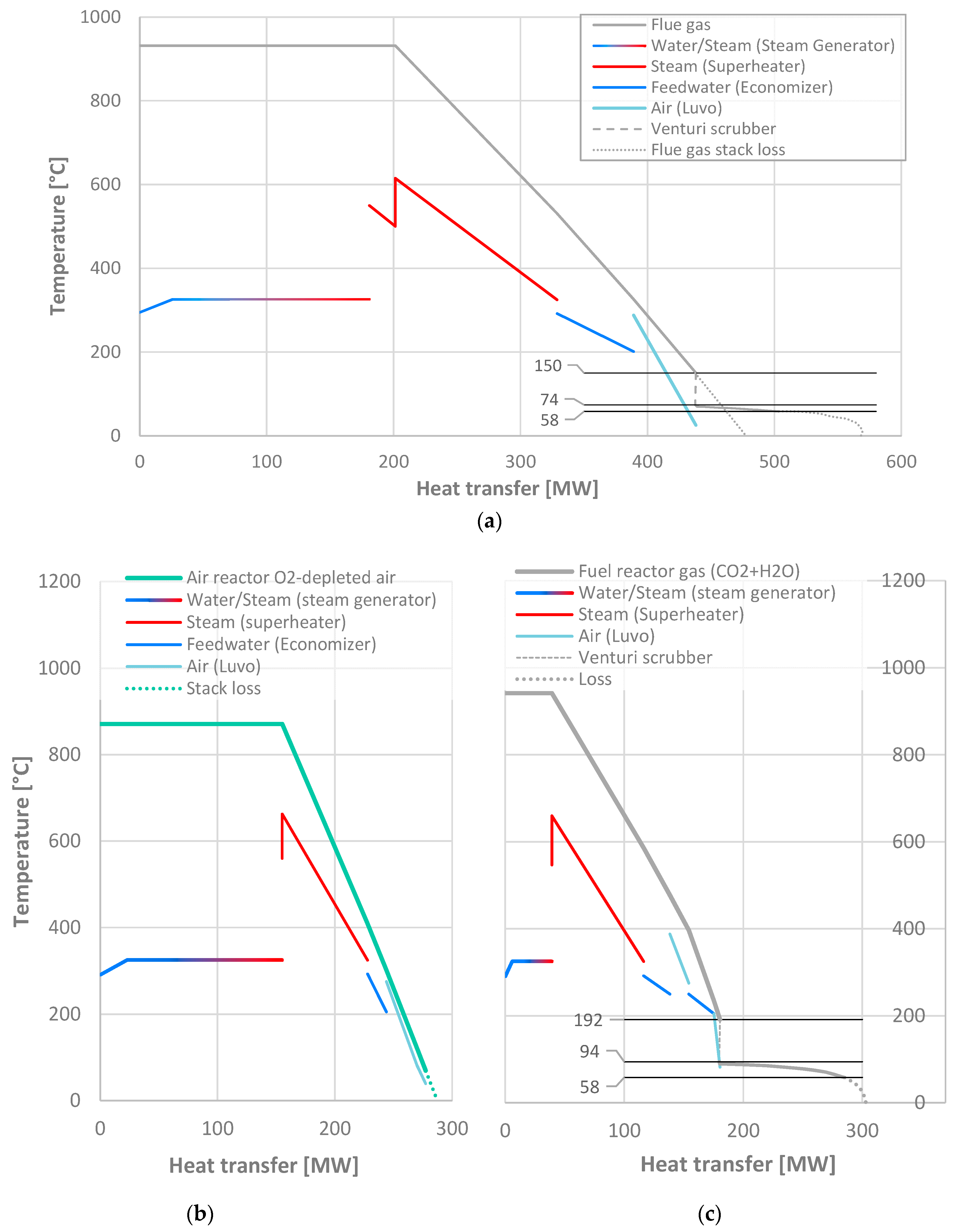
Because the gas flow exiting the CLOU reactor system is split between the relatively clean and benign AR gas stream consisting mainly of oxygen-depleted air, and the FR gas stream with the combustion products including chlorine and alkalis at a higher concentration than in a conventional boiler flue gas, the design of the heat transfer surfaces also differs from the conventional boiler. The temperature diagrams of the reference plant and the CLOU system are shown in
Figure 6.
Combustion air is passed first through the last air heater in the AR back pass; this is to ensure effective cooling of the AR flow, as well as to prevent condensation and corrosion risk in the final FR air heater by preheating the air. In the FR, gas is kept at 190 °C before the flue gas condensers, and the air enters the final, coldest air heater at slightly over 80 °C from the AR. The AR gas is cooled to a stack temperature of 69 °C.
The air intake is also arranged differently in the CLOU plant than in the reference plant: in a typical boiler, combustion air is usually taken from inside the boiler building, warmer than the ambient air. This yields an energy gain for little penalty, as the flue gas exit temperature from the last boiler surface is typically limited by acid condensation risk, not air temperature, and the forced draft fan pressure increase is small enough to render the penalty from compressing slightly warmer air negligible. Neither is true in the case of the CLOU system, however. The cooling of the air reactor gas, consisting mainly of nitrogen and excess oxygen, is practically limited by the availability of low-temperature heat sinks rather than corrosion risk; increasing the temperature of incoming air will thus increase the exit temperature of the AR gas, negating most of the heat gain. As the air blower for the CLOU system also has a pressure ratio of 1.32, for a temperature increase of almost 30 °C and a 5.2 MW power consumption, an increased air temperature would also have a measurable impact on the auxiliary power consumption; thus, it is assumed that warm boiler room air is used for the conventional boiler, and colder ambient air for the CLOU air reactor.
The AR back pass heat exchangers are also designed for clearly smaller temperature differences than those of the FR back pass. While economic optimization was ruled beyond the scope of this study, it is clear that it would be the FR back pass where erosion, corrosion and fouling are expected to be worse problems, and more expensive materials will be needed. By maintaining a higher temperature difference in the FR, the required heat transfer area, and the metal temperature at the heat transfer surface, can both be reduced. The conductances
G (kW/K) required in each of the convective heat transfer surfaces are shown in
Table 4. It is evident that achieving the clearly lower gas exit temperatures in the air reactor requires also clearly higher conductances, and thus more heat transfer area.
3.3. Flue Gas Condensers
Figure 6 shows also the performance of the flue gas condensers (FGC) of the FR exhaust, and in the reference plant if it were so fitted. The very slight move towards the left in the venturi scrubber is due to the heat input into the gas stream. This is due to the spray evaporation into the gas stream being slightly (less than 1 MW in both cases) greater than the impact of reduced sensible enthalpy of the gas due to cooling. An outlet temperature of 58 °C is considered for both the FR gas FGC, and the conventional boiler one. The main limit in lowering the temperature is the availability of suitable low-temperature heat sinks, and the reducing COP of heat pumps if heat is transferred to a warmer heat sink over an increasingly high temperature lift. It can be seen that although the amount of water in the stream entering the VS and exit temperature are almost identical, due to the more concentrated water vapor in the FR gas exhaust stream, the clear majority of all water vapor can be condensed. In terms of remaining water, at 58 °C, only 4.5 kg/s of water vapor remains in the FR stream. If the FGC were to be installed in the reference plant, however, at 58 °C the flue gas would still contain 25 kg/s of water, i.e., the FGC could only condense approximately 1/3 of the water vapor introduced from fuel moisture and hydrogen.
The much reduced heat gain available from the more diluted flue gas stream in a conventional boiler is the main reason the benefit of installing an FGC in a conventional boiler would be much less than in the CLOU plant: 8.5 MW generator power increase, or 13.8% of a 61 MW thermal power gain.
3.4. Carbon Capture Performance
The CLOU reactor system showed high performance with the wood biomass fuel; the volatile matter that was generated during devolatilization was almost completely converted to CO2 and H2O. Only a minor fraction of the char remained unburnt and escaped to the air reactor, resulting in a total CO2 capture efficiency of 97%. Most of the char escaping to the air reactor burned there, and unburned losses were low.
The specific CO2 emissions from the CLOU plant were very low in comparison to those of the reference plant: 13 kgCO2/MWh versus 482 kgCO2/MWh. The specific negative CO2 emissions from the CLOU plant, defined as the ratio of potential CO2 removal rate from the atmosphere and net plant production (electricity + heat), was found to be approximately 470 kgCO2/MWh.
4. Discussion
The CLOU scheme presented above proved to result in a highly effective CO2 capture process, with only a very small efficiency penalty of 0.7%. The low efficiency penalty is particularly noteworthy as it was achieved while shifting the steam cycle operation from mainly back pressure plant towards a condensing one, in a plant with modest live steam pressure and no reheat; this alone without the added parasitic load of the CO2 capture could be expected to clearly reduce the net efficiency. The low efficiency penalty is in large part due to the efficient waste heat recovery system, as well as the comparatively low parasitic load of chemical looping combustion based CCS systems.
The CLOU heat recovery scheme yields an additional 105 MW of low-grade heat from the same 430 MWLHV fuel input as used in the reference plant, corresponding to a 27% increase over the conventional reference plant. Although the low-grade heat enables only an additional 18 MW of gross power generation, this is enough to almost offset the additional parasitic load caused by the CCS scheme. The effective heat recovery is made possible by the way the CLOU system separates the exhaust gas flow into two streams: one with the oxygen-depleted air, and the other with undiluted combustion products. This increases both the heat recovery temperature and the fraction of the flue gas moisture that can be condensed, as well as enabling the oxygen-depleted air to be cooled to a lower temperature without low-temperature corrosion risk.
While still relatively uncommon, such a heat recovery scheme could also be added to a conventional boiler. The approximately 16% of additional heat recovery achieved in the reference plant falls in the range of typical values obtained for biomass-fired boilers in an earlier study considering heat-only boilers [
35]. In the CHP plant studied here, the DH return water could be heated only by approximately 20 °C by condensing heat recovery from conventional CFB boiler flue gas. With the 5.5 MW additional net power generation from flue gas condensation, the advantage of the reference case over the CLOU plant would grow to 8.6 MW of net power generation and 2% of net efficiency. This, too, is still a very low figure for performance loss caused by a carbon capture solution. The efficiency loss amounts to an order of magnitude less than what was recently obtained for industrial steam cycle CHP BECCS implementation based on conventional post-combustion amine scrubbing CCS technology [
36]. Even though the comparability of the results is limited due to the different configurations of the plants, the magnitude of the difference gives a strong indication of a considerable efficiency advantage in favor of the CLOU technology.
Calcium looping (CaL) and oxy-combustion technologies are other alternatives for BECCS, sharing some advantages with chemical looping, but also with notable differences. Oxy-combustion, as the name suggests, involves burning the fuel with oxygen instead of air. This yields a flue gas stream not diluted with nitrogen, very similar to the FR gas stream from the CLOU reactor system. CaL, on the other hand, is a post-combustion technology, where the combustor resembling a conventional boiler is followed by a twin-reactor system of calciner and carbonator. This, too, yields a stream with more concentrated water vapor from the calciner. One clear disadvantage is that, with separate combustor, calciner, and carbonator, three CFB reactors are required.
Another disadvantage of particularly the oxy-combustion, but to some extent also CaL, is the requirement for oxygen. Producing the oxygen requires an air separation unit, which introduces a significant additional parasitic load. Recently, calcium looping and oxy-combustion were compared for CHP generation in a plant only slightly larger than the one considered in this study [
37]. Although the plant differs from the one considered here enough to make direct comparisons of results infeasible, the impact of the air separation unit on the parasitic load becomes clear: in oxy-combustion the magnitude is broadly similar to that of the CO
2-CPU, and in CaL, approximately half of it.
During the last ten years, a firm base has been achieved in the development of chemical looping processes for solid fuels, including experience gained from experimental chemical looping units ranging from 0.5 to 4 MW
th [
6]. The CLOU reactor system presented in this paper has significant similarities with the well-known and commercially available CFB combustion technology for solid fuels. Thus, retrofitting a circulating fluidized bed with chemical looping by adding two reactors side by side can be seen as a promising option in the next stage of the scale-up process.
5. Conclusions
The studied system appears to offer an efficient pathway to utilize biomass resources in a sustainable and efficient manner while removing CO2 (net) from the atmosphere. A very small net efficiency penalty of less than 1% point was achieved in comparison to the conventional reference plant operating with the same fuel input and the same heat production, even though the CLOU plant operation was shifted from mostly back pressure towards inherently less efficient condensing cycle.
The good performance was mainly due to two reasons. Firstly, the CLOU system provides an opportunity for highly efficient heat recovery due to the separate streams of combustion products and oxygen-depleted air. Secondly, the parasitic load of the CCS scheme is low, limited to the unavoidable CO2 compression and purification unit and a fairly modest increase of fan power and heat recovery system power consumption. There is no need for solvent regeneration or producing oxygen in an air separation unit, which typically introduce significant heat or power demands in alternative CCS solutions.
Based on the results presented in this study, the overall performance of CLOU-based BECCS schemes is considered potentially superior to many alternatives, but definitive conclusions cannot be drawn yet based on these initial results. Further studies are required to develop a better understanding of the advantages and disadvantages of alternative technologies, and to evaluate the suitability for different applications with different heat consumers, as well as different plant configurations. Furthermore, the successful implementation of CO2 capture technologies in the power and heat sector will depend not only on the technical performance, but also on the economics. Future work will include a detailed economic analysis of the proposed CLOU-CHP plant, as well as considering seasonal variations in DH load and temperature levels.