Abstract
This paper presents the results of studies on the possibility of using lignite to produce blast furnace coke. The aim of the investigation was to evaluate the influence of lignite addition (direct addition or incorporated into briquettes) on the textural, structural and quality parameters (NSC-CRI and CSR) of blast furnace coke. It was found that the introduction of lignite in briquettes (4.5% addition) allows coke to be produced that is characterized by equally high NSC parameters as for coke obtained without lignite addition for standard top-charged operation.
Keywords:
coke; lignite; brown coal; briquetting; coke making; coke strength; coke reactivity; CSR; CRI 1. Introduction
The decreasing supply and increasing price of prime coking coals forces coke manufacturers to look for new solutions to enhance the quality and economics of the coke-making process [1,2,3,4]. Coke is an essential raw material in the iron- and steel-making sectors [5], where in the blast furnace process it plays the role of a fuel, a reductant agent and a permeable grid supporting a column of input materials. Approximately 75% of the cost of coke production is the cost of coals used for coking blend preparation. Nowadays, common practice in the coke-making industry demands low-cost coal blends that produce a high-quality coke and ensure a safe exploitation of coke-oven batteries. One of the methods of improving the economic efficiency of coke production is to introduce cheaper components into coal blends, such as semi-soft coals, non-caking coals or other carbon-bearing materials (coke breeze, charcoal, biomass, plastic wastes, etc.) [6,7,8,9,10,11,12,13,14,15,16,17,18,19,20,21]. However, the proportion of these is very limited—as a rule it should not exceed a few percent. In general, an increase in the proportion of the aforementioned materials is always detrimental to coke quality, due to their deleterious effect on fluidity development, providing worse (compared to hard coking coals) or non-fusible constituents and decreasing the coal charge bulk density. Some of them are donors of weakly ordered, highly reactive carbon structures. All these elements are crucial in shaping the technological parameters. To minimize or even eliminate these drawbacks is to simultaneously introduce techniques for increasing coal charge bulk density, which has a positive impact on coke quality (particularly mechanical strength), and thus allows for the use of a higher proportion of weaker constituents in the coking blend [22,23]. As a result of increasing the bulk density, we can improve the quality of the coke or maintain its quality with an increased content of lower quality blend components, which can reduce its cost. Improvement of coal charge bulk density can be obtained in a number of ways: adjusting the grain size distribution or moisture content (drying), adding oil or mechanical treatment, (e.g., partial briquetting or stamping). The most efficient method is to use coal stamping—stamp charging technology—enabling an increase in charge density by 30–40% compared to top-charged coke-oven batteries. Unfortunately, the use of this technology is possible only in the case of the construction of new coke-oven batteries or reconstruction of old ones (change of technology from top charging to stamp charging). Other methods can be applied to existing top-charged batteries that are already in operation. Briquetting processes are widely used to compact various types of granular materials [24,25,26,27]. Coke production using the technology of partial briquetting of coal feed consists of briquetting (compacting) a part of the coal blend (usually 20–30%) and then mixing this part with the remaining part which has not been compacted. The compacting process is usually carried out using hydraulic roll presses with an adjustable downforce. As a rule, a few percent of a binder can be added to obtain the appropriate mechanical strength of the briquettes produced, e.g., coal tar or coal tar pitch. Recipes (proportions of individual components) for the part of the blend to be briquetted and the loose (uncompacted) part, may be the same or different. The technology of partial briquetting of the coal feed has found industrial application in Asian countries, especially in Japan but also in India and South Korea [3,28]. In recent years, the interest in applying briquetting technology to the production of coke has increased [3,11,19,20,21,22,29,30,31,32,33,34,35]. Montiano et al. [23] carried out a comparison of the direct addition of biomass (chestnut sawdust) to coking blends and its addition with the use of briquetting (as one of the ingredients). They concluded that direct addition of biomass causes a decrease in the coal charge bulk density. Increased coal charge bulk density due to the addition of briquettes results in the formation of cokes with lower porosity. In most cases, the mechanical strengths of cokes produced with briquettes were better. Regarding the aspect of coke reactivity, it was possible to maintain the coke quality with the use of 10–15% briquettes (depending on the coking coals used). In another study, Montiano et al. [19] investigated the influence of briquette composition and size on the quality of the resulting cokes for three coking blends, two type of biomass (chestnut and pine sawdust) and two sizes of briquettes (with masses of 1 g and 4 g). In general, the addition of briquettes containing sawdust, regardless of their origin, had detrimental effects on the cold mechanical strength, coke reactivity index (CRI) and post-reaction strength (CSR). Nomura [3] studied the influence of different sized briquettes for three coking blends on the productivity, coke quality parameters and pushing performance on a laboratory scale. The whole mass of the coal blend was subjected to briquetting. In each case, the quality of coke (DI15150 and CSR) and the productivity were improved. The best results from the point of view of productivity were obtained for large briquettes. The pushing performance of coke produced from the coal briquette charging process depends on the experimental conditions—in this case, the best results were also obtained for larger sized briquettes. Florentino-Madiedo et al. [33] studied the reactivity of biomass-containing briquettes. Different briquettes based on coal, four species of biomass and four binders were produced. They found that depending on the biomass and binder used, coke had a different reactivity. According to the authors, optical anisotropy index (OAI) of obtained cokes is the parameter which best explains the reactivity results. In other research, Florentino-Madiedo et al. [34] studied the effect of briquette composition on coking pressure generation. It was found that for the addition of all tested briquettes, regardless of their quantity (up to 20%), the coking pressure was reduced. In addition, positive effect of bulk density on coke quality allows the introduction to the coking blend of a greater amount of additives, like non-coking coal. In a recently published work, Florentino-Madiedo et al. [35] stated that briquettes produced from the coking blend with addition of various biomass and binders were characterized with different density and that the cokes obtained had different strength. They also found, that the strength of cokes were correlated to density and volatile matter content of briquettes produced and textural composition of produced coke.
Some attempts to produce coke with the use of lignites (brown coals) were carried out [30,32,36]. Mori et al. [30] studied the preparation of high-strength coke via carbonization of hot-briquetted Victorian brown coal. Application of mechanical pressures of 64–128 MPa at temperatures of 130–200 °C allowed coke to be produced with a tensile strength (in cold conditions) of 28–37 MPa, which is five or six times higher than that of conventional metallurgical coke. Mollah et al. [32] studied the preparation of coke from brown coal (Loy Yang coal and brown coal briquettes). The quality parameters of obtained cokes (chars) were compared to blast furnace (BF) coke from Japan (Nippon Steel Company). It was found that cokes obtained from brown coal briquettes (regardless of the carbonization process conditions) had lower compressive strength than blast furnace coke. In the case of cokes prepared from coal, compressive strengths were always more than twice as high (compared to BF coke). The surface areas of the obtained cokes were around 40 times higher than those of BF cokes. A similar effect was observed for reactivity—cokes were more than six times more reactive, and chars produced from briquettes were more reactive than those from coal. Additionally, cokes produced from brown coals were characterized by worse carbon matrix organization (a higher interlayer space d002 and lower values of carbon stack height Lc). The possibility of obtaining domestic coke for heating purposes was investigated by Karcz and Rozwadowski [36]. The authors added a certain amount (5–20%) of soft brown coal to semi-soft coals (gas-coking coals). Prepared blends were carbonized under various bulk densities in order to simulate different conditions (top- and stamp-charged batteries). They concluded that the addition of brown coal positively influenced coke reactivity. Moreover, it has been found that the bulk density increase has a beneficial effect on the mechanical properties of the obtained cokes.
Interest in using lignites (brown coals) for coke production results from its availability and its much lower price than the coals usually used, i.e., hard coking coals and semi-soft coking coals. The use of lignites in blast furnace coke production could decrease the total cost of coal blend preparation. Based on the above-mentioned literature reports, it can be concluded that cokes (chars) obtained only from lignites are characterized by high reactivity—much higher than typical blast furnace cokes. It seems that it would be beneficial to introduce lignite in smaller quantities. In addition, the introduction of methods to improve the density of the charge could compensate for the unfavorable impact of the addition of lignite.
To the best of the authors’ knowledge, there is a lack of information about the possibility of producing blast furnace coke with the use of a certain amount of lignite. In particular, this gap concerns the impact of lignite addition on the most important parameters currently used for coke quality evaluation—NSC (Nippon Steel Corporation), coke reactivity index (CRI) and coke strength after reaction (CSR) [37,38,39,40]. Furthermore, there is a lack of data on the impact of lignite addition on the textural and structural properties of coke, which are crucial for coke quality parameters. The aim of the investigation was to evaluate the influence of lignite addition (direct addition and incorporated into briquettes) on the textural, structural and quality (NSC) parameters of blast furnace coke.
2. Materials and Methods
Coking tests on a four-component coking blend with the addition of lignite (BC) were carried out. Five blends were prepared. The first blend was a base blend without the addition of BC. The second and third blends had 4.5% and 9% (respectively, dry basis) of BC added directly. The fourth and fifth blends had 4.5% and 9% addition of BC incorporated into briquettes. Briquettes were prepared using a Komarek B050 roller press (Figure 1). The proportions of BC in the briquetted blends were 15% and 30% respectively (dry basis). The mass share of the briquettes added to the coking blend was 30%, and therefore the proportions of BC with respect to the whole blend were 4.5% and 9%. The investigated blend was similar to a blend used in industrial practice for blast furnace coke production. The blend was prepared from coals originating from the mines of the Upper Silesian Coal Basin (Poland), which are the basic components of the coking blends used in Polish coking plants. The basic properties of the prepared blend and BC are presented in Table 1.

Figure 1.
General view of prepared briquettes.

Table 1.
Properties of used coking blend and brown coal.
The analyses of the prepared blend were performed according to the Polish standards for moisture content (Ma), PN-ISO 589:2006; for ash content (Aa), PN-ISO 1171:2002 and for volatile matter content (VMdaf), ISO 562:2010. Carbon (Ct), hydrogen (Ht) and nitrogen (Nt) content was determined according to ISO 29,541:2010 standard. Total sulfur content (St) was determined according to ISO 19,579:2006. The prepared coal charges were carbonized in a Karbotest apparatus (Figure 2).
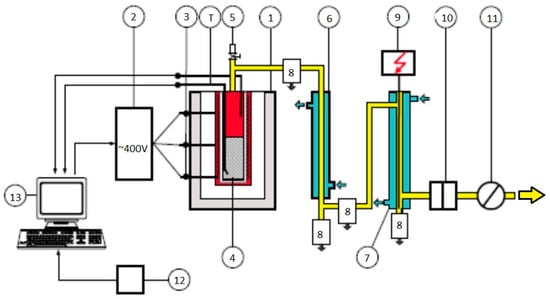
Figure 2.
Scheme of the Karbotest apparatus. (1—Furnace; 2—control system; 3—heating sections; 4—retort with heating head; 5—safety valve; 6—water cooler; 7—electrostatic precipitator; 8—receptacles; 9—high voltage power supply adaptor; 10—filter; 11—gas meter; 12—balance; 13—computer). Reprinted from The Fuel, Vol. 196, Piechaczek M., Mianowski A., Coke optical texture as the fractal object, Pages No. 59–68, Copyright (2017), with permission from Elsevier.
The Karbotest apparatus is a vertical tubular oven for producing metallurgical coke under laboratory conditions that are close to an industrial scale. The coal blend, weighing 4 kg, was gravity charged to a steel retort (depth 850 mm and inner diameter 150 mm). The retort was introduced to the hot oven and heated up until the temperature in the center of the charge reached 950 °C. The cokes were kept at the final coking temperature until gas production dropped below 0.5 dm3/min. Then, the oven was turned off, and the retort remained in the oven for self-cooling to ambient temperature. The next day, after the retort had cooled, the coke was removed. The NSC parameters CRI and CSR were determined according to ISO: 2006. Microscopic studies of the coals and resulting cokes were performed using a polarized microscope (Axio Imager M1 m, Carl Zeiss, Germany, MSP 400-J&M) with monochromatic polarized (for cokes) and unpolarized (for coals) light, at a magnification of ×500 under oil immersion. Grained coal (≤1.00 mm in diameter) was embedded in acrylic resin and then polished according to the procedures recommended by the International Committee for Coal and Organic Petrology (ICCP). The petrographic analysis of hard coals, including a maceral group analysis and determination of the mean random vitrinite reflectance, was performed according to ISO 7404:2009. Coke optical anisotropy was evaluated using the fibrosity index Wx (according to the method described by Piechaczek et al. [40]). For this purpose, determination of the textural components of coke was carried out according to ASTM D5061-07. The method for qualitative and quantitative analysis of coke optical textures was performed with the assumption that the textures delivered from the brown coal that were isotropic and unfused with the coke matrix, were regarded as organic inerts (filler phase). A Micromeritics 3Flex analyzer was used to assess the porous structure of the coke. This measures the physisorption and has appropriate software for analyzing the results obtained. As a result of the analysis of N2 and CO2 sorption at 77 and 273 K respectively, the values for the volume and specific surface area of the pores were determined. Before the measurement, the samples were suitably prepared by crushing (3.15–5 mm) and vacuum degassing at 350 °C for 4 h. N2 surface area was assessed using the BET (Brunauer–Emmett–Teller) equation. CO2 surface area was assessed using the Dubinin–Radushkevich equation. By means of CO2 sorption, the CO2 micropore surface area (SDR) and micropore volume ((CO2) VMICRO) were determined. Using N2 sorption, the micropore volume ((N2) VMICRO), mesopore volume ((N2) VMESO), macropore volume ((N2) VMACRO), surface area SBET and total pore volume (((N2) VTOTAL) were determined. For the measurement of real density (ρt) and total pore volume ((He) VTOTAL), an AccuPyc II 1340 helium pycnometer from Micromeritics (USA) was used. For the measurement of apparent density (ρapp) a GeoPyc 1350 from Micromeritics was used. Total porosity (P) was obtained via the formula:
The results obtained are presented in Table 2.

Table 2.
Coal blend and coke properties.
3. Discussion
3.1. Coal Charge Bulk Density
The bulk density of the coal charge plays an important role in aspects of coke quality and productivity in coke-oven batteries. It can clearly be seen from Table 2 and Figure 3 that the directly added brown coal caused a decrease in coal charge bulk density. The dry bulk density, which is important in terms of the coke-oven chamber’s productivity, decreased gradually from 701.4 kg/m3 for the standard blend (without BC) to 636.8 kg/m3 for a 9% addition of BC (9.2% decrease). This is mainly due to the lower apparent density of brown coal particles. On the other hand, lower bulk density can also be a result of the finer granulation of BC and its surface properties—a higher wettability than coking blends. Finer granulation, and in consequence higher surface area of coal particles, acts against particle reorganization during the charging operation. This effect can be intensified by the higher wettability of BC particles, which promotes the formation of stronger forces (liquid bridges) between particles and also counteracts dense packing of the coal charge. Introducing briquettes to the coking blend caused an increase in bulk density to 766.8 and 733.7 kg/m3 for 4.5% and 9% BC additions (increases of 9.3% and 4.6%), respectively. This results mainly from the higher density of briquettes compared to the uncompacted part of the coking blend. The density of briquettes with 15% BC was 1094 kg/m3 (932.1 kg/m3 dry basis) and with 30% BC it was 1069 kg/m3 (845.6 kg/m3 dry basis).
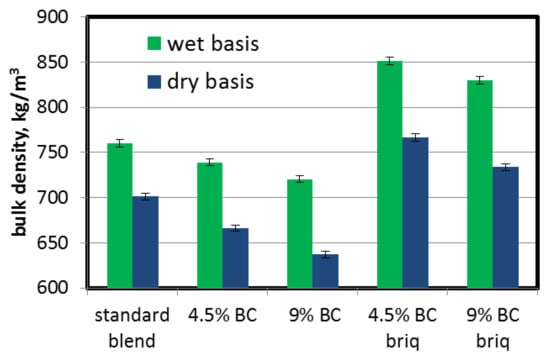
Figure 3.
Influence of lignite addition on coal charge bulk density.
3.2. Coke Porous Structure
Parameters determined using the N2 adsorption method allow assessment of the porous structure of coke with respect to the micropores (<2 nm), mesopores (2–50 nm) and some macropores (~50–150 nm). The method allows the assessment of micropores with dimensions no smaller than ~1.5 nm. The area of micropores with dimensions <1.5 nm, in particular slit-shaped micropores (<0.6–0.7 nm) can only be determined using CO2. The diffusion of nitrogen into the microporous structure is limited due to the fact that nitrogen at 77 K has lower kinetic energy than carbon dioxide at 273 K [41]. For this reason, both methods provide complementary information on the structure of coke with respect to the micropores and mesopores. Both the increase in the volume of micropores and mesopores and the associated surface area can significantly affect the reactivity of coke. A larger surface area means better accessibility of the coke matrix for the gasification agent, i.e., CO2. The dependence of the reactivity on the volume and surface area of the micropores has been noted in various studies [42,43]. Some relations between coke reactivity and SBET, calculated based on N2 adsorption, can be found in [44,45] The nature of the porous structure, including the total pore volume, size distribution and morphology, has a significant impact on the strength and reactivity of the coke [46,47]. Generally, the coke strength decreases with an increase in the total pore volume [48]. An increase in the total pore volume and average pore size increases the reactivity of coke [47].
3.3. Carbon Dioxide Adsorption
To assess the micropore volume and surface area, CO2 sorption was used. The ranges of determined micropore volumes and their surface areas were 2.20–5.40 (×10−3) cm3/g and 19.4–34.9 m2/g respectively. The addition of brown coal to the coking blend increased the CO2 micropore volume and micropore surface area of the cokes obtained (Figure 4). No significant differences between coke produced with direct addition and with a partially briquetted blend were observed; thus, the briquetting operation had no effect on micropore volume development. The maximum increase of CO2 micropore volume was from 0.022 cm3/g for the standard blend without BC to 0.052 for 9% BC addition (briquetted blend). A similar trend was observed for the micropore surface area assessed using the Dubinin–‘Radushkevich equation (Figure 5). In general, brown coal particles do not undergo a plastic stage, therefore pore structure development is mainly due to devolatilization. Tomaszewicz and Mianowski [49] observed that the volumes of micropores and the surface areas of micropores of chars made from brown coal are much higher than those obtained from hard coals (from a few to a dozen or so times higher). Based on this, we can conclude that BC, and in consequence coke (char) from BC particles produced during the pyrolysis process, provides carbon structures with a high micropore volume and surface area which are scattered throughout the entire volume of the coke matrix. Even a small addition of BC resulted in a more than twofold increase in micropore volume and almost doubled the micropore surface area.
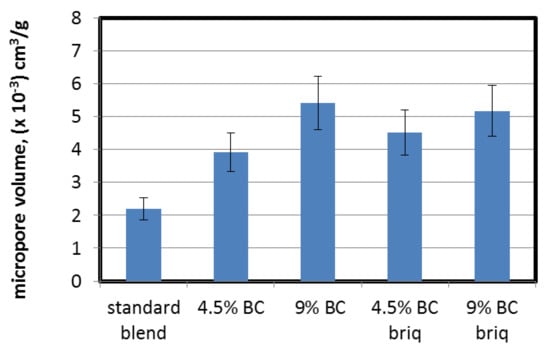
Figure 4.
Influence of lignite addition on CO2 micropore volume of cokes.
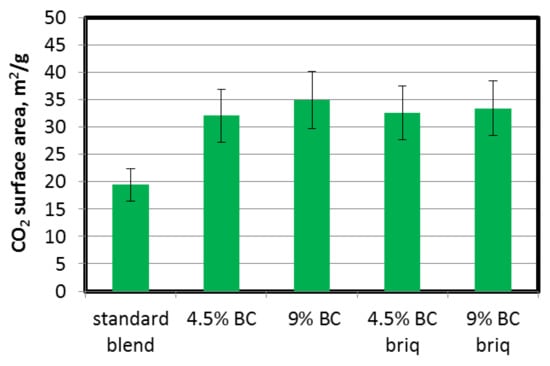
Figure 5.
Influence of lignite addition on CO2 surface area of coke.
3.4. Nitrogen Adsorption
Addition of BC to the coking blend did not cause any significant changes in the porous structure in the range of 1.5–150 nm (micropore and mesopore volume, measured by N2 adsorption) (Figure 6, Table 2). Changes in the values of micropores and mesopores were in the range 0.178–0.233 (×10−3) cm3/g and 0.876–0.985 (×10−3) cm3/g, respectively. The range of SBET was 0.723–0.811 m2/g. Higher values for the SDR surface area (CO2 surface area) than for the SBET (obtained for N2) may indicate that the coke samples have slit-shaped pores with dimensions <0.6–0.7 nm, unavailable for N2 molecules at 77 K but available for CO2 at 273 K. Based on the data, it can be concluded that parameters related to pore sizes 1.5–150 nm were quite similar. Some variation was observed in the case of 9% direct BC addition, but when we take into consideration total pore volume (as the sum of micropores, mesopores and macropores) measured by N2 sorption, it was at a similar level (Table 2). Taking into account the results obtained in the CO2 sorption tests, it was to be expected that in the case of N2 sorption, the volume and surface area would also increase. However, this was not the case. This is probably due to the properties of the coal used. The same coal was used in [49]. In that study, it was noted that the single char from Turów lignite was characterized by a strongly developed microporous structure (assessed by CO2 sorption). However, the parameters of the porous structure determined by means of N2 sorption strongly differed from other brown coal chars and were closer to those of chars produced from hard coals. Therefore, in our case, such small additions of brown coal (4.5% and 9%) could not have a significant impact on the parameters of coke produced from blends.
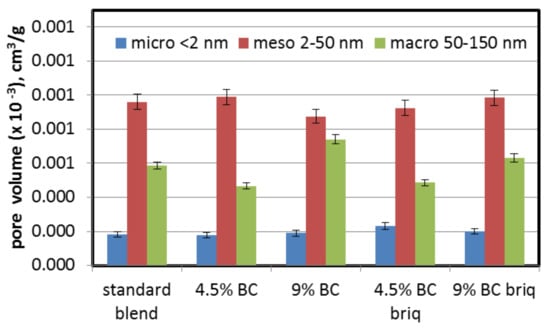
Figure 6.
Influence of lignite addition on volume of pores measured via N2 sorption.
3.5. Total Porosity of Coke
Cokes produced with the use of brown coal are characterized by a higher total pore volume, lower apparent density and in consequence, higher total porosity (Table 2 and Figure 7). The addition of brown coal caused an increase in total porosity from 46.4% (standard blend) to 48.4% for 9% direct BC addition. As a result of the partial briquetting (more tightly packed coal grains), a decrease in total porosity was noted. For blends with BC addition, cokes obtained from briquetted blends were characterized by lower porosity than those produced from blends with direct BC addition. The total porosity of coke from the briquetted blend with 4.5% BC was even lower than that of coke from the standard blend. The difference of 0.9% does not seem impressive, but it should be borne in mind that the porosity of cokes produced from stamp-charged blends is only a few percentage points lower than cokes produced from top-charged blends [50]. The difference in the density of these two systems can be up to 200–350 kg/m3. The difference in the density of the tested blends was around 65 kg/m3, so reducing the porosity by almost one unit seems to be quite significant.
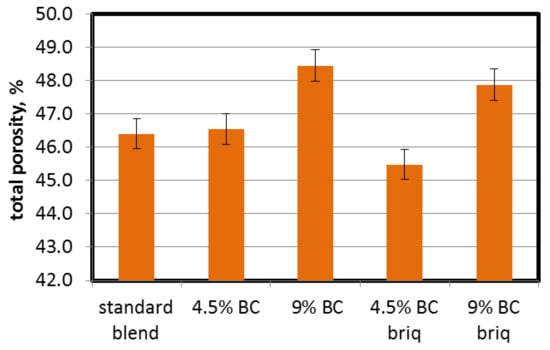
Figure 7.
Influence of lignite addition on total porosity of cokes.
The formation of new pores, as well as the development of pores already existing in the grains of carbonized coals, and in consequence in coke, is determined by the possibility of increasing the volume of these grains during the plastic phase [51]. This increase results from both the grain size and carbon properties, but also from the possibility of grain expansion under the conditions of the carbonization process. Grain expansion is primarily due to the empty space between the grains.
3.6. Coke Optical Anisotropy
The basic structural units (BSUs) of carbonaceous materials including hard coal and coke are the so-called graphite-like crystals made of polyaromatic planes (lamellae), either alone or stacked, consisting of two to three approximately parallel layers [52,53]. Each of the planes consists of four to 10 aromatic rings with a diameter of about 1 nm. Between the individual BSUs, there are cross-linkages (aliphatic, oxygen) limiting their mobility and ability to group. Heat supplied to the pyrolysis process and the associated increase in temperature causes cracking of cross-linkages between the units. The mobility of units obtained in this way (plastic state) allows molecular ordering and the development of the coke microtexture. The BSUs present in the coal blend are regrouped in parallel to form larger areas with a local MOD orientation (LMO). Domain sizes can range from a few nanometers to dozens of micrometers or even more. MOD domains greater than around 0.5 μm can be identified using optical microscopy. The degree of ordering in the optical texture of coke is determined by qualitative and quantitative analysis of the optical textures and by measuring the reflectance of the coke matrix [54,55,56].
Coke optical anisotropy corresponds with coke reactivity (CRI) and strength (CSR). Based on the analysis of optical textures, appropriate indicators can be determined to make the interpretation of results clearer, e.g., OTI (optical texture index) or Wx (fibrosity index) [40,54,55,56]. In this study, the fibrosity index Wx was used. The calculation of the fibrosity index is based on the results of a qualitative and quantitative analysis carried out according to the ASTM method for a metallurgical coke. As brown coal is not a typical component of a coking blend for metallurgical coke production, the optical textures derived from the brown coal components that were not fused to the coke matrix and were isotropic (Figure 8) were treated as a filler phase (organic inerts) in the calculation algorithm.
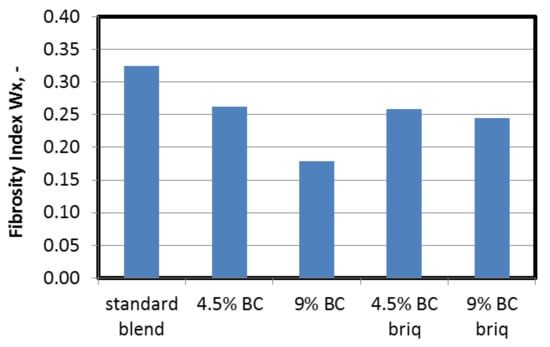
Figure 8.
Influence of lignite addition on fibrosity index Wx of cokes.
As a result of the addition of lignite into the coking blends, a decrease in the anisotropy of the cokes, as described by the fibrosity index, was observed (Figure 8). A 4.5% share of BC in the coking blend resulted in a decrease in fibrosity index from 0.32 (for the standard blend) to 0.26. For a 9% addition, it decreased to 0.18% lignite does not exhibit plasticity; it can be practically treated as an inert agent, which in turn causes an increase in the amount of so-called filler phase and a reduction in the binder phase. It will also play the role of a hydrogen donor, changing the plasticity of the accompanying constituents of a blend and their molecular ordering conditions. Proportions visible in the optical textures are presented in Table 3 and the optical texture photomicrographs in Figure 9.

Table 3.
Qualitative and quantitative analysis of the coke optical texture and calculation of the fibrosity index Wx.
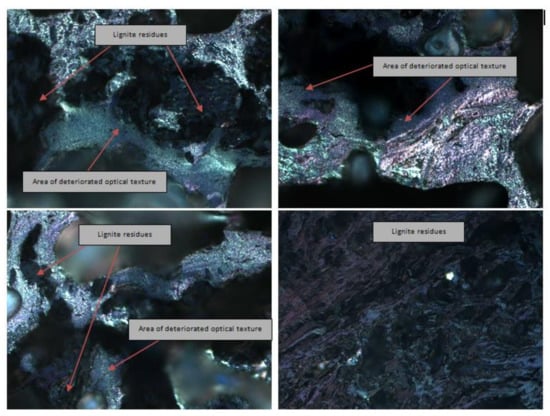
Figure 9.
Exemplary photomicrographs of lignite residues in coke.
In general, the value of the fibrosity index of cokes made from blends with 4.5% addition of BC (briquetted or loose charge) are virtually the same. This means that the addition of 4.5% of brown coal into the coal blend changes the coke quality in the same way, whether the brown coal is compacted or loose. A significant difference is visible when the brown coal content in a blend is increased to 9%. In this situation, compacting/briquetting of the brown coal additive makes a big difference to the resulting coke’s quality. The addition of 9% of brown coal in a loose form causes a deterioration of the coke matrix ordering, resulting in the lowest calculated fibrosity index, Wx = 0.18. The same amount of brown coal content used in a blend (9%) as briquettes does not affect the quality of the coke as negatively (Wx = 0.245). Comparing the results of fibrosity indices, it is clear that briquetting a brown coal enables the addition of higher amounts of this component to the coking blend, up to 4.5%, without a large deterioration in coke quality as measured by the fibrosity index. This phenomenon was proved by other quality parameters of coke. It was observed that the coke sample made of coal blend containing 9% of loose brown coal, showed a higher quantity of inorganic inerts. This situation could be an effect of a non-homogenous distribution of mineral matter on the surface of the microscopic block. In general, coal and coke are considered non-homogeneous materials. Taking into consideration the distribution of inorganics in the organic matter, it is obvious that it has a very local and random character. This non-homogeneity is partially reduced by grinding, blending and representative sampling, but it is still possible for this effect to occur.
3.7. Coke Technological Parameters
As might be expected, the introduction of brown coal to the coking blend impaired the coke quality parameters. Direct addition of 4.5% of BC caused an increase in coke reactivity index (CRI) from 34.1% to 38.2%. Further addition of BC (up to 9%) resulted in an increase in CRI to 42.2%. Gasification of coke with carbon dioxide is destructive to its porous structure, thus also affecting the CSR parameter. Direct addition of BC in proportions of 4.5% and 9% caused a decrease in CSR from 49.1% to 41.4% and 34.5%, respectively. Increased reactivity could result from higher micropore volumes and surface areas of cokes obtained from blends with the addition of BC. Higher micropore volumes and surface areas create easier access for carbon CO2 to the coke matrix. Moreover, the coke matrix of cokes obtained from blends with BC, are characterized by a lower degree of optical anisotropy, which can also affect coke reactivity [6,46,48,53,54,57,58,59]. When the coke microtexture is built of large objects (domains), more carbon atoms are inside them, making access to CO2 molecules more difficult. This type of texture has lower reactivity (CRI) and its strength (CSR) is higher. Whereas if the texture is formed of small domains, most of the carbon atoms are located on their edges, which makes them more accessible to CO2, which in turn increases the reactivity [6].
In this experiment most of the brown coal residues in the coke matrix gave isotropic, poorly fused textures that were highly reactive.
It can be clearly seen that incorporation of the briquetting process positively influences the coke quality parameters (Figure 10). The quality parameters (both CRI and CSR) of coke produced from a partially briquetted blend (4.5% share of BC) were at a level similar to the standard blend, i.e., CRI = 34.7 and CSR = 50.0. In the case of 9% BC addition, the CRI and CSR parameters were significantly reduced (CRI = 39.0, CSR = 44.0) but were better than those obtained for direct addition of BC (CRI = 42.2, CSR = 34.5). The values of these parameters seem too low from the industrial point of view. However, on the basis of many years of experience, transition coefficients have been developed from the laboratory scale to the industrial scale (for the PWR 63 top charged coke oven batteries): industrial CRI = (0.85–0.9) × laboratory CRI; industrial CSR = (1.14–1.18) × laboratory CSR [60]. On their basis, it may be assumed that the values of coke quality parameters at the industrial scale should be more favorable. Obviously, from the point of view of industrial use, an important issue is the impact of the addition of briquettes, and in consequence—bulk density of coal charge, on the coking pressure. Excessive pressure may result in operational problems and contribute to faster degradation of ceramic brickwork of coke ovens. In the case under study, a component that is an inert in the pyrolysis process was introduced which should compensate the effect of increased bulk density. The addition of materials which act as an inert is one of the methods for controlling the coking pressure [61,62,63,64,65]. In the case of biomass, its addition both in loose form and in briquettes resulted in a reduction in coking pressure [34,64]. Taking into consideration aforementioned information, incorporation of brown coal to the coking blend may reduce coking pressure due not only to the “inert” effect but also due to the “fissure effect”. Higher volatile matter content of this type of coal, may result in more fissures in the semi-coke, which may facilitate the release of gas from the plastic layer adjacent to the semi-coke. However, in order to confirm the above assumptions, it is necessary to perform a test on a movable wall oven. Thanks to the use of the briquetting method, the detrimental effect of BC addition was minimized. The increased bulk density of the coal charge due to briquetting can enhance particle adhesion, which improves sintering conditions. In various studies [57,58], together with a decrease in total porosity caused by the increase of the charge density, an increase in the coke wall thickness was also noted, which positively affects the mechanical properties of the porous coke structure.
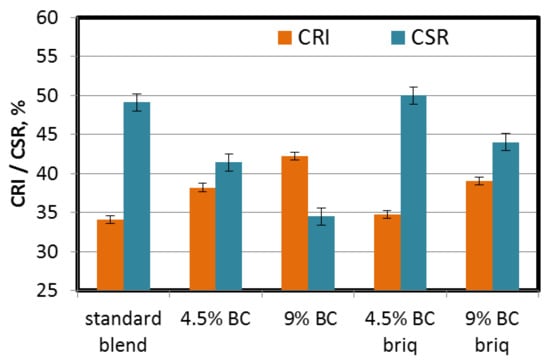
Figure 10.
Influence of lignite addition on coke reactivity index (CRI) and coke strength after reaction (CSR) of cokes.
The improved quality parameters for cokes from briquetted charges could also be a result of slightly lower total porosity, which in general influences the reactivity and strength of coke [47,59]. Pores of larger size also play a transport role for CO2, and therefore they may influence the coke reactivity.
4. Conclusions
The aim of the investigation was to evaluate the influence of lignite addition (direct addition and incorporated into briquettes) on the textural, structural and quality parameters (NSC) of blast furnace coke. The results are summarized as follows.
- -
- The addition of a lignite in loose form to the coking blend, causes significant deterioration in the coke quality parameters: an increase in the volume and area of micropores (measured by CO2 sorption), an increase in the total pore volume and total porosity, a decrease in coke optical anisotropy and a deterioration in NSC parameters (CRI and CSR).
- -
- Incorporation of the briquetting process positively influenced the coke quality parameters. It was found that the total pore volume and total porosity were slightly decreased. Thanks to the use of the partial briquetting method, coke with equally high quality parameters as without the addition of lignite was produced.
Author Contributions
Conceptualization, M.R. and R.B.; Methodology, M.R., R.B. and M.W.; Formal analysis, M.R., R.B. and M.W.; Investigation, M.R., R.B. and M.W.; Writing–original draft preparation, M.R., R.B. and M.W.; Writing–review and editing, M.R., R.B. and M.W. All authors have read and agreed to the published version of the manuscript.
Funding
This study was carried out within the framework of Project funding from the European Union’s Research Fund for Coal and Steel (RFCS) research program under grant agreements No.RFCR-CT-2014-00006: Developing uses of alternative raw materials in cokemaking; acronym: ALTERAMA.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Karcz, A.; Strugała, A. Increasing chances of utilizing the domestic coking coal resources through technological operations in coal blend preparation. Gospod. Surowcami Miner. Miner. Resour. Manag. 2008, 24, 5–18, (in Polish with English abstract). [Google Scholar]
- Nomura, S. Coal briquette carbonization in a slot-type coke oven. Fuel 2016, 185, 649–655. [Google Scholar] [CrossRef]
- Flores, B.D.; Flores, I.V.; Guerrero, A.; Orellana, D.R.; Pohlmann, J.G.; Diez, M.A.; Borrego, A.G.; Osório, E.; Vilela, A.C.F. Effect of charcoal blending with a vitrinite rich coking coal on coke reactivity. Fuel Process. Technol. 2017, 155, 97–105. [Google Scholar] [CrossRef]
- Żarczyński, P.; Strugała, A. Studies on the Possibility of Extending Coal Resources for Coke Production through the Application of Coal Predrying. Energy Fuels 2018, 32, 5666–5676. [Google Scholar] [CrossRef]
- Ahmed, H. New Trends in the Application of Carbon-Bearing Materials in Blast Furnace Iron-Making. Minerals 2018, 8, 561. [Google Scholar] [CrossRef]
- Smędowski, Ł.; Krzesińska, M. Molecular oriented domains (MOD) and their effect on technological parameters within the structure of cokes produced from binary and ternary coal blends. Int. J. Coal Geol. 2013, 111, 90–97. [Google Scholar] [CrossRef]
- Castro Díaz, M.; Zhao, H.; Kokonya, S.; Dufour, A.; Snape, C.E. The effect of biomass on fluidity development in coking blends using high-temperature SAOS rheometry. Energy Fuels 2012, 26, 1767–1775. [Google Scholar] [CrossRef]
- Kokonya, S.; Castro Díaz, M.; Barriocanal, C.; Snape, C.E. An investigation into the effect of fast heating on fluidity development and coke quality for blends of coal and biomass. Biomass Bioenergy 2013, 56, 295–306. [Google Scholar] [CrossRef]
- Diez, M.A.; Alvarez, R.; Fernández, M. Biomass derived products as modifiers of the rheological properties of coking coals. Fuel 2012, 96, 306–313. [Google Scholar] [CrossRef]
- Krzesińska, M.; Szeluga, U.; Smędowski, Ł.; Majewska, J.; Pusz, S.; Czajkowska, S.; Kwiecińska, B. TGA and DMA studies of blends from very good coking Zofiówka coal and various carbon additives: Weakly coking coals, industrial coke and carbonized plants. Int. J. Coal Geol. 2010, 81, 293–300. [Google Scholar] [CrossRef]
- Diez, M.A.; Alvarez, R.; Climadevilla, J.L.G. Briquetting of carbon-containing wastes from steelmaking for metallurgical coke production. Fuel 2013, 114, 216–223. [Google Scholar] [CrossRef]
- Melendi, S.; Diez, M.A.; Alvarez, R.; Barriocanal, C. Relevance of the composition of municipal plastic wastes for metallurgical coke production. Fuel 2011, 90, 1431–1438. [Google Scholar] [CrossRef]
- MacPhee, J.A.; Gransden, J.F.; Giroux, L.; Price, J.T. Possible CO2 mitigation via addition of charcoal to coking coal blends. Fuel Process. Technol. 2009, 90, 16–20. [Google Scholar] [CrossRef]
- Alvarez, R.; Barriocanal, C.; Díez, M.A.; Cimadevilla, J.L.G.; Casal, M.D.; Canga, C.S. Recycling of hazardous waste materials in the coking process. Environ. Sci. Technol. 2004, 38, 1611–1615. [Google Scholar] [CrossRef] [PubMed]
- Fernández, A.M.; Barriocanal, C.; Díez, M.A.; Alvarez, R. Influence of additives of different origins on thermoplastic properties of coal. Fuel 2009, 88, 2365–2372. [Google Scholar] [CrossRef]
- Diez, M.A.; Alvarez, R.; Melendi, S.; Barriocanal, C. Feedstock recycling of plastic wastes/oil mixtures in cokemaking. Fuel 2009, 88, 1937–1944. [Google Scholar] [CrossRef]
- Díaz, M.C.; Edecki, L.; Steel, K.M.; Patrick, J.W.; Snape, C.E. Determination of the effects caused by different polymers on coal fluidity during carbonisation using high-temperature 1H NMR and rheometry. Energy Fuels 2008, 22, 471–479. [Google Scholar] [CrossRef]
- Collin, G.; Bujnowska, B.; Polaczek, J. Co-coking of coal with pitches and waste plastics. Fuel Process. Technol. 1997, 47, 179–184. [Google Scholar] [CrossRef]
- Montiano, M.G.; Diaz-Faes, E.; Barriocanal, C. Partial briquetting vs direct addition of biomass in coking blends. Fuel 2014, 137, 313–320. [Google Scholar] [CrossRef]
- Montiano, M.G.; Diaz-Faes, E.; Barriocanal, C. Effect of briquette composition and size on the quality of the resulting coke. Fuel 2016, 185, 649–655. [Google Scholar] [CrossRef]
- Nomura, S. Recent developments in cokemaking technologies in Japan. Fuel Process. Technol. 2017, 159, 1–8. [Google Scholar] [CrossRef]
- Sobolewski, A.; Rejdak, M.; Czaplicki, A.; Janusz, M.; Mianowski, A. The effect of coal charge preparation on coke quality. Przemysł Chem. 2014, 93, 2103–2110, (in Polish with English abstract). [Google Scholar]
- Rejdak, M.; Wasielewski, R. Mechanical compaction of coking coals for carbonization in stamp-charging coke ovens. Physicochem. Probl. Miner. Process. 2015, 51, 151–161. [Google Scholar] [CrossRef]
- Borowski, G.; Hycnar, J. Utilization of Fine Coal Waste as a Fuel Briquettes. Int. J. Coal Prep. Util. 2013, 33, 204. [Google Scholar] [CrossRef]
- Rejdak, M.; Robak, J.; Czardybon, A.; Ignasiak, K.; Fudała, P. Research on the Production of Composite Fuel on the Basis of Fine-Grained Coal Fractions and Biomass—The Impact of Process Parameters and the Type of Binder on the Quality of Briquettes Produced. Minerals 2020, 10, 31. [Google Scholar] [CrossRef]
- Li, Y.; Han, Y.; Sun, Y.; Gao, P.; Li, Y.; Gong, G. Growth Behavior and Size Characterization of Metallic Iron Particles in Coal-Based Reduction of Oolitic Hematite–Coal Composite Briquettes. Minerals 2018, 8, 177. [Google Scholar] [CrossRef]
- Pang, L.; Yang, Y.; Wu, L.; Wang, F.; Meng, H. Effect of Particle Sizes on the Physical and Mechanical Properties of Briquettes. Energies 2019, 12, 3618. [Google Scholar] [CrossRef]
- Kurunov, I.; Lizogub, P.; Golubev, O. Coal Preparation, Coking, and Slaking in China and Japan. Coke Chem. 2010, 9, 22–27. [Google Scholar] [CrossRef]
- Fehse, F.; Rosin, K.; Schröder, H.W.; Kim, R.; Spöttle, M.; Repke, J.U. Influence of briquetting and coking parameters on the lump coke production using non-caking coals. Fuel 2017, 203, 915–923. [Google Scholar] [CrossRef]
- Mori, A.; Kubo, S.; Kudo, S.; Norinaga, K.; Kanai, T.; Aoki, H.; Hayashi, J.-I. Preparation of high-strength coke by carbonization of hot-briquetted Victorian brown coal. Energy Fuel 2011, 26, 296–301. [Google Scholar] [CrossRef]
- Zubkova, V.; Strójwąs, A.; Strojanowska, M.; Kowalczyk, J. The influence of composition of coal briquettes on changes in volume of the heated coal charge. Fuel Process. Technol. 2014, 128, 265–275. [Google Scholar] [CrossRef]
- Mamun Mollah, M.; Jackson, W.R.; Marshall, M.; Chaffee, A.L. An attempt to produce blast furnace coke from Victorian brown coal. Fuel 2015, 148, 104–111. [Google Scholar] [CrossRef]
- Florentino-Madiedo, L.; Diaz-Faes, E.; Barriocanal, C. Reactivity of biomass containing briquettes for metallurgical coke production. Fuel Process. Technol. 2019, 193, 212–220. [Google Scholar] [CrossRef]
- Florentino-Madiedo, L.; Diaz-Faes, E.; Barriocanal, C. The effect of briquette composition on coking pressure generation. Fuel 2019, 258, 116128. [Google Scholar] [CrossRef]
- Florentino-Madiedo, L.; Diaz-Faes, E.; Barriocanal, C. Mechanical strength of bio-coke from briquettes. Renew. Energy 2020, 146, 1717–1724. [Google Scholar] [CrossRef]
- Karcz, A.; Rozwadowski, A. The pyrolysis examinations for blends of bituminous coal and soft brown coal. Karbo 2002, 6, 171–175, (in Polish with English abstract). [Google Scholar]
- Li, K.; Khanna, R.; Zhang, J.; Liu, Z.; Sahajwalla, V.; Yang, T.; Deven, K. The evolution of structural order, microstructure and mineral matter of metallurgical coke in a blast furnace: A review. Fuel 2014, 133, 194–215. [Google Scholar] [CrossRef]
- Nag, D.; Haldar, S.K.; Choudhary, P.K.; Banerjee, P.K. Prediction of Coke CSR from Ash Chemistry of Coal Blend. Int. J. Coal Prep. Util. 2009, 29, 243–250. [Google Scholar] [CrossRef]
- Tiwari, H.P.; Haldar, S.K.; Das, A.; Mishra, P.; Kumar, A.; Khattri, P. Potential Use of High Ash Indian Medium Coking Coal in Stamp Charged Coke Making. Int. J. Coal Prep. Util. 2017, 39, 101–111. [Google Scholar] [CrossRef]
- Piechaczek, M.; Mianowski, A.; Sobolewski, A. The original concept of description of the coke optical texture. Int. J. Coal Geol. 2015, 139, 84–190. [Google Scholar] [CrossRef]
- Lozano-Castello, D.; Cazorla-Amor, D.; Linares-Solano, A. Usefulness of CO2 adsorption at 273 K for the characterization of porous carbons. Carbon 2004, 42, 1231–1236. [Google Scholar] [CrossRef]
- Grigore, M.; Sakurows, R.; French, D.; Sahajwalla, S. Properties and CO2 reactivity of the inert and reactive maceral derived components in cokes. Int. J. Coal Geol. 2012, 98, 1–9. [Google Scholar] [CrossRef]
- Diez, M.A.; Borrego, A.G. Evaluation of CO2-reactivity patterns in cokes from coal and woody biomass blends. Fuel 2013, 113, 59–68. [Google Scholar] [CrossRef]
- Zhang, S.; Peng, L.; Rui, G.; Pengfei, L.; Yinhua, L. Preparation of high strength and highly reactive coke by the addition of steel slag. Coke Chem. 2014, 57, 391–397. [Google Scholar] [CrossRef]
- Krzesińska, M.; Pusz, S.; Smędowski, Ł. Characterization of the porous structure of cokes produced from the blends of three Polish bituminous coking coals. Int. J. Coal Geol. 2009, 78, 169–176. [Google Scholar] [CrossRef]
- Koba, K.; Sakata, K.; Ida, S. Gasification studies of cokes from coals. The effects of carbonization pressure on optical texture and porosity. Fuel 1981, 60, 499–506. [Google Scholar] [CrossRef]
- Patrick, J.W.; Walker, A. Macroporosity in cokes: Its significance, measurement, and control. Carbon 1989, 27, 117–123. [Google Scholar] [CrossRef]
- Marsh, H. Metallurgical coke: Formation, structure and properties. Ironmak. Conf. Proc. 1982, 4, 2–10. [Google Scholar]
- Tomaszewicz, M.; Mianowski, A. Char structure dependence on formation enthalpy of parent coal. Fuel 2017, 199, 380–393. [Google Scholar] [CrossRef]
- Rejdak, M. Influence of Raw Material Properties and Stamping Operation on the Mechanical Strength of Stamped Coal Cake and the Quality of Obtained Coke. Ph.D. Thesis, University of Science and Technology, AGH Kraków, Kraków, Poland, 2018. (in Polish with English abstract). [Google Scholar]
- Strugała, A. The Role of Material and Technological Factors in the Formation Process of Coke Porous Structure; AGH University Press: Kraków, Poland, 2006; (in Polish with English abstract). [Google Scholar]
- Oberlin, A. High resolution TEM studies of carbonization and graphitization. Chem. Phys. Carbon 1989, 22, 1–143. [Google Scholar]
- Rouzaud, J.N.; Vogt, D.; Oberlin, A. Coke properties and their microtexture; Part I: Micro textural analysis: A guide for cokemaking. Fuel Process. Technol. 1988, 20, 143–154. [Google Scholar] [CrossRef]
- Suarez-Ruiz, I.; Garcia, A.B. Optical parameters as a toll to study the microtextural evolution of carbonized anthracites during high temperature treatment. Energy Fuels 2007, 21, 2935–2941. [Google Scholar] [CrossRef]
- Pusz, S.; Kwiecińska, B.; Koszorek, A.; Krzesińska, M.; Pilawa, B. Relationships between the optical reflectance of coal blends and the microscopic characteristics of their cokes. Int. J. Coal Geol. 2009, 77, 356–362. [Google Scholar] [CrossRef]
- Flores, B.; Borrego, A.; Diez, M.A.; Da Silva, G.; Zymla, V.; Vilela, A.; Osorio, E. How coke optical texture became a relevant tool for understanding coal blending and coke quality. Fuel Process. Technol. 2017, 164, 13–23. [Google Scholar] [CrossRef]
- Nyathi, M.S.; Kruse, R.; Mastalerz, M.; Blish, D. Impact of Oven Bulk Density and Coking Rate on Stamp-Charged Metallurgical Coke Structural Properties. Energy Fuels 2013, 27, 7876–7884. [Google Scholar] [CrossRef]
- Cui, P.; Qu, L.; Ling, Q.; Cheng, L.; Cao, Y. Effects of Coal Moisture Control and Coal Briquette Technology on Structure and Reactivity of Cokes. Coke Chem. 2015, 5, 162–169. [Google Scholar] [CrossRef]
- Marsh, H. Introduction to Carbon Science: Coal to Coke Conversion; Butterworths: London, UK, 1989. [Google Scholar]
- Żarczyński, P. Technological and Economic Evaluation of the Pre-Drying Operation of the Coal Charge in Zdzieszowice Coking Plant. Ph.D. Thesis, University of Science and Technology, AGH Kraków, Kraków, Poland, 2015. (in Polish with English abstract). [Google Scholar]
- Nomura, S.; Mahoney, M.; Fukuda, K.; Kato, K.; Le Bas, A.; McGuire, S. The mechanism of coking pressure generation I: Effect of high volatile coking coal, semi-anthracite and coke breeze on coking pressure and plastic coal layer permeability. Fuel 2010, 89, 1549–1556. [Google Scholar] [CrossRef]
- Mahoney, M.; Nomura, S.; Fukuda, K.; Kato, K.; Le Bas, A.; Jenkins, D.R.; McGuire, S. The mechanism of coking pressure generation II: Effect of high volatile matter coking coal, semi-anthracite and coke breeze on coking pressure and contraction. Fuel 2010, 89, 1557–1565. [Google Scholar] [CrossRef]
- Nomura, S. Effect of Coal Briquette Size on Coke Quality and Coal Bulk Density in Coke Oven, 2019). ISIJ Int. 2019, 59, 1512–1518. [Google Scholar] [CrossRef]
- Florentino-Madiedo, L.; Casal, D.; Díaz-Faes, E.; Barriocanal, C. Effect of sawdust addition on coking pressure produced by two low vol bituminous coals. J. Anal. Appl. Pyrolysis 2017, 127, 369–376. [Google Scholar] [CrossRef]
- Fernández, A.M.; Barriocanal, C.; Alvarez, R. The effect of additives on coking pressure and coke quality. Fuel 2012, 95, 642–647. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).