Development of Full-Cycle Utilization of Chlorella sorokiniana Microalgae Biomass for Environmental and Food Purposes
Abstract
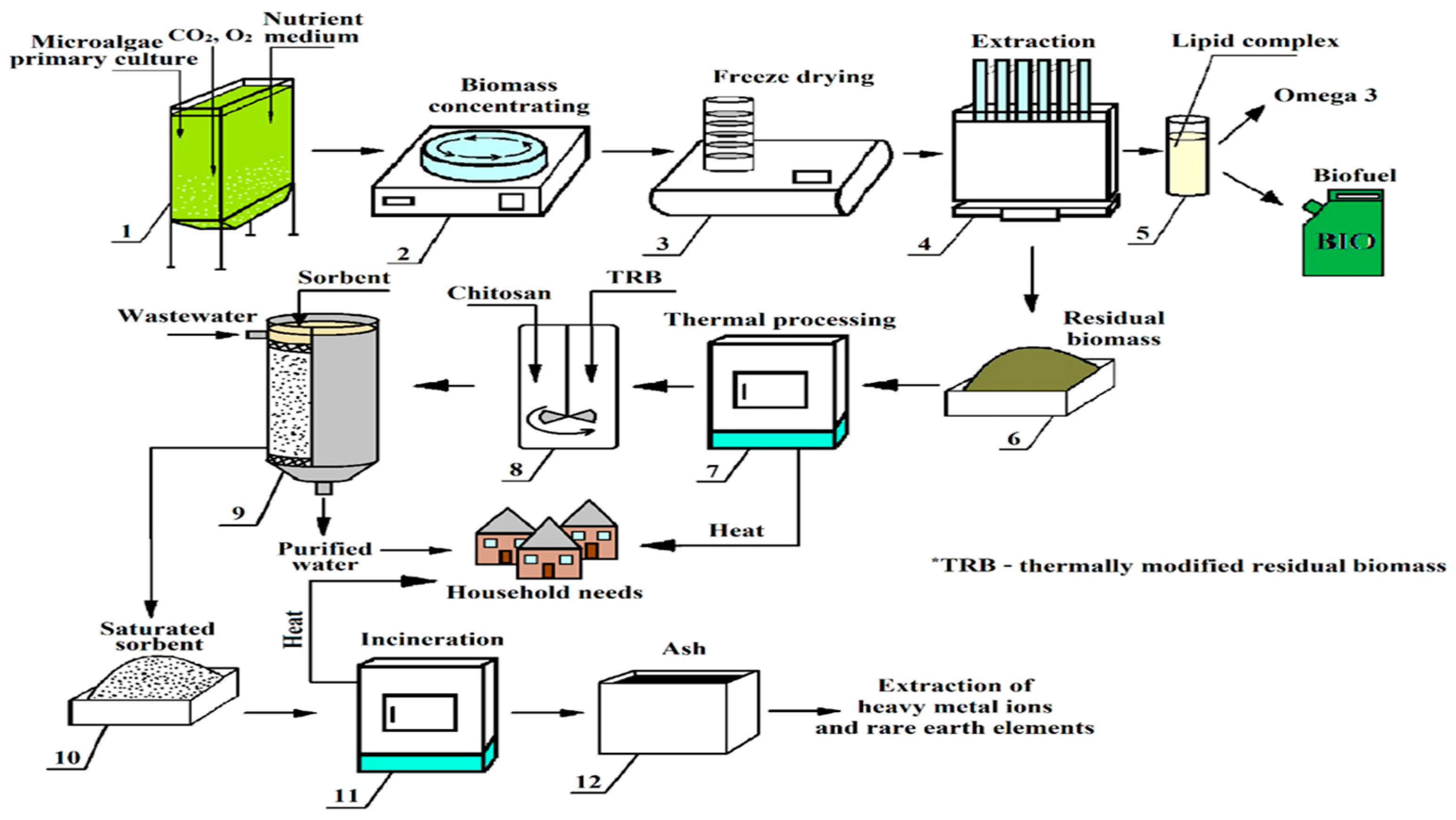
1. Introduction
- Lipids, which, depending on the fatty acid composition, can be sources of Omega-3, Omega-6 [40] or biofuel;
- Biosorbents for metal biomining;
- Enterosorbents for detoxification.
2. Materials and Methods
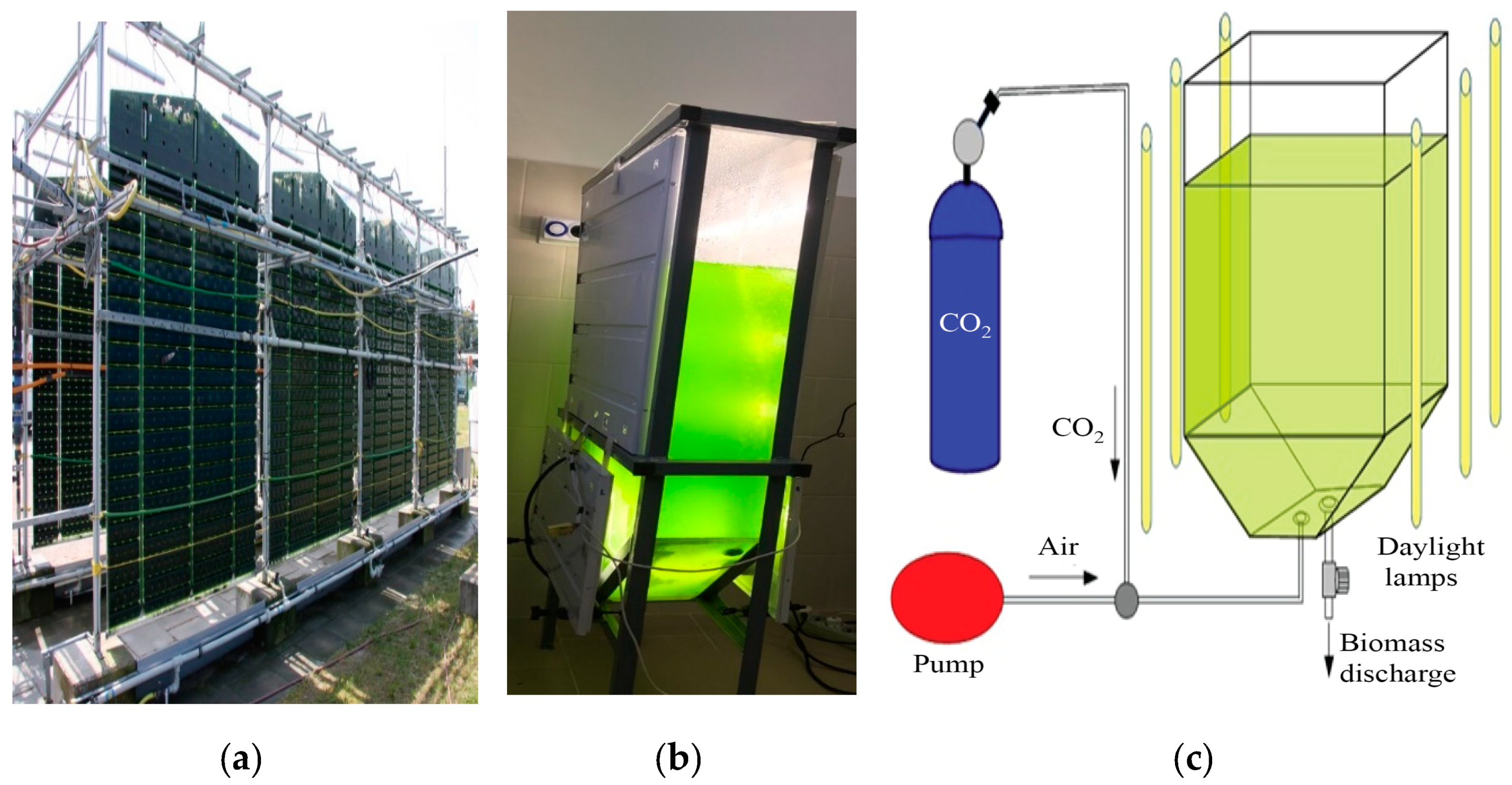
2.1. Cultivation of Microalgae Biomass
2.2. Obtaining Dry Biomass
2.3. Determination of Specific Surface Area
2.4. IR Spectra
2.5. Lipid Extraction and Determination of Their Composition
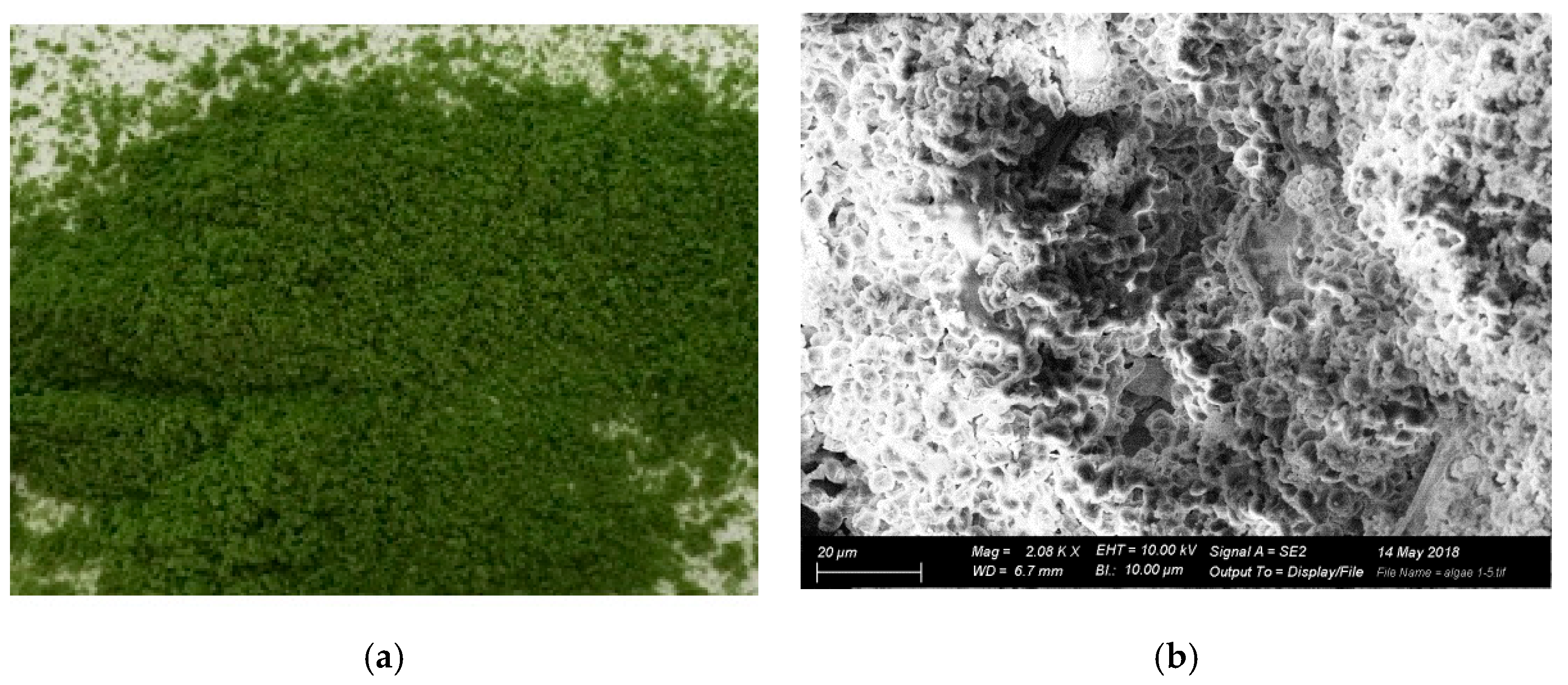
2.6. Microstructural Studies
2.7. Obtaining Sorbents and Studying Their Sorption Properties
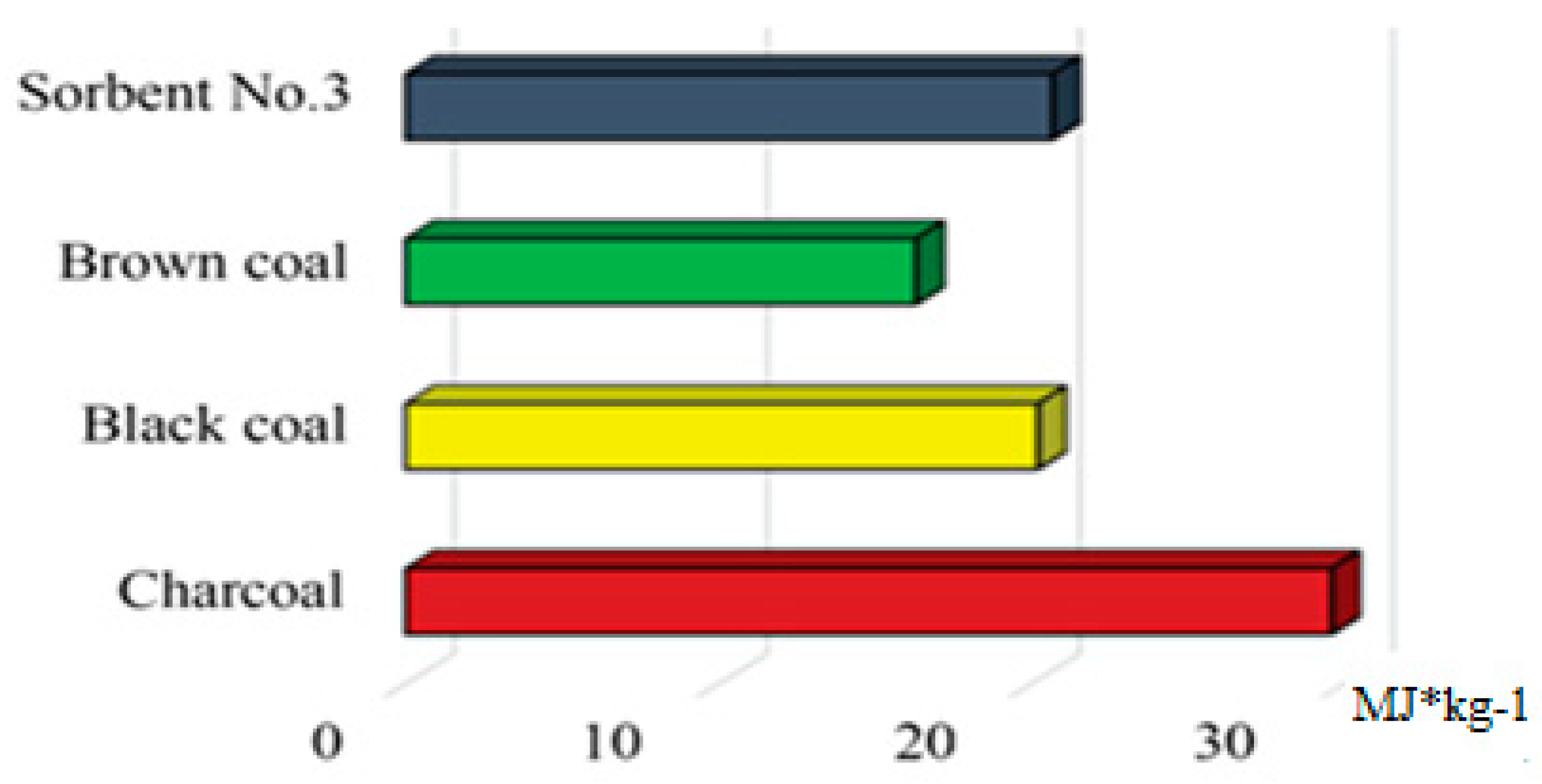
2.8. Determination of Specific Heat of Combustion
3. Results
- Sterols Rf = 0.19;
- Fatty acids Rf = 0.39;
- Triglycerides Rf = 0.60;
- Long-chain aldehydes Rf = 0.73.
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Akubude, V.C.; Nwaigwe, K.N.; Dintwa, E. Production of biodiesel from microalgae via nanocatalyzed transesterification process: A review. Mater. Sci. Energy Technol. 2019, 2, 216–225. [Google Scholar] [CrossRef]
- Rezania, S.; Oryani, B.; Park, J.; Hashemi, B.; Yadav, K.K.; Kwon, E.E.; Hur, J.; Cho, J. Review on transesterification of non-edible sources for biodiesel production with a focus on economic aspects, fuel properties and by-product applications. Energy Convers. Manag. 2019, 201, 112155. [Google Scholar] [CrossRef]
- Al Afif, R.; Linke, B. Biogas production from three-phase olive mill solid waste in lab-scale continuously stirred tank reactor. Energy 2019, 171, 1046–1052. [Google Scholar] [CrossRef]
- Pramanik, S.K.; Suja, F.B.; Zain, S.; Pramanik, B.K. The anaerobic digestion process of biogas production from food waste: Prospects and constraints. Bioresour. Technol. Rep. 2019, 8, 100310. [Google Scholar] [CrossRef]
- Al Afif, R.; Amon, T. Mesophilic anaerobic co-digestion of cow manure with three-phase olive mill solid waste. Energy Sources Part A Recovery Util. Environ. Eff. 2018. [Google Scholar] [CrossRef]
- Rasapoor, M.; Young, B.; Brar, R.; Sarmah, A.; Zhuang, W.Q.; Baroutian, S. Recognizing the challenges of anaerobic digestion: Critical steps toward improving biogas generation. Fuel 2020, 261, 116497. [Google Scholar] [CrossRef]
- Sun, X.; Atiyeh, H.K.; Huhnke, R.L.; Tanner, R.S. Syngas fermentation process development for production of biofuels and chemicals: A review. Bioresour. Technol. Rep. 2019, 7, 100279. [Google Scholar] [CrossRef]
- Al Afif, R.; Wendland, M.; Amon, T.; Pfeifer, C. Supercritical carbon dioxide enhanced pre-treatment of cotton stalks for methane production. Energy 2020, 194. [Google Scholar] [CrossRef]
- Li, P.; Sakuragi, K.; Makino, H. Extraction techniques in sustainable biofuel production: A concise review. Fuel Process. Technol. 2019, 193, 295–303. [Google Scholar] [CrossRef]
- Shen, Y. A review on hydrothermal carbonization of biomass and plastic wastes to energy products. Biomass Bioenergy 2020, 134, 105479. [Google Scholar] [CrossRef]
- Sharma, H.B.; Sarmah, A.K.; Dubey, B. Hydrothermal carbonization of renewable waste biomass for solid biofuel production: A discussion on process mechanism, the influence of process parameters, environmental performance and fuel properties of hydrochar. Renew. Sustain. Energy Rev. 2020, 123, 109761. [Google Scholar] [CrossRef]
- Seyedsadr, S.; Al Afif, R.; Pfeifer, C. Hydrothermal carbonization of agricultural residues: A case study of the farm residues -based biogas plants. Carbon Resour. Convers. 2018, 1, 81–85. [Google Scholar] [CrossRef]
- Wang, L.; Chang, Y.; Li, A. Hydrothermal carbonization for energy-efficient processing of sewage sludge: A review. Renew. Sustain. Energy Rev. 2019, 108, 423–440. [Google Scholar] [CrossRef]
- Uzoejinwa, B.B.; He, X.; Wang, S.; Abomohra, A.; Hu, Y.; Wang, Q. Co-pyrolysis of biomass and waste plastics as a thermochemical conversion technology for high-grade biofuel production: Recent progress and future directions elsewhere worldwide. Energy Convers. Manag. 2018, 163, 468–492. [Google Scholar] [CrossRef]
- Al Afif, R.; Anayah, S.; Pfeifer, P. Batch pyrolysis of cotton stalks for evaluation of biochar energy potential. Renew. Energy 2020, 147, 2250–2258. [Google Scholar] [CrossRef]
- Al Afif, R.; Pfeifer, C.; Pröll, T. Bioenergy recovery from cotton stalk. In Cotton Research; IntechOpen: London, UK, 2019. [Google Scholar] [CrossRef]
- Schaffer, S.; Pröll, T.; Al Afif, R.; Pfeifer, C. A mass- and energy balance-based process modelling study for the pyrolysis of cotton stalks with char utilization for sustainable soil enhancement and carbon storage. Biomass Bioenergy 2019, 120, 281–290. [Google Scholar] [CrossRef]
- Pröll, T.; Al Afif, R.; Schaffer, S.; Pfeifer, C. Reduced local emissions and long-term carbon storage through pyrolysis of agricultural waste and application of pyrolysis char for soil improvement. Energy Procedia 2017, 114, 6057–6066. [Google Scholar] [CrossRef]
- Yang, C.; Li, R.; Zhang, B.; Qiu, Q.; Wang, B.; Yang, H.; Ding, Y.; Wang, C. Pyrolysis of microalgae: A critical review. Fuel Process. Technol. 2019, 186, 53–72. [Google Scholar] [CrossRef]
- Vineet Sing, S.; Ming, Z.; Fennel, P.S.; Shah, N.; Anthony, E.J. Progress in biofuel production from gasification. Prog. Energy Combust. Sci. 2017, 61, 189–248. [Google Scholar] [CrossRef]
- Widjaya, E.R.; Chen, G.; Bowtell, L.; Hills, C. Gasification of non-woody biomass: A literature review. Renew. Sustain. Energy Rev. 2018, 89, 184–193. [Google Scholar] [CrossRef]
- Karl, J.; Pröll, T. Steam gasification of biomass in dual fluidized bed gasifiers: A review. Renew. Sustain. Energy Rev. 2018, 98, 64–78. [Google Scholar] [CrossRef]
- Sansaniwal, S.K.; Rosen, M.A.; Tyagi, S.K. Global challenges in the sustainable development of biomass gasification: An overview. Renew. Sustain. Energy Rev. 2017, 80, 23–43. [Google Scholar] [CrossRef]
- Kim, J.Y.; Lee, H.W.; Lee, S.M.; Jae, J.; Park, Y.K. Overview of the recent advances in lignocellulose liquefaction for producing biofuels, bio-based materials and chemicals. Bioresour. Technol. 2019, 279, 373–384. [Google Scholar] [CrossRef]
- Perkins, G.; Batalha, N.; Kumar, A.; Bhaskar, T.; Konarova, M. Recent advances in liquefaction technologies for production of liquid hydrocarbon fuels from biomass and carbonaceous wastes. Renew. Sustain. Energy Rev. 2019, 115, 109400. [Google Scholar] [CrossRef]
- Gu, X.; Martinez-Fernandez, J.S.; Pang, N.; Fu, X.; Chen, S. Recent development of hydrothermal liquefaction for algal biorefinery. Renew. Sustain. Energy Rev. 2020, 121, 109707. [Google Scholar] [CrossRef]
- Vassilev, S.V.; Vassileva, C.G. Composition, properties and challenges of algae biomass for biofuel application: An overview. Fuel 2016, 181, 1–33. [Google Scholar] [CrossRef]
- Mathimani, T.; Pugazhendhi, A. Utilization of algae for biofuel, bio-products and bio-remediation. Biocatal. Agric. Biotechnol. 2019, 17, 326–330. [Google Scholar] [CrossRef]
- Pragya, N.; Pandey, K.K.; Sahoo, P.K. A review on harvesting, oil extraction and biofuels production technologies from microalgae. Renew. Sustain. Energy Rev. 2013, 24, 159–171. [Google Scholar] [CrossRef]
- Lee, X.J.; Ong, H.C.; Gan, Y.Y.; Chen, W.H.; Mahlia, T.M.I. State of art review on conventional and advanced pyrolysis of macroalgae and microalgae for biochar, bio-oil and bio-syngas production. Energy Convers. Manag. 2020, 210, 112707. [Google Scholar] [CrossRef]
- Mandika, I.; Cheirsilp, B.; Srinuanpan, S.; Maneechote, W.; Boonsawang, P.; Prasertsan, P.; Sirisansaneeyakuld, S. Zero-waste biorefinery of oleaginous microalgae as promising sources of biofuels and biochemicals through direct transesterification and acid hydrolysis. Process Biochem. 2020. [Google Scholar] [CrossRef]
- Chew, K.W.; Yap, J.Y.; Show, P.L.; Suan, N.H.; Juan, J.C.; Ling, T.C.; Lee, D.-J.; Chang, J.-S. Microalgae biorefinery: High value products perspectives. Bioresour. Technol. 2017, 229, 53–62. [Google Scholar] [CrossRef] [PubMed]
- Venugopal, V. Functional Foods and Nutraceuticals: Marine Products for Healthcare: Functional and Bioactive Nutraceutical Compounds from the Ocean; CRC Press: Boca Raton, FL, USA, 2008; p. 528. [Google Scholar]
- Kotrbáček, V.; Doubek, J.; Doucha, J. The chlorococcalean alga Chlorella in animal nutrition: a review. J Appl Psychol. 2015, 27, 2173–2180. [Google Scholar]
- Kunitsyn, M.V. Utilization of a Suspension of Planktonic Strain of Chlorella as a Dietary Supplement to Food. RU Patent 2013112810A, 20 May 2015. [Google Scholar]
- Posten, C.; Walter, C. Microalgal Biotechnology: Integration and Economy. Available online: https://www.degruyter.com/view/title/125819 (accessed on 14 March 2020).
- Richman, A. The new age of Marine Nutraceuticals. Nutraceuticals World 2013, 16, 40–48. [Google Scholar]
- Wong, K.H.; Cheung, P.C.K. Nutritional evaluation of some subtropical red and green seaweeds: Part I—Proximate composition, amino acid profiles and some physico-chemical properties. Food Chem. 2000, 71, 475–482. [Google Scholar] [CrossRef]
- Politaeva, N.A.; Smyatskaya, Y.u.A.; Kuznetsova, T.A.; Olshanskaya, L.N.; Valiev, R.S.h. Cultivation and Use of Chlorella Microalgae and Higher Aquatic Plant Lemna Duckweed; Nauka: Saratov, Russia, 2017; p. 125. ISBN 978-5-9999-2883-2. (In Russian) [Google Scholar]
- Menegazzo, M.L. Fonseca G.G., Biomass recovery and lipid extraction processes for microalgae biofuels production: A review. Renew. Sustain. Energy Rev. 2019, 107, 87–107. [Google Scholar] [CrossRef]
- Kobayashi, N.; Noel, E.A.; Barnes, A.; Watson, A.; Rosenberg, J.N.; Erickson, G.; Oyler, G.A. Characterization of three Chlorella sorokiniana strains in anaerobic digested effluent from cattle manure. Bioresour. Technol. 2013, 150, 377–386. [Google Scholar] [CrossRef] [PubMed]
- Chader, S.; Mahmah, B.; Chetehouna, K.; Mignolet, E. Biodiesel production using Chlorella sorokiniana a green microalga. Rev. Energies Renouv. 2011, 14, 21–26. [Google Scholar]
- Blinová, L.; Bartošová, A.; Gerulová Blinová, K. Cultivation of microalgae (Chlorella vulgaris) for biodiesel production. Res. Pap. Fac. Mater. Sci. Technol. Slovak Univ. Technol. 2015, 23, 87–95. [Google Scholar] [CrossRef]
- Işıldar, A.; van Hullebusch, E.D.; Lenz, M.; Du Laing, G.; Marra, A.; Cesaro, A.; Panda, S.; Akcil, A.; Kucuker, M.A.; Kuchta, K. Biotechnological strategies for the recovery of valuable and critical raw materials from waste electrical and electronic equipment (WEEE)—A review. J. Hazard. Mater. 2018. [Google Scholar] [CrossRef]
- Johnson, D.B. Biomining–biotechnologies for extracting and recovering metals from ores and waste materials. Curr. Opin. Biotechnol. 2014, 30, 24–31. [Google Scholar] [CrossRef]
- Brierley, C.L.; Brierley, J.A. Progress in bioleaching: Part B: Applications of microbial processes by the minerals industries. Appl. Microbiol. Biotechnol. 2013, 97, 7543–7552. [Google Scholar] [CrossRef] [PubMed]
- Kucuker, M.A.; Kuchta, K. Biomining—Biotechnological Systems (Bioleaching and Biosorption) for the Extraction and Recovery of Metals from Secondary Sources. Glob. Nest J. 2017, 20, 28–33. [Google Scholar]
- Farooq, U.; Kozinski, J.A.; Khan, M.A.; Athar, M. Biosorption of heavy metal ions using wheat based biosorbents—A review of the recent literature. Bioresour. Technol. 2010, 101, 5043–5053. [Google Scholar] [CrossRef]
- Dhankhar, R.; Hooda, A. Fungal biosorption—An alternative to meet the challenges of heavy metal pollution in aqueous solutions. Environ. Technol. 2011, 32, 467–491. [Google Scholar] [CrossRef] [PubMed]
- Dave, S.R.; Sodha, A.B.; Tipre, D.R. Microbial technology for metal recovery from e-waste printed circuit boards. J. Bacteriol. Mycol. 2018, 6, 241–247. [Google Scholar] [CrossRef]
- Kucuker, M.A.; Wieczorek, N.; Kuchta, K.; Copty, N.K. Biosorption of neodymium on Chlorella vulgaris in aqueous solution obtained from hard disk drive magnets. PLoS ONE 2017, 12, e0175255. [Google Scholar] [CrossRef] [PubMed]
- Petrovič, A.; Simonič, M. Removal of heavy metal ions from drinking water by alginate-immobilised Chlorella sorokiniana. Int. J. Environ. Sci. Technol. 2016, 13, 1761–1780. [Google Scholar] [CrossRef]
- Akhtar, N.; Iqbal, M.; Zafar, S.I.; Iqbal, J. Biosorption characteristics of unicellular green alga Chlorella sorokiniana immobilized in loofa sponge for removal of Cr(III). J. Environ. Sci. 2008, 20, 231–239. [Google Scholar] [CrossRef]
- Ardila, L.; Godoy, R.; Montenegro, L. Sorption capacity measurement of Chlorella vulgaris and Scenedesmus acutus to remove chromium from tannery waste water. IOP Conf. Ser. Earth Environ. Sci. 2017, 83, 1–16. [Google Scholar] [CrossRef]
- Zhou, W.; Cheng, Y.; Li, Y.; Wan, Y.; Liu, Y.; Lin, X.; Ruan, R. Novel fungal pelletization-assisted technology for algae harvesting and wastewater treatmen. Appl. Biochem. Biotechnol. 2012, 167, 214–228. [Google Scholar] [CrossRef]
- Sobgaida, N.A.; Olshanskaya, L.N.; Makarova, Y.u.A. The effect of wheat husk modification on its sorption properties to Pb2+, Cd2+, Zn2+ and Cu2+ ions. Izv. Vyss. Uchebnykh Zaved. Seriya Khimiya Khimicheskaya Tekhnologiya 2010, 53, 36–40. [Google Scholar]
- Taranovskaya, E.A.; Sobgaida, N.A.; Markina, D.V. Technology for Obtaining and Using Granulated Absorbents Based on Chitosan. Chem. Pet. Eng. 2016, 52, 357–361. [Google Scholar] [CrossRef]
- Politaeva, N.A.; Slugin, V.V.; Taranovskaya, E.A.; Soloviev, M.A.; Zakharevich, A.M. Granulated sorption materials for waste waters purufucation from zink ions (Zn2+). Izv. Vyss. Uchebnykh Zaved. Seriya Khimiya Khimicheskaya Tekhnologiya 2017, 60, 85–90. [Google Scholar] [CrossRef]
- Politaeva, N.A.; Smyatskaya, Y.A.; Tatarintseva, E.A. Tatarintseva Using Adsorption Material Based on the Residual Biomass of Chlorella sorokiniana Microalgae for Wastewater Purification to Remove Heavy Metal Ions. Chem. Pet. Eng. 2020, 55, 907–912. [Google Scholar] [CrossRef]
- Adarme-Vega, T.C.; Lim, D.K.; Timmins, M.; Vernen, F.; Li, Y.; Schenk, P.M. Microalgal biofactories: A promising approach towards sustainable omega-3 fatty acid production. Microb. Cell Fact. 2012, 11, 96. [Google Scholar] [CrossRef]
- Simopoulos, A.P. Omega-3 fatty acids in inflammation and autoimmune diseases. J. Am. Coll. Nutr. 2002, 21, 494–505. [Google Scholar] [CrossRef] [PubMed]
- Suliman, K.; Rabeea, S.; Wasim, S.; Ghulam, N.; Khizar, H.; Pengfei, D.; Lunguang, Y. Biodiesel Production From Algae to Overcome the Energy Crisis. HAYATI J. Biosci. 2017, 24, 163–167. [Google Scholar] [CrossRef]
- Dada, A.O.; Olalekan, A.P.; Olatunya, A.M. Isotherm and thermodynamic studies of Cd (II) removal process using chemically modified lignocellulosic adsorbent. Appl. Chem. 2012, 3, 38–45. [Google Scholar]
- Physical Encyclopedia. Available online: https://www.prlib.ru/en/history/619024 (accessed on 1 April 2020).
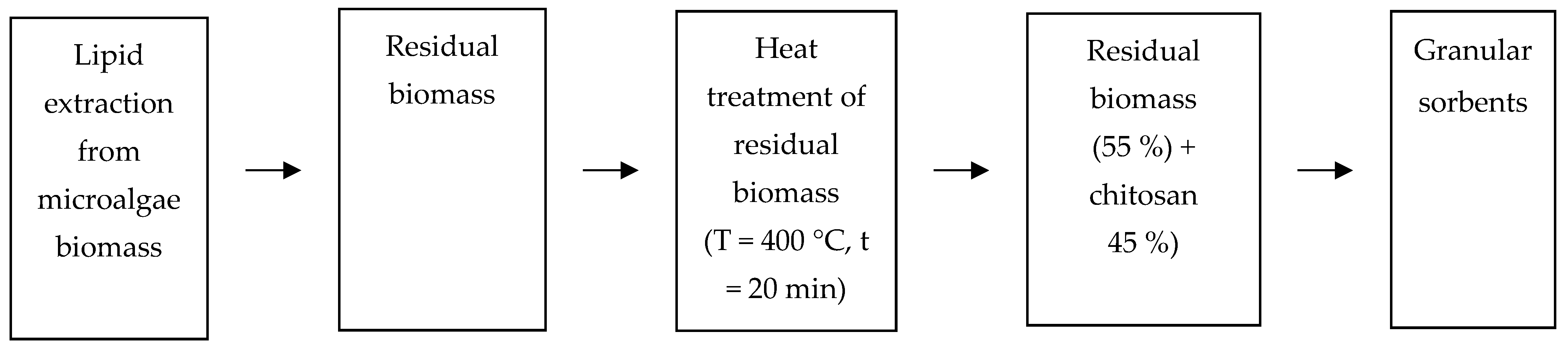
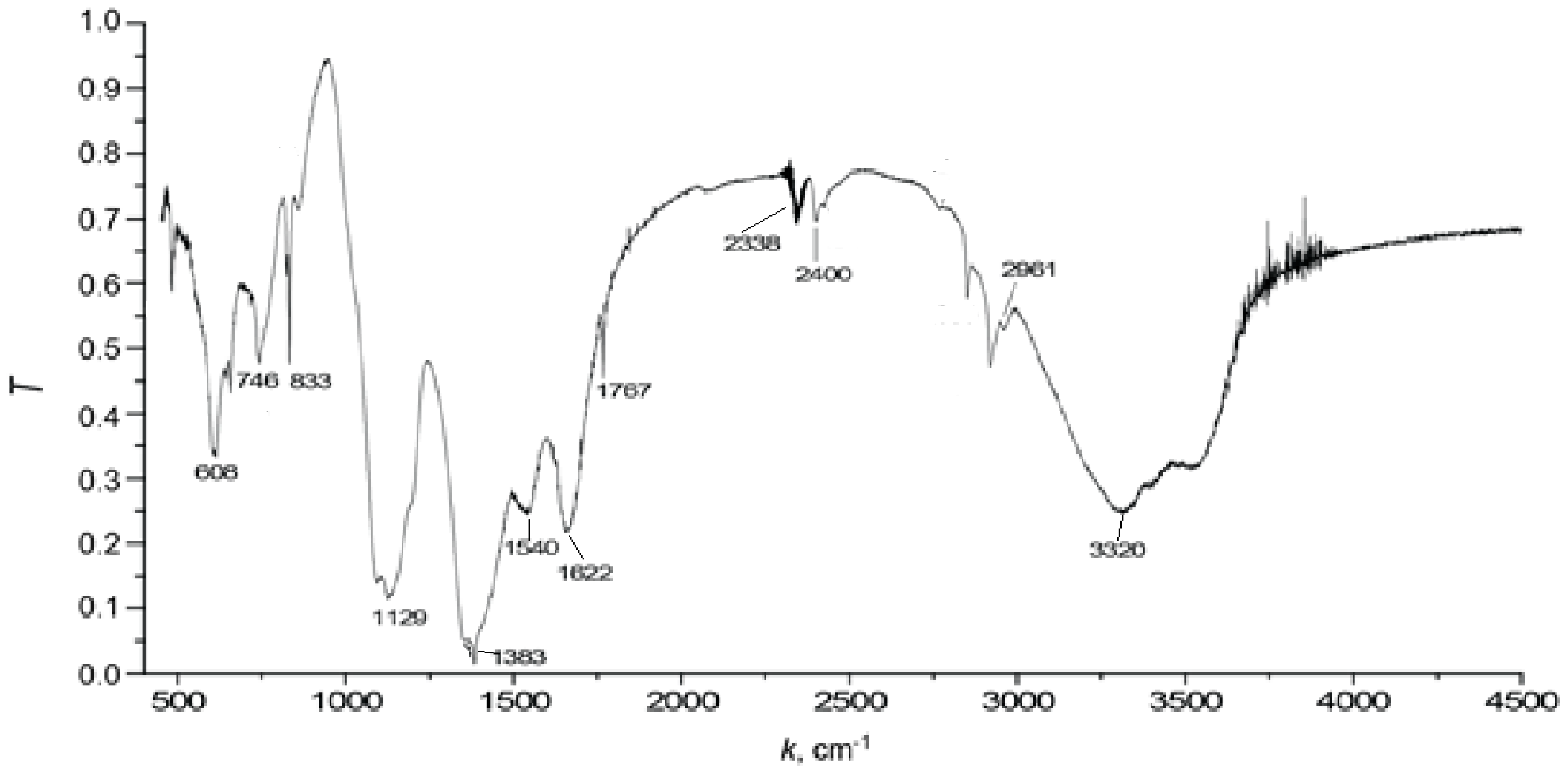

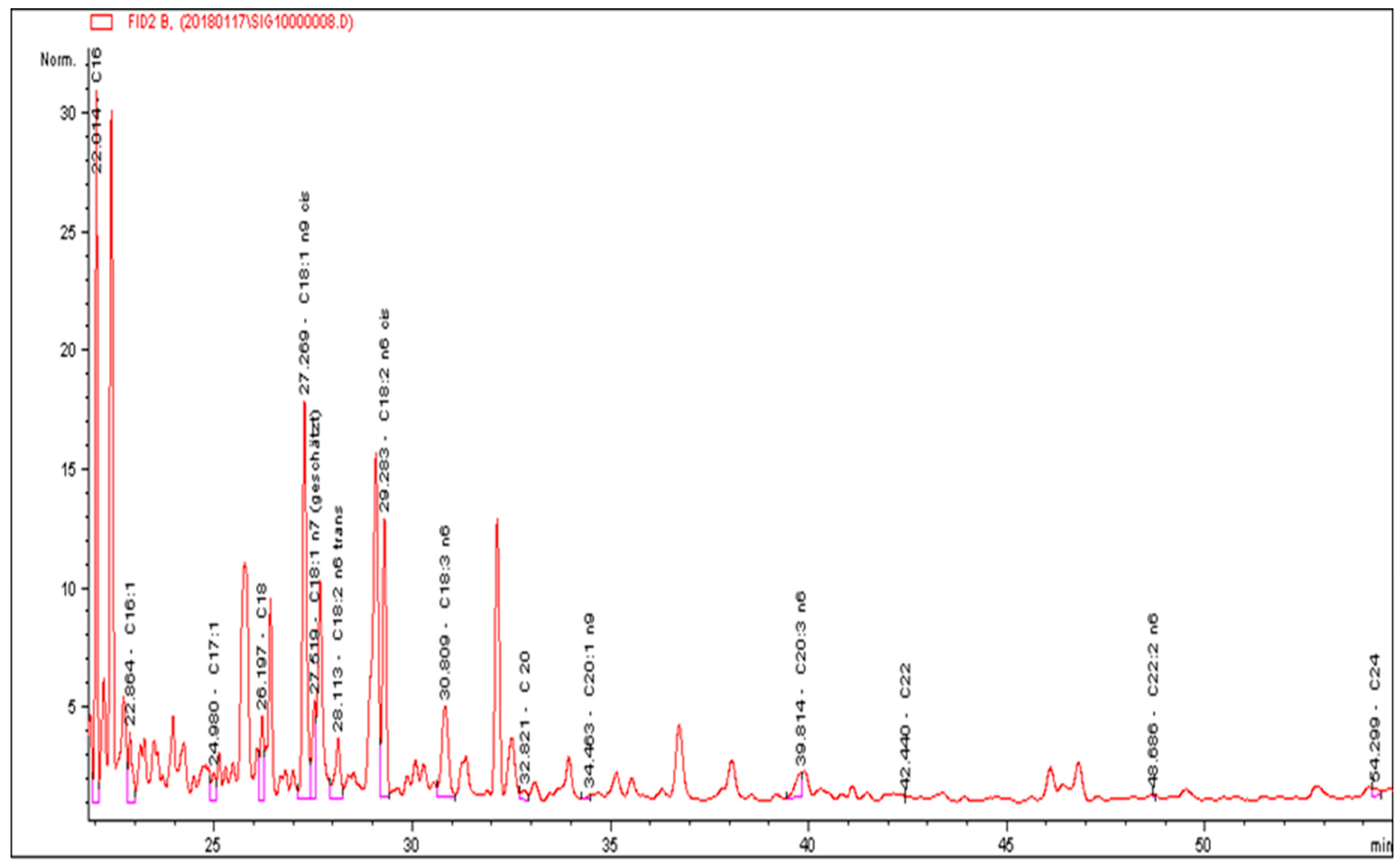

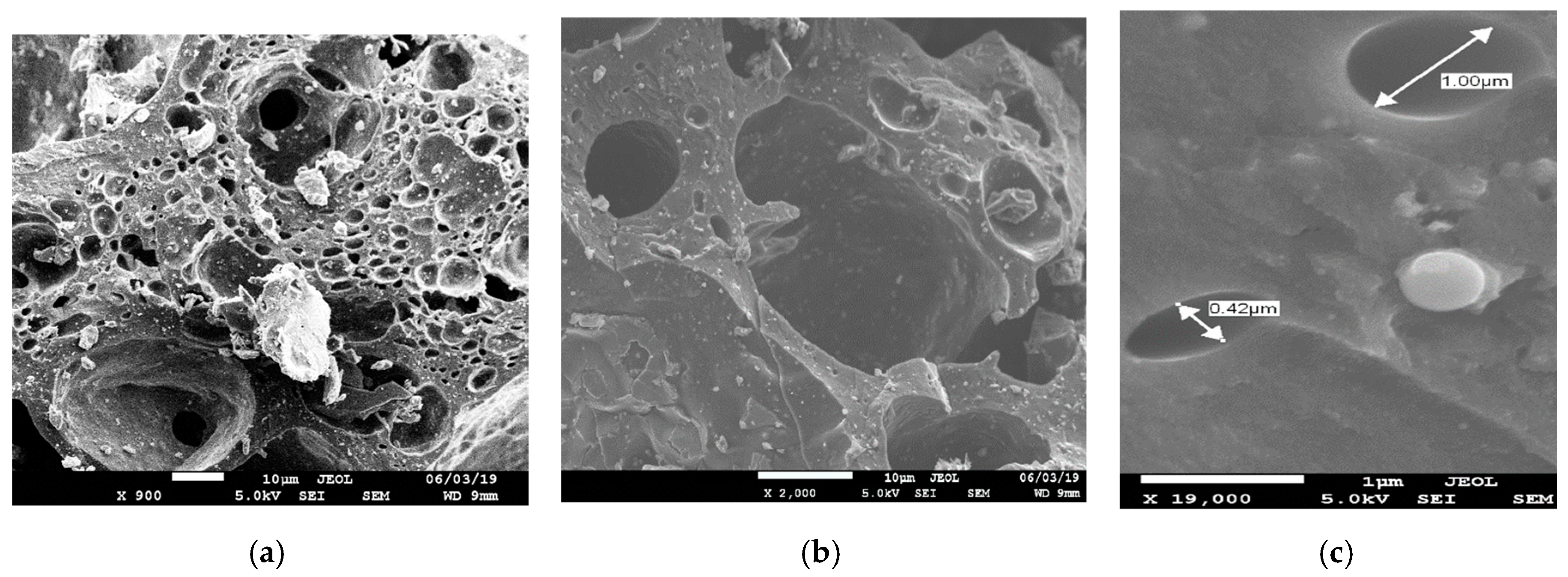
| Wavelength, cm−1 | Functional Groups |
|---|---|
| 1000–1100 | Oxygen-containing C-O bond groups |
| 1300–1400 | Deformation vibration of NH-groups |
| 1600 | Carboxyl groups C=O, |
| 2800–2900 | Asymmetric bonds of CH2 groups |
| 3100–3600 | Valence vibrations of OH groups |
| N0 | Component | Percentage, wt % |
|---|---|---|
| 1 | C16 Palmitic | 14.74 |
| 2 | C16:1 Palmitoleic | 1.37 |
| 3 | C17 Heptadecanoic | 2.69 |
| 4 | C 17:1 Heptadecenoic | 0.45 |
| 5 | C 18:1 n9 cis Oleinic | 18.90 |
| 6 | C18-2- n6 trans γ-Linolenic | 2.90 |
| 7 | C18-2 n6-cis Linolenic | 7.34 |
| 8 | C18:3 n6 Linolenic | 6.88 |
| 9 | C18:3 n3 α-Linolenic | 44.01 |
| 10 | C20:3 Eicosatrienoic | 0.41 |
| 11 | C22 Behenic | 0.31 |
| 13 | Total | 100.00 |
| Sorbent | Sorption Capacity, A, mg/g | Specific Surface m2/g | Pore Volume, cm3/g | Average Pore Size, nm | Attrition, % Not More Than 0.5% | |||
|---|---|---|---|---|---|---|---|---|
| Zn2+ | Cd2+ | Cu2+ | Total A, mg/g | |||||
| 1 | 102.5 | 62.7 | 51.2 | 216.4 | 75.5 | 0.012 | 4–6 | - |
| 2 | 132.6 | 68.3 | 54.5 | 255.4 | 28.7 | 0.098 | 5–20 | - |
| 3 | 177.3 | 73.9 | 98.5 | 349.7 | 5.2 | 0.014 | 2–8 | 0.3 |
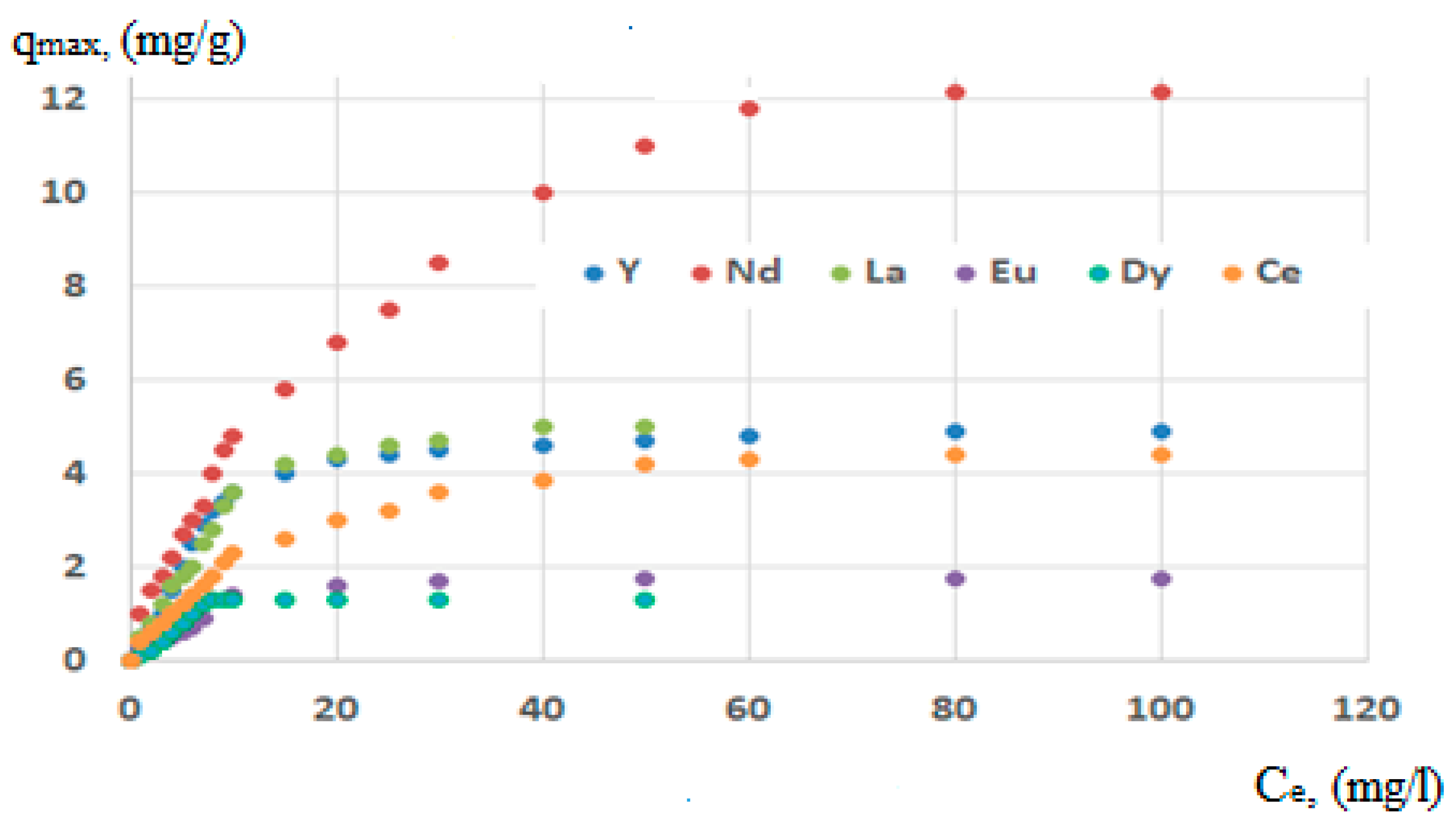
| Elements | Langmuir | Freundlich | |||
|---|---|---|---|---|---|
| qmax (mg/g) | R2 | 1/n | KF | R2 | |
| Ce (Cerium) | 4.41 | 0.996 | 0.22 | 1.76 | 0.939 |
| Dy (Dysprosium) | 1.33 | 0.996 | 0.35 | 0.72 | 0.840 |
| Eu (Europium) | 1.74 | 0.991 | 0.33 | 0.94 | 0.920 |
| La (Lanthanum) | 5.02 | 0.999 | 0.31 | 2.03 | 0,928 |
| Y (Yttrium) | 4.85 | 0.999 | 0.30 | 1.74 | 0.880 |
| Nd (Neodymium) | 12.18 | 0.997 | 0.27 | 3.41 | 0.875 |
| Calculated ∑ qmax (mg/g) | 31.9 | ||||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Politaeva, N.; Smyatskaya, Y.; Al Afif, R.; Pfeifer, C.; Mukhametova, L. Development of Full-Cycle Utilization of Chlorella sorokiniana Microalgae Biomass for Environmental and Food Purposes. Energies 2020, 13, 2648. https://doi.org/10.3390/en13102648
Politaeva N, Smyatskaya Y, Al Afif R, Pfeifer C, Mukhametova L. Development of Full-Cycle Utilization of Chlorella sorokiniana Microalgae Biomass for Environmental and Food Purposes. Energies. 2020; 13(10):2648. https://doi.org/10.3390/en13102648
Chicago/Turabian StylePolitaeva, Natalia, Yulia Smyatskaya, Rafat Al Afif, Christoph Pfeifer, and Liliya Mukhametova. 2020. "Development of Full-Cycle Utilization of Chlorella sorokiniana Microalgae Biomass for Environmental and Food Purposes" Energies 13, no. 10: 2648. https://doi.org/10.3390/en13102648
APA StylePolitaeva, N., Smyatskaya, Y., Al Afif, R., Pfeifer, C., & Mukhametova, L. (2020). Development of Full-Cycle Utilization of Chlorella sorokiniana Microalgae Biomass for Environmental and Food Purposes. Energies, 13(10), 2648. https://doi.org/10.3390/en13102648







