Production of Biodiesel from Castor Oil: A Review
Abstract
1. Introduction
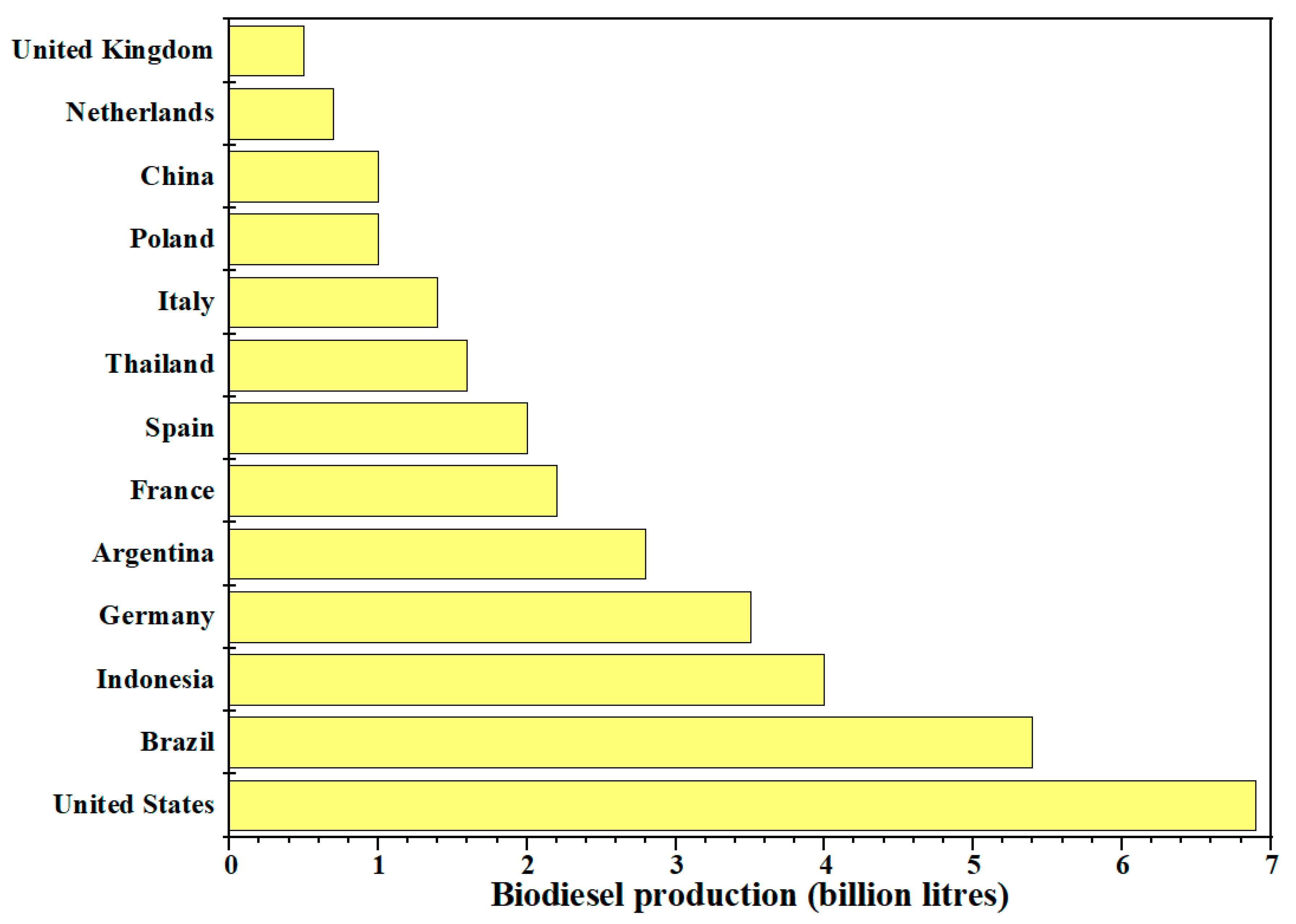
2. Vegetable Oils to Produce Biodiesel
3. Oil Extraction Processes
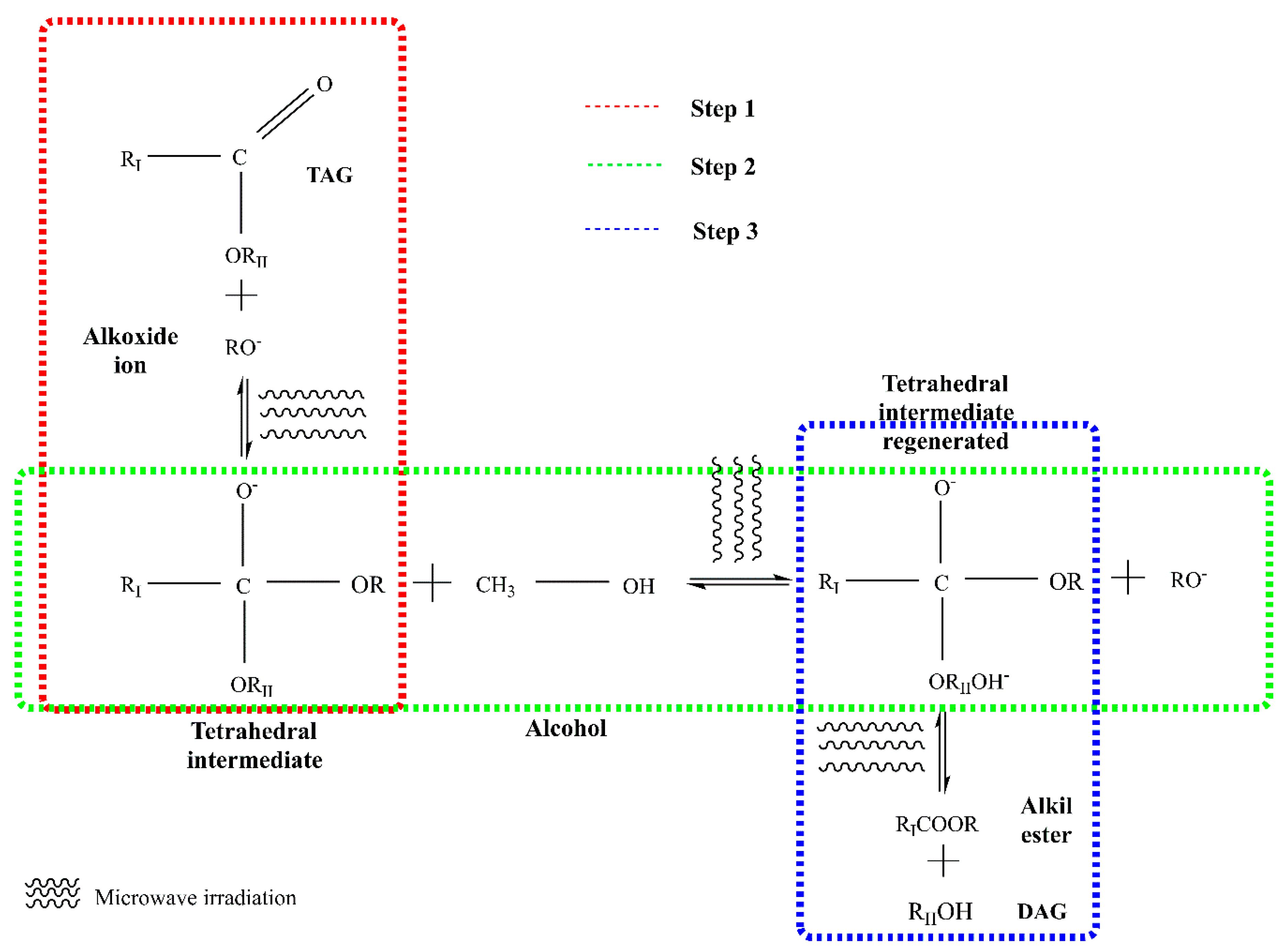
4. Transesterification
4.1. Chemical Processes: Homogeneous and Heterogeneous Catalysis
4.1.1. Homogeneous Catalysis
4.1.2. Heterogeneous Catalysis
4.1.3. Parameters Affecting Transesterification Reactions
4.2. Biological Transesterification
4.3. Transesterification Using Ultrasound
4.4. Membrane Reactors
4.5. Microwave
5. Castor Oil Biodiesel Production and Features
6. Biodiesel Up-Grading
7. By-Products of Biodiesel Production
7.1. Residual Cake
7.2. Glycerol
8. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Chen, S.-Y.; Lao-ubol, S.; Mochizuki, T.; Abe, Y.; Toba, M.; Yoshimura, Y. Production of Jatropha biodiesel fuel over sulfonic acid-based solid acids. Bioresour. Technol. 2014, 157, 346–350. [Google Scholar] [CrossRef] [PubMed]
- Keera, S.T.; Sabagh, S.M.E.; Taman, A.R. Castor oil biodiesel production and optimization. Egypt. J. Pet. 2018, 27, 979–984. [Google Scholar] [CrossRef]
- Panagiotopoulos, I.A.; Pasias, S.; Bakker, R.R.; de Vrije, T.; Papayannakos, N.; Claassen, P.A.M.; Koukios, E.G. Biodiesel and biohydrogen production from cotton-seed cake in a biorefinery concept. Bioresour. Technol. 2013, 136, 78–86. [Google Scholar] [CrossRef] [PubMed]
- Adesanya, V.O.; Cadena, E.; Scott, S.A.; Smith, A.G. Life cycle assessment on microalgal biodiesel production using a hybrid cultivation system. Bioresour. Technol. 2014, 163, 343–355. [Google Scholar] [CrossRef]
- Acharya, N.; Nanda, P.; Panda, S.; Acharya, S. A comparative study of stability characteristics of mahua and jatropha biodiesel and their blends. J. King Saud Univ. - Eng. Sci. 2019, 31, 184–190. [Google Scholar] [CrossRef]
- Kayode, B.; Hart, A. An overview of transesterification methods for producing biodiesel from waste vegetable oils. Biofuels 2019, 10, 419–437. [Google Scholar] [CrossRef]
- Callegari, A.; Bolognesi, S.; Cecconet, D.; Capodaglio, A.G. Production technologies, current role, and future prospects of biofuels feedstocks: A state-of-the-art review. Crit. Rev. Environ. Sci. Technol. 2020, 50, 384–436. [Google Scholar] [CrossRef]
- Bauddh, K.; Singh, K.; Singh, B.; Singh, R.P. Ricinus communis: A robust plant for bio-energy and phytoremediation of toxic metals from contaminated soil. Ecol. Eng. 2015, 84, 640–652. [Google Scholar] [CrossRef]
- McKeon, T.A. Castor (Ricinus communis L.). In Industrial Oil Crops; Elsevier: Amsterdam, The Netherlands, 2016; pp. 75–112. ISBN 978-1-893997-98-1. [Google Scholar]
- Yan, J.; Zheng, X.; Du, L.; Li, S. Integrated lipase production and in situ biodiesel synthesis in a recombinant Pichia pastoris yeast: An efficient dual biocatalytic system composed of cell free enzymes and whole cell catalysts. Biotechnol. Biofuels 2014, 7, 55. [Google Scholar] [CrossRef]
- Ramachandran, K.; Sivakumar, P.; Suganya, T.; Renganathan, S. Production of biodiesel from mixed waste vegetable oil using an aluminium hydrogen sulphate as a heterogeneous acid catalyst. Bioresour. Technol. 2011, 102, 7289–7293. [Google Scholar] [CrossRef]
- Issariyakul, T.; Dalai, A.K. Biodiesel from vegetable oils. Renew. Sustain. Energy Rev. 2014, 31, 446–471. [Google Scholar] [CrossRef]
- Poli, J.S.; da Silva, M.A.N.; Siqueira, E.P.; Pasa, V.M.D.; Rosa, C.A.; Valente, P. Microbial lipid produced by Yarrowia lipolytica QU21 using industrial waste: A potential feedstock for biodiesel production. Bioresour. Technol. 2014, 161, 320–326. [Google Scholar] [CrossRef] [PubMed]
- Aranda-Rickert, A.; Morzán, L.; Fracchia, S. Seed oil content and fatty acid profiles of five Euphorbiaceae species from arid regions in Argentina with potential as biodiesel source. Seed Sci. Res. 2011, 21, 63–68. [Google Scholar] [CrossRef]
- Datta, A.; Mandal, B.K. Use of Jatropha Biodiesel as a Future Sustainable Fuel. Energy Technol. Policy 2014, 1, 8–14. [Google Scholar] [CrossRef]
- Sanghamitra, K.; Oramas, R.V.; Prasad, R.N. Comparative Yeild and Oil Quality of Toxic and Non-Toxic Mexican Jatropha curcas Grown in the Same Agroclimatic Conditions. Am. J. Plant Sci. 2014, 05, 230–234. [Google Scholar] [CrossRef][Green Version]
- Sagiroglu, A.; Ozcan, H.M.; Isbilir, S.S.; Paluzar, H.; Toprakkiran, N.M. Alkali Catalysis of Different Vegetable Oils for Comparisons of Their Biodiesel Productivity. J. Sustain. Bioenergy Syst. 2013, 3, 79–85. [Google Scholar] [CrossRef][Green Version]
- Pinzi, S.; Leiva, D.; López-García, I.; Redel-Macías, M.D.; Dorado, M.P. Latest trends in feedstocks for biodiesel production. Biofuels Bioprod. Biorefining 2014, 8, 126–143. [Google Scholar] [CrossRef]
- Raiol, L.C.B.; Kuss, F.; Silva, A.G.M.; Soares, B.C.; de Souza, K.D.S.; Colodo, J.C.N.; de Brito Lourenço Júnior, J.; de Ávila, S.C. Nutrient intake and digestibility of the lipid residue of biodiesel from palm oil in sheep. Rev. Bras. Zootec. 2012, 41, 2364–2368. [Google Scholar] [CrossRef][Green Version]
- Li, M.; Zheng, Y.; Chen, Y.; Zhu, X. Biodiesel production from waste cooking oil using a heterogeneous catalyst from pyrolyzed rice husk. Bioresour. Technol. 2014, 154, 345–348. [Google Scholar] [CrossRef]
- Buy Organic & Kosher Castor Oil - Drums, Pails, & Bags | 18c. Available online: https://www.ah18c.com/en-us/castor-oil/castor-oil-castor%20oil?returnurl=%2fen-us%2fcastor-oil%2f (accessed on 27 April 2020).
- Jatropha Oil Crude Jatropha Oil/crude Jatropha Oil Subsidize Prices In Sa - Buy Canola Oil Biodiesel, Jatropha Curcas Oil, Crude Coconut Oil Product on Alibaba.com. Available online: https://www.alibaba.com/product-detail/Jatropha-Oil-Crude-Jatropha-Oil-Crude_62015801964.html?spm=a2700.7724857.normalList.27.182b26c93tbdv0&bypass=true (accessed on 27 April 2020).
- International Prices of Oilseeds and Oilmeals Unchanged, Those of Vegetable Oils Appreciate Further | Food Price Monitoring and Analysis (FPMA) | Food and Agriculture Organization of the United Nations. Available online: http://www.fao.org/giews/food-prices/international-prices/detail/en/c/1181877/ (accessed on 20 April 2020).
- Used cooking oil (uco) -International Market Price- B2B- Import Export- Manufacturers, Wholesalers- Sell Leads, Offers- Free B2B Website- Free B2B Directory- Free Listing- Suppliers, Exporters.2139. Available online: http://www.importexportplatform.com/2139_used_cooking_oil_(uco)_sellers.html (accessed on 27 April 2020).
- Aguirre, A.; Peiru, S.; Eberhardt, F.; Vetcher, L.; Cabrera, R.; Menzella, H.G. Enzymatic hydrolysis of steryl glucosides, major contaminants of vegetable oil-derived biodiesel. Appl. Microbiol. Biotechnol. 2014, 98, 4033–4040. [Google Scholar] [CrossRef]
- Hincapié, G.; Moreno, A.; López, D. Transesterificación de aceite de higuerilla crudo utilizando catalizadores heterogéneos-estudio preliminar. Dyna 2011, 78, 176–181. [Google Scholar]
- Mutlu, H.; Meier, M.A.R. Castor oil as a renewable resource for the chemical industry. Eur. J. Lipid Sci. Technol. 2010, 112, 10–30. [Google Scholar] [CrossRef]
- Rajkumari, K.; Rokhum, L. A sustainable protocol for production of biodiesel by transesterification of soybean oil using banana trunk ash as a heterogeneous catalyst. Biomass Convers. Biorefinery 2020. [Google Scholar] [CrossRef]
- Rial, R.C.; de Freitas, O.N.; Nazário, C.E.D.; Viana, L.H. Biodiesel from soybean oil using Porcine pancreas lipase immobilized on a new support: P-nitrobenzyl cellulose xanthate. Renew. Energy 2020, 149, 970–979. [Google Scholar] [CrossRef]
- Avramović, J.M.; Stamenković, O.S.; Todorović, Z.B.; Lazić, M.L.; Veljković, V.B. The optimization of the ultrasound-assisted base-catalyzed sunflower oil methanolysis by a full factorial design. Fuel Process. Technol. 2010, 91, 1551–1557. [Google Scholar] [CrossRef]
- Da Rós, P.C.M.; Freitas, L.; Perez, V.H.; de Castro, H.F. Enzymatic synthesis of biodiesel from palm oil assisted by microwave irradiation. Bioprocess Biosyst. Eng. 2013, 36, 443–451. [Google Scholar] [CrossRef] [PubMed]
- Batista, A.C.F.; Vieira, A.T.; Rodrigues, H.S.; Silva, T.A.; Assunção, R.M.N.; Beluomini, M.A.; Rezende, H.P.; Hernandez-Terrones, M.G. Production and physicochemical characterization of methylic and ethylic biodiesel from canola oil/obtenção e caracterização do biodiesel de canola pelas rotas metílica e etílica. Rev. Bras. Eng. Biossistemas 2014, 8, 289. [Google Scholar] [CrossRef]
- Mata, T.M.; Martins, A.A.; Sikdar, S.K.; Costa, C.A.V. Sustainability considerations of biodiesel based on supply chain analysis. Clean Technol. Environ. Policy 2011, 13, 655–671. [Google Scholar] [CrossRef]
- Ali, M.; Watson, I.A. Comparison of oil extraction methods, energy analysis and biodiesel production from flax seeds: Microwave, ultrasonic and solvent extraction methods for flax seeds. Int. J. Energy Res. 2014, 38, 614–625. [Google Scholar] [CrossRef]
- Fore, S.R.; Porter, P.; Lazarus, W. Net energy balance of small-scale on-farm biodiesel production from canola and soybean. Biomass Bioenergy 2011, 35, 2234–2244. [Google Scholar] [CrossRef]
- Parawira, W. Biodiesel production from Jatropha curcas: A review. Sci. Res. Essays 2010, 5, 1796–1808. [Google Scholar]
- Jokić, S.; Nagy, B.; Zeković, Z.; Vidović, S.; Bilić, M.; Velić, D.; Simándi, B. Effects of supercritical CO2 extraction parameters on soybean oil yield. Food Bioprod. Process. 2012, 90, 693–699. [Google Scholar] [CrossRef]
- Sangaletti-Gerhard, N.; Romanelli, T.L.; de Souza Vieira, T.M.F.; Navia, R.; Regitano-d’Arce, M.A.B. Energy flow in the soybean biodiesel production chain using ethanol as solvent extraction of oil from soybeans. Biomass Bioenergy 2014, 66, 39–48. [Google Scholar] [CrossRef]
- Sawada, M.M.; Venâncio, L.L.; Toda, T.A.; Rodrigues, C.E.C. Effects of different alcoholic extraction conditions on soybean oil yield, fatty acid composition and protein solubility of defatted meal. Food Res. Int. 2014, 62, 662–670. [Google Scholar] [CrossRef]
- Danlami, J.M.; Arsad, A.; Zaini, M.A.A. Characterization and process optimization of castor oil (Ricinus communis L.) extracted by the soxhlet method using polar and non-polar solvents. J. Taiwan Inst. Chem. Eng. 2015, 47, 99–104. [Google Scholar] [CrossRef]
- del Valle, J.M.; Núñez, G.A.; Aravena, R.I. Supercritical CO2 oilseed extraction in multi-vessel plants. 1. Minimization of operational cost. J. Supercrit. Fluids 2014, 92, 197–207. [Google Scholar] [CrossRef]
- Núñez, G.A.; del Valle, J.M. Supercritical CO2 oilseed extraction in multi-vessel plants. 2. Effect of number and geometry of extractors on production cost. J. Supercrit. Fluids 2014, 92, 324–334. [Google Scholar] [CrossRef]
- Kanitkar, A.; Sabliov, C.M.; Balasubramanian, S.; Lima, M.; Boldor, D. Microwave-Assisted Extraction of Soybean and Rice Bran Oil: Yield and Extraction Kinetics. Trans. ASABE 2011, 54, 1387–1394. [Google Scholar] [CrossRef]
- Elango, R.K.; Sathiasivan, K.; Muthukumaran, C.; Thangavelu, V.; Rajesh, M.; Tamilarasan, K. Transesterification of castor oil for biodiesel production: Process optimization and characterization. Microchem. J. 2019, 145, 1162–1168. [Google Scholar] [CrossRef]
- Meneghetti, S.M.P.; Meneghetti, M.R.; Wolf, C.R.; Silva, E.C.; Lima, G.E.S.; de Lira Silva, L.; Serra, T.M.; Cauduro, F.; de Oliveira, L.G. Biodiesel from Castor Oil: A Comparison of Ethanolysis versus Methanolysis. Energy Fuels 2006, 20, 2262–2265. [Google Scholar] [CrossRef]
- Cavalcante, K.S.B.; Penha, M.N.C.; Mendonça, K.K.M.; Louzeiro, H.C.; Vasconcelos, A.C.S.; Maciel, A.P.; de Souza, A.G.; Silva, F.C. Optimization of transesterification of castor oil with ethanol using a central composite rotatable design (CCRD). Fuel 2010, 89, 1172–1176. [Google Scholar] [CrossRef]
- Eze, V.C.; Phan, A.N.; Harvey, A.P. A more robust model of the biodiesel reaction, allowing identification of process conditions for significantly enhanced rate and water tolerance. Bioresour. Technol. 2014, 156, 222–231. [Google Scholar] [CrossRef]
- Aransiola, E.F.; Ojumu, T.V.; Oyekola, O.O.; Madzimbamuto, T.F.; Ikhu-Omoregbe, D.I.O. A review of current technology for biodiesel production: State of the art. Biomass Bioenergy 2014, 61, 276–297. [Google Scholar] [CrossRef]
- Cancela, A.; Maceiras, R.; Urrejola, S.; Sanchez, A. Microwave-Assisted Transesterification of Macroalgae. Energies 2012, 5, 862–871. [Google Scholar] [CrossRef]
- Dias, J.M.; Araújo, J.M.; Costa, J.F.; Alvim-Ferraz, M.C.M.; Almeida, M.F. Biodiesel production from raw castor oil. Energy 2013, 53, 58–66. [Google Scholar] [CrossRef]
- Bateni, H.; Saraeian, A.; Able, C.; Karimi, K. Biodiesel Purification and Upgrading Technologies. In Biodiesel; Tabatabaei, M., Aghbashlo, M., Eds.; Springer International Publishing: Cham, Switzerland, 2019; Volume 8, pp. 57–100. ISBN 978-3-030-00984-7. [Google Scholar]
- Devi, B.L.A.P.; Reddy, T.V.K.; Lakshmi, K.V.; Prasad, R.B.N. A green recyclable SO3H-carbon catalyst derived from glycerol for the production of biodiesel from FFA-containing karanja (Pongamia glabra) oil in a single step. Bioresour. Technol. 2014, 153, 370–373. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.; Qiu, F.; Yang, D.; Ye, B. Preparation of biodiesel from soybean oil catalyzed by Al-Ca hydrotalcite loaded with K2CO3 as heterogeneous solid base catalyst. Fuel Process. Technol. 2014, 126, 383–391. [Google Scholar] [CrossRef]
- Badday, A.S.; Abdullah, A.Z.; Lee, K.-T. Transesterification of crude Jatropha oil by activated carbon-supported heteropolyacid catalyst in an ultrasound-assisted reactor system. Renew. Energy 2014, 62, 10–17. [Google Scholar] [CrossRef]
- Du, L.; Ding, S.; Li, Z.; Lv, E.; Lu, J.; Ding, J. Transesterification of castor oil to biodiesel using NaY zeolite-supported La2O3 catalysts. Energy Convers. Manag. 2018, 173, 728–734. [Google Scholar] [CrossRef]
- Baskar, G.; Selvakumari, I.A.E.; Aiswarya, R. Biodiesel production from castor oil using heterogeneous Ni doped ZnO nanocatalyst. Bioresour. Technol. 2018, 250, 793–798. [Google Scholar] [CrossRef]
- Kai, T.; Mak, G.L.; Wada, S.; Nakazato, T.; Takanashi, H.; Uemura, Y. Production of biodiesel fuel from canola oil with dimethyl carbonate using an active sodium methoxide catalyst prepared by crystallization. Bioresour. Technol. 2014, 163, 360–363. [Google Scholar] [CrossRef] [PubMed]
- Ganesan, D.; Rajendran, A.; Thangavelu, V. An overview on the recent advances in the transesterification of vegetable oils for biodiesel production using chemical and biocatalysts. Rev. Environ. Sci. Biotechnol. 2009, 8, 367–394. [Google Scholar] [CrossRef]
- Aboelazayem, O.; El-Gendy, N.S.; Abdel-Rehim, A.A.; Ashour, F.; Sadek, M.A. Biodiesel production from castor oil in Egypt: Process optimisation, kinetic study, diesel engine performance and exhaust emissions analysis. Energy 2018, 157, 843–852. [Google Scholar] [CrossRef]
- Sánchez, N.; Encinar, J.R.; Nogales, S.; González, J. Biodiesel Production from Castor Oil by Two-Step Catalytic Transesterification: Optimization of the Process and Economic Assessment. Catalysts 2019, 9, 864. [Google Scholar] [CrossRef]
- Zeng, D.; Li, R.; Feng, M.; Fang, T. Continuous Esterification of Free Fatty Acids in Crude Biodiesel by an Integrated Process of Supercritical Methanol and Sodium Methoxide Catalyst. Appl. Biochem. Biotechnol. 2014, 174, 1484–1495. [Google Scholar] [CrossRef]
- Kumar, D.; Das, T.; Giri, B.S.; Verma, B. Preparation and characterization of novel hybrid bio-support material immobilized from Pseudomonas cepacia lipase and its application to enhance biodiesel production. Renew. Energy 2020, 147, 11–24. [Google Scholar] [CrossRef]
- Andrade, T.A.; Martín, M.; Errico, M.; Christensen, K.V. Biodiesel production catalyzed by liquid and immobilized enzymes: Optimization and economic analysis. Chem. Eng. Res. Des. 2019, 141, 1–14. [Google Scholar] [CrossRef]
- Amini, Z.; Ilham, Z.; Ong, H.C.; Mazaheri, H.; Chen, W.-H. State of the art and prospective of lipase-catalyzed transesterification reaction for biodiesel production. Energy Convers. Manag. 2017, 141, 339–353. [Google Scholar] [CrossRef]
- Jiang, Y.; Liu, X.; Chen, Y.; Zhou, L.; He, Y.; Ma, L.; Gao, J. Pickering emulsion stabilized by lipase-containing periodic mesoporous organosilica particles: A robust biocatalyst system for biodiesel production. Bioresour. Technol. 2014, 153, 278–283. [Google Scholar] [CrossRef]
- Lam, M.K.; Lee, K.T.; Mohamed, A.R. Homogeneous, heterogeneous and enzymatic catalysis for transesterification of high free fatty acid oil (waste cooking oil) to biodiesel: A review. Biotechnol. Adv. 2010, 28, 500–518. [Google Scholar] [CrossRef]
- Suganya, T.; Kasirajan, R.; Renganathan, S. Ultrasound-enhanced rapid in situ transesterification of marine macroalgae Enteromorpha compressa for biodiesel production. Bioresour. Technol. 2014, 156, 283–290. [Google Scholar] [CrossRef] [PubMed]
- Elizabeth Grant, G.; Gnaneswar Gude, V. Kinetics of ultrasonic transesterification of waste cooking oil. Environ. Prog. Sustain. Energy 2014, 33, 1051–1058. [Google Scholar] [CrossRef]
- Sabzimaleki, M.; Ghobadian, B.; Farsibaf, M.M.; Najafi, G.; Soufi, M.D.; Ardebili, S.M.S. Optimization of Biodiesel Ultrasound-Assisted Synthesis from Castor Oil Using Response Surface Methodology (RSM). Chem. Prod. Process Model. 2015, 10, 123–133. [Google Scholar] [CrossRef]
- Zhang, X.; Yan, S.; Tyagi, R.D.; Surampalli, R.Y.; Valéro, J.R. Ultrasonication aided in-situ transesterification of microbial lipids to biodiesel. Bioresour. Technol. 2014, 169, 175–180. [Google Scholar] [CrossRef] [PubMed]
- Shuit, S.H.; Ong, Y.T.; Lee, K.T.; Subhash, B.; Tan, S.H. Membrane technology as a promising alternative in biodiesel production: A review. Biotechnol. Adv. 2012, 30, 1364–1380. [Google Scholar] [CrossRef]
- Kumar, R.; Ghosh, A.K.; Pal, P. Sustainable Production of Biofuels through Membrane-Integrated Systems. Sep. Purif. Rev. 2019, 1–22. [Google Scholar] [CrossRef]
- Zahan, K.A.; Kano, M. Technological Progress in Biodiesel Production: An Overview on Different Types of Reactors. Energy Procedia 2019, 156, 452–457. [Google Scholar] [CrossRef]
- Xu, W.; Gao, L.; Wang, S.; Xiao, G. Biodiesel production in a membrane reactor using MCM-41 supported solid acid catalyst. Bioresour. Technol. 2014, 159, 286–291. [Google Scholar] [CrossRef]
- Gude, V.; Patil, P.; Martinez-Guerra, E.; Deng, S.; Nirmalakhandan, N. Microwave energy potential for biodiesel production. Sustain. Chem. Process. 2013, 1, 5. [Google Scholar] [CrossRef]
- Nayak, S.N.; Bhasin, C.P.; Nayak, M.G. A review on microwave-assisted transesterification processes using various catalytic and non-catalytic systems. Renew. Energy 2019, 143, 1366–1387. [Google Scholar] [CrossRef]
- Thakkar, K.; Shah, K.; Kodgire, P.; Kachhwaha, S.S. In-situ reactive extraction of castor seeds for biodiesel production using the coordinated ultrasound – microwave irradiation: Process optimization and kinetic modeling. Ultrason. Sonochem. 2019, 50, 6–14. [Google Scholar] [CrossRef] [PubMed]
- Patil, P.; Gude, V.G.; Pinappu, S.; Deng, S. Transesterification kinetics of Camelina sativa oil on metal oxide catalysts under conventional and microwave heating conditions. Chem. Eng. J. 2011, 168, 1296–1300. [Google Scholar] [CrossRef]
- Mazubert, A.; Taylor, C.; Aubin, J.; Poux, M. Key role of temperature monitoring in interpretation of microwave effect on transesterification and esterification reactions for biodiesel production. Bioresour. Technol. 2014, 161, 270–279. [Google Scholar] [CrossRef] [PubMed]
- Zhang, A.; Wang, Q.; He, Y.; Lai, P.; Miu, Y.; Xiao, Z. Preparation of Biodiesel Based on Alkaline Ionic Liquid [Bmim]OH Catalyzed Castor Oil. IOP Conf. Ser. Mater. Sci. Eng. 2020, 729, 012048. [Google Scholar] [CrossRef]
- Molefe, M.; Nkazi, D.; Mukaya, H.E. Method Selection for Biojet and Biogasoline Fuel Production from Castor Oil: A Review. Energy Fuels 2019, 33, 5918–5932. [Google Scholar] [CrossRef]
- Kaur, R.; Bhaskar, T. Chapter 11—Potential of castor plant (Ricinus communis) for production of biofuels, chemicals, and value-added products. In Waste Biorefinery; Bhaskar, T., Pandey, A., Rene, E.R., Tsang, D.C.W., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 269–310. ISBN 978-0-12-818228-4. [Google Scholar]
- Schmutzler, L.O.F. (54) PROCESS FOR REFINING GLYCERIDE OIL. 4. Available online: https://patents.google.com/patent/US5248799A/en (accessed on 27 February 2020).
- Atadashi, I.M.; Aroua, M.K.; Aziz, A.R.A.; Sulaiman, N.M.N. Membrane biodiesel production and refining technology: A critical review. Renew. Sustain. Energy Rev. 2011, 15, 5051–5062. [Google Scholar] [CrossRef]
- Shrirame, H.Y.; Panwar, N.L.; Bamniya, B.R. Bio Diesel from Castor Oil—A Green Energy Option. Low Carbon Econ. 2011, 2, 1–6. [Google Scholar] [CrossRef]
- Tesfa, B.; Mishra, R.; Gu, F.; Powles, N. Prediction models for density and viscosity of biodiesel and their effects on fuel supply system in CI engines. Renew. Energy 2010, 35, 2752–2760. [Google Scholar] [CrossRef]
- Dwivedi, G.; Sharma, M.P. Impact of cold flow properties of biodiesel on engine performance. Renew. Sustain. Energy Rev. 2014, 31, 650–656. [Google Scholar] [CrossRef]
- Hajlari, S.A.; Najafi, B.; Ardabili, S.F. Castor oil, a source for biodiesel production and its impact on the diesel engine performance. Renew. Energy Focus 2019, 28, 1–10. [Google Scholar] [CrossRef]
- Banerjee, A.; Varshney, D.; Kumar, S.; Chaudhary, P.; Gupta, V.K. Biodiesel production from castor oil: ANN modeling and kinetic parameter estimation. Int. J. Ind. Chem. 2017, 8, 253–262. [Google Scholar] [CrossRef]
- Bueno, A.V.; Pereira, M.P.B.; de Oliveira Pontes, J.V.; de Luna, F.M.T.; Cavalcante, C.L. Performance and emissions characteristics of castor oil biodiesel fuel blends. Appl. Therm. Eng. 2017, 125, 559–566. [Google Scholar] [CrossRef]
- Atabani, A.E.; Silitonga, A.S.; Ong, H.C.; Mahlia, T.M.I.; Masjuki, H.H.; Badruddin, I.A.; Fayaz, H. Non-edible vegetable oils: A critical evaluation of oil extraction, fatty acid compositions, biodiesel production, characteristics, engine performance and emissions production. Renew. Sustain. Energy Rev. 2013, 18, 211–245. [Google Scholar] [CrossRef]
- Gomes, M.G.; Santos, D.Q.; de Morais, L.C.; Pasquini, D. Purification of biodiesel by dry washing, employing starch and cellulose as natural adsorbents. Fuel 2015, 155, 1–6. [Google Scholar] [CrossRef]
- Fan, X.; Burton, R.; Austic, G. Preparation and characterization of biodiesel produced from fish oil. Chem. Technol. Fuels Oils 2010, 46, 287–293. [Google Scholar] [CrossRef]
- Tyagi, O.S.; Atray, N.; Kumar, B.; Datta, A. Production, characterization and development of standards for biodiesel—A review. MAPAN 2010, 25, 197–218. [Google Scholar] [CrossRef]
- Çetinkaya, M.; Karaosmanoǧlu, F. Optimization of Base-Catalyzed Transesterification Reaction of Used Cooking Oil. Energy Fuels 2004, 18, 1888–1895. [Google Scholar] [CrossRef]
- Maceiras, R.; Rivero, J.J.; Cancela, M.A.; Urrejola, S.; Sanchez, A. Development and modeling of production of biodiesel from sunflower oil. Chem. Technol. Fuels Oils 2010, 46, 154–159. [Google Scholar] [CrossRef]
- Berrios, M.; Skelton, R.L. Comparison of purification methods for biodiesel. Chem. Eng. J. 2008, 144, 459–465. [Google Scholar] [CrossRef]
- Vasconcelos, A.F.F.; Dantas, M.B.; Filho, M.G.R.; Rosenhaim, R.; Cavalcanti, E.H.S.; Filho, N.R.A.; Sinfrônio, F.S.M.; Santos, I.M.G.; Souza, A.G. Influence of drying processes on oxidative stability of ethyl corn biodiesel by differential scanning calorimetry. J. Therm. Anal. Calorim. 2009, 97, 657–660. [Google Scholar] [CrossRef]
- Predojević, Z.J. The production of biodiesel from waste frying oils: A comparison of different purification steps. Fuel 2008, 87, 3522–3528. [Google Scholar] [CrossRef]
- Stojkovič, G.; Plazl, I.; Žnidaršič-Plazl, P. l-Malic acid production within a microreactor with surface immobilised fumarase. Microfluid. Nanofluidics 2011, 10, 627–635. [Google Scholar] [CrossRef]
- He, W.; Fang, Z.; Ji, D.; Zhang, K.; Guo, K. Optimization of biodiesel production by continuous microflow system with online separation. Monatshefte Für Chem. Chem. Mon. 2014, 145, 223–227. [Google Scholar] [CrossRef]
- Chatzifragkou, A.; Papanikolaou, S.; Kopsahelis, N.; Kachrimanidou, V.; Dorado, M.P.; Koutinas, A.A. Biorefinery development through utilization of biodiesel industry by-products as sole fermentation feedstock for 1,3-propanediol production. Bioresour. Technol. 2014, 159, 167–175. [Google Scholar] [CrossRef]
- Chambi, H.N.M.; Lacerda, R.S.; Makishi, G.L.A.; Bittante, A.M.Q.B.; Gomide, C.A.; Sobral, P.J.A. Protein extracted from castor bean (Ricinus communis L.) cake in high pH results in films with improved physical properties. Ind. Crops Prod. 2014, 61, 217–224. [Google Scholar] [CrossRef]
- Abada, E.; Al-Fifi, Z.; Osman, M. Bioethanol production with carboxymethylcellulase of Pseudomonas poae using castor bean (Ricinus communis L.) cake. Saudi J. Biol. Sci. 2019, 26, 866–871. [Google Scholar] [CrossRef]
- Pedroso, L.A.; Campos, V.P.; Pedroso, M.P.; Barros, A.F.; Freire, E.S.; Resende, F.M. Volatile organic compounds produced by castor bean cake incorporated into the soil exhibit toxic activity against Meloidogyne incognita. Pest Manag. Sci. 2019, 75, 476–483. [Google Scholar] [CrossRef]
- Nicory, I.M.C.; de Carvalho, G.G.P.; Ribeiro, O.L.; Santos, S.A.; da Silva, F.F.; Silva, R.R.; Costa Lopes, L.S.; Souza, F.N.C.; de Freitas, J.E., Jr. Productive and metabolic parameters in lambs fed diets with castor seed meal. Livest. Sci. 2015, 181, 171–178. [Google Scholar] [CrossRef]
- Akande, T.O.; Odunsi, A.A.; Akinfala, E.O. A review of nutritional and toxicological implications of castor bean ( R. communis L.) meal in animal feeding systems. J. Anim. Physiol. Anim. Nutr. 2016, 100, 201–210. [Google Scholar] [CrossRef]
- Araújo, R.A.D.; Neiva, J.N.M.; Rogério, M.C.P.; Pimentel, P.G.; Furtado, R.N.; Mariz, L.D.S.; Cândido, M.J.D.; Pompeu, R.C.F.F. Ingestive behavior and physiological parameters of lactating goats fed diets containing detoxified castor cake. Biol. Rhythm Res. 2019, 1–11. [Google Scholar] [CrossRef]
- Anastácio, G.S.; Santos, K.O.; Suarez, P.A.Z.; Torres, F.A.G.; De Marco, J.L.; Parachin, N.S. Utilization of glycerin byproduct derived from soybean oil biodiesel as a carbon source for heterologous protein production in Pichia pastoris. Bioresour. Technol. 2014, 152, 505–510. [Google Scholar] [CrossRef]
- Pflügl, S.; Marx, H.; Mattanovich, D.; Sauer, M. Heading for an economic industrial upgrading of crude glycerol from biodiesel production to 1,3-propanediol by Lactobacillus diolivorans. Bioresour. Technol. 2014, 152, 499–504. [Google Scholar] [CrossRef]
- Sarma, S.J.; Brar, S.K.; Le Bihan, Y.; Buelna, G.; Soccol, C.R. Mitigation of the inhibitory effect of soap by magnesium salt treatment of crude glycerol—A novel approach for enhanced biohydrogen production from the biodiesel industry waste. Bioresour. Technol. 2014, 151, 49–53. [Google Scholar] [CrossRef]
- Duarte, S.H.; Ansolin, M.; Maugeri, F. Cultivation of Candida sp. LEB-M3 in glycerol: Lipid accumulation and prediction of biodiesel quality parameters. Bioresour. Technol. 2014, 161, 416–422. [Google Scholar] [CrossRef]
- Pott, R.W.M.; Howe, C.J.; Dennis, J.S. The purification of crude glycerol derived from biodiesel manufacture and its use as a substrate by Rhodopseudomonas palustris to produce hydrogen. Bioresour. Technol. 2014, 152, 464–470. [Google Scholar] [CrossRef]
- Zhou, Y.; Nie, K.; Zhang, X.; Liu, S.; Wang, M.; Deng, L.; Wang, F.; Tan, T. Production of fumaric acid from biodiesel-derived crude glycerol by Rhizopus arrhizus. Bioresour. Technol. 2014, 163, 48–53. [Google Scholar] [CrossRef]

| Feedstock Source (Oil Content, %) | Characteristics | Advantages | Disadvantages | Ref. |
|---|---|---|---|---|
| Castor beans (46–55) | Liquid at room temperature, light yellow color, and slightly pungent *US$824/tonne of oil | Transesterification can be performed at room temperature Miscible in alcohol Non-edible Low acid value High flashpoint | Generation of toxic solid waste High viscosity Decrease fuel atomization | [2,14] |
| Jatropha (34–60) | Colorless after extraction (fresh) and pale yellow after standing time, liquid at room temperature **US$250/tonne of oil | Biodiesel obtained is stable during storage Non-edible High cetane number, good oxidation stability, low viscosity | Engine corrosion due to free fatty acids Generation of toxic solid waste High cloud point Not suitable at low temperature High acid value | [15,16] |
| Soybean (12–22) | Fresh has a pale light color, and dark after storage, liquid at room temperature ***US$746/tonne of oil | The yield of 98% crude biodiesel in refined oils High thermal stability Low viscosity | The high cost of production Biodiesel production in long-term is unsustainable Edible High acid value | [12,17] |
| Sunflower (38–50) | Refined has a clear and vaguely yellowish-brown color Liquid at room temperature ***US$689/tonne of oil | Low viscosity | Used to produce food and fiber Biodiesel production in long-term is unsustainable Edible High acid value | [17,18] |
| Palm (18–40) | Semi-solid at room temperature, reddish and clear color, depending on extraction source (pulp or kernel) ***US$535/tonne of oil | 96% yield of crude biodiesel in refined oils Cheap feedstock Good oxidation stability Acceptable ratio of saponification High flashpoint | High cloud point Conversion to biodiesel may not be sustainable long term Edible | [12,19] |
| Used cooking oil | Depends on the cooking process can vary yellow to dark brown, liquid at room temperature ****US$500/tonne of oil | Low cloud point Environmentally friendly Low price of feedstock Non-edible High thermal stability | High ratio of acid esterification High ratio of saponification High acid value | [18,20] |
| Oil | Fatty Acid Composition (wt%) | Ref. | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Saturated | Monounsaturated | Polyunsaturated | |||||||
| C14:0 | C16:0 | C18:0 | C18:1 cis-9 | C18:1* | C18:2 | C18:3 | Others | ||
| Castor | - | 1.1 | 1.0 | 3.3 | 87.7 | 4.7 | 0.7 | 1.5 | [27] |
| Jatropha | - | 12.80 | 6.20 | 39.94 | - | 45.40 | - | <1.0 | [16] |
| Soybean | - | 11.46 | 3.08 | 23.30 | - | 53.32 | 0.31 | 8.53 | [28,29] |
| Sunflower | 0.08 | 8.03 | 3.26 | 29.27 | - | 59.32 | - | 0.04 | [30] |
| Palm | 0 | 46.8 | 3.80 | 37.60 | - | 10.50 | - | 1.3 | [31] |
| Canola | - | 3.90 | 1.10 | 64.40 | - | 20.4 | 9.60 | 0.6 | [32] |
| Method | Conditions | Yield | Biodiesel Features | Ref. |
|---|---|---|---|---|
| Alkaline transesterification | 1:12 oil:methanol, potassium hydroxide 1.25%wt 60 °C 60 min | 94.9% | High density and flashpoint Low sulfur content Yellow color | [44] |
| Alkaline transesterification | 1:5.4 oil:methanol, potassium hydroxide 0.73%wt 64 °C 2.5 h | 97.8% | - | [45] |
| Alkaline transesterification | 1:8.24 oil:methanol, potassium hydroxide 1.45%wt 35.5 °C 40 min | 93.2% | Low acid value | [50] |
| Alkaline transesterification | 0.29:1 oil:ethanol, potassium hydroxide 1%wt 62.5 °C 226 min | 85% | High density and flashpoint | [51] |
| Alkaline transesterification | 1:6 oil:ethanol Potassium hydroxide 1%wt 55–65 °C 2–8 h | 43.3–74.1% | Low acid value High flashpoint | [50] |
| Parameter | Effect | Ref. |
|---|---|---|
| Temperature | Depending on the catalyst, temperature affects the reaction rate. The temperature range is 25–120 °C, being optimal around 60 °C | [59] |
| Alcohol triglyceride molar ratio | The stoichiometric ratio needs a 3:1 alcohol:oil molar ratio to produce three moles of alkyl esters of fatty acids and one mole of glycerol. To perform the best transesterification reaction is necessary for an alcohol excess to promote the product’s equilibrium. However, residual alcohol interferes during glycerol separation from biodiesel because it is highly soluble in alcohol | [2] |
| Time | Has been reported that longer reaction times increase the conversion of fatty acids to fatty acid methyl esters | [51] |
| Catalyst | Alkaline catalysts increase the reaction rates in comparison with an acid catalyst. However, when the vegetable oil has a high free fatty acid content as well as high water content, an acid transesterification is recommended because soap is not formed | [60] |
| Moisture content | For alkaline catalysis, both compounds (triglycerides and alcohol) need to be anhydrous, due to water induces saponification | [44] |
| Content of free fatty acids | In the alkaline catalysis, the content of free fatty acids should be as low as 0.5% w/w of oil, to avoid the formation of soaps. | [61] |
| Biocatalyst | Advantages | Disadvantages | Ref. |
|---|---|---|---|
| Free enzymes | Low energy consumption, highly selective, efficient catalytic activity, environmentally friendly | Unstable Difficult to be reused Costly | [63] |
| Immobilized enzymes | Stability and reusability | Costly Low reaction rate Extra steps during the recovery process Loss of enzyme activity | [62] |
| Whole-cell | Simple preparation Purification and immobilization not required | Low activity Easy inactivation Low rate of recovery and reusability | [64] |
| Parameter | Zhang et al. [80] | Molefe et al. [81] | Kaur and Bhaskar [82] |
|---|---|---|---|
| Acid value /(mg.g−1) | 1 | 2.07 | <4 |
| Saponification value /(mg.g−1) | 180 | 175 | 178 |
| Iodine value (g/100 g) | 86 | 84 | 85 |
| Refractive index (n20 D) | 1.48 | 1.48 | 1.47 |
| Relative density (g/cm3) | 0.956 | 0.961 | 0.965 |
| Flashpoint (℃) | 322 | 145 | 229 |
| Specific heat (kJ/kg/K) | nd | 0.089 | 0.089 |
| Ricinic acid (%wt) | 88 | 89.5 | 87–90 |
| Oleic acid (%wt) | 7 | 3 | 2–7 |
| Linoleic acid (%wt) | 5 | 4 | 1–5 |
| Linolenic acid (%wt) | 1 | 0.3 | nd |
| Product | Kinematic Viscosity (mm2/s at 40 °C) | Density (kg/m3) | Acid Value (mgKOH/g) | Flash Point (°C) | Water Content (%) | Calorific Value (MJ/kg) | Cetane Number | Cloud Point (°C) | Pour Point (°C) | Iodine Value (gI2/100 g) | Ref |
|---|---|---|---|---|---|---|---|---|---|---|---|
| *Biodiesel from castor oil | 23 | 960 | 13.12 | 262 | nd | 30.18 | nd | 3 | −13 | nd | [62] |
| 14 | 926 | nd | 164 | nd | 37.90 | nd | −23 | nd | nd | [88] | |
| 15 | 946 | 0.63 | 194 | 0.15 | 38.34 | 43 | nd | −30 | nd | [44] | |
| 26 | 961 | 1.19 | nd | 0.31 | nd | nd | nd | nd | 80.5 | [60] | |
| 18 | 920 | 0.25 | 170 | 0.006 | 39.5 | nd | nd | nd | nd | [89] | |
| 14 | 923 | nd | 273.1 | nd | 37.34 | 50 | nd | nd | 83.40 | [90] | |
| **Biodiesel according ASTM D 6751–06a | 1.9–6.0 | 860–900 | ≤0.50 | ≥130 | ≤0.50 | nd | ≥47 | −3–−12 | −15–10 | nd | [51] |
| **Biodiesel according EN 14214 | 3.5–5.0 | 860–900 | ≤0.50 | ≥101 | ≤0.50 | nd | ≥51 | nd | nd | nd | [51] |
| ***Fossil Diesel | 2.5–4.5 | 820–860 | nd | 68–80 | nd | ≥45.56 | ≥46 | −15–5 | −35–15 | nd | [91] |
| Impurities | Effect | Ref. |
|---|---|---|
| Free fatty acids (FFA) | Corrosion Low oxidation stability | [92,93] |
| Water | Corrosion | |
| Methanol | Low values of viscosity and density Low flashpoint Corrosion of aluminum and zinc surfaces | [93] |
| Glycerides | High viscosity Carbon residues in the injection system Crystallization | [94] |
| Metals (soap, catalyst) | Carbon residues in the injection system Filter fuel system blockage Power loss | [95] |
| Glycerol | Problems caused by sediment accumulation Increased emission of acrolein and aldehydes | [61] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Osorio-González, C.S.; Gómez-Falcon, N.; Sandoval-Salas, F.; Saini, R.; Brar, S.K.; Ramírez, A.A. Production of Biodiesel from Castor Oil: A Review. Energies 2020, 13, 2467. https://doi.org/10.3390/en13102467
Osorio-González CS, Gómez-Falcon N, Sandoval-Salas F, Saini R, Brar SK, Ramírez AA. Production of Biodiesel from Castor Oil: A Review. Energies. 2020; 13(10):2467. https://doi.org/10.3390/en13102467
Chicago/Turabian StyleOsorio-González, Carlos S., Natali Gómez-Falcon, Fabiola Sandoval-Salas, Rahul Saini, Satinder K. Brar, and Antonio Avalos Ramírez. 2020. "Production of Biodiesel from Castor Oil: A Review" Energies 13, no. 10: 2467. https://doi.org/10.3390/en13102467
APA StyleOsorio-González, C. S., Gómez-Falcon, N., Sandoval-Salas, F., Saini, R., Brar, S. K., & Ramírez, A. A. (2020). Production of Biodiesel from Castor Oil: A Review. Energies, 13(10), 2467. https://doi.org/10.3390/en13102467









