Preparation and Properties of Capric–Myristic Acid/Expanded Graphite Composite Phase Change Materials for Latent Heat Thermal Energy Storage
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Capric–Myristic Acid Binary Eutectic Solution
2.3. Preparation of CA–MA/EG CPCMs
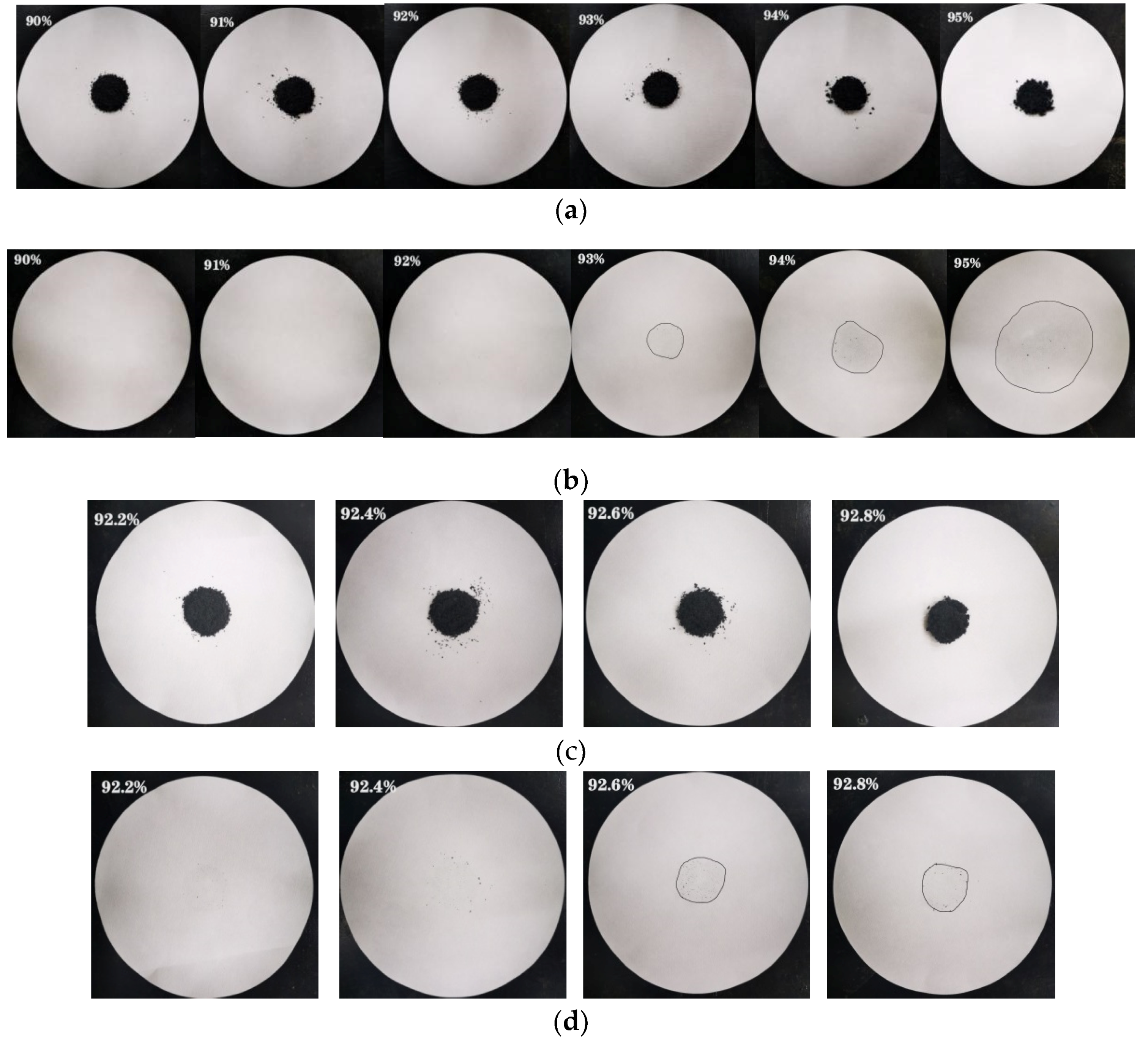
2.4. Experiment of Maximum Mass Absorption Ratio of the CA–MA in the CA–MA/EG CPCMs
2.5. Thermal Conductivities Test of CA–MA/EG CPCMs
2.6. Characterization
3. Results and Discussion
3.1. Maximum Mass Absorption Ratio of the CA–MA in the CA–MA/EG CPCMs
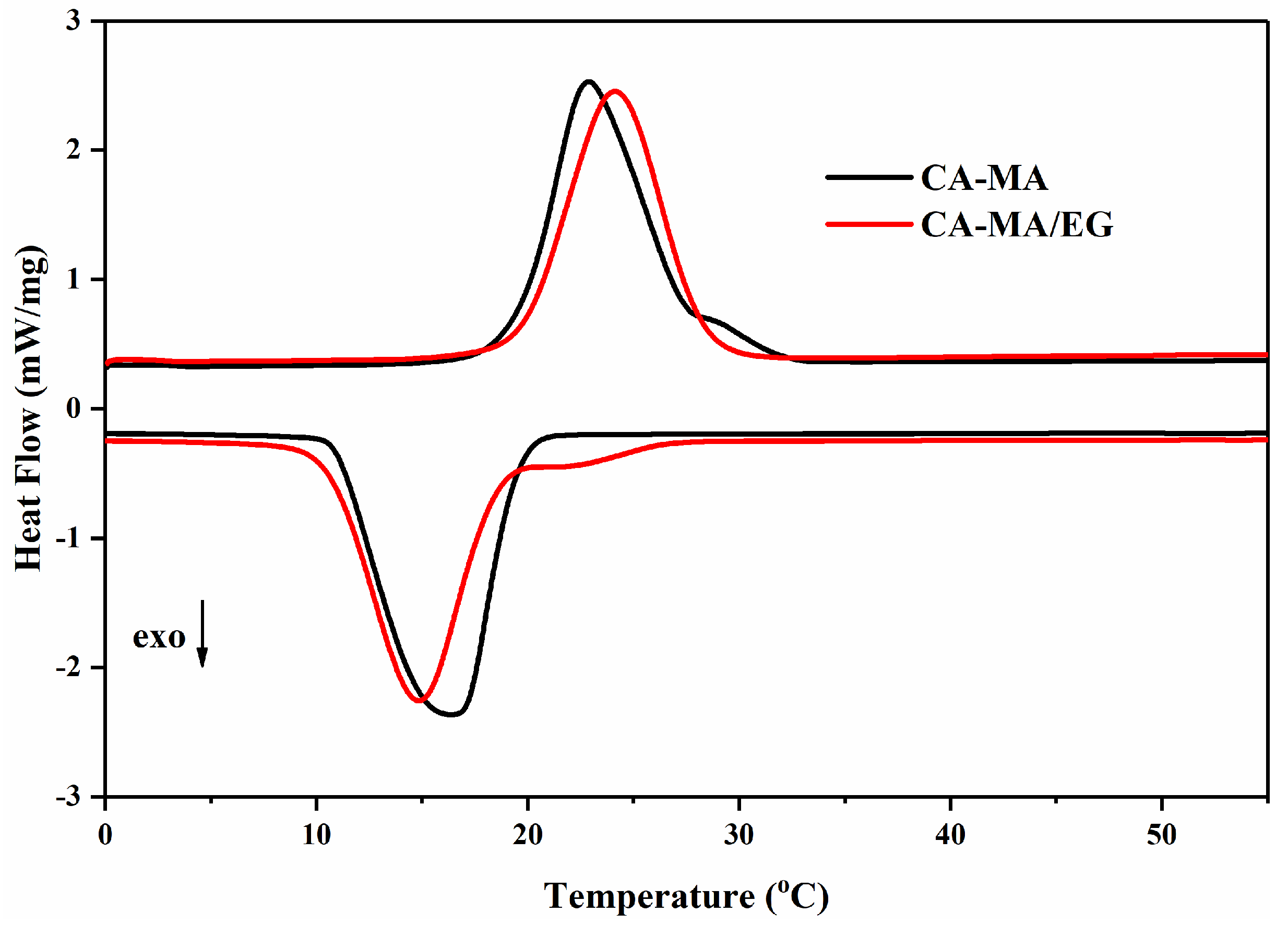
3.2. Thermal Properties of the CPCMs
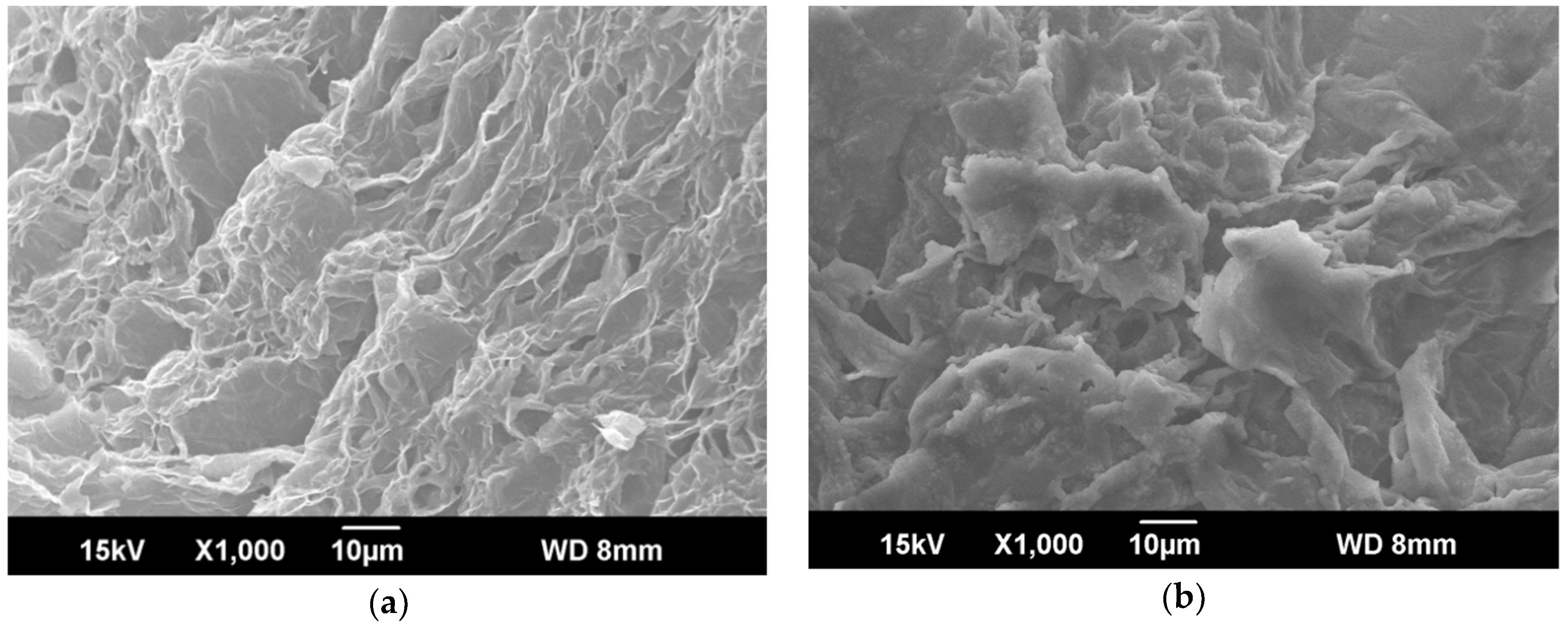
3.3. Microstructure of EG and CA–MA/EG Composite PCMs
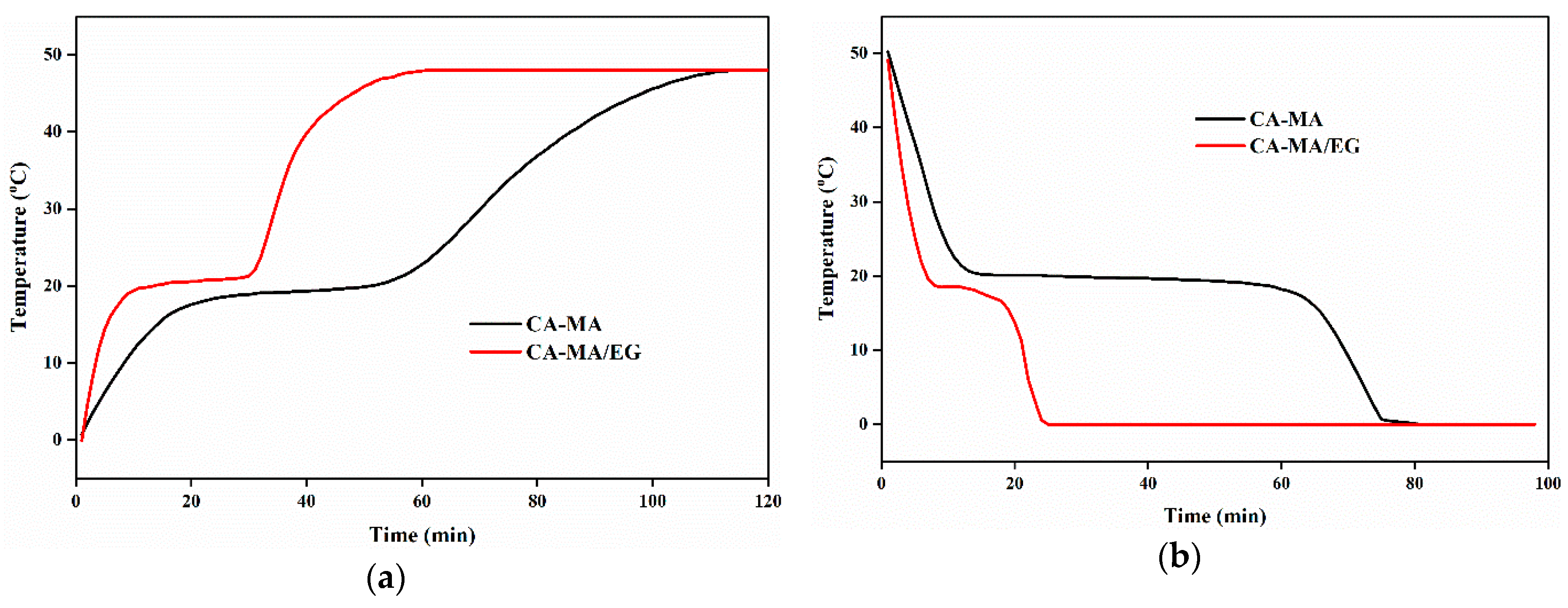
3.4. Improvement of the Thermal Performance of the CPCMs
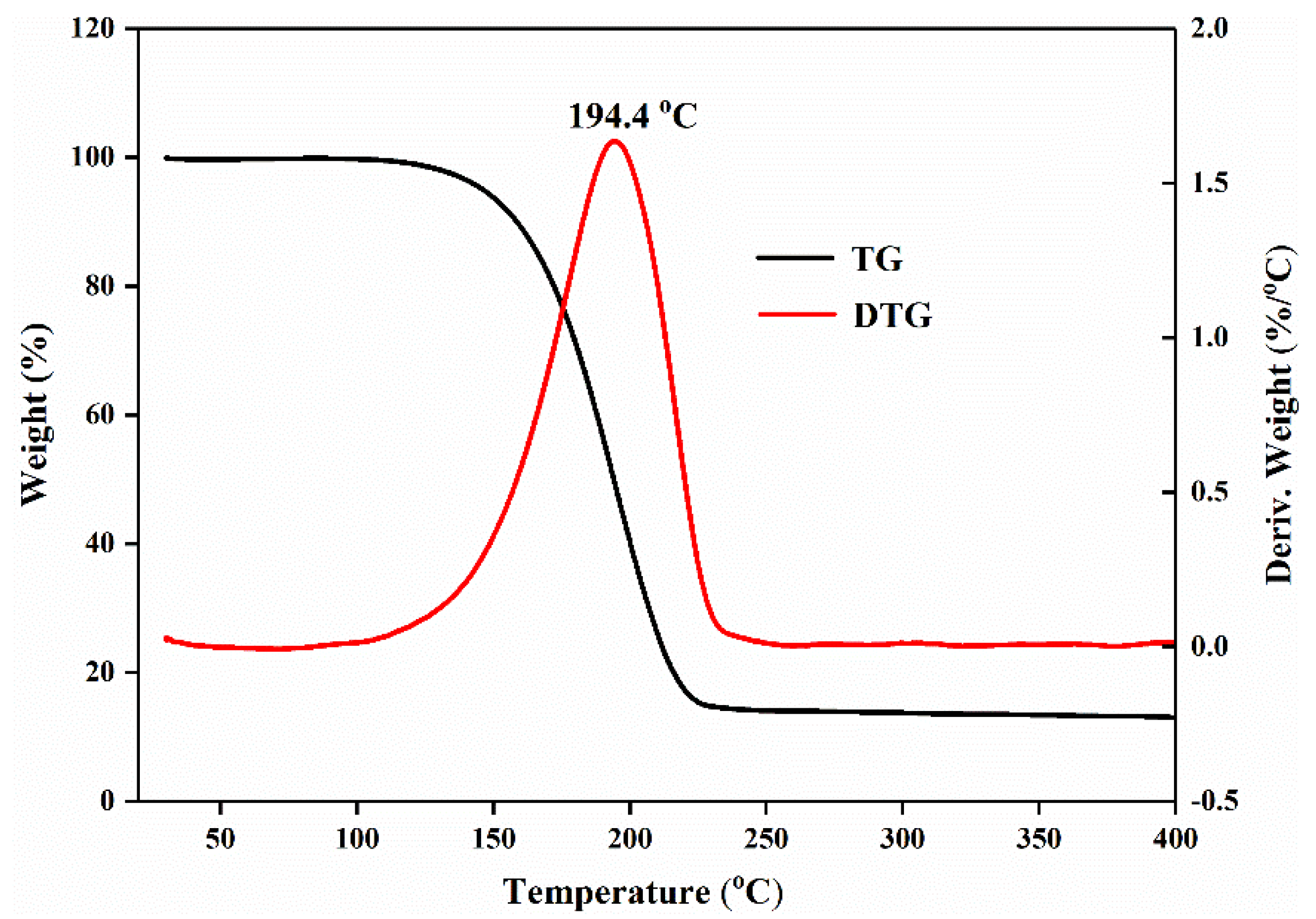
3.5. Thermo-Stability and Thermo-Reliability of the CPCMs
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Akeiber, H.; Nejat, P.; Majid, M.Z.A.; Wahid, M.A.; Jomehzadeh, F.; Zeynali Famileh, I.; Calautit, J.K.; Hughes, B.R.; Zaki, S.A. A Review on Phase Change Material (PCM) for Sustainable Passive Cooling in Building Envelopes. Renew. Sustain. Energy Rev. 2016, 60, 1470–1497. [Google Scholar] [CrossRef]
- Mofijur, M.; Mahlia, T.M.I.; Silitonga, A.S.; Ong, H.C.; Silakhori, M.; Hasan, M.H.; Putra, N.; Rahman, S.M. Phase Change Materials (PCM) for Solar Energy Usages and Storage: An Overview. Energies 2019, 12, 3167. [Google Scholar] [CrossRef] [Green Version]
- He, H.; Zhao, P.; Yue, Q.; Gao, B.; Yue, D.; Li, Q. A Novel Polynary Fatty Acid/Sludge Ceramsite Composite Phase Change Materials and Its Applications in Building Energy Conservation. Renew. Energy 2015, 76, 45–52. [Google Scholar] [CrossRef]
- Pereira da Cunha, J.; Eames, P. Thermal Energy Storage for Low and Medium Temperature Applications Using Phase Change Materials—A Review. Appl. Energy 2016, 177, 227–238. [Google Scholar] [CrossRef] [Green Version]
- Zou, T.; Fu, W.; Liang, X.; Wang, S.; Gao, X.; Zhang, Z.; Fang, Y. Preparation and Performance of Modified Calcium Chloride Hexahydrate Composite Phase Change Material for Air-Conditioning Cold Storage. Int. J. Refrig. 2018, 95, 175–181. [Google Scholar] [CrossRef]
- Kenisarin, M.M. Thermophysical Properties of Some Organic Phase Change Materials for Latent Heat Storage. A Review. Sol. Energy 2014, 107, 553–575. [Google Scholar] [CrossRef]
- Mohamed, S.A.; Al-Sulaiman, F.A.; Ibrahim, N.I.; Zahir, M.H.; Al-Ahmed, A.; Saidur, R.; Yılbaş, B.S.; Sahin, A.Z. A Review on Current Status and Challenges of Inorganic Phase Change Materials for Thermal Energy Storage Systems. Renew. Sustain. Energy Rev. 2017, 70, 1072–1089. [Google Scholar] [CrossRef]
- Zhang, P.; Xiao, X.; Ma, Z.W. A Review of the Composite Phase Change Materials: Fabrication, Characterization, Mathematical Modeling and Application to Performance Enhancement. Appl. Energy 2016, 165, 472–510. [Google Scholar] [CrossRef]
- Şahan, N.; Fois, M.; Paksoy, H. Improving Thermal Conductivity Phase Change Materials—A Study of Paraffin Nanomagnetite Composites. Sol. Energy Mater. Sol. Cells 2015, 137, 61–67. [Google Scholar] [CrossRef]
- Yuan, Y.; Zhang, N.; Tao, W.; Cao, X.; He, Y. Fatty Acids as Phase Change Materials: A Review. Renew. Sustain. Energy Rev. 2014, 29, 482–498. [Google Scholar] [CrossRef]
- Sarı, A.; Karaipekli, A. Preparation, Thermal Properties and Thermal Reliability of Palmitic Acid/Expanded Graphite Composite as Form-Stable PCM for Thermal Energy Storage. Sol. Energy Mater. Sol. Cells 2009, 93, 571–576. [Google Scholar] [CrossRef]
- Kahwaji, S.; White, M.A. Prediction of the Properties of Eutectic Fatty Acid Phase Change Materials. Thermochim. Acta 2018, 660, 94–100. [Google Scholar] [CrossRef]
- Zhao, P.; Yue, Q.; He, H.; Gao, B.; Wang, Y.; Li, Q. Study on Phase Diagram of Fatty Acids Mixtures to Determine Eutectic Temperatures and the Corresponding Mixing Proportions. Appl. Energy 2014, 115, 483–490. [Google Scholar] [CrossRef]
- Ke, H. Phase Diagrams, Eutectic Mass Ratios and Thermal Energy Storage Properties of Multiple Fatty Acid Eutectics as Novel Solid-Liquid Phase Change Materials for Storage and Retrieval of Thermal Energy. Appl. Therm. Eng. 2017, 113, 1319–1331. [Google Scholar] [CrossRef]
- Paul, A.; Shi, L.; Bielawski, C.W. A Eutectic Mixture of Galactitol and Mannitol as a Phase Change Material for Latent Heat Storage. Energy Convers. Manag. 2015, 103, 139–146. [Google Scholar] [CrossRef] [Green Version]
- Diarce, G.; Gandarias, I.; Campos-Celador, Á.; García-Romero, A.; Griesser, U.J. Eutectic Mixtures of Sugar Alcohols for Thermal Energy Storage in the 50–90 °C Temperature Range. Sol. Energy Mater. Sol. Cells 2015, 134, 215–226. [Google Scholar] [CrossRef]
- Han, L.; Ma, G.; Xie, S.; Sun, J.; Jia, Y.; Jing, Y. Thermal Properties and Stabilities of the Eutectic Mixture: 1,6-Hexanediol/Lauric Acid as a Phase Change Material for Thermal Energy Storage. Appl. Therm. Eng. 2017, 116, 153–159. [Google Scholar] [CrossRef]
- Gunasekara, S.N.; Martin, V.; Chiu, J.N. Phase Equilibrium in the Design of Phase Change Materials for Thermal Energy Storage: State-of-the-Art. Renew. Sustain. Energy Rev. 2017, 73, 558–581. [Google Scholar] [CrossRef]
- Saeed, R.M.; Schlegel, J.P.; Castano, C.; Sawafta, R.; Kuturu, V. Preparation and Thermal Performance of Methyl Palmitate and Lauric Acid Eutectic Mixture as Phase Change Material (PCM). J. Energy Storage 2017, 13, 418–424. [Google Scholar] [CrossRef]
- Fan, L.; Khodadadi, J.M. Thermal Conductivity Enhancement of Phase Change Materials for Thermal Energy Storage: A Review. Renew. Sustain. Energy Rev. 2011, 15, 24–46. [Google Scholar] [CrossRef]
- Wu, S.; Yan, T.; Kuai, Z.; Pan, W. Thermal Conductivity Enhancement on Phase Change Materials for Thermal Energy Storage: A Review. Energy Storage Mater. 2020, 25, 251–295. [Google Scholar] [CrossRef]
- Xu, T.; Chen, Q.; Huang, G.; Zhang, Z.; Gao, X.; Lu, S. Preparation and Thermal Energy Storage Properties of D-Mannitol/Expanded Graphite Composite Phase Change Material. Sol. Energy Mater. Sol. Cells 2016, 155, 141–146. [Google Scholar] [CrossRef]
- Karaipekli, A.; Sarı, A.; Kaygusuz, K. Thermal Conductivity Improvement of Stearic Acid Using Expanded Graphite and Carbon Fiber for Energy Storage Applications. Renew. Energy 2007, 32, 2201–2210. [Google Scholar] [CrossRef]
- Ramakrishnan, S.; Sanjayan, J.; Wang, X.; Alam, M.; Wilson, J. A Novel Paraffin/Expanded Perlite Composite Phase Change Material for Prevention of PCM Leakage in Cementitious Composites. Appl. Energy 2015, 157, 85–94. [Google Scholar] [CrossRef]
- Tang, F.; Su, D.; Tang, Y.; Fang, G. Synthesis and Thermal Properties of Fatty Acid Eutectics and Diatomite Composites as Shape-Stabilized Phase Change Materials with Enhanced Thermal Conductivity. Sol. Energy Mater. Sol. Cells 2015, 141, 218–224. [Google Scholar] [CrossRef]
- Wang, T.; Wang, S.; Luo, R.; Zhu, C.; Akiyama, T.; Zhang, Z. Microencapsulation of Phase Change Materials with Binary Cores and Calcium Carbonate Shell for Thermal Energy Storage. Appl. Energy 2016, 171, 113–119. [Google Scholar] [CrossRef]
- Wu, Y.; Wang, T. Preparation and Characterization of Hydrated Salts/Silica Composite as Shape-Stabilized Phase Change Material via Sol–Gel Process. Thermochim. Acta 2014, 591, 10–15. [Google Scholar] [CrossRef]
- Fu, X.; Liu, Z.; Wu, B.; Wang, J.; Lei, J. Preparation and Thermal Properties of Stearic Acid/Diatomite Composites as Form-Stable Phase Change Materials for Thermal Energy Storage via Direct Impregnation Method. J. Therm. Anal. Calorim. 2016, 123, 1173–1181. [Google Scholar] [CrossRef]
- He, Y.; Zhang, X.; Zhang, Y.; Song, Q.; Liao, X. Utilization of Lauric Acid-Myristic Acid/Expanded Graphite Phase Change Materials to Improve Thermal Properties of Cement Mortar. Energy Build. 2016, 133, 547–558. [Google Scholar] [CrossRef]
- Wei, H.; Xie, X.; Li, X.; Lin, X. Preparation and Characterization of Capric-Myristic-Stearic Acid Eutectic Mixture/Modified Expanded Vermiculite Composite as a Form-Stable Phase Change Material. Appl. Energy 2016, 178, 616–623. [Google Scholar] [CrossRef]
- Wu, S.; Li, T.X.; Yan, T.; Dai, Y.J.; Wang, R.Z. High Performance Form-Stable Expanded Graphite/Stearic Acid Composite Phase Change Material for Modular Thermal Energy Storage. Int. J. Heat Mass Transf. 2016, 102, 733–744. [Google Scholar] [CrossRef]
- Lin, Y.; Jia, Y.; Alva, G.; Fang, G. Review on Thermal Conductivity Enhancement, Thermal Properties and Applications of Phase Change Materials in Thermal Energy Storage. Renew. Sustain. Energy Rev. 2018, 82, 2730–2742. [Google Scholar] [CrossRef]
- Bose, P.; Amirtham, V.A. A Review on Thermal Conductivity Enhancement of Paraffinwax as Latent Heat Energy Storage Material. Renew. Sustain. Energy Rev. 2016, 65, 81–100. [Google Scholar] [CrossRef]
- Zhou, D.; Zhou, Y.; Yuan, J.; Liu, Y. Palmitic Acid-Stearic Acid/Expanded Graphite as Form-Stable Composite Phase-Change Material for Latent Heat Thermal Energy Storage. J. Nanomater. 2020, 2020, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Yang, X.; Yuan, Y.; Zhang, N.; Cao, X.; Liu, C. Preparation and Properties of Myristic–Palmitic–Stearic Acid/Expanded Graphite Composites as Phase Change Materials for Energy Storage. Sol. Energy 2014, 99, 259–266. [Google Scholar] [CrossRef]
- Liu, C.; Yuan, Y.; Zhang, N.; Cao, X.; Yang, X. A Novel PCM of Lauric–Myristic–Stearic Acid/Expanded Graphite Composite for Thermal Energy Storage. Mater. Lett. 2014, 120, 43–46. [Google Scholar] [CrossRef]
- Zhang, H.; Gao, X.; Chen, C.; Xu, T.; Fang, Y.; Zhang, Z. A Capric–Palmitic–Stearic Acid Ternary Eutectic Mixture/Expanded Graphite Composite Phase Change Material for Thermal Energy Storage. Compos. Part A Appl. Sci. Manuf. 2016, 87, 138–145. [Google Scholar] [CrossRef]
- Zhu, H.; Zhang, P.; Meng, Z.; Li, M. Thermal Characterization of Lauric–Stearic Acid/Expanded Graphite Eutectic Mixture as Phase Change Materials. J. Nanosci. Nanotechnol. 2015, 15, 3288–3294. [Google Scholar] [CrossRef]
- Zhang, N.; Yuan, Y.; Wang, X.; Cao, X.; Yang, X.; Hu, S. Preparation and Characterization of Lauric–Myristic–Palmitic Acid Ternary Eutectic Mixtures/Expanded Graphite Composite Phase Change Material for Thermal Energy Storage. Chem. Eng. J. 2013, 231, 214–219. [Google Scholar] [CrossRef]
- Ma, F.; Li, Y.; Cheng, L.; Chen, M. Preparation and properties of octadecane–palmitic acid/expanded graphite phase change energy storage materials. J. Aeronaut. Mater. 2010, 30, 66–69. [Google Scholar] [CrossRef]
- Zhou, D.; Shi, C.; Yuan, W. Research on the Applicability of Solar Energy-Ground Source Heat Pump in Different Regions of China. In Proceedings of the 2011 Second International Conference on Digital Manufacturing & Automation, Zhangjiajie, China, 5–7 August 2011; pp. 1026–1029. [Google Scholar] [CrossRef]
- Zhou, D.; Zhou, Y.; Liu, Y.; Luo, X.; Yuan, J. Preparation and Performance of Capric-Myristic Acid Binary Eutectic Mixtures for Latent Heat Thermal Energy Storages. J. Nanomater. 2019, 2019, 1–9. [Google Scholar] [CrossRef]
| PCMs/CPCMs | Melting | Freezing | References | ||
|---|---|---|---|---|---|
| Temperature (°C) | Latent Heat (J/g) | Temperature (°C) | Latent Heat (J/g) | ||
| PA–SA | 54.81 | 187.0 | 54.06 | 179.7 | [34] |
| PA–SA/EG | 55.18 | 176.2 | 54.91 | 175.6 | |
| MA–PA–SA | 41.72 | 163.5 | 42.38 | 159.8 | [35] |
| MA–PA–SA/EG | 41.64 | 153.5 | 42.99 | 151.4 | |
| LA–MA–SA | 29.29 | 140.9 | 28.38 | 137.2 | [36] |
| LA–MA–SA/EG | 29.05 | 137.1 | 29.38 | 131.3 | |
| CA–PA–SA | 18.90 | 147.2 | 16.73 | 142.3 | [37] |
| CA–PA–SA/EG | 21.33 | 131.7 | 19.01 | 127.2 | |
| LA–SA | 35.54 | 159.9 | 34.36 | / | [38] |
| LA–SA/EG | 35.69 | 143.4 | 34.28 | / | |
| LA–MA–PA | 31.41 | 145.8 | / | / | [39] |
| LA–MA–PA/EG | 30.94 | 135.9 | / | / | |
| OC–PA | 28.78 | 181.6 | / | / | [40] |
| OC–PA/EG | 29.18 | 160.7 | / | / | |
| CA–MA | 19.45 | 150.9 | 18.34 | 149.2 | This study |
| CA–MA/EG | 19.78 | 137.3 | 18.85 | 139.8 | |
| PCM | Melting | Freezing | ||||
|---|---|---|---|---|---|---|
| Phase Change Temperature (°C) | Peak Temperature (°C) | Phase Change Latent Heat (J/g) | Phase Change Temperature (°C) | Peak Temperature (°C) | Phase Change Latent Heat (J/g) | |
| CA–MA | 19.45 ± 0.05 | 22.92 ± 0.05 | 150.9 ± 0.2 | 18.34 ± 0.05 | 16.45 ± 0.05 | 149.2 ± 0.1 |
| CA–MA/EG | 19.78 ± 0.05 | 24.15 ± 0.05 | 137.3 ± 0.1 | 18.85 ± 0.05 | 14.83 ± 0.05 | 139.8 ± 0.1 |
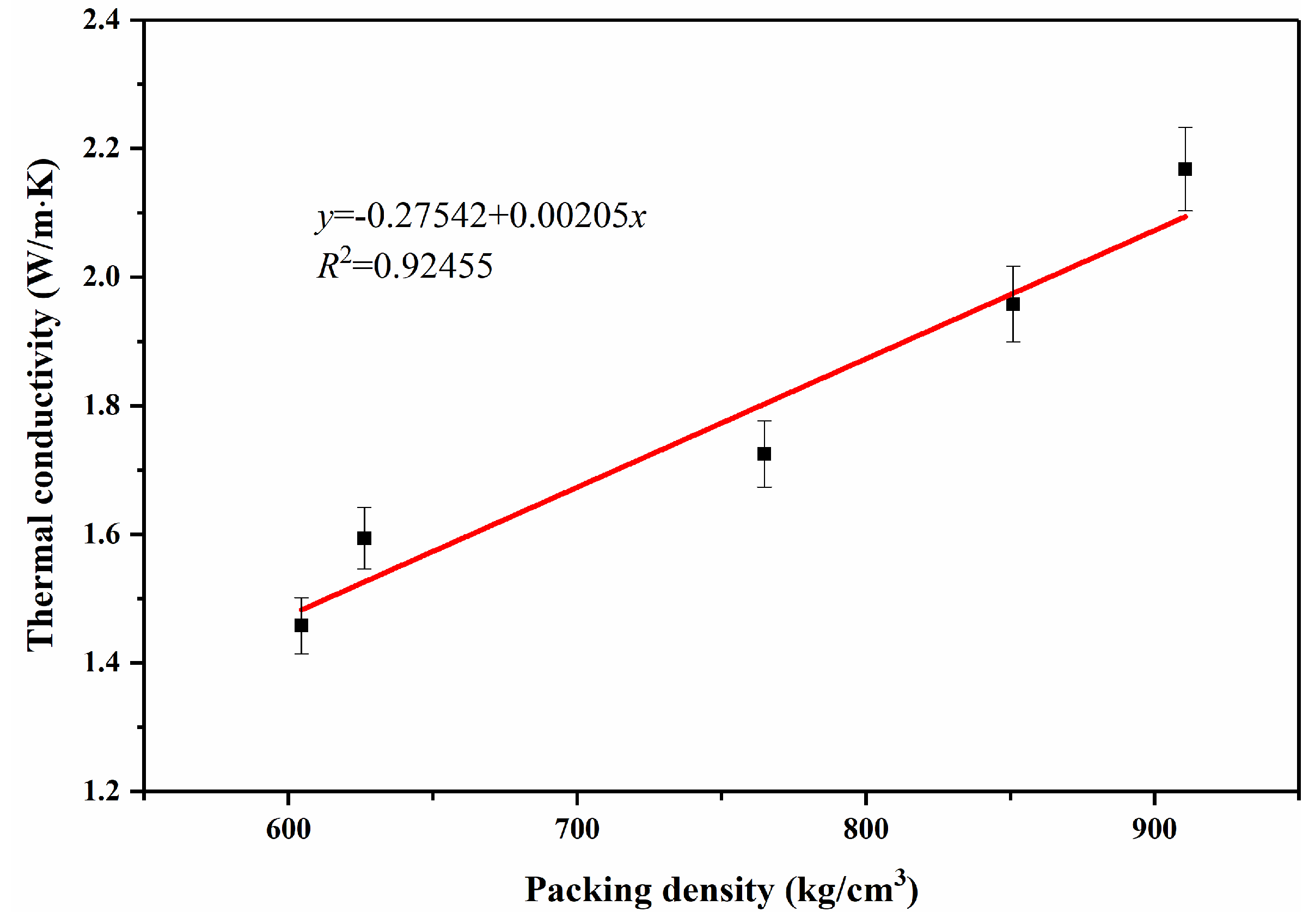
| PCM | Mass (g) | Thickness (mm) | Diameter (mm) | Density (kg/m3) | Thermal Conductivity (W/m·K) |
| 1.6654 | 3.92 | 29.92 | 604.42±0.03 | 1.46 ± 0.04 | |
| 1.4601 | 3.33 | 29.88 | 625.26±0.03 | 1.59 ± 0.05 | |
| 2.1612 | 4.01 | 29.96 | 764.75±0.03 | 1.73 ± 0.05 | |
| 2.2549 | 4.22 | 28.28 | 850.90±0.02 | 1.96 ± 0.06 | |
| 2.0588 | 3.23 | 29.86 | 910.65±0.02 | 2.17 ± 0.07 |
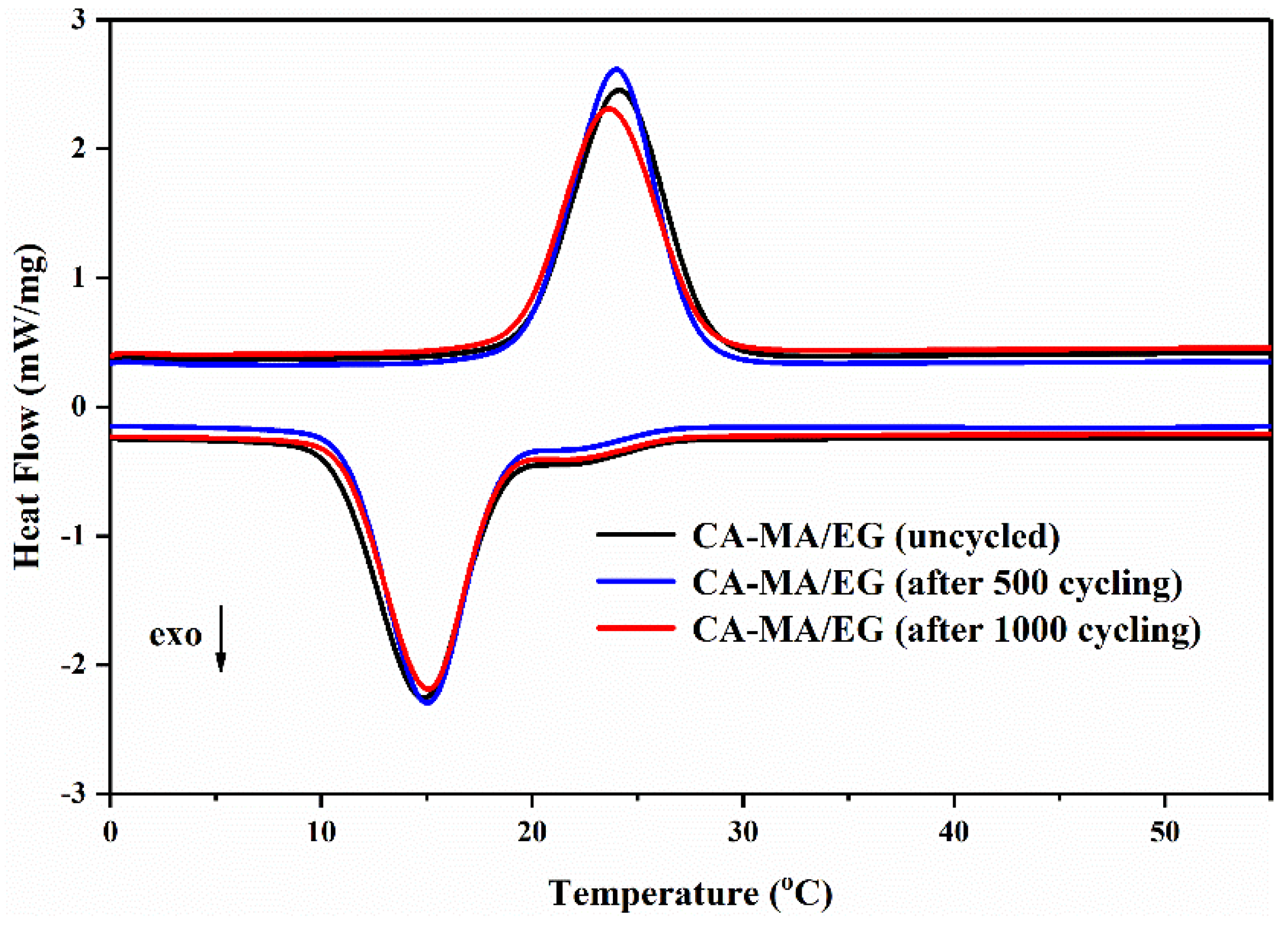
| Number of Thermal Cycling | Melting | Freezing | ||
|---|---|---|---|---|
| Phase Change Temperature (°C) | Phase Change Latent Heat (J/g) | Phase Change Temperature (°C) | Phase Change Latent Heat (J/g) | |
| 0 | 19.78 ± 0.05 | 137.3 ± 0.1 | 18.85 ± 0.05 | 139.8 ± 0.1 |
| 500 | 19.62 ± 0.05 | 134.5 ± 0.1 | 18.73 ± 0.05 | 138.1 ± 0.1 |
| 1000 | 19.42 ± 0.05 | 134.5 ± 0.1 | 18.64 ± 0.05 | 128.7 ± 0.1 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, D.; Yuan, J.; Zhou, Y.; Liu, Y. Preparation and Properties of Capric–Myristic Acid/Expanded Graphite Composite Phase Change Materials for Latent Heat Thermal Energy Storage. Energies 2020, 13, 2462. https://doi.org/10.3390/en13102462
Zhou D, Yuan J, Zhou Y, Liu Y. Preparation and Properties of Capric–Myristic Acid/Expanded Graphite Composite Phase Change Materials for Latent Heat Thermal Energy Storage. Energies. 2020; 13(10):2462. https://doi.org/10.3390/en13102462
Chicago/Turabian StyleZhou, Dongyi, Jiawei Yuan, Yuhong Zhou, and Yicai Liu. 2020. "Preparation and Properties of Capric–Myristic Acid/Expanded Graphite Composite Phase Change Materials for Latent Heat Thermal Energy Storage" Energies 13, no. 10: 2462. https://doi.org/10.3390/en13102462
APA StyleZhou, D., Yuan, J., Zhou, Y., & Liu, Y. (2020). Preparation and Properties of Capric–Myristic Acid/Expanded Graphite Composite Phase Change Materials for Latent Heat Thermal Energy Storage. Energies, 13(10), 2462. https://doi.org/10.3390/en13102462





