“Paper Dye-Sensitized Solar Cell” Based on Carbon-Nanotube-Composite Papers
Abstract
1. Introduction
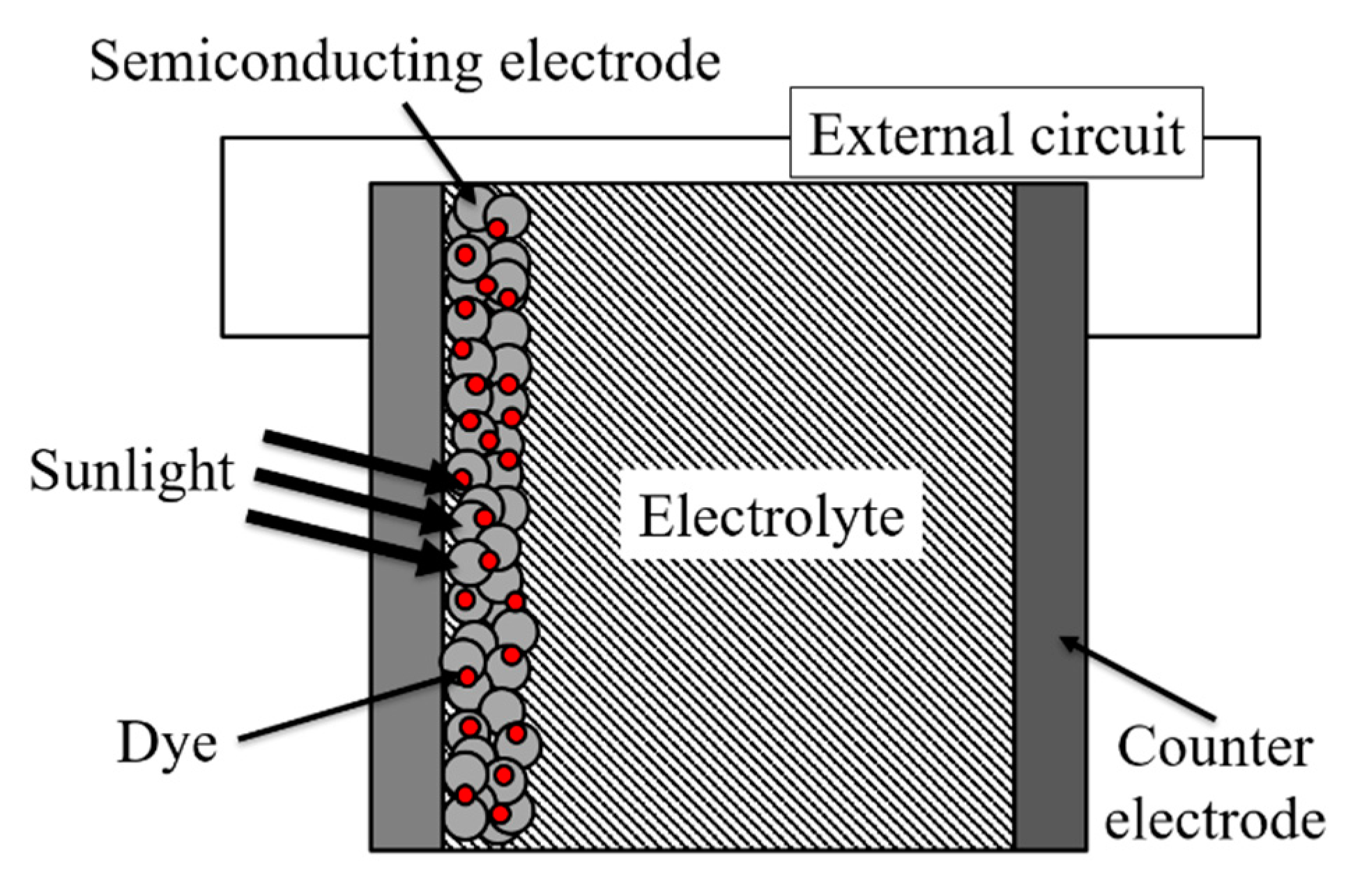
2. Dye-Sensitized Solar Cell
2.1. Principle of Dye-Sensitized Solar Cell
2.2. Evaluation Method for Dye-Sensitized Solar Cell
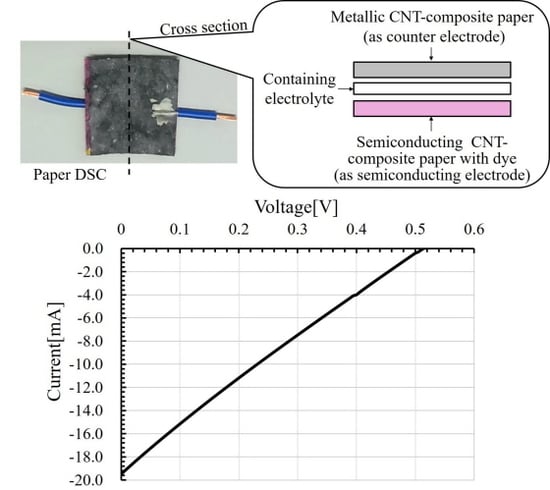
3. Experimental Method and Results
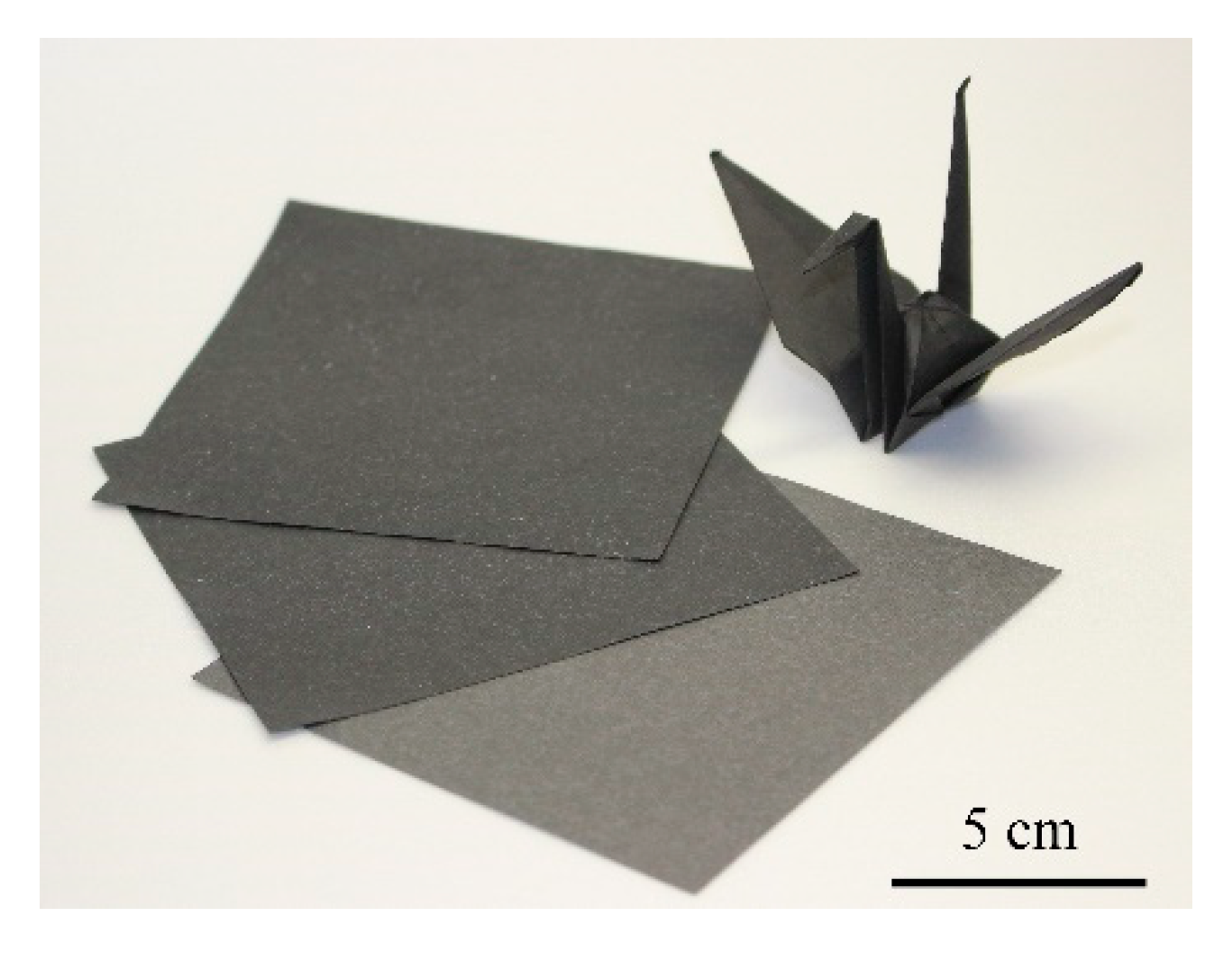
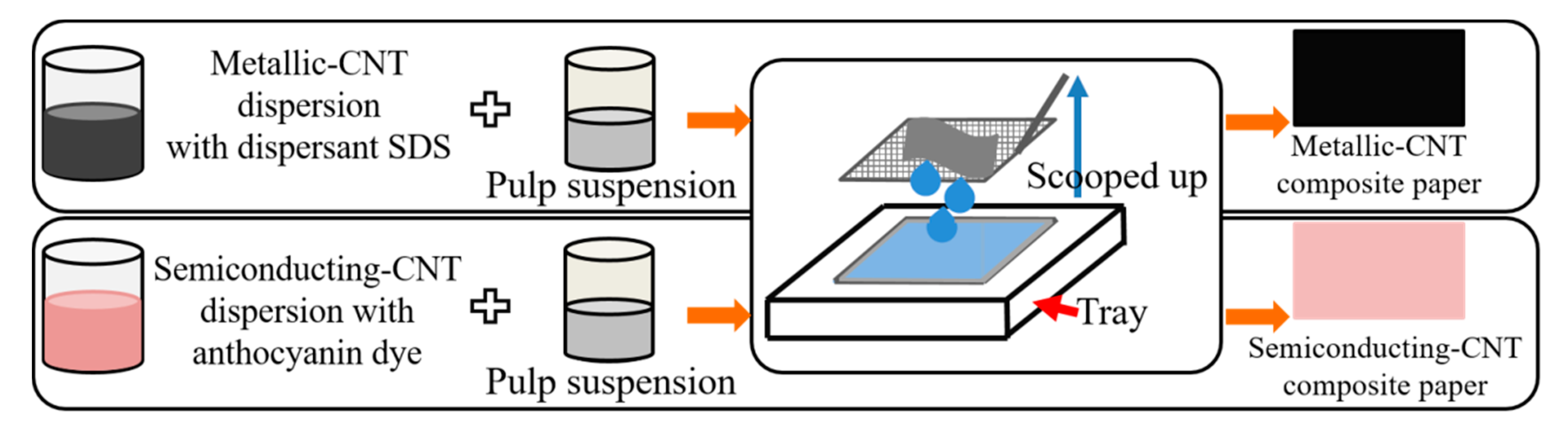
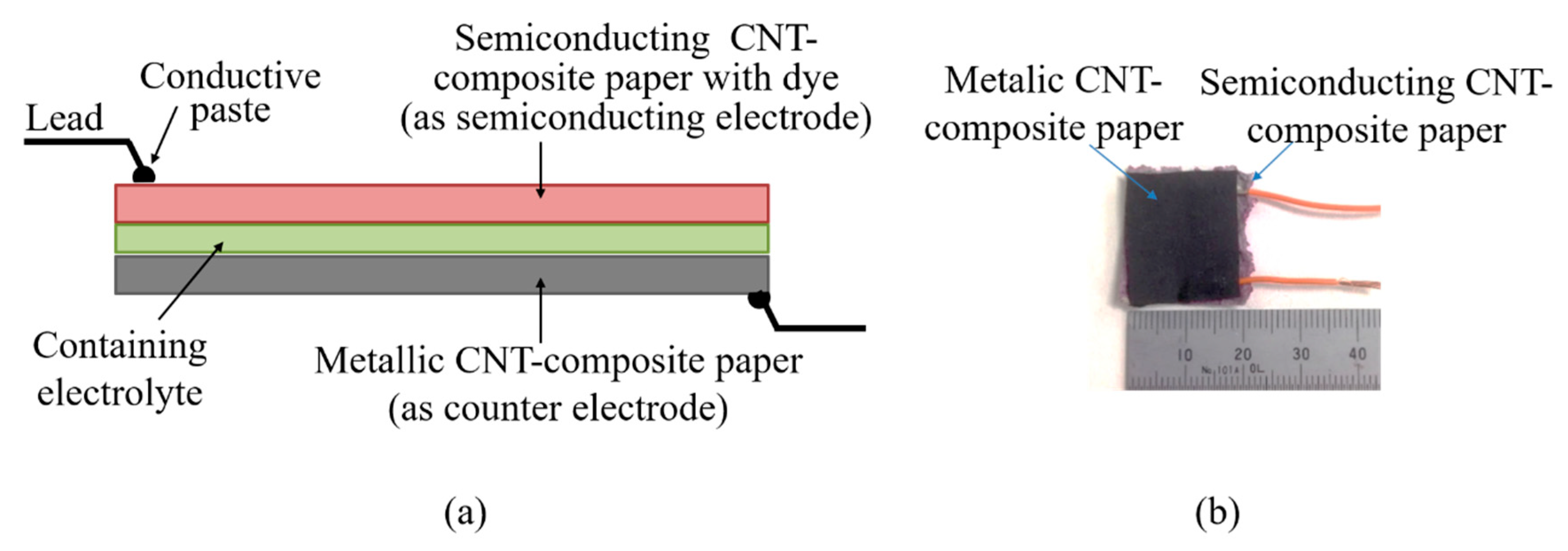
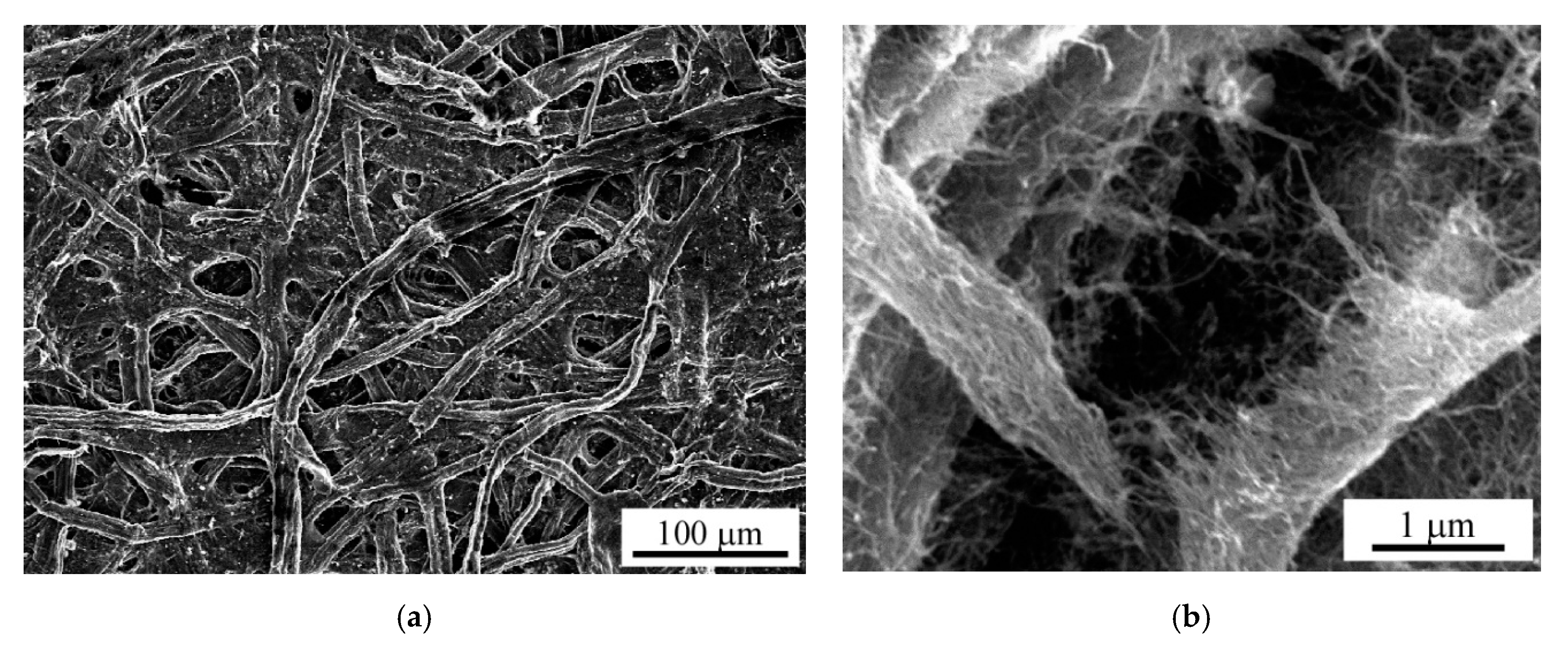
3.1. Method for Preparing Carbon Nanotube-Composite Papers
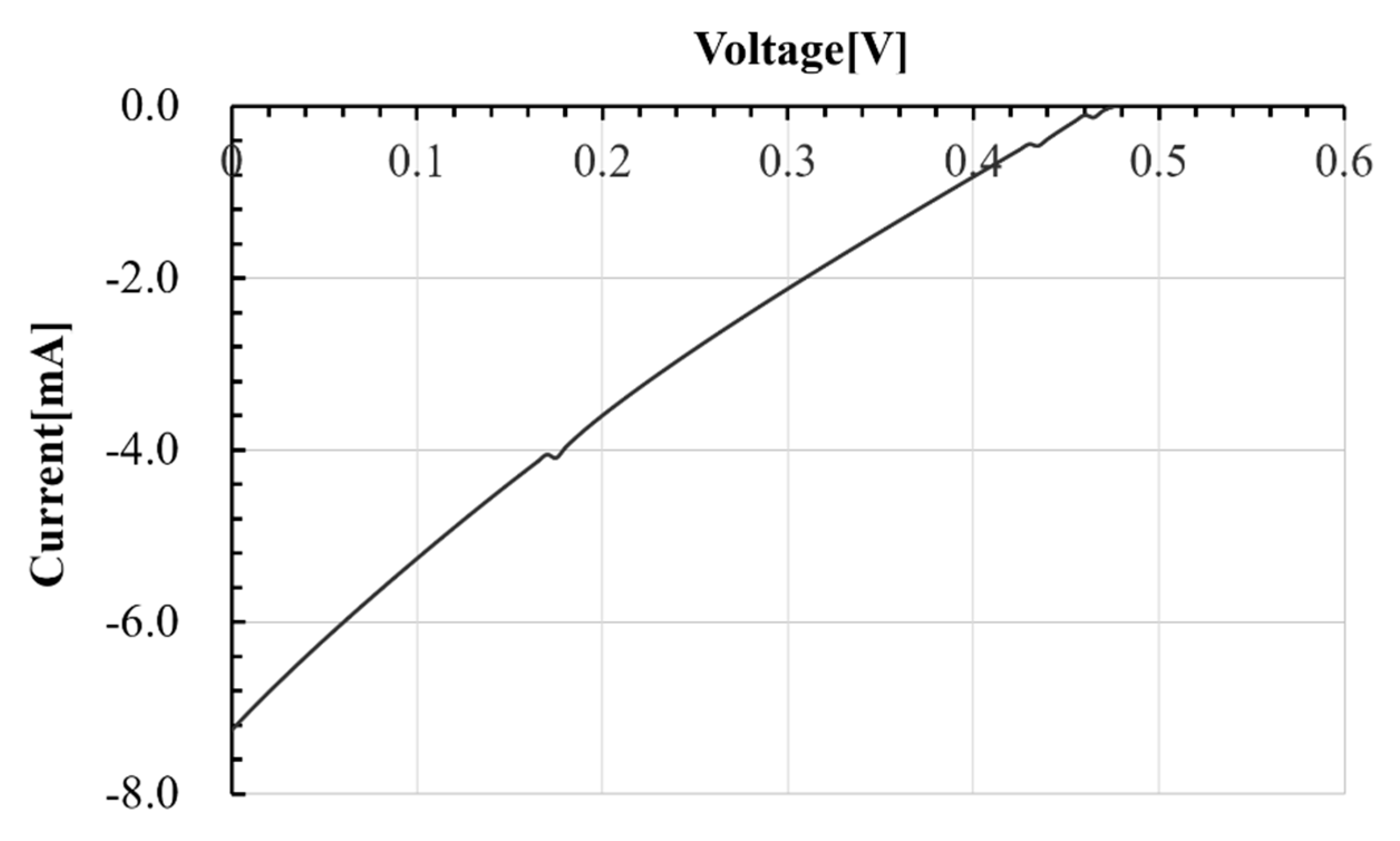
3.2. Method of Fabricating Original Dye-Sensitized Solar Cell and Its Evaluation
3.3. Approach to Improving Conversion Efficiency and Fill Factor of Our Paper Dye-Sensitized Solar Cell
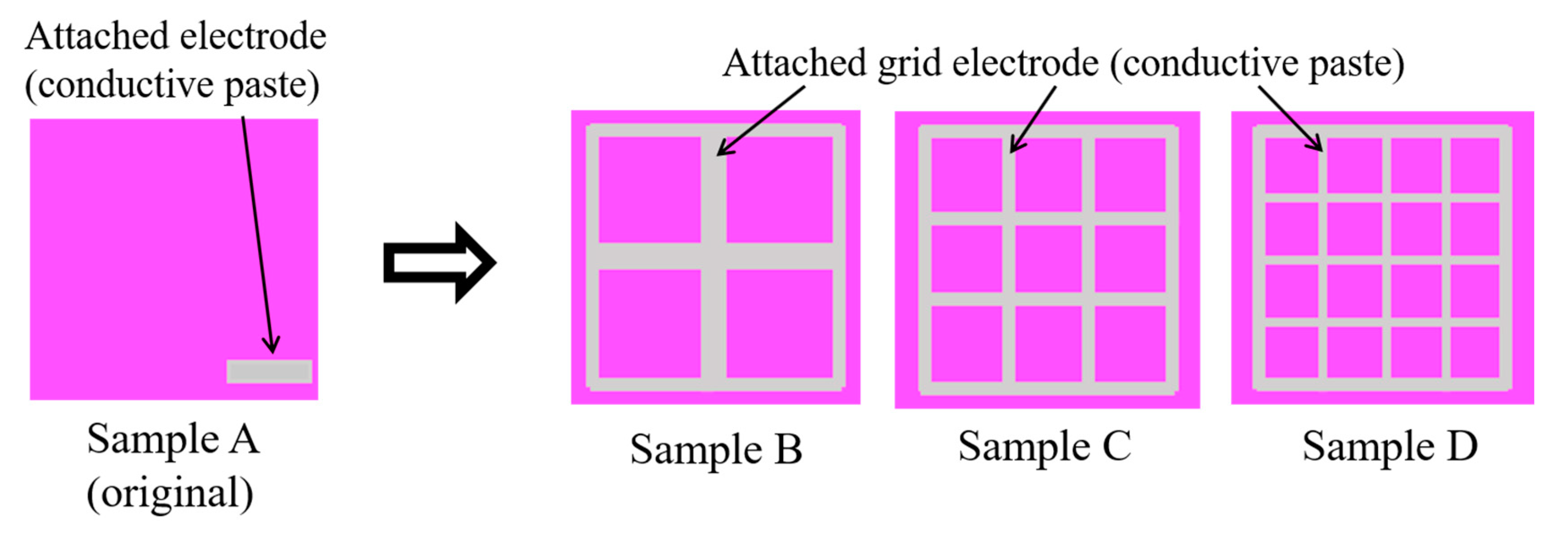
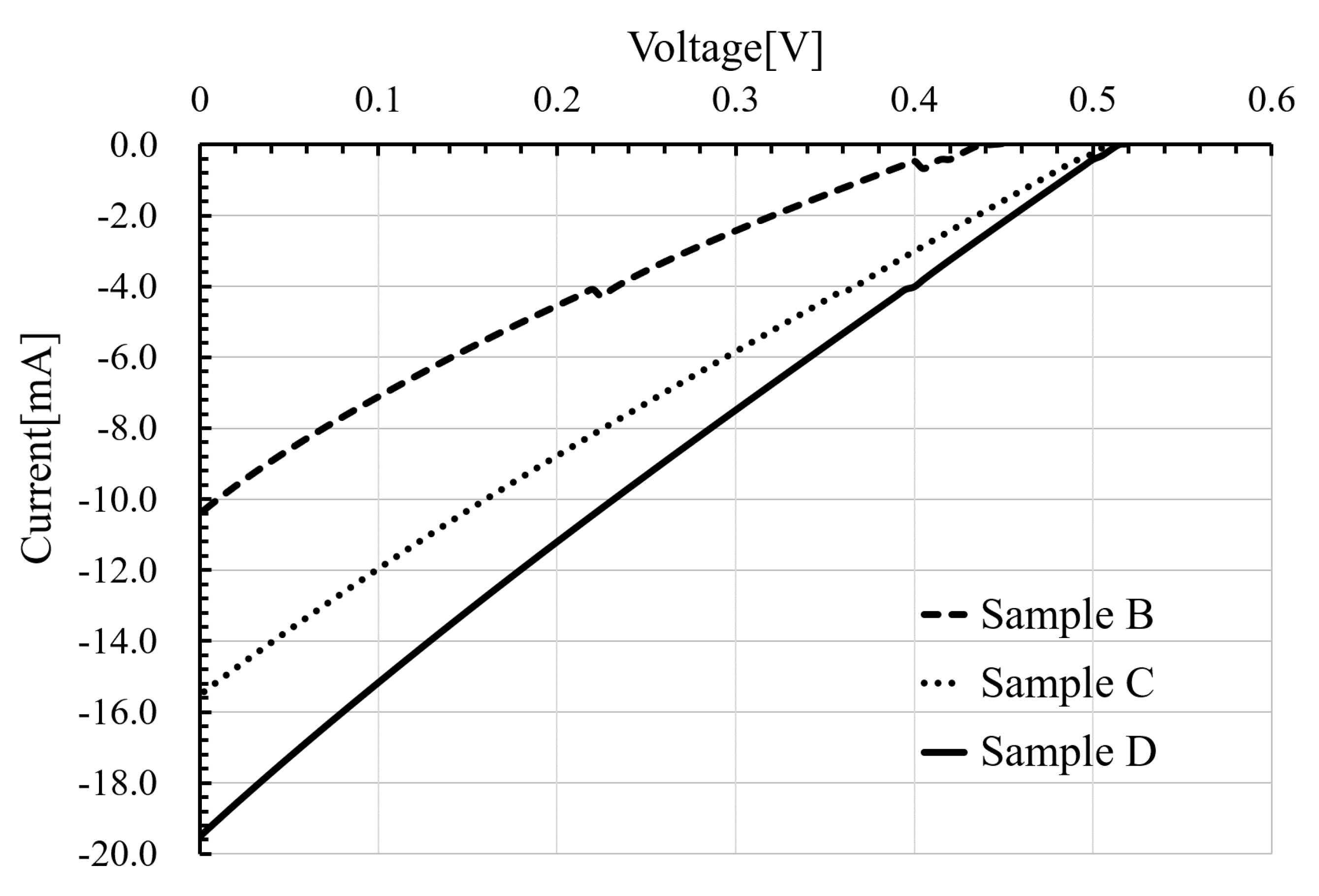
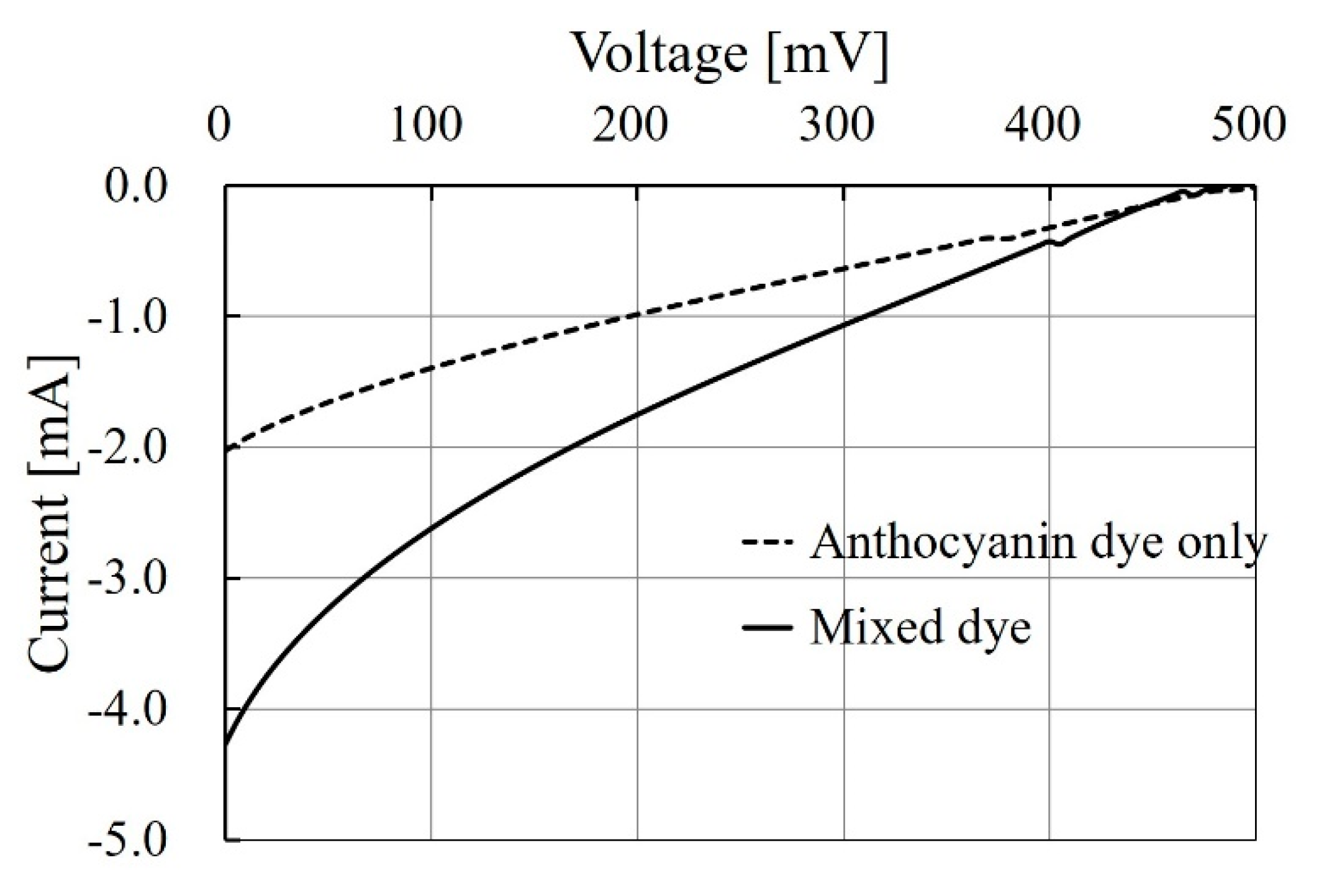
3.3.1. Changing Shape of Attached Electrodes
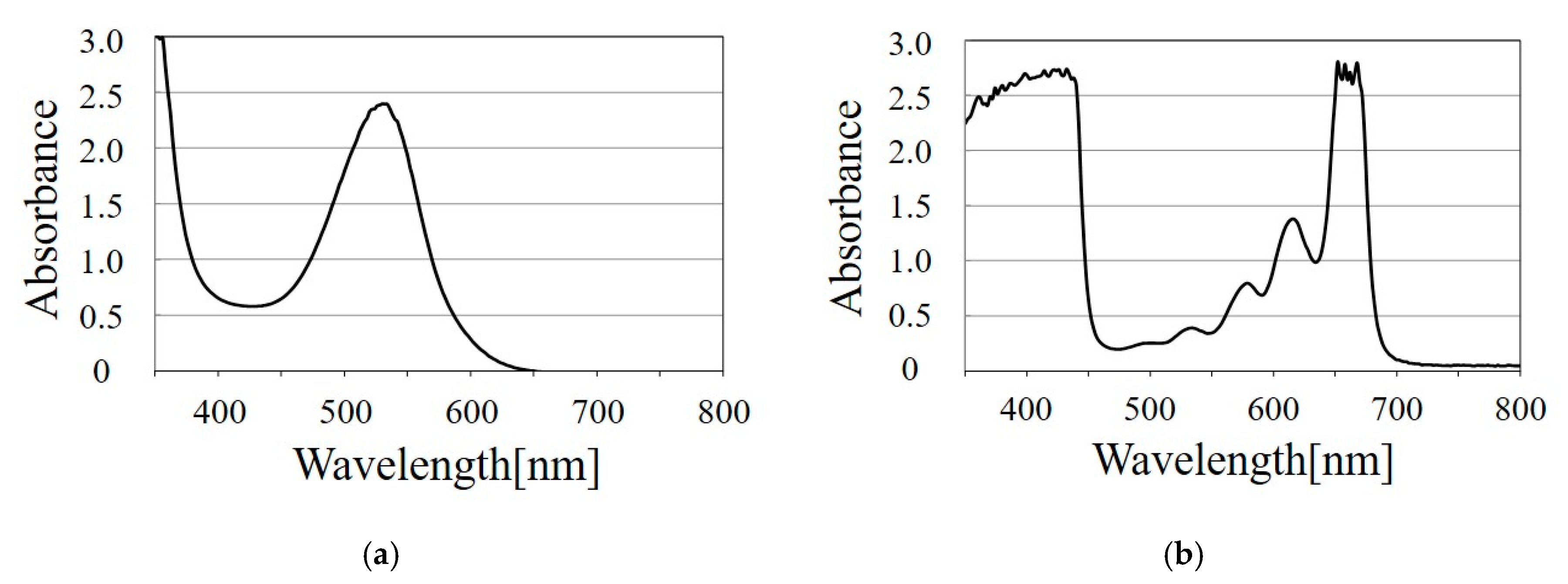
3.3.2. Using More than Two Dyes
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- O’Regan, B.; Grätzel, M. A low-cost, high-efficiency solar cell based on dye-sensitized colloidal TiO2 films. Nature 1991, 353, 737–740. [Google Scholar] [CrossRef]
- Grätzel, M. Dye-sensitized solar cells. J. Photochem. Photobiol. Photochem. Rev. C 2003, 4, 145–153. [Google Scholar] [CrossRef]
- García-Salinas, M.J.; Ariza, M.J. Optimizing a simple natural dye production method for dye-sensitized solar cells: Examples for betalain (bougainvillea and beetroot extracts) and anthocyanin dyes. Appl. Sci. 2019, 9, 2515. [Google Scholar] [CrossRef]
- Cari, C.; Khairuddin; Septiawan, T.Y.; Suciatmoko, P.M.; Kurniawan, D.; Supriyanto, A. The preparation of natural dye for dye-sensitized solar cell (DSSC). AIP Conf. Proc. 2018, 2014, 020106. [Google Scholar] [CrossRef]
- Sathyajothi, S.; Jayavel, R.; Dhanemozhi, A.C. The fabrication of natural dye sensitized solar cell (Dssc) based on TiO2 using henna and beetroot dye extracts. Mater. Today Proc. 2017, 4, 668–676. [Google Scholar] [CrossRef]
- Tahir, D.; Satriani, W.; Gareso, P.L.; Abdullah, B. Dye sensitized solar cell (DSSC) with natural dyes extracted from Jatropha leaves and purple Chrysanthemum flowers as sensitizer. J. Phys. Conf. Ser. 2018, 979, 012056. [Google Scholar] [CrossRef]
- Xin, X.; He, M.; Han, W.; Jung, J.; Lin, Z. Low-cost Copper Zinc Tin Sulfide counter electrodes for high-efficiency dye-sensitized solar cells. Angew. Chem. Int. Ed. 2011, 50, 11739–11742. [Google Scholar] [CrossRef]
- Matsunaga, Y.; Oya, T. Development of paint-type dye-sensitized solar cell using carbon nanotube paint. J. Nanotechnol. 2019, 2019, 5081034. [Google Scholar] [CrossRef]
- Iijima, S. Helical microtubules of graphitic carbon. Nature 1991, 354, 56–58. [Google Scholar] [CrossRef]
- Oya, T.; Ogino, T. Production of electrically conductive paper by adding carbon nanotubes. Carbon 2008, 46, 169–171. [Google Scholar] [CrossRef]
- Iijima, R.; Oya, T. Aiming to improve gate controllability of paper transistors using carbon-nanotube-composite papers by using ionic liquid. In Proceedings of the 45th International Conference on Micro & Nano Engineering (MNE 2019), Rhodes, Greece, 23–26 September 2019. [Google Scholar]
- Kawata, K.; Oya, T. Development and evaluation of “thermoelectric power generating paper” using carbon nanotube-composite paper. Jpn. J. Appl. Phys. 2017, 56, 06GE10. [Google Scholar] [CrossRef]
- Abdou, E.M.; Hafez, H.S.; Bakir, E.; Abdel-Mottaleb, M.S.A. Photostability of low cost dye-sensitized solar cells based on natural and synthetic dyes. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2013, 115, 202–207. [Google Scholar] [CrossRef] [PubMed]
- Romero, H.E.; Sumanasekera, G.U.; Mahan, G.D.; Eklund, P.C. Thermoelectric power of single-walled carbon nanotube films. Phys. Rev. B 2002, 65, 205410. [Google Scholar] [CrossRef]
- Fan, W.; Chu, Z.Z.; Wang, F.Z.; Zhang, C.; Chen, L.; Tang, Y.W.; Zou, D.C. Wire-shaped flexible dye-sensitized solar sells. Adv. Mater. 2008, 20, 592–595. [Google Scholar] [CrossRef]
- Fan, X.; Wang, F.; Chu, Z.; Chen, L.; Zhang, C.; Zou, D. Conduct. Mesh Based Flex. Dye Sensitized Sol. Cells. Appl. Phys. Lett. 2007, 90, 073501. [Google Scholar] [CrossRef]
- Chang, H.; Cho, K.-C.; Chen, T.-L.; Chu, K.-H.; Jiang, L.-J. Preparation and characterization of anthocyanin dye and counter electrode thin film with carbon nanotubes for dye-sensitized solar cells. Mater. Trans. 2011, 52, 1977–1982. [Google Scholar] [CrossRef]
- Iguchi, K.; Sugiyama, S.; Oya, T. Development of “paper dye sensitized solar cell” using carbon-nanotube-composite paper with mixed dyes. In Proceedings of the 9th International Symposium on Atomic Level Characterizations for New Materials and Devices 13 (ALC 13), Hawaii, HI, USA, 2–6 December 2013. [Google Scholar]
- Ito, S.; Murakami, T.; Comte, P.; Liska, P.; Grätzel, C.; Nazeeruddin, M.K.; Grätzel, M. Fabrication of thin film dye sensitized solar cells with solar to electric power conversion efficiency over 10%. Thin Solid Film 2008, 516, 4613–4619. [Google Scholar] [CrossRef]
- Chiba, Y.; Islam, A.; Watanabe, Y.; Komiya, R.; Koide, N.; Han, L. Dye-sensitized solar cells with conversion efficiency of 11.1%. Jpn. J. Appl. Phys. 2006, 45, L638–L640. [Google Scholar] [CrossRef]
- Mathew, S.; Yella, A.; Gao, P.; Humphry-Baker, R.; Curchod, B.F.E.; Ashari-Astani, N.; Tavernelli, I.; Rothlisberger, U.; Nazeeruddin, M.K.; Grätzel, M. Dye-sensitized solar cells with 13% efficiency achieved through the molecular engineering of porphyrin sensitizers. Nat. Chem. 2014, 6, 242–247. [Google Scholar] [CrossRef]
- Zheng, M.; Jagota, A.; Strano, M.S.; Santos, A.P.; Barone, P.; Chou, S.G.; Diner, B.A.; Dresselhaus, M.S.; Mclean, R.S.; Onoa, G.B.; et al. Structure-based carbon nanotube sorting by sequence-dependent DNA assembly. Science 2003, 302, 1545–1548. [Google Scholar] [CrossRef]
- Nihei, M.; Kawabata, A.; Kondo, D.; Horibe, M.; Sato, S.; Awano, Y. Electrical properties of carbon nanotube bundles for future via interconnects. Jpn. J. Appl. Phys. 2005, 44, 1626–1628. [Google Scholar] [CrossRef]
- Hung, N.T.; Nugraha, A.R.; Hasdeo, E.H.; Dresselhaus, M.S.; Saito, R. Diameter dependence of thermoelectric power of semiconducting carbon nanotubes. Phys. Rev. B 2015, 92, 165426. [Google Scholar] [CrossRef]
- Oshima, Y.; Kitamura, Y.; Maniwa, Y.; Yanagi, K. Fabrication of thermoelectric devices using precisely Fermi level-tuned semiconducting single-wall carbon nanotubes. Appl. Phys. Lett. 2015, 107, 043106. [Google Scholar] [CrossRef]
- Fugetsu, B.; Sano, E.; Sunada, M.; Sambongi, Y.; Shibuya, T.; Wang, X.; Hiraki, T. Electrical conductivity and electromagnetic interference shielding efficiency of carbon nanoube/cellulose composite paper. Carbon 2008, 46, 1256–1258. [Google Scholar] [CrossRef]
- Nakamura, G.; Narimatsu, K.; Niidome, T.; Nakashima, N. Green tea solution individually solubilizes single-walled carbon nanotubes. Chem. Lett. 2007, 36, 1140–1141. [Google Scholar] [CrossRef]
- Fujitsuka, Y.; Oya, T. Fabrication of Aligned-carbon-nanotube-composite paper with high and anisotropic conductivity. J. Nanotechnol. 2012, 2012, 819281. [Google Scholar] [CrossRef]
- Chang, H.; Lo, Y.J. Pomegranate leaves and mulberry fruit as natural sensitizers for dye-sensitized solar cells. Sol. Energy 2010, 84, 1833–1837. [Google Scholar] [CrossRef]
- Giusti, M.M.; Wrolstad, R.E. Characterization and measurement of Anthocyanins by UV-visible spectroscopy. In Current Protocols in Food Analytical Chemistry; Giusti, M.M., Wrolstad, R.E., Eds.; John Wiley and Sons: Hoboken, NJ, USA, 2001; pp. 1–13. [Google Scholar]
- Harborne, J.B. Spectral methods of characterizing anthocyanins. Biochem. J. 1958, 70, 22–28. [Google Scholar] [CrossRef]
- Khwanchit, W.; Meeyoo, V.; Chavadej, S. Dye-sensitized solar cell using natural dyes extracted from rosella and blue pea flowers. Sol. Energy Mater. Sol. Cells 2007, 91, 566–571. [Google Scholar] [CrossRef]
- Ozuomba, J.O.; Okoli, L.U.; Ekpunobi, A.J. The performance and stability of anthocyanin local dye as a photosensitizer for DSSCs. Adv. Appl. Sci. Res. 2013, 4, 60–69. [Google Scholar]
- Zhan, X.; Tan, A.; Domercq, B.; An, Z.; Zhang, X.; Barlow, S.; Li, Y.; Zhu, D.; Kippelen, B.; Marder, S.R. A high-mobility electron-transport polymer with broad absorption and its use in field-effect transistors and all-polymer solar cells. J. Am. Chem. Soc. 2007, 129, 7246–7247. [Google Scholar] [CrossRef] [PubMed]
- Goncalves, L.M.; de Bermudez, V.Z.; Ribeiro, H.A.; Mendes, A.M. Dye-sensitized solar cells: A safe bet for the future. Energy Environ. Sci. 2008, 1, 655–667. [Google Scholar] [CrossRef]
- Wang, P.; Zakeeruddin, S.M.; Moser, J.E.; Nazeeruddin, M.K.; Sekiguchi, T.; Grätzel, M. A stable quasi-solid-state dye-sensitized solar cell with an amphiphilic ruthenium sensitizer and polymer gel electrolyte. Nat. Mater. 2003, 2, 402–407. [Google Scholar] [CrossRef] [PubMed]
- Kubo, W.; Kambe, S.; Nakade, S.; Kitamura, T.; Hanabusa, K.; Wada, Y.; Yanagida, S. Photocurrent-determining processes in quasi-solid-state dye-sensitized solar cells using ionic gel electrolytes. J. Phys. Chem. B 2003, 107, 4374–4381. [Google Scholar] [CrossRef]
- Kubo, W.; Murakoshi, K.; Kitamura, T.; Yoshida, S.; Haruki, M.; Hanabusa, K.; Shirai, H.; Wada, Y.; Yanagida, S. Quasi-solid-state dye-sensitized TiO2 solar cells: effective charge transport in mesoporous space filled with gel electrolytes containing Iodide and Iodine. J. Phys. Chem. B 2001, 105, 12809–12815. [Google Scholar] [CrossRef]
- Salvador, G.P.; Pugliese, D.; Bella, F.; Chiappone, A.; Sacco, A.; Bianco, S.; Quaglio, M. New insights in long-term photovoltaic performance characterization of cellulose-based gel electrolytes for stable dye-sensitized solar cells. Electrochim. Acta 2014, 146, 44–51. [Google Scholar] [CrossRef]
- Ragoussi, M.-E.; Ince, M.; Torres, T. Recent advances in Phthalocyanine-based sensitizers for dye-sensitized solar cells. Eur. J. Org. Chem. 2013, 29, 6475–6489. [Google Scholar] [CrossRef]
- Wanga, Z.-S.; Kawauchi, H.; Kashima, T.; Arakawa, H. Significant influence of TiO2 photoelectrode morphology on the energy conversion efficiency of N719 dye-sensitized solar cell. Coord. Chem. Rev. 2004, 248, 1381–1389. [Google Scholar] [CrossRef]
- Chen, C.Y.; Wu, S.J.; Wu, C.G.; Chen, J.G.; Ho, K.C. A Ruthenium complex with superhigh light-harvesting capacity for dye-sensitized solar cells. Angew. Chem. 2006, 118, 5954–5957. [Google Scholar] [CrossRef]


| Sample A | New Samples with Different Grid Patterns | |||
|---|---|---|---|---|
| B | C | D | ||
| Short circuit current Isc (mA) | 7.26 | 10.41 | 15.51 | 19.50 |
| Conversion efficiency η (%) | 0.23 | 0.24 | 0.46 | 0.58 |
| I:II | 1:4 | 2:3 | 3:2 | 4:1 | Only I | Only II |
|---|---|---|---|---|---|---|
| Absorption efficiency [%] | 80.0 | 68.4 | 84.0 | 73.1 | 48.3 | 61.1 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ogata, Y.; Iguchi, K.; Oya, T. “Paper Dye-Sensitized Solar Cell” Based on Carbon-Nanotube-Composite Papers. Energies 2020, 13, 57. https://doi.org/10.3390/en13010057
Ogata Y, Iguchi K, Oya T. “Paper Dye-Sensitized Solar Cell” Based on Carbon-Nanotube-Composite Papers. Energies. 2020; 13(1):57. https://doi.org/10.3390/en13010057
Chicago/Turabian StyleOgata, Yuya, Kodai Iguchi, and Takahide Oya. 2020. "“Paper Dye-Sensitized Solar Cell” Based on Carbon-Nanotube-Composite Papers" Energies 13, no. 1: 57. https://doi.org/10.3390/en13010057
APA StyleOgata, Y., Iguchi, K., & Oya, T. (2020). “Paper Dye-Sensitized Solar Cell” Based on Carbon-Nanotube-Composite Papers. Energies, 13(1), 57. https://doi.org/10.3390/en13010057