The Impact of Biodiesel Fuel on Ethanol/Diesel Blends
Abstract
1. Introduction
2. Model
3. Results
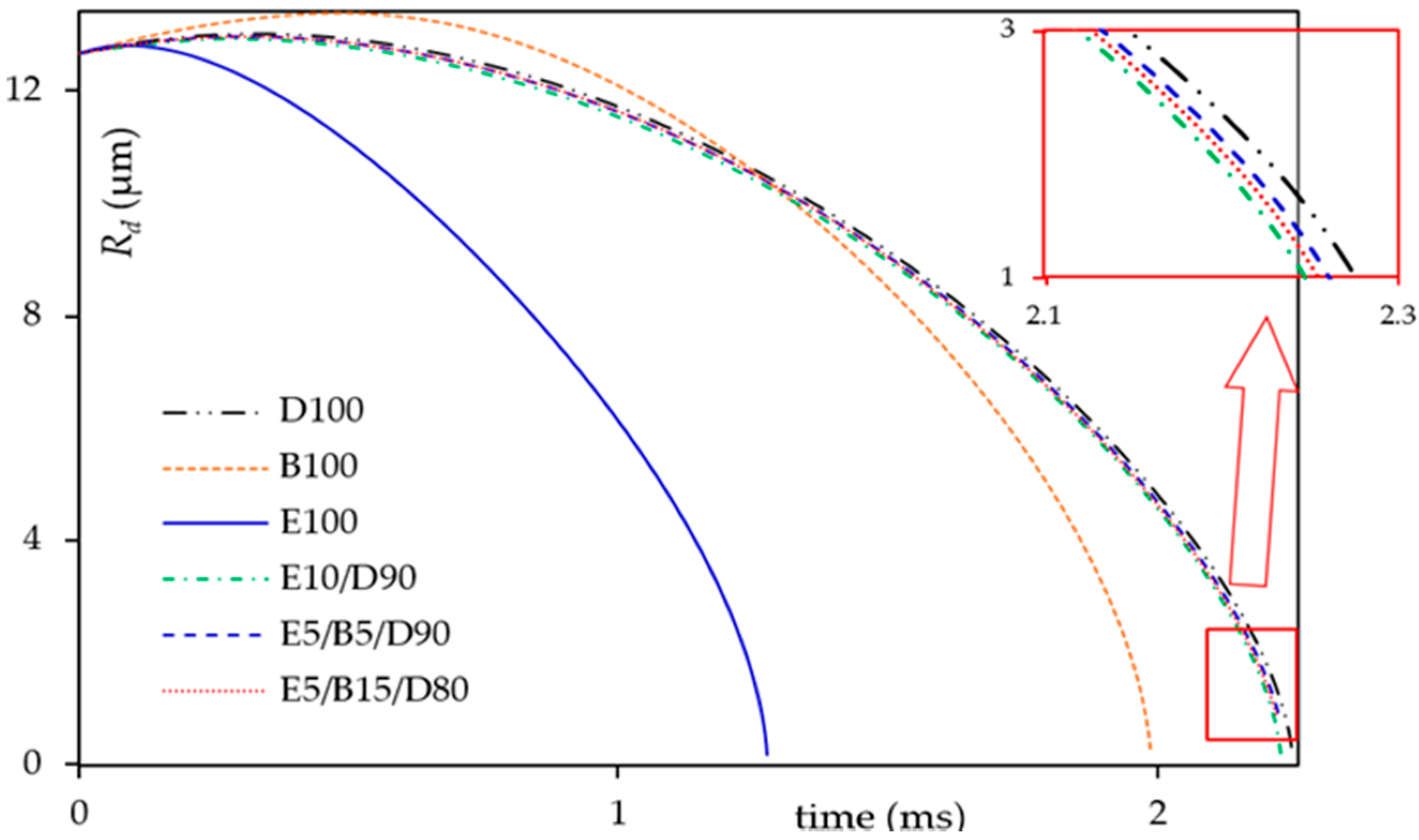
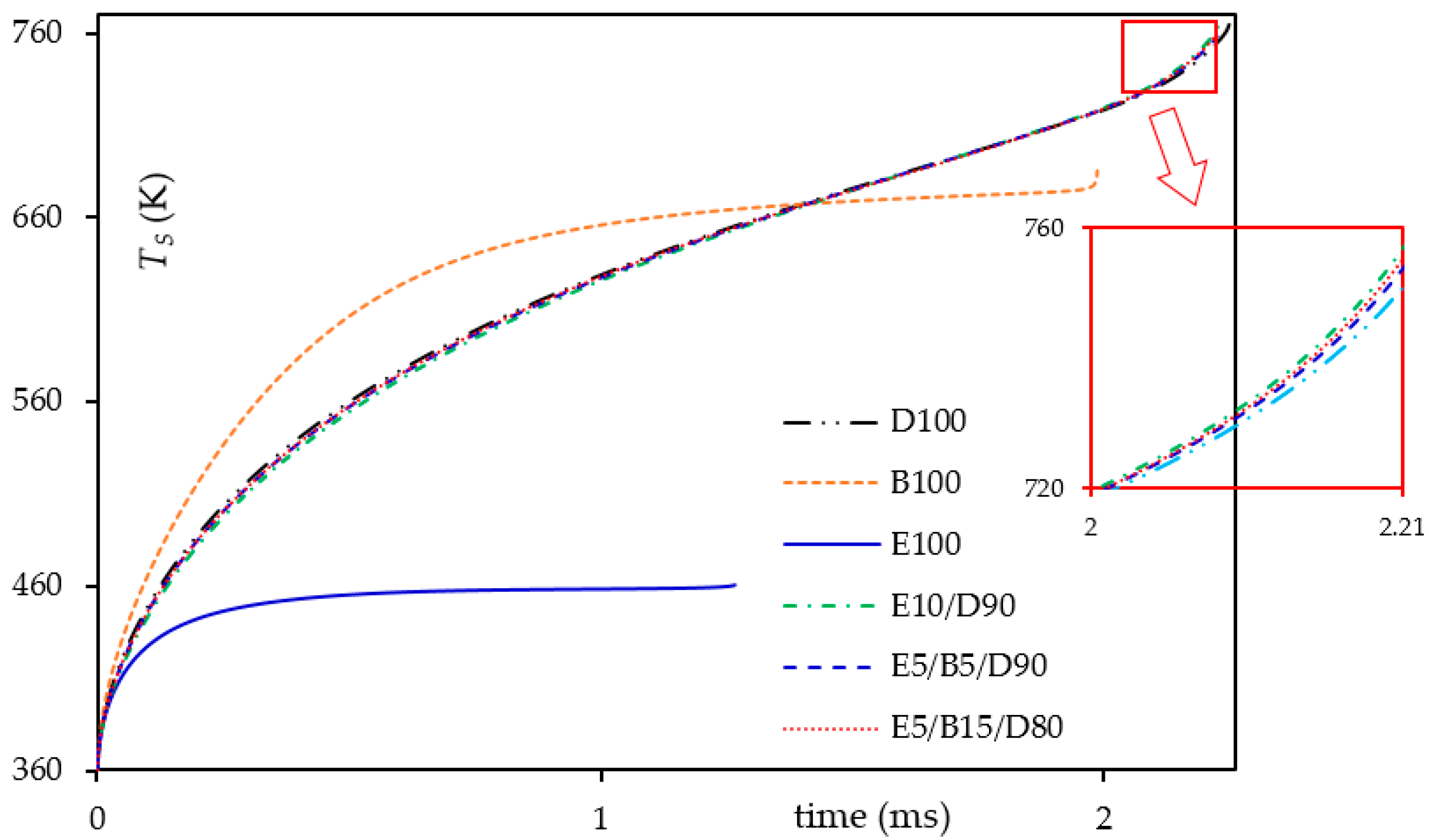
3.1. Heating and Evaporation
3.2. Cetane Number and Viscosity
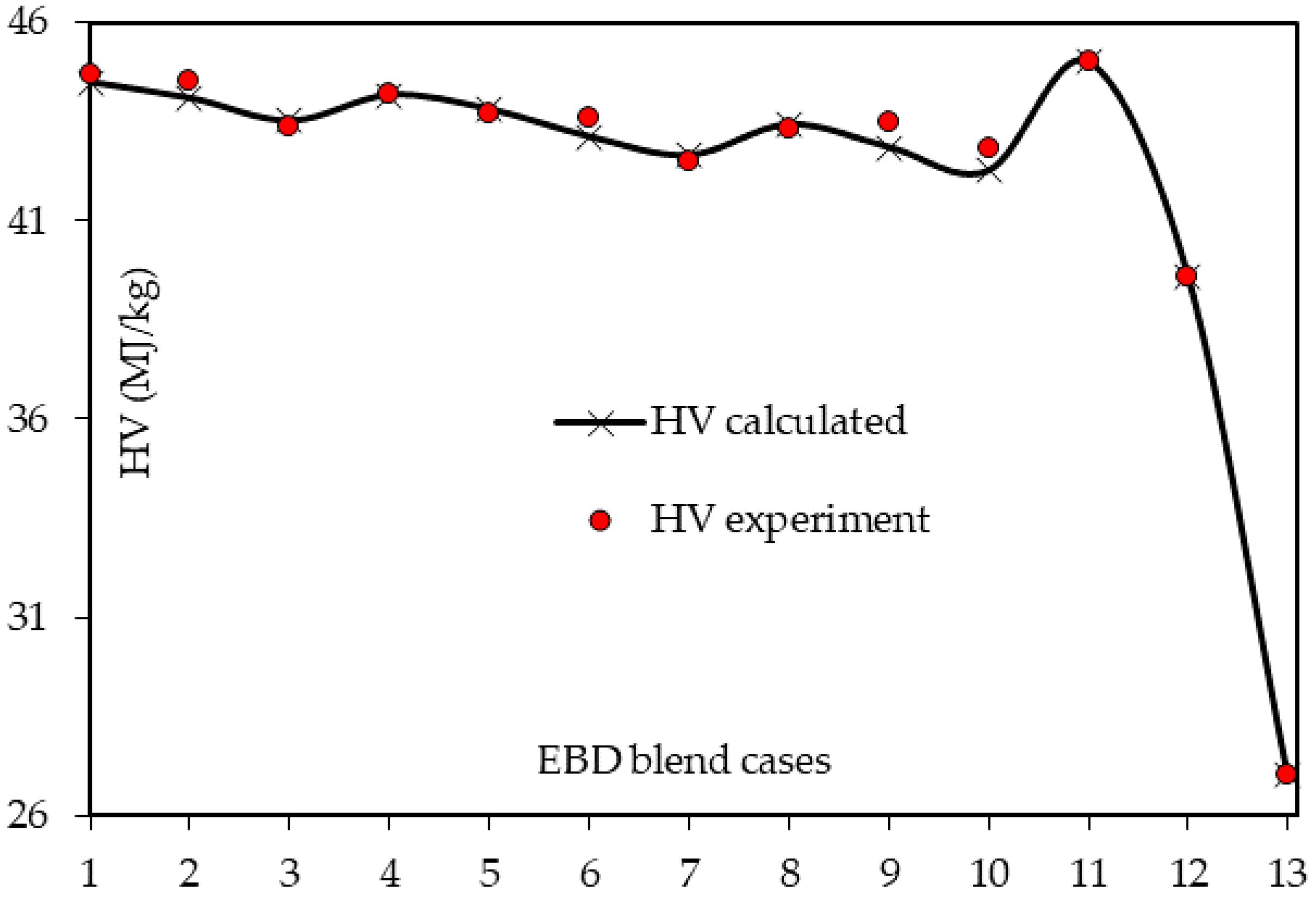
3.3. Heating Value
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
| Name | Group | Group Number | ||
|---|---|---|---|---|
| alkanes | CH3 | 1 | 0.9011 | 0.848 |
| CH2 | 1 | 0.6744 | 0.540 | |
| CH | 1 | 0.4469 | 0.228 | |
| olefins | CH2=CH | 2 | 1.3454 | 1.176 |
| benzenes | ACH | 3 | 0.5313 | 0.400 |
| alkylbenzenes | ACCH3 | 4 | 1.2663 | 0.968 |
| ACCH2 | 4 | 1.0396 | 0.660 | |
| ACCH | 4 | 0.8121 | 0.348 | |
| ethanol | OH | 5 | 1.0000 | 1.200 |
| methyl esters | CH2COO | 11 | 1.6764 | 1.188 |
| Group Number | 2 | 3 | 4 | 5 | 11 | |
|---|---|---|---|---|---|---|
| = 1 | 0.0 | 86.02 | 61.13 | 76.50 | 986.5 | 232.11 |
| 2 | −35.36 | 0.0 | 38.81 | 74.15 | 524.1 | 37.85 |
| 3 | −11.12 | 3.446 | 0.0 | 167.0 | 636.1 | 5.994 |
| 4 | −69.70 | −113.6 | −146.8 | 0.0 | 803.2 | 5688 |
| 5 | 156.4 | 457.0 | 89.6 | 25.82 | 0.0 | 101.1 |
| 11 | 114.8 | 132.1 | 85.84 | −170.0 | 245.4 | 0.0 |
References
- Kalghatgi, G. Is it really the end of internal combustion engines and petroleum in transport? Appl. Energy 2018, 225, 965–974. [Google Scholar] [CrossRef]
- Kalghatgi, G.; Levinsky, H.; Colket, M. Future transportation fuels. Prog. Energy Combust. Sci. 2018, 69, 103–105. [Google Scholar] [CrossRef]
- Ali, O.; Mamat, R.; Najafi, G.; Yusaf, T.; Safieddin Ardebili, S. Optimization of biodiesel-diesel blended fuel properties and engine performance with ether additive using statistical analysis and response surface methods. Energies 2015, 8, 14136–14150. [Google Scholar] [CrossRef]
- Abdul Rahim, N.; Mohd Jaafar, M.; Sapee, S.; Elraheem, H. Effect on particulate and gas emissions by combusting biodiesel blend fuels made from different plant oil feedstocks in a liquid fuel burner. Energies 2016, 9, 659. [Google Scholar] [CrossRef]
- Qasim, M.; Ansari, T.M.; Hussain, M. Combustion, performance, and emission evaluation of a diesel engine with biodiesel like fuel blends derived from a mixture of pakistani waste canola and waste transformer oils. Energies 2017, 10, 1023. [Google Scholar] [CrossRef]
- Jaliliantabar, F.; Ghobadian, B.; Najafi, G.; Yusaf, T. Artificial neural network modeling and sensitivity analysis of performance and emissions in a compression ignition engine using biodiesel fuel. Energies 2018, 11, 2410. [Google Scholar] [CrossRef]
- Department for Transport, UK New Regulations to Double the Use of Sustainable Renewable Fuels by 2020. Available online: https://www.gov.uk/government/news/new-regulations-to-double-the-use-of-sustainable-renewable-fuels-by-2020 (accessed on 22 February 2019).
- Imdadul, H.K.; Masjuki, H.H.; Kalam, M.A.; Zulkifli, N.W.M.; Alabdulkarem, A.; Rashed, M.M.; Teoh, Y.H.; How, H.G. Higher alcohol–biodiesel–diesel blends: An approach for improving the performance, emission, and combustion of a light-duty diesel engine. Energy Convers. Manag. 2016, 111, 174–185. [Google Scholar] [CrossRef]
- Li, L.; Wang, J.; Wang, Z.; Xiao, J. Combustion and emission characteristics of diesel engine fueled with diesel/biodiesel/pentanol fuel blends. Fuel 2015, 156, 211–218. [Google Scholar] [CrossRef]
- Novaes, T.L.C.C.; Henríquez, J.R.; Ochoa, A.A.V. Numerical simulation of the performance of a diesel cycle operating with diesel-biodiesel mixtures. Energy Convers. Manag. 2019, 180, 990–1000. [Google Scholar] [CrossRef]
- Satgé de Caro, P. Interest of combining an additive with diesel–ethanol blends for use in diesel engines. Fuel 2001, 80, 565–574. [Google Scholar] [CrossRef]
- Li, D.; Zhen, H.; Li, X.; Zhang, W.; Yang, J. Physico-chemical properties of ethanol–diesel blend fuel and its effect on performance and emissions of diesel engines. Renew. Energy 2005, 30, 967–976. [Google Scholar] [CrossRef]
- Torres-Jimenez, E.; Jerman, M.S.; Gregorc, A.; Lisec, I.; Dorado, M.P.; Kegl, B. Physical and chemical properties of ethanol–diesel fuel blends. Fuel 2011, 90, 795–802. [Google Scholar] [CrossRef]
- Tutak, W.; Lukács, K.; Szwaja, S.; Bereczky, Á. Alcohol–diesel fuel combustion in the compression ignition engine. Fuel 2015, 154, 196–206. [Google Scholar] [CrossRef]
- He, B.-Q.; Shuai, S.-J.; Wang, J.-X.; He, H. The effect of ethanol blended diesel fuels on emissions from a diesel engine. Atmos. Environ. 2003, 37, 4965–4971. [Google Scholar] [CrossRef]
- Pidol, L.; Lecointe, B.; Starck, L.; Jeuland, N. Ethanol–biodiesel–Diesel fuel blends: Performances and emissions in conventional Diesel and advanced Low Temperature Combustions. Fuel 2012, 93, 329–338. [Google Scholar] [CrossRef]
- Kwanchareon, P.; Luengnaruemitchai, A.; Jai-In, S. Solubility of a diesel–biodiesel–ethanol blend, its fuel properties, and its emission characteristics from diesel engine. Fuel 2007, 86, 1053–1061. [Google Scholar] [CrossRef]
- Kim, H.J.; Park, S.H. Optimization study on exhaust emissions and fuel consumption in a dimethyl ether (DME) fueled diesel engine. Fuel 2016, 182, 541–549. [Google Scholar] [CrossRef]
- Shahir, S.A.; Masjuki, H.H.; Kalam, M.A.; Imran, A.; Fattah, I.M.R.; Sanjid, A. Feasibility of diesel–biodiesel–ethanol/bioethanol blend as existing CI engine fuel: An assessment of properties, material compatibility, safety and combustion. Renew. Sustain. Energy Rev. 2014, 32, 379–395. [Google Scholar] [CrossRef]
- Silveira, M.B.; do Carmo, F.R.; Santiago-Aguiar, R.S.; de Sant’Ana, H.B. Ab–diesel: Liquid–liquid equilibrium and volumetric transport properties. Fuel 2014, 119, 292–300. [Google Scholar] [CrossRef]
- Beatrice, C.; Napolitano, P.; Guido, C. Injection parameter optimization by DoE of a light-duty diesel engine fed by Bio-ethanol/RME/diesel blend. Appl. Energy 2014, 113, 373–384. [Google Scholar] [CrossRef]
- Fang, Q.; Fang, J.; Zhuang, J.; Huang, Z. Effects of ethanol–diesel–biodiesel blends on combustion and emissions in premixed low temperature combustion. Appl. Therm. Eng. 2013, 54, 541–548. [Google Scholar] [CrossRef]
- Aydın, F.; Öğüt, H. Effects of using ethanol-biodiesel-diesel fuel in single cylinder diesel engine to engine performance and emissions. Renew. Energy 2017, 103, 688–694. [Google Scholar] [CrossRef]
- Noorollahi, Y.; Azadbakht, M.; Ghobadian, B. The effect of different diesterol (diesel–biodiesel–ethanol) blends on small air-cooled diesel engine performance and its exhaust gases. Energy 2018, 142, 196–200. [Google Scholar] [CrossRef]
- Tse, H.; Leung, C.W.; Cheung, C.S. Investigation on the combustion characteristics and particulate emissions from a diesel engine fueled with diesel-biodiesel-ethanol blends. Energy 2015, 83, 343–350. [Google Scholar] [CrossRef]
- Labeckas, G.; Slavinskas, S.; Mažeika, M. The effect of ethanol–diesel–biodiesel blends on combustion, performance and emissions of a direct injection diesel engine. Energy Convers. Manag. 2014, 79, 698–720. [Google Scholar] [CrossRef]
- Shahir, S.A.; Masjuki, H.H.; Kalam, M.A.; Imran, A.; Ashraful, A.M. Performance and emission assessment of diesel–biodiesel–ethanol/bioethanol blend as a fuel in diesel engines: A review. Renew. Sustain. Energy Rev. 2015, 48, 62–78. [Google Scholar] [CrossRef]
- Mofijur, M.; Rasul, M.G.; Hyde, J.; Azad, A.K.; Mamat, R.; Bhuiya, M.M.K. Role of biofuel and their binary (diesel–biodiesel) and ternary (ethanol–biodiesel–diesel) blends on internal combustion engines emission reduction. Renew. Sustain. Energy Rev. 2016, 53, 265–278. [Google Scholar] [CrossRef]
- Sazhin, S.S. Droplets and Sprays; Springer: London, UK, 2014; ISBN 978-1-4471-6385-5. [Google Scholar]
- Al Qubeissi, M. Predictions of droplet heating and evaporation: An application to biodiesel, diesel, gasoline and blended fuels. Appl. Therm. Eng. 2018, 136, 260–267. [Google Scholar] [CrossRef]
- Sazhin, S.S. Modelling of fuel droplet heating and evaporation: Recent results and unsolved problems. Fuel 2017, 196, 69–101. [Google Scholar] [CrossRef]
- Sazhin, S.S. Advanced models of fuel droplet heating and evaporation. Prog. Energy Combust. Sci. 2006, 32, 162–214. [Google Scholar] [CrossRef]
- Sazhin, S.S.; Elwardany, A.E.; Krutitskii, P.A.; Deprédurand, V.; Castanet, G.; Lemoine, F.; Sazhina, E.M.; Heikal, M.R. Multi-component droplet heating and evaporation: Numerical simulation versus experimental data. Int. J. Therm. Sci. 2011, 50, 1164–1180. [Google Scholar] [CrossRef]
- Elwardany, A.E.; Sazhin, S.S.; Im, H.G. A new formulation of physical surrogates of FACE A gasoline fuel based on heating and evaporation characteristics. Fuel 2016, 176, 56–62. [Google Scholar] [CrossRef]
- Al Qubeissi, M.; Al-Esawi, N.; Sazhin, S.S.; Ghaleeh, M. Ethanol/gasoline droplet heating and evaporation: Effects of fuel blends and ambient conditions. Energy Fuels 2018, 32, 6498–6506. [Google Scholar] [CrossRef]
- Al-Esawi, N.; Al Qubeissi, M.; Whitaker, R.; Sazhin, S.S. Blended E85–Diesel fuel droplet heating and evaporation. Energy Fuels 2019, 33, 2477–2488. [Google Scholar] [CrossRef]
- Al Qubeissi, M.; Sazhin, S.S.; Elwardany, A.E. Modelling of blended Diesel and biodiesel fuel droplet heating and evaporation. Fuel 2017, 187, 349–355. [Google Scholar] [CrossRef]
- Abramzon, B.; Sirignano, W.A. Droplet vaporization model for spray combustion calculations. Int. J. Heat Mass Transf. 1989, 32, 1605–1618. [Google Scholar] [CrossRef]
- Al-Esawi, N.; Al Qubeissi, M.; Sazhin, S.S.; Whitaker, R. The impacts of the activity coefficient on heating and evaporation of ethanol/gasoline fuel blends. Int. Commun. Heat Mass Transf. 2018, 98, 177–182. [Google Scholar] [CrossRef]
- Poling, B.E.; Prausnitz, J.M.; O’Connell, J.P. The Properties of Gases and Liquids; McGraw-Hill: New York, NY, USA, 2001; ISBN 0-07-011682-2. [Google Scholar]
- Sazhin, S.S.; Al Qubeissi, M.; Nasiri, R.; Gun’ko, V.M.; Elwardany, A.E.; Lemoine, F.; Grisch, F.; Heikal, M.R. A multi-dimensional quasi-discrete model for the analysis of Diesel fuel droplet heating and evaporation. Fuel 2014, 129, 238–266. [Google Scholar] [CrossRef]
- Sazhin, S.S.; Al Qubeissi, M.; Kolodnytska, R.; Elwardany, A.E.; Nasiri, R.; Heikal, M.R. Modelling of biodiesel fuel droplet heating and evaporation. Fuel 2014, 115, 559–572. [Google Scholar] [CrossRef]
- Al Qubeissi, M.; Sazhin, S.S.; Crua, C.; Turner, J.; Heikal, M.R. Modelling of biodiesel fuel droplet heating and evaporation: Effects of fuel composition. Fuel 2015, 154, 308–318. [Google Scholar] [CrossRef]
- Pitz, W.J.; Mueller, C.J. Recent progress in the development of diesel surrogate fuels. Prog. Energy Combust. Sci. 2011, 37, 330–350. [Google Scholar] [CrossRef]
- Alptekin, E.; Canakci, M. Characterization of the key fuel properties of methyl ester–diesel fuel blends. Fuel 2009, 88, 75–80. [Google Scholar] [CrossRef]
- Zöldy, M. Ethanol–biodiesel–diesel blends as a diesel extender option on compression ignition engines. Transport 2011, 26, 303–309. [Google Scholar] [CrossRef]
- Ghosh, P.; Jaffe, S.B. Detailed composition-based model for predicting the cetane number of diesel fuels. Ind. Eng. Chem. Res. 2006, 45, 346–351. [Google Scholar] [CrossRef]
- Santana, R.; Do, P.; Santikunaporn, M.; Alvarez, W.; Taylor, J.; Sughrue, E.; Resasco, D. Evaluation of different reaction strategies for the improvement of cetane number in diesel fuels. Fuel 2006, 85, 643–656. [Google Scholar] [CrossRef]
- Qian, Y.; Yu, L.; Li, Z.; Zhang, Y.; Xu, L.; Zhou, Q.; Han, D.; Lu, X. A new methodology for diesel surrogate fuel formulation: Bridging fuel fundamental properties and real engine combustion characteristics. Energy 2018, 148, 424–447. [Google Scholar] [CrossRef]
- Creton, B.; Dartiguelongue, C.; de Bruin, T.; Toulhoat, H. Prediction of the Cetane Number of Diesel Compounds Using the Quantitative Structure Property Relationship. Energy Fuels 2010, 24, 5396–5403. [Google Scholar] [CrossRef]
- Lapuerta, M.; Rodríguez-Fernández, J.; de Mora, E.F. Correlation for the estimation of the cetane number of biodiesel fuels and implications on the iodine number. Energy Policy 2009, 37, 4337–4344. [Google Scholar] [CrossRef]
- Tong, D.; Hu, C.; Jiang, K.; Li, Y. Cetane number prediction of biodiesel from the composition of the fatty acid methyl esters. J. Am. Oil Chem. Soc. 2011, 88, 415–423. [Google Scholar] [CrossRef]
- Shinde, S.; Yadav, S.D. Theoretical properties prediction of diesel-biodiesel-DEE blend as a fuel for C.I. engine with required modifications for optimum performance. Int. J. Curr. Eng. Technol. 2016, 6, 1562–1567. [Google Scholar]
- Grabar, I.G.; Kolodnytska, R.V.; Semenov, V.G. Biofuel Based on Oil for Diesel Engines; ZDTU: Zhytomyr, Ukraine, 2011. (In Ukrainian) [Google Scholar]
| Groups | ||
|---|---|---|
| n-alkanes | 15.94 | 0.5212 |
| iso-alkanes | 31.32 | 7.3717 |
| cycloalkanes | 15.99 | 0.0727 |
| bicycloalkanes | 7.53 | 0.0727 |
| aromatics | 12.84 | 3.1967 |
| tetralines | 10.39 | 3.1967 |
| naphthalenes | 5.97 | 0.0727 |
| EBD vol.% | [53] | [46] |
|---|---|---|
| D100 | 54.5 | 54.5 |
| B100 | 56.4 | 56.4 |
| E100 | 8.0 | 8.0 |
| E10/D90 | 49.8 | 48.6 |
| E5/B5/D90 | 52.3 | 54.4 |
| E5/B15/D90 | 52.5 | 55.0 |
| EBD vol.% | |
|---|---|
| D100 | 3.51 |
| B100 | 3.59 |
| E100 | 0. 81 |
| E10/D90 | 3.27 |
| E5/B5/D90 | 3.46 |
| E5/B15/D80 | 3.44 |
| Sample | %D | %B | %E |
|---|---|---|---|
| 1 | 90 | 10 | 0 |
| 2 | 90 | 5 | 5 |
| 3 | 90 | 0 | 10 |
| 4 | 85 | 15 | 0 |
| 5 | 85 | 10 | 5 |
| 6 | 85 | 5 | 10 |
| 7 | 85 | 0 | 15 |
| 8 | 80 | 15 | 5 |
| 9 | 80 | 10 | 10 |
| 10 | 80 | 5 | 15 |
| 11 | 100 | 0 | 0 |
| 12 | 0 | 100 | 0 |
| 13 | 0 | 0 | 100 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Esawi, N.; Al Qubeissi, M.; Kolodnytska, R. The Impact of Biodiesel Fuel on Ethanol/Diesel Blends. Energies 2019, 12, 1804. https://doi.org/10.3390/en12091804
Al-Esawi N, Al Qubeissi M, Kolodnytska R. The Impact of Biodiesel Fuel on Ethanol/Diesel Blends. Energies. 2019; 12(9):1804. https://doi.org/10.3390/en12091804
Chicago/Turabian StyleAl-Esawi, Nawar, Mansour Al Qubeissi, and Ruslana Kolodnytska. 2019. "The Impact of Biodiesel Fuel on Ethanol/Diesel Blends" Energies 12, no. 9: 1804. https://doi.org/10.3390/en12091804
APA StyleAl-Esawi, N., Al Qubeissi, M., & Kolodnytska, R. (2019). The Impact of Biodiesel Fuel on Ethanol/Diesel Blends. Energies, 12(9), 1804. https://doi.org/10.3390/en12091804






