Abstract
Sugarcane bagasse is the major by-product of the sugarcane industry and, due to its abundant availability, it has been extensively studied for lignocellulosic bioconversion in the production of bioethanol and other value-added commercial products. In the study presented herein, a combined pretreatment using sulfolane, TiO2 and alkali microwave irradiation (MW-A) was assessed for the dissolution of lignin prior to enzymatic saccharification of holocellulose. Total reducing sugars (TRS) and saccharinic acid yields were investigated. The increase in NaOH concentration up to 5% and in temperature from 120 °C to 140 °C were found to have a positive influence on both yields. While increasing the reaction time from 5 to 60 min only led to an increase in TRS yield <2%, a reaction time of 30 min almost doubled the saccharinic acids production. TRS yields and saccharinic acid production were approximately 5% and 33% higher when the sulfolane-TiO2 reaction medium was used, as compared to MW-A in water, reaching up to 64.8% and 15.24 g/L of saccharinic acids, respectively. The proposed MW-A pretreatment may hold promise for industrial applications, given the good TRS yields obtained, and the associated enzyme and time/energy savings. The use of sulfolane-TiO2 reaction medium is encouraged if saccharinic acids are to be recovered too.
1. Introduction
In recent decades, several strategies for the valorization of biomass and its associated waste have been extensively researched for the production of energy and bioproducts. In this context, second-generation (2G) bioethanol from lignocellulosic biomass as a raw material has gained interest in the development and application of sustainable technologies [1].
Lignocellulosic materials are complex structures formed mainly by cellulose, hemicellulose and lignin. For lignocellulosic ethanol production, three steps are required: (i) a pretreatment, which converts the recalcitrant lignocellulosic structure into cellulosic and hemicellulosic intermediates; (ii) an enzymatic hydrolysis, in which enzymes hydrolyze cellulose and hemicellulose polymers to fermentable sugars such as glucose and xylose, respectively; and (iii) a fermentation stage, in which ethanol is produced by microorganisms.
Thus, the goal of the pretreatment is to disrupt the polymeric components of the lignocellulosic matrix, increasing availability of cellulose and hemicellulose so that they can be converted into monosaccharides and bioethanol in subsequent steps. Nonetheless, lignin is a recalcitrant structure that hinders the enzymes accessibility to aforementioned polysaccharides [2]. Hence, one of the most challenging stages in the second-generation ethanol production process is the pretreatment (dissolution of lignin) prior to enzymatic saccharification of holocellulose [3].
A number of different strategies have been assayed to disrupt the macromolecular structure that binds the matrix of cellulose, hemicellulose and lignin [4]. The choice of an appropriate pretreatment—according to the physical and chemical characteristics of the biomass to be converted into ethanol—is essential for the fractionation of lignocellulosic biomass, lignin removal, the reduction of cellulose crystallinity and the increase in material porosity, resulting in an increase in the amount of fermentable sugars released to the medium [5].
Numerous technologies involving physical and chemical pretreatments—applied separately, simultaneously, or in a sequential manner—have been investigated [6]. Physical pretreatments include: mechanical comminution [7], thermal methods (pyrolysis, steam explosion and hydrothermal) [8,9], microwave irradiation [10] and ultrasonic treatment [11]. On the other hand, chemical pretreatments that make use of acidic catalysts [12], alkalis [13], ionic liquids [14] and organosolvs [15] have also been received extensive attention in the literature.
Combined physical-chemical pretreatments have been reported to have a synergistic effect on the dissolution of lignin, improving subsequent enzymatic saccharification [16,17]. In this regard, microwave irradiation combined with chemical catalysts (acids, alkalis, organosolvs and ionic liquids) holds particular promise in comparison with other heating systems [18,19]. The main advantage of microwave irradiation (MWI) pretreatments over those based on conventional heating systems is that a homogeneous distribution of heat in the substrate can be achieved, while the latter generate temperature gradients in the biomass. In the MWI-based approach, the molecular collisions generated by dielectric polarization and rapid heat transfer break the complex structure of lignocellulose, resulting in shorter reaction times and in a higher energy efficiency [7,20].
Apropos of the chemical pretreatments, several studies have reported on the efficiency of orgasolvs for the conversion of lignocellulosic biomass into hydrolyzed cellulose and high-purity lignin, allowing for the recovery of valuable byproducts and improving the economic viability of the overall cellulosic ethanol process [21]. In this type of pretreatment, the lignocellulosic material is subjected to an organic solvent, with or without catalysts [22]. Low-boiling alcohols, such as ethanol and methanol, have been widely used as organosolvs due to their low cost and easy recovery in the process, but they require operating at a high pressure and hence special equipment that is expensive to purchase and operate has to be used [23]. High-boiling alcohols, such as ethylene glycol or glycerol, can also be used at low temperatures and pressure, but their recovery requires more energy [24].
The search for alternative solvents amenable to be used in lignocellulose pretreatments has led to the consideration of sulfolane, which features several interesting properties: it has a high chemical and thermal stability, and presents solvent properties for the extraction of aromatic hydrocarbons [25]. Thus, sulfolane can be used for the delignification of lignocellulosic materials, given the fact that lignin is a complex molecule composed mainly by aromatic alcohols such as p-coumaryl alcohol, coniferyl alcohol and sinapyl alcohol.
Further, during the pretreatment stage, other platform chemicals can be recovered [26]. For instance, saccharinic acids can be used for the preparation of various fine chemicals; as complexing agents for heavy metals [27], radionuclides and other materials; or as energy sources for aerobic and anaerobic bacteria [28].
In the study presented herein, the effect of a combined pretreatment of sugarcane bagasse (SCB) using organosolv (sulfolane) and alkali microwave irradiation was assessed, with the aim of finding the best pretreatment conditions for a more efficient subsequent enzymatic hydrolysis. Secondarily, the production of saccharinic acids during the pretreatment was also monitored.
2. Materials and Methods
2.1. Raw Materials and Reagents
Sugarcane bagasse was supplied by a local sugar production company (Ingenio Azucarero del Norte, Imbabura, Ecuador) and was used as the lignocellulosic raw material. The lignocellulosic material was dried, physically processed (cut, milled) and sieved (<100 µm). Titanium dioxide (TiO2; CAS 1317-70-0; anatase, nanopowder, 99.7%), sulfolane (C4H8O2S; CAS 126-33-0; 99%), 3,5-dinitrosalicylic acid (DNS; CAS 609-99-4; 98%), barbituric acid (C4H4N2O3; CAS 67-52-7; 99%), 2,4,5,6-tetrahydroxyhexanoic acid (C6H12O6; CAS 1518-59-8), sodium hydroxide (NaOH; CAS 1310-73-2; ≥97%), sodium (meta)periodate (NaIO4; CAS 7790-28-5; ≥99.0%) and sodium (meta)arsenite (NaAsO2; CAS 7784-46-5; ≥90%) were purchased from Sigma Aldrich Química S.A. (Madrid, Spain). Phosphoric acid (H3PO4, CAS 7664-38-2; 85%) was supplied by Panreac Química SLU (Castellar del Vallès, Spain). Cellic® Ctec2 y Cellic® Htec2 enzymes were donated by Novozymes (Bagsværd, Denmark).
2.2. Vibrational and SEM Characterization
The vibrational spectra were characterized with a Nicolet iS50 (Thermo Scientific, Waltham, MA, USA) Fourier-Transform Infrared (FTIR) spectrometer equipped with a diamond attenuated total reflection (ATR) module. Spectra were collected in the 400–4000 cm−1 region with a 1 cm−1 spectral resolution, averaging 64 scans.
Scanning electron microscopy (SEM) images were obtained with a FlexSEM 1000 (Hitachi, Chiyoda, Tokyo, Japan) apparatus.
2.3. Organosolv on Alkali Microwave-Assisted Pretreatment
SCB pretreatments were carried out in a Milestone Srl (Sorisole, Italy) Ethos-One microwave oven, with and without sulfolane as an organosolv medium and with TiO2 as a co-catalyst of the reaction. The effect of the organosolv was tested in alkali conditions, for which 0.5%, 1%, 3% and 5% NaOH solutions were tested. The experiment was carried out by mixing 10% (w/v) of SCB (dry biomass, particles < 100 µm), 0.2% (w/v) of TiO2, and a 1:1 (v/v) NaOH solution to sulfolane ratio. The final concentration of sulfolane in the reaction was 50% (v/v) [29]. In pretreatments without organosolv medium, sulfolane and TiO2 were replaced by distilled water. Finally, this mixture was subjected to sonication (with a probe-type UIP1000hdT ultrasonicator; Hielscher, Teltow, Germany; 1000 W, 20 kHz) for 1 min and to irradiation in a microwave oven at different temperatures (120, 130 and 140 °C) and for various reaction times (5, 15, 30, 45 and 60 min). After pretreatment, the solid residue was separated by centrifugation, at 5000 rpm for 10 min. The solid fraction was repeatedly washed with distilled water until a neutral pH was reached, and was dried at 105 °C for 24 h.
2.4. Enzymatic Hydrolysis
The enzymatic hydrolysis of the solid residues obtained from the pretreatments was carried out using Cellic® Ctec2 and Cellic® Htec2 enzymes. For the reactions, a loading of Cellic® Ctec 2 of 10 filterpaper units (FPU)/g of substrate and 20% Cellic® Htec2 (based on the amount of Cellic® Ctec2 loading) was added to a 50 mM sodium citrate buffer (pH 4.8), and then the solid substrate was mixed up to 15% (w/v). Enzymatic hydrolysis experiments were performed in triplicate at 50 °C, 100 rpm for 72 h.
2.5. Total Reducing Sugars Measurements
The total amount of reducing sugars (TRS) in the enzymatic hydrolysates was determined spectrometrically according to the DNS method (Miller, 1959). The hydrolysate was filtered and 2 mL of the filtrate were mixed in a test tube with 2 mL of DNS reagent. The test tube was incubated in a boiling water bath for 10 min and immediately immersed in an ice bath to stop the reaction. The solution of the sample and the DNS reagent was calibrated with distilled water to a final volume of 15 mL and the absorbance of the solution was measured at 540 nm. Glucose was used for the calibration curve. The TRS yield was obtained with Equation (1):
2.6. Saccharinic Acid Measurements
Saccharinic acids were spectrometrically quantified according to the method proposed by Ko [30]. In a tube, 0.2 M sodium (meta)periodate (0.1 mL) and the hydrolysate resulting from SCB alkaline pretreatment (0.2 mL) were mixed. The mixture was left in incubation at room temperature for 20 min, and then sodium arsenite 5% (1 mL) and barbituric acid (1.5 mL) were added. The solution was heated at 100 °C for 30 min, cooled to room temperature, and the absorbance was determined at 486 nm. The same procedure was applied to the standard (2,4,5,6-tetrahydroxyhexanoic acid) to determine the calibration curve.
2.7. Statistical Analysis
The data was statistically processed by using IBM (Armonk, NY, USA) SPSS Statistics v.25 software, performing an analysis of variance (ANOVA). The means of experiments were compared using Tukey’s HSD (honestly significant difference) test with a significance level of 0.05.
3. Results and Discussion
3.1. Vibrational and SEM Characterization
The ATR-FTIR spectra of the untreated and treated SCB samples are depicted in Figure 1. Both pretreated and treated residues exhibited a peak at around 2900 cm−1, which indicates -CH2 stretching; a peak at 897 cm−1, which indicates β-glucosidic linkages (present in cellulose and xylan); and a strong band at 1031 cm−1, attributed to C–O–C stretching from cellulose and hemicelluloses [31].
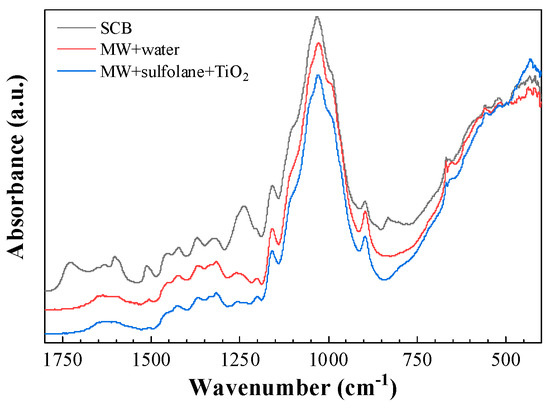
Figure 1.
Attenuated total reflection-Fourier-transform infrared (ATR-FTIR) spectra of sugarcane bagasse (SCB) samples: before treatment (black); after microwave irradiation (MWI) treatment with NaOH 3% at 140 °C for 60 min in the water-based medium (red), and in the sulfolane-based medium (blue).
The treated bagasse presented a notable decrease in the absorbance of peaks attributed to lignin, such as the one at 1729 cm−1, attributed to ferulate and p-coumarate esters [32]; those at 1603 cm−1 and 1513 cm−1, ascribed to the vibration of the aromatic ring [33]; the one at 1374 cm−1, associated with syringyl group [34]; and the one at 832 cm−1, attributed to the C-H out of plane bending vibrations in the p-hydroxyphenyl propane units [35]. While the increased intensity of the band at 898 cm−1 showed an increase in cellulose content due to the delignification, the decrease in intensity of the band at around 1369 cm−1 indicated an increase in amorphous cellulose [36]. Therefore, all these alterations in the FTIR-ATR spectra are consistent with the effectiveness of the two MWI treatments for lignin removal.
Representative SEM micrographs of both untreated and treated SCB are shown in Figure 2. Changes in morphology could be observed after MWI treatment in both media, resulting in a more flaky structure. Nonetheless, they were slightly more evident in the sulfolane-based medium than in the water-based medium, suggesting a higher degree of attack for the former.
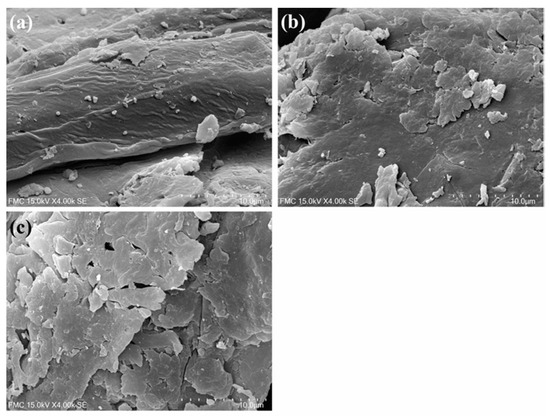
Figure 2.
SEM micrographs of SCB samples at 4000× magnification: (a) before treatment; after MWI treatment with NaOH 3% at 140 °C for 60 min in the (b) water-based medium and in the (c) sulfolane-based medium.
3.2. Influence of Sodium Hydroxide Concentration, Temperature and Reaction Time on TRS Yield
3.2.1. NaOH Concentration
An increase in TRS yields was observed when the alkali concentration was increased from 0.5% to 5%, both with and without organosolv (Table 1). This can be ascribed to the fact that NaOH is one of the strongest base catalysts, and its effectiveness for the pretreatment of lignocellulosic biomass has been evidenced by a higher degree of enzymatic hydrolysis compared to other alkaline pretreatments in the literature [37,38].

Table 1.
Total reducing sugars (TRS) production from enzymatic hydrolysis of SCB for different temperatures, NaOH concentrations and reaction times, with and without sulfolane-TiO2.
The alkaline pretreatment reactions include the dissolution of lignin and hemicellulose, and the de-esterification (saponification) of intermolecular ester bonds cross-linking hemicellulose and lignin. During the NaOH pretreatment reaction, NaOH is dissociated into hydroxide ion (OH−) and sodium ion (Na+) [13], and the increase in the concentration of hydroxide ions increases the rate of the hydrolysis reaction. This is consistent with the results obtained in this study, in which the increase in TRS production was evident when NaOH concentration was increased to 3% and 5%, reaching yields of up to 61.1% and 64.8%, respectively. Significant differences were found for both pretreatments, with sulfolane-TiO2 (Table 2) and without sulfolane-TiO2 (Table A1).

Table 2.
Tukey’s HSD test results for the MW-A + sulfolane-TiO2 pretreatment in relation to the TRS yield (%). Analysis of the differences between the categories with a confidence interval of 95% for: temperature (top), NaOH concentration (center) and time (bottom).
In an investigation carried out by Hoşgün [39], pretreatments at different NaOH concentrations in the 2 to 10% (w/v) range were tested on lignocellulosic material (hazelnut shells). The results evidenced that the increase in NaOH concentration led to greater lignin removal. TRS yields improved up to a NaOH concentration of 6%, but further increase resulted in a decrease in TRS production.
3.2.2. Temperature
In the enzymatic hydrolysis of lignocellulosic biomass (sweet sorghum stalks) pretreated with organosolv using an acid catalyst, Ostovareh et al. [40] reported that an increase in temperature (100–160 °C) improved enzymatic conversion. However, Koo et al. [41] showed that enzymatic hydrolysis was barely affected beyond 140 °C, thus suggesting that the pretreatment would not need to be conducted at higher temperatures.
In the study presented herein, in line with Ostovareh et al. [40], temperature was found to be an important parameter, resulting in significant differences (Table 2 and Table A1): the increase in temperature from 120 °C to 140 °C improved the formation of TRS from enzymatic hydrolysis of SCB by approximately 5%.
3.2.3. Reaction Time
Although significant differences were also found among the five reaction times assayed (Table 2 and Table A1), it is worth noting that it would be parameter that had the smallest influence: the increase in reaction time from 5 to 60 min only resulted in a 1.9% increase in TRS yield.
Wang et al. [42] reported that pretreatments with organosolv (2-propanol) at higher temperatures (ranging from 200 to 220 °C) and longer reaction times (60 and 120 min) in an autoclave facilitated enzymatic hydrolysis. In contrast, the use of MWI—even under milder conditions (in terms of temperatures and reaction times, such as those used as in this study)—would noticeably speed up the process, resulting in TRS yields of up to 62.9% in only 5 min.
In view of aforementioned results, NaOH 5%, 140 °C and 5 min would be the most appropriate conditions for the pretreatment of lignocellulosic biomass with sulfolane in alkaline conditions under MWI, from the point of view of sugar yield.
3.3. Influence of Sodium Hydroxide Concentration, Temperature and Reaction Time on Saccharinic Acids Yield
3.3.1. NaOH Concentration
In a similar fashion to the TRS yield, saccharinic acids production systematically increased as NaOH concentration was increased (Table 3). Significant differences were found between the four assayed concentrations for both pretreatment media: TiO2 + sulfolane (Table 4, top) and water (Table A2). Improvement factors of up to 3.24 and 2.65 were obtained for the water and sulfolane media, respectively, depending on the other two parameters under study.

Table 3.
Saccharinic acids production from enzymatic hydrolysis of SCB for different temperatures, NaOH concentrations and reaction times, with and without sulfolane-TiO2.

Table 4.
Tukey’s HSD test results for the MW-A + sulfolane-TiO2 pretreatment in relation to the saccharinic acids yield (g/L). Analysis of the differences between the categories with a confidence interval of 95% for: temperature (top), NaOH concentration (center) and time (bottom).
3.3.2. Temperature
Statistically significant differences in the saccharinic acids production were also detected as a function of temperature (Table 4, center; Table A2): Depending on the reaction time and NaOH concentration, improvement factors when the temperature was increased from 120 °C to 140 °C ranged from 1.15 to 2.33 for the water pretreatment medium, and from 1.18 to 1.88 for the sulfolane medium.
3.3.3. Reaction Time
In relation to the effect of reaction time, the behavior differed from that reported above for the TRS yield. In this case, a significant improvement in saccharinic acids production occurred when the reaction time was increased from 5 to 30 min (Table 4, bottom; Table A2). The enhancement factor was in the 1.2–2.9 range for the water-based medium, and in the 1.4–2.3 range for the sulfolane medium, depending on temperature and NaOH concentration. Further increase of the reaction time (to 45 and 60 min) did not lead to additional saccharinic acids production.
Taking all parameters into account, the best combination in terms of saccharinic acid production was NaOH 5%, 140 °C and 30 min. In these conditions, 11.31 g/L and 15.08 g/L were obtained for the water-based and the sulfolane-based pretreatments, respectively (i.e., the sulfolane medium resulted in a 33% higher production).
3.4. Effect of the Pretreatment Reaction Medium
3.4.1. TRS Yield
The statistical analysis showed significant differences between the two reaction media (Table 5), indicating that sulfolane-TiO2 promoted the production of TRS, attaining yields of up to 64.8% (vs. yields of up to 59.5% for the pretreatment without the addition of sulfolane-TiO2). This relatively small difference points at the very positive impact of MWI, regardless of the reaction medium.

Table 5.
Tukey’s HSD test results for the TRS yield (%). Analysis of the differences between the two reaction media with a confidence interval of 95%. “1” stands for the pretreatment medium with sulfolane-TiO2 and “2” for the medium without it.
In the studies carried out by Peng et al. [43,44] in which MWI pretreatment was assayed on microcrystalline cellulose, they reported that noticeably shorter times were needed in order to reach a favorable saccharides yield. Moreover, in the assays conducted by Lai et al. [45], and Podschun et al. [46], pretreatments with different heating systems that included a steam-alkali-chemical combination, conventional thermo-chemical (NaOH) and microwave-alkali (MW-A) pretreatments on lignocellulosic biomass were compared. They demonstrated that the MW-A was the most effective method for the disruption of lignocellulosic materials and for improving enzyme saccharification, suggesting that it may be the best alternative due to its lower energy consumption and shorter reaction times, which make it economically viable in industrial scale processes.
3.4.2. Saccharinic Acids
Apropos of saccharinic acids production, statistically significant differences were also detected between the two reaction media (Table 6), with a higher production of these byproducts for sulfolane-TiO2-based pretreatment.

Table 6.
Tukey’s HSD test results for the saccharinic acids yield (g/L). Analysis of the differences between the two reaction media with a confidence interval of 95%. “1” stands for the pretreatment medium with sulfolane-TiO2 and “2” for the medium without it.
3.5. Comparison with Other Pretreatment Conditions Reported in the Literature
Table 7 shows a comparison of the pretreatment efficiency attained through the proposed procedure vs. those achieved with other pretreatments reported in the literature for SBC. It should be stressed that the lack of a common agreement in relation to the units (results may be expressed in terms of glucose yield, TRS production -in mg/g or in g/L-, etc.) jeopardizes a direct comparison among studies. Comparisons of the yields below should therefore be taken with caution.

Table 7.
Comparison of the yield results presented herein with the reported literature.
In view of Table 7, it may be inferred that higher yields may be attained with other pretreatments, but at the expense of higher enzyme loadings and longer pretreatment times. As noted by Mithra et al. [47], the cost of enzymatic hydrolysis stage is critical in the low-cost production of 2G ethanol. Thus, the proposed MW-A approach, with much shorter processing times, regardless of the chosen reaction medium, may be regarded as an interesting option for industrial applications from the cost-efficiency point of view. Moreover, the system may be supplemented with detoxification chemicals to further reduce cellulase dosage [47,48,49]. Further research to explore this possibility is under way.
4. Conclusions
The use of sulfolane-TiO2 in combination with alkali MWI pretreatment was assessed on SCB. The use of sulfolane as a solvent resulted in 5% higher TRS yields and 33% higher saccharinic acids production than the water-based medium. The former led to TRS yields of up to 64.8% and 15.24 g/L of saccharinic acids, while the water-based treatment led to TRS and saccharinic acid yields of up to 59.5% and 11.31 g/L, respectively. Regardless of the reaction medium, the best results were attained for a NaOH concentration of 5% at a temperature of 140 °C. In relation to the reaction time, 5 min was found to be the best choice for TRS production, while 30 min would optimize saccharinic acids production. While the MW-A in water approach may be preferable with a view to industrial applications due to environmental and cost factors, the sulfolane-TiO2 medium clearly outperforms it if saccharinic acids are to be recovered as high-added value byproducts. Regardless of the chosen reaction medium, the proposed MW-A pretreatment results in high glucose yields with short reaction times and enzyme-associated savings.
Author Contributions
Conceptualization, E.J.C.-B. and J.M.-G.; formal analysis, P.P.-B., E.J.C.-B., J.M.-G. and P.M.-R.; funding acquisition, E.J.C.-B.; investigation, P.P.-B.; methodology, E.J.C.-B. and J.M.-G.; resources, J.M.-G.; supervision, E.J.C.-B. and J.M.-G.; validation, P.M.-R.; visualization, P.M.-R.; writing—original draft, P.P.-B. and P.M.-R.; writing—review & editing, P.M.-R.
Funding
This research was funded by Neotropical Center for Biomass Research (CNIB) of the Pontifical Catholic University of Ecuador (PUCE).
Conflicts of Interest
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results. Mention of trade names or commercial products in this article is solely for the purpose of providing specific information and does not imply recommendation or endorsement.
Appendix A

Table A1.
Tukey’s HSD test results for the MW-A pretreatment in water medium in relation to the TRS yield (%). Analysis of the differences between the categories with a confidence interval of 95% for: temperature (top), NaOH concentration (center) and time (bottom).
Table A1.
Tukey’s HSD test results for the MW-A pretreatment in water medium in relation to the TRS yield (%). Analysis of the differences between the categories with a confidence interval of 95% for: temperature (top), NaOH concentration (center) and time (bottom).
| T (°C) | LS Means | Standard Error | Lower Bound (95%) | Upper Bound (95%) | Groups | ||||
| 120 | 42.921 | 0.159 | 42.608 | 43.235 | A | ||||
| 130 | 45.797 | 0.159 | 45.484 | 46.111 | B | ||||
| 140 | 47.656 | 0.159 | 47.343 | 47.969 | C | ||||
| NaOH (% w/v) | LS Means | Standard Error | Lower Bound (95%) | Upper Bound (95%) | Groups | ||||
| 0.5 | 32.602 | 0.183 | 32.240 | 32.964 | A | ||||
| 1 | 39.566 | 0.183 | 39.204 | 39.928 | B | ||||
| 3 | 52.956 | 0.183 | 52.594 | 53.318 | C | ||||
| 5 | 56.709 | 0.183 | 56.347 | 57.071 | D | ||||
| Time (min) | LS Means | Standard Error | Lower Bound (95%) | Upper Bound (95%) | Groups | ||||
| 15 | 44.471 | 0.205 | 44.066 | 44.875 | A | ||||
| 5 | 44.436 | 0.205 | 44.031 | 44.841 | A | ||||
| 30 | 45.453 | 0.205 | 45.049 | 45.858 | B | ||||
| 45 | 46.321 | 0.205 | 45.916 | 46.726 | C | ||||
| 60 | 46.610 | 0.205 | 46.205 | 47.015 | C | ||||
LS means: least-squares means.

Table A2.
Tukey’s HSD test results for the MW-A pretreatment in water medium in relation to the saccharinic acids yield (g/L). Analysis of the differences between the categories with a confidence interval of 95% for: Temperature (top), NaOH concentration (center) and time (bottom).
Table A2.
Tukey’s HSD test results for the MW-A pretreatment in water medium in relation to the saccharinic acids yield (g/L). Analysis of the differences between the categories with a confidence interval of 95% for: Temperature (top), NaOH concentration (center) and time (bottom).
| T (°C) | LS Means | Standard Error | Lower Bound (95%) | Upper Bound (95%) | Groups | |||
| 120 | 3.188 | 0.015 | 3.160 | 3.217 | A | |||
| 130 | 4.902 | 0.015 | 4.874 | 4.931 | B | |||
| 140 | 5.978 | 0.015 | 5.949 | 6.007 | C | |||
| NaOH (% w/v) | LS Means | Standard Error | Lower Bound (95%) | Upper Bound (95%) | Groups | |||
| 0.5 | 2.945 | 0.017 | 2.911 | 2.978 | A | |||
| 1 | 3.827 | 0.017 | 3.794 | 3.860 | B | |||
| 3 | 4.661 | 0.017 | 4.627 | 4.694 | C | |||
| 5 | 7.326 | 0.017 | 7.293 | 7.359 | D | |||
| Time (min) | LS Means | Standard Error | Lower Bound (95%) | Upper Bound (95%) | Groups | |||
| 5 | 3.046 | 0.019 | 3.008 | 3.083 | A | |||
| 15 | 3.184 | 0.019 | 3.147 | 3.221 | B | |||
| 30 | 5.699 | 0.019 | 5.661 | 5.736 | C | |||
| 45 | 5.743 | 0.019 | 5.706 | 5.780 | C | |||
| 60 | 5.777 | 0.019 | 5.740 | 5.815 | ||||
LS means: least-squares means.
References
- Rastogi, M.; Shrivastava, S. Recent advances in second generation bioethanol production: An insight to pretreatment, saccharification and fermentation processes. Renew. Sustain. Energy Rev. 2017, 80, 330–340. [Google Scholar] [CrossRef]
- Han, X.; Guo, Y.; Liu, X.; Xia, Q.; Wang, Y. Catalytic conversion of lignocellulosic biomass into hydrocarbons: A mini review. Catal. Today 2019, 319, 2–13. [Google Scholar] [CrossRef]
- Karimi, K.; Taherzadeh, M.J. A critical review on analysis in pretreatment of lignocelluloses: Degree of polymerization, adsorption/desorption, and accessibility. Bioresour. Technol. 2016, 203, 348–356. [Google Scholar] [CrossRef] [PubMed]
- Zabed, H.; Sahu, J.N.; Boyce, A.N.; Faruq, G. Fuel ethanol production from lignocellulosic biomass: An overview on feedstocks and technological approaches. Renew. Sustain. Energy Rev. 2016, 66, 751–774. [Google Scholar] [CrossRef]
- Robak, K.; Balcerek, M. Review of Second Generation Bioethanol Production from Residual Biomass. Food Technol. Biotechnol. 2018, 56, 174–187. [Google Scholar] [CrossRef]
- Mosier, N.; Wyman, C.; Dale, B.; Elander, R.; Lee, Y.Y.; Holtzapple, M.; Ladisch, M. Features of promising technologies for pretreatment of lignocellulosic biomass. Bioresour. Technol. 2005, 96, 673–686. [Google Scholar] [CrossRef] [PubMed]
- Sarker, T.C.; Azam, S.M.G.G.; Bonanomi, G. Recent Advances in Sugarcane Industry Solid By-Products Valorization. Waste Biomass Valoriz. 2017, 8, 241–266. [Google Scholar] [CrossRef]
- Kumar, M.; Olajire Oyedun, A.; Kumar, A. A review on the current status of various hydrothermal technologies on biomass feedstock. Renew. Sustain. Energy Rev. 2018, 81, 1742–1770. [Google Scholar] [CrossRef]
- Zhang, X.; Yuan, Q.; Cheng, G. Deconstruction of corncob by steam explosion pretreatment: Correlations between sugar conversion and recalcitrant structures. Carbohydr. Polym. 2017, 156, 351–356. [Google Scholar] [CrossRef] [PubMed]
- Aguilar-Reynosa, A.; Romaní, A.; Ma. Rodríguez-Jasso, R.; Aguilar, C.N.; Garrote, G.; Ruiz, H.A. Microwave heating processing as alternative of pretreatment in second-generation biorefinery: An overview. Energy Convers. Manag. 2017, 136, 50–65. [Google Scholar] [CrossRef]
- Liyakathali, N.A.M.; Muley, P.D.; Aita, G.; Boldor, D. Effect of frequency and reaction time in focused ultrasonic pretreatment of energy cane bagasse for bioethanol production. Bioresour. Technol. 2016, 200, 262–271. [Google Scholar] [CrossRef]
- Nair, R.B.; Kalif, M.; Ferreira, J.A.; Taherzadeh, M.J.; Lennartsson, P.R. Mild-temperature dilute acid pretreatment for integration of first and second generation ethanol processes. Bioresour. Technol. 2017, 245, 145–151. [Google Scholar] [CrossRef]
- Kim, J.S.; Lee, Y.Y.; Kim, T.H. A review on alkaline pretreatment technology for bioconversion of lignocellulosic biomass. Bioresour. Technol. 2016, 199, 42–48. [Google Scholar] [CrossRef]
- Zhang, Q.; Hu, J.; Lee, D.-J. Pretreatment of biomass using ionic liquids: Research updates. Renew. Energy 2017, 111, 77–84. [Google Scholar] [CrossRef]
- Chen, H.; Zhao, J.; Hu, T.; Zhao, X.; Liu, D. A comparison of several organosolv pretreatments for improving the enzymatic hydrolysis of wheat straw: Substrate digestibility, fermentability and structural features. Appl. Energy 2015, 150, 224–232. [Google Scholar] [CrossRef]
- Jönsson, L.J.; Martín, C. Pretreatment of lignocellulose: Formation of inhibitory by-products and strategies for minimizing their effects. Bioresour. Technol. 2016, 199, 103–112. [Google Scholar] [CrossRef]
- Moodley, P.; Kana, E.B.G. Development of a steam or microwave-assisted sequential salt-alkali pretreatment for lignocellulosic waste: Effect on delignification and enzymatic hydrolysis. Energy Convers. Manag. 2017, 148, 801–808. [Google Scholar] [CrossRef]
- Li, H.; Qu, Y.; Yang, Y.; Chang, S.; Xu, J. Microwave irradiation—A green and efficient way to pretreat biomass. Bioresour. Technol. 2016, 199, 34–41. [Google Scholar] [CrossRef]
- Mitani, T. Recent Progress on Microwave Processing of Biomass for Bioenergy Production. J. Jpn. Pet. Inst. 2018, 6, 113–120. [Google Scholar] [CrossRef]
- Zhu, Z.; Rezende, C.A.; Simister, R.; McQueen-Mason, S.J.; Macquarrie, D.J.; Polikarpov, I.; Gomez, L.D. Efficient sugar production from sugarcane bagasse by microwave assisted acid and alkali pretreatment. Biomass Bioenergy 2016, 93, 269–278. [Google Scholar] [CrossRef]
- Santo, M.E.; Rezende, C.A.; Bernardinelli, O.D.; Pereira, N.; Curvelo, A.A.S.; deAzevedo, E.R.; Guimarães, F.E.G.; Polikarpov, I. Structural and compositional changes in sugarcane bagasse subjected to hydrothermal and organosolv pretreatments and their impacts on enzymatic hydrolysis. Ind. Crops Prod. 2018, 113, 64–74. [Google Scholar] [CrossRef]
- Zhou, Z.; Lei, F.; Li, P.; Jiang, J. Lignocellulosic biomass to biofuels and biochemicals: A comprehensive review with a focus on ethanol organosolv pretreatment technology. Biotechnol Bioeng. 2018, 115, 2683–2702. [Google Scholar] [CrossRef] [PubMed]
- Borand, M.N.; Karaosmanoğlu, F. Effects of organosolv pretreatment conditions for lignocellulosic biomass in biorefinery applications: A review. J. Renew. Sustain. Energy 2018, 10, 33104. [Google Scholar] [CrossRef]
- Zhang, K.; Pei, Z.; Wang, D. Organic solvent pretreatment of lignocellulosic biomass for biofuels and biochemicals: A review. Bioresour. Technol. 2016, 199, 21–33. [Google Scholar] [CrossRef] [PubMed]
- Clark, E. Sulfolane and Sulfones. Kirk-Othmer Encycl. Chem. Technol. 2000. [Google Scholar] [CrossRef]
- Almond, M.; Suleiman, M.G.; Hawkins, M.; Winder, D.; Robshaw, T.; Waddoups, M.; Humphreys, P.N.; Laws, A.P. Developing cellulosic waste products as platform chemicals: Protecting group chemistry of α-glucoisosaccharinic acid. Carbohydr. Res. 2018, 455, 97–105. [Google Scholar] [CrossRef]
- Almond, M.; Belton, D.; Humphreys, P.N.; Laws, A.P. A study of the metal binding capacity of saccharinic acids formed during the alkali catalysed decomposition of cellulosic materials: Nickel complexation by glucoisosaccharinic acids and xyloisosaccharinic acids. Carbohydr. Res. 2016, 427, 48–54. [Google Scholar] [CrossRef]
- Popa, V. Biorefining as a possibility to increase the efficiency of pulp and paper. Celul. Şi Hârtie 2014, 63, 3–17. [Google Scholar]
- Teng, N.; Ni, J.; Chen, H.; Ren, Q.; Na, H.; Liu, X.; Zhang, R.; Zhu, J. Initiating Highly Effective Hydrolysis of Regenerated Cellulose by Controlling Transition of Crystal Form with Sulfolane under Microwave Radiation. ACS Sustain. Chem. Eng. 2016, 4, 1507–1511. [Google Scholar] [CrossRef]
- Ko, A.M.Y.; Somers, P.J. An improved method for the automated determination of meta- and iso-saccharinic acids. Carbohydr. Res. 1974, 33, 281–285. [Google Scholar] [CrossRef]
- Li, K.; Kobayashi, T. Applied Environmental Materials Science for Sustainability; IGI Global: Hershey, PA, USA, 2016; Chapter 15; pp. 327–346. [Google Scholar]
- Pan, G.X.; Bolton, J.L.; Leary, G.J. Determination of Ferulic and p-Coumaric Acids in Wheat Straw and the Amounts Released by Mild Acid and Alkaline Peroxide Treatment. J. Agric. Food Chem. 1998, 4, 5283–5288. [Google Scholar] [CrossRef]
- Saw, S.K.; Sarkhel, G.; Choudhury, A. Surface modification of coir fibre involving oxidation of lignins followed by reaction with furfuryl alcohol: Characterization and stability. Appl. Surf. Sci. 2011, 257, 3763–3769. [Google Scholar] [CrossRef]
- Xu, C.; Ferdosian, F. Conversion of Lignin into Bio-Based Chemicals and Materials; Springer: Berlin/Heidelberg, Germany, 2017; p. 164. [Google Scholar]
- Hoareau, W.; Trindade, W.G.; Siegmund, B.; Castellan, A.; Frollini, E. Sugar cane bagasse and curaua lignins oxidized by chlorine dioxide and reacted with furfuryl alcohol: Characterization and stability. Polym. Degrad. Stab. 2004, 86, 567–576. [Google Scholar] [CrossRef]
- Xie, W.-H.; Hu, B.-B.; Zhang, X.-B.; Yi, X.-M.; Tan, W.-X.; Zhu, M.-J. Enhanced Enzymatic Digestibility of Sugarcane Bagasse Pretreated by Ionic Liquids. J. Biobased Mater. Bioenergy 2015, 9, 493–501. [Google Scholar] [CrossRef]
- Chang, M.; Li, D.; Wang, W.; Chen, D.; Zhang, Y.; Hu, H.; Ye, X. Comparison of sodium hydroxide and calcium hydroxide pretreatments on the enzymatic hydrolysis and lignin recovery of sugarcane bagasse. Bioresour. Technol. 2017, 244, 1055–1058. [Google Scholar] [CrossRef]
- Singh, R.; Shukla, A.; Tiwari, S.; Srivastava, M. A review on delignification of lignocellulosic biomass for enhancement of ethanol production potential. Renew. Sustain. Energy Rev. 2014, 32, 713–728. [Google Scholar] [CrossRef]
- Hoşgün, E.Z.; Berikten, D.; Kıvanç, M.; Bozan, B. Ethanol production from hazelnut shells through enzymatic saccharification and fermentation by low-temperature alkali pretreatment. Fuel 2017, 196, 280–287. [Google Scholar] [CrossRef]
- Ostovareh, S.; Karimi, K.; Zamani, A. Efficient conversion of sweet sorghum stalks to biogas and ethanol using organosolv pretreatment. Ind. Crops Prod. 2015, 66, 170–177. [Google Scholar] [CrossRef]
- Koo, B.-W.; Kim, H.-Y.; Park, N.; Lee, S.-M.; Yeo, H.; Choi, I.-G. Organosolv pretreatment of Liriodendron tulipifera and simultaneous saccharification and fermentation for bioethanol production. Biomass Bioenergy 2011, 35, 1833–1840. [Google Scholar] [CrossRef]
- Wang, B.; Shen, X.-J.; Wen, J.-L.; Xiao, L.; Sun, R.-C. Evaluation of organosolv pretreatment on the structural characteristics of lignin polymers and follow-up enzymatic hydrolysis of the substrates from Eucalyptus wood. Int. J. Biol. Macromol. 2017, 97, 447–459. [Google Scholar] [CrossRef]
- Peng, H.; Chen, H.; Qu, Y.; Li, H.; Xu, J. Bioconversion of different sizes of microcrystalline cellulose pretreated by microwave irradiation with/without NaOH. Appl. Energy 2014, 117, 142–148. [Google Scholar] [CrossRef]
- Peng, H.; Li, H.; Luo, H.; Xu, J. A novel combined pretreatment of ball milling and microwave irradiation for enhancing enzymatic hydrolysis of microcrystalline cellulose. Bioresour. Technol. 2013, 130, 81–87. [Google Scholar] [CrossRef]
- Lai, L.W.; Idris, A. Comparison of steam-alkali-chemical and microwave-alkali pretreatment for enhancing the enzymatic saccharification of oil palm trunk. Renew. Energy 2016, 99, 738–746. [Google Scholar] [CrossRef]
- Podschun, J.; Saake, B.; Lehnen, R. Catalytic demethylation of organosolv lignin in aqueous medium using indium triflate under microwave irradiation. React. Funct. Polym. 2017, 119, 82–86. [Google Scholar] [CrossRef]
- Mithra, M.G.; Padmaja, G. Strategies for enzyme saving during saccharification of pretreated lignocellulo-starch biomass: Effect of enzyme dosage and detoxification chemicals. Heliyon 2017, 3, 384. [Google Scholar] [CrossRef][Green Version]
- Brondi, M.G.; Vasconcellos, V.M.; Giordano, R.C.; Farinas, C.S. Alternative Low-Cost Additives to Improve the Saccharification of Lignocellulosic Biomass. Appl. Biochem. Biotechnol. 2019, 187, 461–473. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, S.; Yuan, H.; Lyu, G.; Xie, J. FeCl3-catalyzed ethanol pretreatment of sugarcane bagasse boosts sugar yields with low enzyme loadings and short hydrolysis time. Bioresour. Technol. 2018, 249, 395–401. [Google Scholar] [CrossRef]
- You, Y.; Li, P.; Lei, F.; Xing, Y.; Jiang, J. Optimization of Enzymatic Hydrolysis on Sugarcane Bagasse Pretreated with Soda-Green Liquor and Methanol. J. Biobased Mater. Bioenergy 2017, 11, 433–440. [Google Scholar] [CrossRef]
- Xue, W.; Lei, F.; Li, P.; Jiang, J. Cellulose Accessibility and Zeta potentials of Sugarcane Bagasse Pretreated by Green Liquor and Ethanol for High Hydrolysis Efficiency. BioResources 2018, 13, 1510–1524. [Google Scholar] [CrossRef]
- Zhang, Z.; Doherty, W.O.S.; O’Hara, I.M. Integration of Salt-Induced Phase Separation with Organosolv Pretreatment for Clean Fractionation of Lignocellulosic Biomass. ACS Sustain. Chem. Eng. 2017, 5, 5284–5292. [Google Scholar] [CrossRef]
- Suriyachai, N.; Champreda, V.; Kraikul, N.; Techanan, W.; Laosiripojana, N. Fractionation of lignocellulosic biopolymers from sugarcane bagasse using formic acid-catalyzed organosolv process. 3 Biotech 2018, 8, 221. [Google Scholar] [CrossRef] [PubMed]
- Gurgel, L.V.A.; Pimenta, M.T.B.; Curvelo, A.A.d.S. Ethanol–water organosolv delignification of liquid hot water (LHW) pretreated sugarcane bagasse enhanced by high–pressure carbon dioxide (HP–CO2). Ind. Crops Prod. 2016, 94, 942–950. [Google Scholar] [CrossRef]
- Suriyachai, N.; Champreda, V.; Sakdaronnarong, C.; Shotipruk, A.; Laosiripojana, N. Sequential organosolv fractionation/hydrolysis of sugarcane bagasse: The coupling use of heterogeneous H3PO4-activated carbon as acid promoter and hydrolysis catalyst. Renew. Energy 2017, 113, 1141–1148. [Google Scholar] [CrossRef]
- Timung, R.; Deshavath, N.N.; Goud, V.V.; Dasu, V.V. Effect of Subsequent Dilute Acid and Enzymatic Hydrolysis on Reducing Sugar Production from Sugarcane Bagasse and Spent Citronella Biomass. J. Energy 2016, 2016, 1–12. [Google Scholar] [CrossRef]
- Lv, X.; Lin, J.; Luo, L.; Zhang, D.; Lei, S.; Xiao, W.; Xu, Y.; Gong, Y.; Liu, Z. Enhanced enzymatic saccharification of sugarcane bagasse pretreated by sodium methoxide with glycerol. Bioresour. Technol. 2018, 249, 226–233. [Google Scholar] [CrossRef] [PubMed]
- Wei, W.; Wu, S.; Xu, S. Enhancement of enzymatic saccharification of bagasse by ethanol-based organosolv auto-catalyzed pretreatment. J. Chem. Technol. Biotechnol. 2017, 92, 580–587. [Google Scholar] [CrossRef]
- Rabelo, S.C.; Filho, R.M.; Costa, A.C. A comparison between lime and alkaline hydrogen peroxide pretreatments of sugarcane bagasse for ethanol production. Appl. Biochem. Biotechnol. 2008, 144, 87–100. [Google Scholar] [CrossRef] [PubMed]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).