A Brief Review of Anaerobic Digestion of Algae for Bioenergy
Abstract
1. Introduction
2. Microalgal Wastewater Treatment for Biogas Production
3. Seaweed
4. Seaweed Biogas
5. Challenges to Biogas Production from Macro- and Microalgae
5.1. Algal Composition
5.2. Cell Wall Architecture
5.3. Polysaccharide
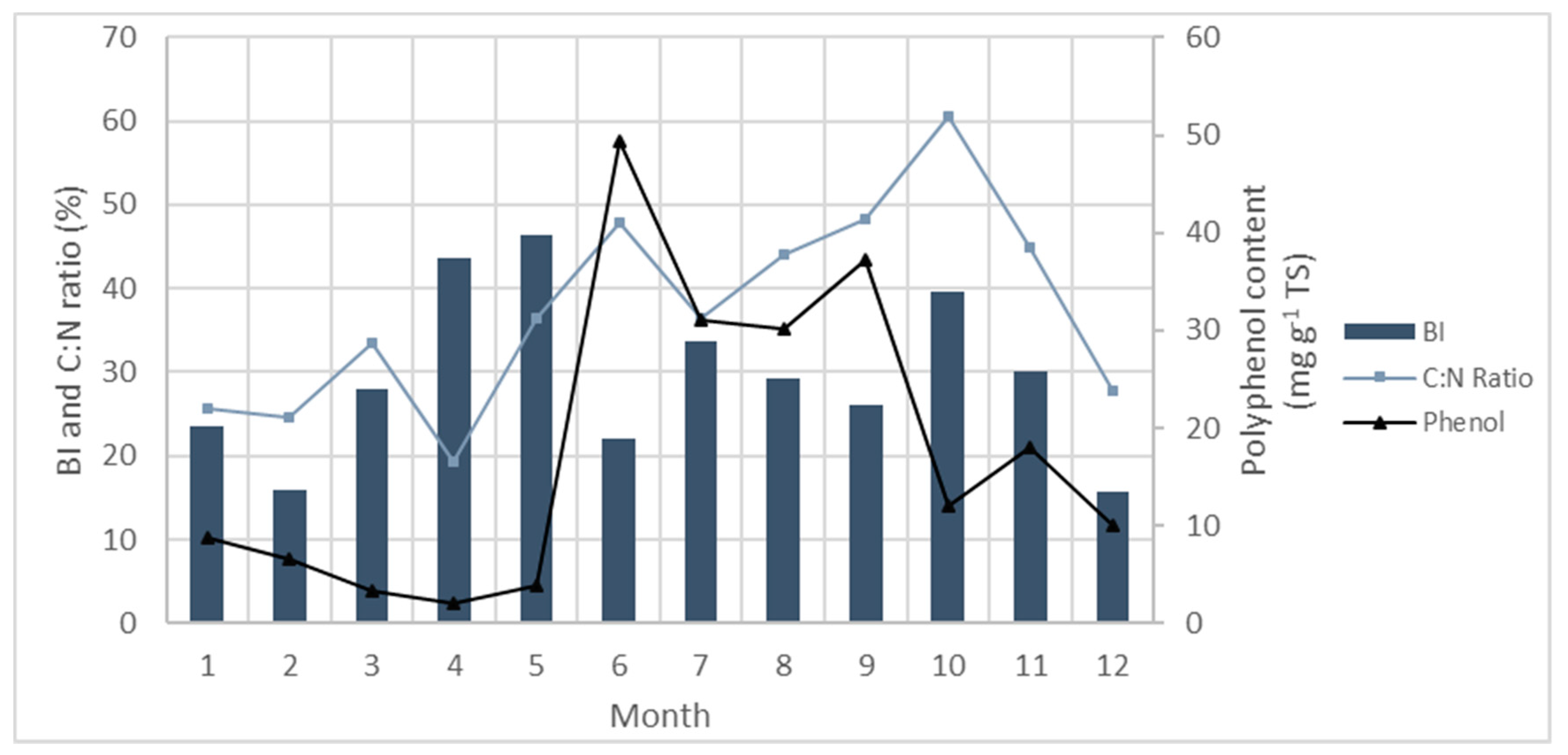
5.4. Phenolics
5.5. Salt
5.6. C:N and Co-digestion
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Chen, H.; Qiu, T.; Rong, J.; He, C.; Wang, Q. Microalgal biofuel revisited: An informatics-based analysis of developments to date and future prospects. Appl. Energy 2015, 155, 585–598. [Google Scholar] [CrossRef]
- Kerrison, P.D.; Stanley, M.S.; Edwards, M.D.; Black, K.D.; Hughes, A.D. The cultivation of European kelp for bioenergy: Site and species selection. Biomass Bioenergy 2015, 80, 229–242. [Google Scholar] [CrossRef]
- Milledge, J.J.; Harvey, P.J. Potential process ‘hurdles’ in the use of macroalgae as feedstock for biofuel production in the British Isles. J. Chem. Technol. Biotechnol. 2016, 91, 2221–2234. [Google Scholar] [CrossRef] [PubMed]
- Milledge, J.J.; Heaven, S. Methods of energy extraction from microalgal biomass: A review. Rev. Environ. Sci. Biotechnol. 2014, 13, 301–320. [Google Scholar] [CrossRef]
- Milledge, J.J.; Smith, B.; Dyer, P.; Harvey, P. Macroalgae-Derived Biofuel: A Review of Methods of Energy Extraction from Seaweed Biomass. Energies 2014, 7, 7194–7222. [Google Scholar] [CrossRef]
- Sills, D.L.; Paramita, V.; Franke, M.J.; Johnson, M.C.; Akabas, T.M.; Greene, C.H.; Tester, J.W. Quantitative Uncertainty Analysis of Life Cycle Assessment for Algal Biofuel Production. Environ. Sci. Technol. 2012, 47, 687–694. [Google Scholar] [CrossRef] [PubMed]
- Fasaei, F.; Bitter, J.H.; Slegers, P.M.; van Boxtel, A.J.B. Techno-economic evaluation of microalgae harvesting and dewatering systems. Algal Res. 2018, 31, 347–362. [Google Scholar] [CrossRef]
- Collet, P.; Helias, A.; Lardon, L.; Steyer, J.P.; Bernard, O. Recommendations for Life Cycle Assessment of algal fuels. Appl. Energy 2015, 154, 1089–1102. [Google Scholar] [CrossRef]
- Milledge, J.J.; Heaven, S. A review of the harvesting of micro-algae for biofuel production. Rev. Environ. Sci. Biotechnol. 2013, 12, 165–178. [Google Scholar] [CrossRef]
- Wang, S.-K.; Stiles, A.R.; Guo, C.; Liu, C.-Z. Harvesting microalgae by magnetic separation: A review. Algal Res. 2015, 9, 178–185. [Google Scholar] [CrossRef]
- Molina Grima, E.; Belarbi, E.-H.; Acien-Fernandez, F.G.; Robles-Medina, A.; Yusuf, C. Recovery of microalgal biomass and metabolites: Process options and economics. Biotechnol. Adv. 2003, 20, 491–515. [Google Scholar] [CrossRef]
- Zamalloa, C.; Vulsteke, E.; Albrecht, J.; Verstraete, W. The techno-economic potential of renewable energy through the anaerobic digestion of microalgae. Bioresour. Technol. 2011, 102, 1149–1158. [Google Scholar] [CrossRef]
- Uduman, N.; Qi, Y.; Danquah, M.K.; Forde, G.M.; Hoadley, A. Dewatering of microalgal cultures: A major bottleneck to algae-based fuels. J. Renew. Sustain. Energy 2010, 2, 012701. [Google Scholar] [CrossRef]
- Reynolds, C.S. The Ecology of Freshwater Phytoplankton; Cambridge University Press: Cambridge, UK, 1984. [Google Scholar]
- Edzwald, J.K. Algae, bubbles, coagulants, and dissolved air flotation. Water Sci. Technol. 1993, 27, 67–81. [Google Scholar] [CrossRef]
- Moraine, R.; Shelef, G.; Meydan, A.; Levi, A. Algal single cell protein from wastewater-treatment and renovation process. Biotechnol. Bioeng. 1979, 21, 1191–1207. [Google Scholar] [CrossRef]
- Packer, M. Algal capture of carbon dioxide; biomass generation as a tool for greenhouse gas mitigation with reference to New Zealand energy strategy and policy. Energy Policy 2009, 37, 3428–3437. [Google Scholar] [CrossRef]
- Bruton, T.; Lyons, H.; Lerat, Y.; Stanley, M.; Rasmussen, M.B. A Review of the Potential of Marine Algae as a Source of Biofuel in Ireland; Sustainable Energy Ireland: Dublin, Ireland, 2009. [Google Scholar]
- Milledge, J.J.; Staple, A.; Harvey, P. Pyrolysis of Invasive Seaweed Species. In Proceedings of the British Phycological Society Annual Meeting, Galway, Ireland, 25–27 June 2014. [Google Scholar]
- Horn, S.V. Bioenergy from Brown Seaweeds; Norwegian University of Science and Technology (NTNU): Trondheim, Norway, 2000. [Google Scholar]
- Murphy, F.; Devlin, G.; Deverell, R.; McDonnell, K. Biofuel Production in Ireland—An Approach to 2020 Targets with a Focus on Algal Biomass. Energies 2013, 6, 6391–6412. [Google Scholar] [CrossRef]
- Milledge, J.J.; Staple, A.; Harvey, P. Slow Pyrolysis as a Method for the Destruction of Japanese Wireweed, Sargassum muticum. Environ. Nat. Resour. Res. 2015, 5, 28–36. [Google Scholar] [CrossRef]
- Seaweed Sustainability: Food and Non-Food Applications, 1st ed.; Tiwari, B., Troy, D., Eds.; Academic Press: Amsterdam, The Nertherlands, 2015. [Google Scholar]
- Peu, P.; Sassi, J.F.; Girault, R.; Picard, S.; Saint-Cast, P.; Béline, F.; Dabert, P. Sulphur fate and anaerobic biodegradation potential during co-digestion of seaweed biomass (Ulva sp.) with pig slurry. Bioresour. Technol. 2011, 102, 10794–10802. [Google Scholar] [CrossRef]
- Vanegas, C.H.; Bartlett, J. Green energy from marine algae: Biogas production and composition from the anaerobic digestion of Irish seaweed species. Environ. Technol. 2013, 34, 2277–2283. [Google Scholar] [CrossRef]
- Barbot, Y.; Al-Ghaili, H.; Benz, R. A Review on the Valorization of Macroalgal Wastes for Biomethane Production. Mar. Drugs 2016, 14, 120. [Google Scholar] [CrossRef] [PubMed]
- Aresta, M.; Dibenedetto, A.; Barberio, G. Utilization of macro-algae for enhanced CO2 fixation and biofuels production: Development of a computing software for an LCA study. Fuel Process. Technol. 2005, 86, 1679–1693. [Google Scholar] [CrossRef]
- Allen, E.; Wall, D.M.; Herrmann, C.; Xia, A.; Murphy, J.D. What is the gross energy yield of third generation gaseous biofuel sourced from seaweed? Energy 2015, 81, 352–360. [Google Scholar] [CrossRef]
- Sutherland, A.; Varela, J. Comparison of various microbial inocula for the efficient anaerobic digestion of Laminaria hyperborea. BMC Biotechnol. 2014, 14, 7. [Google Scholar] [CrossRef] [PubMed]
- Huesemann, M.; Roesjadi, G.; Benemann, J.; Metting, F.B. Biofuels from Microalgae and Seaweeds. In Biomass to Biofuels; Blackwell Publishing Ltd.: Oxford, UK, 2010; pp. 165–184. [Google Scholar]
- Lewis, J.; Salam, F.; Slack, N.; Winton, M.; Hobson, L. Product Options for the Processing of Marine Macro-Algae—Summary Report; The Crown Estates: London, UK, 2011. [Google Scholar]
- Clarens, A.F.; Nassau, H.; Resurreccion, E.P.; White, M.A.; Colosi, L.M. Environmental Impacts of Algae-Derived Biodiesel and Bioelectricity for Transportation. Environ. Sci. Technol. 2011, 45, 7554–7560. [Google Scholar] [CrossRef] [PubMed]
- Cleveland, C.J.; O’Connor, P.A. Energy Return on Investment (EROI) of Oil Shale. Sustainability 2011, 3, 2307–2322. [Google Scholar] [CrossRef]
- Beal, C.M. Algal Biofuels: Energy and Water. In Proceedings of the WEG Symposium, Austin, TX, USA, 21–22 January 2011. [Google Scholar]
- Mulder, K.; Hagens, N.J. Energy return on investment: Toward a consistent framework. Ambio 2008, 37, 74–79. [Google Scholar] [CrossRef]
- Hall, C.A.S.; Klitgaard, K.A. Energy and the Wealth of Nations: Understanding the Biophysical Economy; Springer: New York, NY, USA, 2012. [Google Scholar]
- Twidell, J.; Weir, T. Renewable Energy Sources, 2nd ed.; Taylor & Francis: London, UK, 2006. [Google Scholar]
- Alvarado-Morales, M.; Boldrin, A.; Karakashev, D.B.; Holdt, S.L.; Angelidaki, I.; Astrup, T. Life cycle assessment of biofuel production from brown seaweed in Nordic conditions. Bioresour. Technol. 2013, 129, 92–99. [Google Scholar] [CrossRef]
- Aitken, D.; Bulboa, C.; Godoy-Faundez, A.; Turrion-Gomez, J.L.; Antizar-Ladislao, B. Life cycle assessment of macroalgae cultivation and processing for biofuel production. J. Clean. Prod. 2014, 75, 45–56. [Google Scholar] [CrossRef]
- Aitken, D. An Assessment of the Sustainability of Bioenergy Production from Algal Feedstock; The University of Edinburgh: Edinburgh, UK, 2014. [Google Scholar]
- Pechsiri, J.S.; Thomas, J.-B.E.; Risén, E.; Ribeiro, M.S.; Malmström, M.E.; Nylund, G.M.; Jansson, A.; Welander, U.; Pavia, H.; Gröndahl, F. Energy performance and greenhouse gas emissions of kelp cultivation for biogas and fertilizer recovery in Sweden. Sci. Total Environ. 2016, 573, 347–355. [Google Scholar] [CrossRef] [PubMed]
- Maneein, S.; Milledge, J.J.; Nielsen, B.V.; Harvey, P.J. A Review of Seaweed Pre-Treatment Methods for Enhanced Biofuel Production by Anaerobic Digestion or Fermentation. Fermentation 2018, 4, 100. [Google Scholar] [CrossRef]
- Chen, H.; Zhou, D.; Luo, G.; Zhang, S.; Chen, J. Macroalgae for biofuels production: Progress and perspectives. Renew. Sustain. Energy Rev. 2015, 47, 427–437. [Google Scholar] [CrossRef]
- Lundquist, T.J.; Woertz, I.C.; Quinn, N.W.T.; Benemann, J.R. A Realistic Technology and Engineering Assessment of Algae Biofuel Production; Energy Biosciences Institute: Berkeley, CA, USA, 2010. [Google Scholar]
- Ross, A.B.; Jones, J.M.; Kubacki, M.L.; Bridgeman, T. Classification of macroalgae as fuel and its thermochemical behaviour. Bioresour. Technol. 2008, 99, 6494–6504. [Google Scholar] [CrossRef] [PubMed]
- Milledge, J.J.; Harvey, P.J. Anaerobic digestion and gasification of seaweed. In Grand Challenges in Marine Biotechnology; Rampelotto, P.H., Trincone, A., Eds.; Spinger: Cham, Switzerland, 2018. [Google Scholar]
- Chisti, Y. Biodiesel from microalgae. Biotechnol. Adv. 2007, 25, 294–306. [Google Scholar] [CrossRef]
- Islam, M.A.; Heimann, K.; Brown, R.J. Microalgae biodiesel: Current status and future needs for engine performance and emissions. Renew. Sustain. Energy Rev. 2017, 79, 1160–1170. [Google Scholar] [CrossRef]
- Lardon, L.; Helias, A.; Sialve, B.; Stayer, J.P.; Bernard, O. Life-Cycle Assessment of Biodiesel Production from Microalgae. Environ. Sci. Technol. 2009, 43, 6475–6481. [Google Scholar] [CrossRef]
- Liu, X.; Clarens, A.F.; Colosi, L.M. Meta-model of Algae Bio Energy Life Cycles (MABEL). In Proceedings of the LCA XI Conference, Chicago, IL, USA, 6 October 2011. [Google Scholar]
- Garofalo, R. Algae and Aquatic Biomass for a Sustainable Production of 2nd Generation Biofuels. Deliverables 3.3 and 3.5 Lifecycle Assessment and Environmental Assessment; Aqua Fuels: Brussels, Belgium, 2011. [Google Scholar]
- ter Veld, F. Beyond the Fossil Fuel Era: On the Feasibility of Sustainable Electricity Generation Using Biogas from Microalgae. Energy Fuels 2012, 26, 3882–3890. [Google Scholar] [CrossRef]
- Milledge, J.J.; Heaven, S. Energy Balance of Biogas Production from Microalgae: Effect of Harvesting Method, Multiple Raceways, Scale of Plant and Combined Heat and Power Generation. J. Mar. Sci. Eng. 2017, 5, 9. [Google Scholar] [CrossRef]
- Milledge, J.J.; Heaven, S. Energy Balance of Biogas Production from Microalgae: Development of an Energy and Mass Balance Model. Curr. Biotechnol. 2015, 4, 554–567. [Google Scholar] [CrossRef]
- Gouveia, L. Microalgae as a Feedstock for Biofuels; Springer: Heidelberg, Germany, 2011. [Google Scholar]
- Park, J.B.K.; Craggs, R.J.; Shilton, A.N. Wastewater treatment high rate algal ponds for biofuel production. Bioresour. Technol. 2011, 102, 35–42. [Google Scholar] [CrossRef]
- Oswald, W.J. Large-scale algal culture systems (engineering aspects). In Micro-algal Biotechnology; Borowitzka, M.A., Borowitzka, L.J., Eds.; Cambridge University Press: Cambridge, UK, 1988. [Google Scholar]
- Bowyer, J.; Howe, J.; Levins, R.; Groot, H.; Fernholz, K.; Pepke, E.; Henderson, C. Third Generation Biofuels Implications for Wood-Derived Fuels. Available online: http://www.dovetailinc.org/report_pdfs/2018/dovetail3gbiofuel0218.pdf (accessed on 28 February 2019).
- Green, F.B.; Lundquist, T.J.; Oswald, W.J. Energetics of advanced integrated waste-water pond systems. Water Sci. Technol. 1995, 31, 9–20. [Google Scholar] [CrossRef]
- Goldman, J.C. Outdoor algal mass cultures—I. Applications. Water Res. 1979, 13, 1–19. [Google Scholar] [CrossRef]
- Buhr, H.O.; Miller, S.B. A dynamic model of the high rate algal bacterial waste water treatment pond. Water Res. 1983, 17, 29–38. [Google Scholar] [CrossRef]
- Jimoh, T.A.; Cowan, A.K. Extracellular polymeric substance production in high rate algal oxidation ponds. Water Sci. Technol. 2017, 76, 2647–2654. [Google Scholar] [CrossRef]
- Williams, P.J.l.B.; Laurens, L.M.L. Microalgae as biodiesel & biomass feedstocks: Review & analysis of the biochemistry, energetics & economics. Energy Environ. Sci. 2010, 3, 554–590. [Google Scholar]
- Singh, A.K.; Sharma, N.; Farooqi, H.; Abdin, M.Z.; Mock, T.; Kumar, S. Phycoremediation of municipal wastewater by microalgae to produce biofuel. Int. J. Phytoremediation 2017, 19, 805–812. [Google Scholar] [CrossRef]
- Benemann, J.; Koopman, B.; Weissman, J.; Eisenberg, D.; Goebel, R. Development of microalgae harvesting and high-rate pond technologies in California. In Algae Biomass; Shelef, G., Soeder, C.J., Eds.; Elsevier: Amsterdam, The Nertherlands, 1980. [Google Scholar]
- James, S.C.; Boriah, V. Modeling Algae Growth in an Open-Channel Raceway. J. Comput. Biol. 2010, 17, 895–906. [Google Scholar] [CrossRef]
- Sheehan, J.; Dunahay, T.; Benemann, J.; Roessler, P. A Look Back at the US Department of Energy’s Aquatic Species Program—Biodiesel from Algae; NREL/TP-580-24190; National Renewable Energy Laboratory NREL: Golden, CO, USA, 1998; p. 7.
- Benemann, J.; Oswald, W.J. Systems and Economic Analysis of Microalgae Ponds for Conversion of CO2 to Biomass; Pittsburgh Energy Technology Centre: Pittsburgh, PA, USA, 1996. [Google Scholar]
- Olguín, E.J. Phycoremediation: Key issues for cost-effective nutrient removal processes. Biotechnol. Adv. 2003, 22, 81–91. [Google Scholar] [CrossRef]
- Borowitzka, M.A. Commercial production of microalgae: Ponds, tanks, tubes and fermenters. J. Biotechnol. 1999, 70, 313–321. [Google Scholar] [CrossRef]
- Brennan, L.; Owende, P. Biofuels from microalgae—A review of technologies for production, processing, and extractions of biofuels and co-products. Renew. Sustain. Energy Rev. 2010, 14, 557–577. [Google Scholar] [CrossRef]
- Jimenez, C.; Cossio, B.R.; Niell, F.X. Relationship between physicochemical variables and productivity in open ponds for the production of Spirulina: A predictive model of algal yield. Aquaculture 2003, 221, 331–345. [Google Scholar] [CrossRef]
- Johnson, D.A.; Weissman, J.C.; Goebel, R.P. An Outdoor Test Facility for Large Scale Production of Microalgae; SERI/TP-231-3325; SERI: Golden, CO, USA, 1988. [Google Scholar]
- Aquafuels. Algae and Aquatic Biomass for a Sustainable Production of 2nd Generation Biofuels; Aquafuels: Brussels, Belgium, 2011. [Google Scholar]
- Weissman, J.C.; Tillett, D.M.; Goebel, R.P. Design and Operation of an Outdoor Microalgae Test Facility; SERI/STR-232-3569; SERI: Golden, CO, USA, 1989. [Google Scholar]
- Terry, K.L.; Raymond, L.P. System-design for the autotrophic production of microalgae. Enzym. Microb. Technol. 1985, 7, 474–487. [Google Scholar] [CrossRef]
- Borowitzka, M.A. Culturing of Microalgae in Outdoor Ponds. In Algal Culturing Techniques; Andersen, R.A., Ed.; Elsevier: London, UK, 2005. [Google Scholar]
- Chiaramonti, D.; Prussi, M.; Casini, D.; Tredici, M.R.; Rodolfi, L.; Bassi, N.; Zittelli, G.C.; Bondioli, P. Review of energy balance in raceway ponds for microalgae cultivation: Re-thinking a traditional system is possible. Appl. Energy 2013, 102, 101–111. [Google Scholar] [CrossRef]
- Craggs, R.; Park, J.; Heubeck, S.; Sutherland, D. High rate algal pond systems for low-energy wastewater treatment, nutrient recovery and energy production. N. Z. J. Bot. 2014, 52, 60–73. [Google Scholar] [CrossRef]
- Ahrens, T.; Sander, H. Microalgae in Waste Water Treatment; Green Gold from Sludge? Bioforum Eur. 2010, 14, 16–18. [Google Scholar]
- Kazamia, E.; Aldridge, D.C.; Smith, A.G. Synthetic ecology—A way forward for sustainable algal biofuel production? J. Biotechnol. 2012, 162, 163–169. [Google Scholar] [CrossRef]
- Smith, V.H.; Sturm, B.S.M.; de Noyelles, F.J.; Billings, S.A. The ecology of algal biodiesel production. Trends Ecol. Evol. 2010, 25, 301–309. [Google Scholar] [CrossRef]
- Godwin, C.M.; Lashaway, A.R.; Hietala, D.C.; Savage, P.E.; Cardinale, B.J. Biodiversity improves the ecological design of sustainable biofuel systems. Glob. Chang. Biol. Bioenergy 2018, 10, 752–765. [Google Scholar] [CrossRef]
- Committee on the Sustainable Development of Algal Biofuels. Sustainable Development of Algal Biofuels in the United States; The National Academies Press: Washington, DC, USA, 2012. [Google Scholar]
- Kazamia, E.; Riseley, A.S.; Howe, C.J.; Smith, A.G. An Engineered Community Approach for Industrial Cultivation of Microalgae. Ind. Biotechnol. 2014, 10, 184–190. [Google Scholar] [CrossRef] [PubMed]
- Mambo, P.; Westensee, D.; Zuma, B.; Cowan, A.K. The Belmont Valley integrated algae pond system in retrospect. Water SA 2014, 40. [Google Scholar] [CrossRef]
- Singh, A.; Olsen, S.I. A critical review of biochemical conversion, sustainability and life cycle assessment of algal biofuels. Appl. Energy 2011, 88, 3548–3555. [Google Scholar] [CrossRef]
- Ward, A.J.; Lewis, D.M.; Green, B. Anaerobic digestion of algae biomass: A review. Algal Res.-Biomass Biofuels Bioprod. 2014, 5, 204–214. [Google Scholar] [CrossRef]
- Golueke, C.G.; Oswald, W.J. Biological conversion of light energy to the chemical energy of methane. Appl. Microbiol. 1959, 7, 219–227. [Google Scholar] [PubMed]
- Tran, K.C. Anaerobic Digestion of Microalgal Biomass: Effects of Solid Concentration and Pre-Treatment. Ph.D. Thesis, University of Southampton, Southampton, UK, 2017. [Google Scholar]
- Glaz, P.; Bartosiewicz, M.; Laurion, I.; Reichwaldt, E.S.; Maranger, R.; Ghadouani, A. Greenhouse gas emissions from waste stabilisation ponds in Western Australia and Quebec (Canada). Water Res. 2016, 101, 64–74. [Google Scholar] [CrossRef]
- All-gas. All-gas Newsletter 11/2017. Available online: http://www.all-gas.eu/documents/1509955/1514023/Newsletter_2017.11_eng.pdf/629a9f4c-17e4-4cca-0b8f-b1b8bbe855ca (accessed on 17 January 2019).
- Maga, D. Life cycle assessment of biomethane produced from microalgae grown in municipal waste water. Biomass Convers. Biorefinery 2017, 7, 1–10. [Google Scholar] [CrossRef]
- Benzie, J.A.H.; Hynes, S. Bioenergy Production by Anaerobic Digestion: Using Agricultural Biomass and Organic Wastes. In Bioenergy Production by Anaerobic Digestion; Korres, N., O’Kiely, P., Benzie, J., West, J., Eds.; Routledge: London, UK, 2013. [Google Scholar]
- Food and Agriculture Organisation of the United Nations. Statistical Collections. Available online: http://www.fao.org/fishery/statistics/collections/en (accessed on 17 December 2018).
- Xia, A.; Cheng, J.; Murphy, J.D. Innovation in biological production and upgrading of methane and hydrogen for use as gaseous transport biofuel. Biotechnol. Adv. 2016, 34, 451–472. [Google Scholar] [CrossRef] [PubMed]
- Herrmann, C.; FitzGerald, J.; O’Shea, R.; Xia, A.; O’Kiely, P.; Murphy, J.D. Ensiling of seaweed for a seaweed biofuel industry. Bioresour. Technol. 2015, 196, 301–313. [Google Scholar] [CrossRef]
- Sode, S.; Bruhn, A.; Balsby, T.J.S.; Larsen, M.M.; Gotfredsen, A.; Rasmussen, M.B. Bioremediation of reject water from anaerobically digested waste water sludge with macroalgae (Ulva lactuca, Chlorophyta). Bioresour. Technol. 2013, 146, 426–435. [Google Scholar] [CrossRef] [PubMed]
- Bruhn, A.; Dahl, J.; Nielsen, H.B.; Nikolaisen, L.; Rasmussen, M.B.; Markager, S.; Olesen, B.; Arias, C.; Jensen, P.D. Bioenergy potential of Ulva lactuca: Biomass yield, methane production and combustion. Bioresour. Technol. 2011, 102, 2595–2604. [Google Scholar] [CrossRef]
- Nikolaisen, L.; Daugbjerg Jensen, P.; Svane Bech, K.; Dahl, J.; Busk, J.; Brødsgaard, T.; Schmidt, E.R. Energy Production from Marine Biomass (Ulva lactuca); Danish Technological Institute: Taastrup, Denmark, 2011. [Google Scholar]
- Chynoweth, D.P.; Owens, J.M.; Legrand, R. Renewable methane from anaerobic digestion of biomass. Renew. Energy 2001, 22, 1–8. [Google Scholar] [CrossRef]
- Jingura, R.M.; Kamusoko, R. Methods for determination of biomethane potential of feedstocks: A review. Biofuel Res. J. 2017, 4, 573–586. [Google Scholar] [CrossRef]
- HELCOM. HELCOM Thematic Assessment of Eutrophication 2011–2016; Baltic Marine Environment Protection Commission—HELCOM Helsinki: Helsinki, Finland, 2018. [Google Scholar]
- Fox, S.E.; Stieve, E.; Valiela, I.; Hauxwell, J.; McClelland, J. Macrophyte abundance in Waquoit Bay: Effects of land-derived nitrogen loads on seasonal and multi-year biomass patterns. Estuar. Coasts 2008, 31, 532–541. [Google Scholar] [CrossRef]
- Duarte, C.M.; Wu, J.; Xiao, X.; Bruhn, A.; Krause-Jensen, D. Can seaweed farming play a role in climate change mitigation and adaptation? Front. Mar. Sci. 2017, 4. [Google Scholar] [CrossRef]
- Pastare, L.; Romagnoli, F.; Lauka, D.; Dzene, I.; Kuznecova, T. Sustainable Use Of Macro-Algae For Biogas Production in Latvian Conditions: A Preliminary Study Through an Integrated MCA and LCA Approach. Environ. Clim. Technol. 2014, 13, 44. [Google Scholar] [CrossRef]
- Balina, K.; Romagnoli, F.; Blumberga, D. Seaweed biorefinery concept for sustainable use of marine resources. Energy Procedia 2017, 128, 504–511. [Google Scholar] [CrossRef]
- Mhatre, A.; Gore, S.; Mhatre, A.; Trivedi, N.; Sharma, M.; Pandit, R.; Anil, A.; Lali, A. Effect of multiple product extractions on bio-methane potential of marine macrophytic green alga Ulva lactuca. Renew. Energy 2019, 132, 742–751. [Google Scholar] [CrossRef]
- Tedesco, S.; Daniels, S. Optimisation of biogas generation from brown seaweed residues: Compositional and geographical parameters affecting the viability of a biorefinery concept. Appl. Energy 2018, 228, 712–723. [Google Scholar] [CrossRef]
- Rajendran, K.; Browne, J.D.; Murphy, J.D. What is the level of incentivisation required for biomethane upgrading technologies with carbon capture and reuse? Renew. Energy 2019, 133, 951–963. [Google Scholar] [CrossRef]
- European Academies’ Science Advisory Council. Negative Emission Technologies: What Role in Meeting Paris Agreement Targets? Leopoldina: Halle, Germany, 2018. [Google Scholar]
- Mayfield, S.P. Consortium for Algal Biofuel Commercialization (CAB-COMM) Final Report; EE0003373; UC San Diego: La Jolla, CA, USA, 2015; 69p. [Google Scholar]
- Astals, S.; Musenze, R.S.; Bai, X.; Tannock, S.; Tait, S.; Pratt, S.; Jensen, P.D. Anaerobic co-digestion of pig manure and algae: Impact of intracellular algal products recovery on co-digestion performance. Bioresour. Technol. 2015, 181, 97–104. [Google Scholar] [CrossRef]
- Banks, C.; Zhang, Y. Optimising Inputs and Outputs from Anaerobic Digestion Processes—Technical Report; Defra: Southampton, UK, 2010. [Google Scholar]
- Golueke, C.G.; Oswald, W.J.; Gotaas, H.B. Anaerobic digestion of algae. Appl. Microbiol. 1957, 5, 47–55. [Google Scholar] [PubMed]
- Nallathambi Gunaseelan, V. Anaerobic digestion of biomass for methane production: A review. Biomass Bioenergy 1997, 13, 83–114. [Google Scholar] [CrossRef]
- Nguyen, H.; Heaven, S.; Banks, C. Energy potential from the anaerobic digestion of food waste in municipal solid waste stream of urban areas in Vietnam. Int. J. Energy Environ. Eng. 2014, 5, 365–374. [Google Scholar] [CrossRef]
- Jard, G.; Marfaing, H.; Carrere, H.; Delgenes, J.P.; Steyer, J.P.; Dumas, C. French Brittany macroalgae screening: Composition and methane potential for potential alternative sources of energy and products. Bioresour. Technol. 2013, 144, 492–498. [Google Scholar] [CrossRef]
- Soto, M.; Vazquez, M.A.; de Vega, A.; Vilarino, J.M.; Fernandez, G.; de Vicente, M.E. Methane potential and anaerobic treatment feasibility of Sargassum muticum. Bioresour Technol 2015, 189, 53–61. [Google Scholar] [CrossRef] [PubMed]
- Milledge, J.J.; Harvey, P.J. Ensilage and anaerobic digestion of Sargassum muticum. J. Appl. Phycol. 2016, 28, 3021–3030. [Google Scholar] [CrossRef]
- Roberts, K.P.; Heaven, S.; Banks, C.J. Comparative testing of energy yields from micro-algal biomass cultures processed via anaerobic digestion. Renew. Energy 2016, 87 Pt 1, 744–753. [Google Scholar] [CrossRef]
- Passos, F.; Gutiérrez, R.; Brockmann, D.; Steyer, J.-P.; García, J.; Ferrer, I. Microalgae production in wastewater treatment systems, anaerobic digestion and modelling using ADM1. Algal Res. 2015, 10, 55–63. [Google Scholar] [CrossRef]
- Fernandez-Rodriguez, M.J.; Rincon, B.; Fermoso, F.G.; Jimenez, A.M.; Borja, R. Assessment of two-phase olive mill solid waste and microalgae co-digestion to improve methane production and process kinetics. Bioresour. Technol. 2014, 157, 263–269. [Google Scholar] [CrossRef]
- Mussgnug, J.H.; Klassen, V.; Schluter, A.; Kruse, O. Microalgae as substrates for fermentative biogas production in a combined biorefinery concept. J. Biotechnol. 2010, 150, 51–56. [Google Scholar] [CrossRef]
- McKennedy, J.; Sherlock, O. Anaerobic digestion of marine macroalgae: A review. Renew. Sustain. Energy Rev. 2015, 52, 1781–1790. [Google Scholar] [CrossRef]
- Samson, R.; LeDuy, A. Improved performance of anaerobic digestion of Spirulina maxima algal biomass by addition of carbon-rich wastes. Biotechnol. Lett. 1983, 5, 677–682. [Google Scholar] [CrossRef]
- Gonzalez-Fernandez, C.; Sialve, B.; Bernet, N.; Steyer, J.P. Impact of microalgae characteristics on their conversion to biofuel. Part II: Focus on biomethane production. Biofuels Bioprod. Biorefining 2012, 6, 205–218. [Google Scholar] [CrossRef]
- Park, S.; Li, Y.B. Evaluation of methane production and macronutrient degradation in the anaerobic co-digestion of algae biomass residue and lipid waste. Bioresour. Technol. 2012, 111, 42–48. [Google Scholar] [CrossRef]
- Weiland, P. Biogas production: Current state and perspectives. Appl. Microbiol. Biotechnol. 2010, 85, 849–860. [Google Scholar] [CrossRef] [PubMed]
- Heaven, S.; Milledge, J.; Zhang, Y. Comments on ‘Anaerobic digestion of microalgae as a necessary step to make microalgal biodiesel sustainable’. Biotechnol. Adv. 2011, 29, 164–167. [Google Scholar] [CrossRef]
- Øverland, M.; Mydland, L.T.; Skrede, A. Marine macroalgae as sources of protein and bioactive compounds in feed for monogastric animals. J. Sci. Food Agric. 2019, 99, 13–24. [Google Scholar] [CrossRef]
- Becker, E.W. Microalgae. Biotechnology & Microbiology; Cambridge University Press: Cambridge, UK, 1994. [Google Scholar]
- Leal, M.C.; Munro, M.H.G.; Blunt, J.W.; Puga, J.; Jesus, B.; Calado, R.; Rosa, R.; Madeira, C. Biogeography and biodiscovery hotspots of macroalgal marine natural products. Nat. Prod. Rep. 2013, 30, 1380–1390. [Google Scholar] [CrossRef] [PubMed]
- Tabassum, M.R.; Xia, A.; Murphy, J.D. Biomethane production from various segments of brown seaweed. Energy Conv. Manag. 2018, 174, 855–862. [Google Scholar] [CrossRef]
- Monlau, F.; Sambusiti, C.; Barakat, A.; Quéméneur, M.; Trably, E.; Steyer, J.P.; Carrère, H. Do furanic and phenolic compounds of lignocellulosic and algae biomass hydrolyzate inhibit anaerobic mixed cultures? A comprehensive review. Biotechnol. Adv. 2014, 32, 934–951. [Google Scholar] [CrossRef]
- Streefland, M. Algae and Aquatic Biomass for a Sustainable Production of 2nd Generation Biofuels—Deliverable 1.5- Report on Biofuel Production Processes from Micro, Macroalgae and Other Aquatic Biomass; AquaFUELs: Brussels, Belgium, 2010. [Google Scholar]
- Campbell, P.K.; Beer, T.; Batten, D. Greenhouse Gas Sequestration by Algae-Energy & Greenhouse Gas Life Cycle Studies. In Proceedings of the 6th Australian Conference on Life Cycle Assessment, Melbourne, Australia, 17–19 February 2009. [Google Scholar]
- Gavrilescu, M.; Chisti, Y. Biotechnology—A sustainable alternative for chemical industry. Biotechnol. Adv. 2005, 23, 471–499. [Google Scholar] [CrossRef]
- Tamiya, H. Mass Culture of Algae. Annu. Rev. Plant Physiol. 1957, 8, 309–334. [Google Scholar] [CrossRef]
- Chisti, Y. Constraints to commercialization of algal fuels. J. Biotechnol. 2013, 167, 201–214. [Google Scholar] [CrossRef]
- Harris, P.W.; McCabe, B.K. Review of pre-treatments used in anaerobic digestion and their potential application in high-fat cattle slaughterhouse wastewater. Appl. Energy 2015, 155, 560–575. [Google Scholar] [CrossRef]
- Long, J.H.; Aziz, T.N.; Reyes, F.L.d.L.; Ducoste, J.J. Anaerobic co-digestion of fat, oil, and grease (FOG): A review of gas production and process limitations. Process Saf. Environ. Prot. 2012, 90, 231–245. [Google Scholar] [CrossRef]
- Jing, H.; Yu, D.; Xia, L.; Yunfei, Z.; Nengmin, Z.; Xiaobo, Y. A Review of Process Limitations and Microbial Community in Anaerobic Digestion of Fat, Oil, and Grease (Fog). Res. Rev. J. Microbiol. Biotechnol. 2016, 5. [Google Scholar]
- Dasa, K.T.; Westman, S.Y.; Millati, R.; Cahyanto, M.N.; Taherzadeh, M.J.; Niklasson, C. Inhibitory Effect of Long-Chain Fatty Acids on Biogas Production and the Protective Effect of Membrane Bioreactor. Biomed Res. Int. 2016, 2016, 7263974. [Google Scholar] [CrossRef]
- Molina-Alcaide, E.; Carro, M.D.; Roleda, M.Y.; Weisbjerg, M.R.; Lind, V.; Novoa-Garrido, M. In vitro ruminal fermentation and methane production of different seaweed species. Anim. Feed Sci. Technol. 2017, 228, 1–12. [Google Scholar] [CrossRef]
- Roy, S.S.; Pal, R. Microalgae in Aquaculture: A Review with Special References to Nutritional Value and Fish Dietetics. Proc. Zool. Soc. 2015, 68, 1–8. [Google Scholar] [CrossRef]
- Becker, E.W. Micro-algae as a source of protein. Biotechnol. Adv. 2007, 25, 207–210. [Google Scholar] [CrossRef]
- Metcalf, L.; Tchobanoglous, G. Wastewater Engineering: Treatment, Disposal, Reuse (Metcalf & Eddy); McGraw-Hill: New York, NY, USA, 1972. [Google Scholar]
- Rittmann, B.E.; McCarty, P.L. Environmental Biotechnology: Principles and Applications; McGraw-Hill: Singapore, 2001. [Google Scholar]
- Tian, H.; Fotidis, I.A.; Mancini, E.; Treu, L.; Mahdy, A.; Ballesteros, M.; González-Fernández, C.; Angelidaki, I. Acclimation to extremely high ammonia levels in continuous biomethanation process and the associated microbial community dynamics. Bioresour. Technol. 2018, 247, 616–623. [Google Scholar] [CrossRef]
- Jung, K.A.; Lim, S.R.; Kim, Y.; Park, J.M. Potentials of macroalgae as feedstocks for biorefinery. Bioresour. Technol. 2013, 135, 182–190. [Google Scholar] [CrossRef] [PubMed]
- Kostas, E.T.; White, D.A.; Du, C.; Cook, D.J. Selection of yeast strains for bioethanol production from UK seaweeds. J. Appl. Phycol. 2016, 28, 1427–1441. [Google Scholar] [CrossRef] [PubMed]
- Anastasakis, K.; Ross, A.B. Hydrothermal liquefaction of the brown macro-alga Laminaria Saccharina: Effect of reaction conditions on product distribution and composition. Bioresour. Technol. 2011, 102, 4876–4883. [Google Scholar] [CrossRef]
- Hilton, M.G.; Archer, D.B. Anaerobic digestion of a sulfate-rich molasses wastewater: Inhibition of hydrogen sulfide production. Biotechnol. Bioeng. 1988, 31, 885–888. [Google Scholar] [CrossRef] [PubMed]
- Tokusoglu, O.; Unal, M.K. Biomass nutrient profiles of three microalgae: Spirulina platensis, Chlorella vulgaris, and Isochrisis galbana. J. Food Sci. 2003, 68, 1144–1148. [Google Scholar] [CrossRef]
- Brown, M.R.; Jeffrey, S.W. The amino acid and gross composition of marine diatoms potentially useful for mariculture. J. Appl. Phycol. 1995, 7, 521–527. [Google Scholar] [CrossRef]
- Zeraatkar, A.K.; Ahmadzadeh, H.; Talebi, A.F.; Moheimani, N.R.; McHenry, M.P. Potential use of algae for heavy metal bioremediation, a critical review. J. Environ. Manag. 2016, 181, 817–831. [Google Scholar] [CrossRef] [PubMed]
- Besada, V.; Andrade, J.M.; Schultze, F.; Gonzalez, J.J. Heavy metals in edible seaweeds commercialised for human consumption. J. Mar. Syst. 2009, 75, 305–313. [Google Scholar] [CrossRef]
- Santos-Ballardo, D.U.; Rossi, S.; Reyes-Moreno, C.; Valdez-Ortiz, A. Microalgae potential as a biogas source: Current status, restraints and future trends. Rev. Environ. Sci. Biotechnol. 2016, 1–22. [Google Scholar] [CrossRef]
- Tarchevsky, I.A.; Marchenko, G.N. Cellulose: Biosynthesis and Structure; Springer: Berlin, Germany, 1991. [Google Scholar]
- Domozych, D.S. Algal Cell Walls. In eLS; John Wiley & Sons Ltd.: Chichester, UK, 2011. [Google Scholar]
- Cheng, Y.-S.; Zheng, Y.; Labavitch, J.M.; VanderGheynst, J.S. The impact of cell wall carbohydrate composition on the chitosan flocculation of Chlorella. Process Biochem. 2011, 46, 1927–1933. [Google Scholar] [CrossRef]
- Peeler, T.C.; Stephenson, M.B.; Einspahr, K.J.; Thompson, G.A. Lipid characterization of an enriched plasma-membrane fraction of Dunaliella salina grown in media of varying salinity. Plant Physiol. 1989, 89, 970–976. [Google Scholar] [CrossRef]
- Ben-Amotz, A.; Polle, J.E.W.; Subba Rao, D.V. The alga Dunaliella: Biodiversity, Physiology, Genomics and Biotechnology; Science Publishers: Enfleld, NJ, USA, 2009. [Google Scholar]
- Xu, Y.; Milledge, J.J.; Abubakar, A.; Swamy, R.; Bailey, D.; Harvey, P.J. Effects of centrifugal stress on cell disruption and glycerol leakage from Dunaliella salina. Microalgae Biotechnol. 2015, 1, 20–27. [Google Scholar] [CrossRef]
- Wang, J.-K.; Seibert, M. Prospects for commercial production of diatoms. Biotechnol. Biofuels 2017, 10, 16. [Google Scholar] [CrossRef]
- Royal Society. Ocean Acidification due to Increasing Atmospheric Carbon Dioxide; Policy Document 12/050; Royal Society: London, UK, 2005. [Google Scholar]
- Ganesh Saratale, R.; Kumar, G.; Banu, R.; Xia, A.; Periyasamy, S.; Dattatraya Saratale, G. A critical review on anaerobic digestion of microalgae and macroalgae and co-digestion of biomass for enhanced methane generation. Bioresour. Technol. 2018, 262, 319–332. [Google Scholar] [CrossRef]
- Szwaja, S.; Dębowski, M.; Zieliński, M.; Kisielewska, M.; Stańczyk-Mazanek, E.; Sikorska, M. Influence of a light source on microalgae growth and subsequent anaerobic digestion of harvested biomass. Biomass Bioenergy 2016, 91, 243–249. [Google Scholar] [CrossRef]
- Fermoso, F.G.; Beltran, C.; Jimenez, A.; Fernandez, M.J.; Rincon, B.; Borja, R.; Jeison, D. Screening of biomethane production potential from dominant microalgae. J. Environ. Sci. Health Part A-Toxic/Hazard. Subst. Environ. Eng. 2016, 51, 1062–1067. [Google Scholar] [CrossRef]
- Dixon, C.; Wilken, L.R. Green microalgae biomolecule separations and recovery. Bioresour. Bioprocess. 2018, 5, 14. [Google Scholar] [CrossRef]
- Gruber-Brunhumer, M.R.; Jerney, J.; Zohar, E.; Nussbaumer, M.; Hieger, C.; Bromberger, P.; Bochmann, G.; Jirsa, F.; Schagerl, M.; Obbard, J.P.; et al. Associated effects of storage and mechanical pre-treatments of microalgae biomass on biomethane yields in anaerobic digestion. Biomass Bioenergy 2016, 93, 259–268. [Google Scholar] [CrossRef]
- Kloareg, B.; Quatrano, R.S. Structure of the cell walls of marine algae and ecophysiological functions of the matrix polysaccharides. Oceanogr. Mar. Biol. 1988, 26, 259–315. [Google Scholar]
- Hurd, C.L.; Harrison, P.J.; Bischof, K.; Lobban, C.S. Seaweed Ecology and Physiology, 2nd ed.; Cambridge University Press: Cambridge, UK, 2014. [Google Scholar]
- Baldan, B.; Andolfo, P.; Navazio, L.; Tolomio, C.; Mariani, P. Cellulose in algal cell wall: An “in situ” localization. Eur. J. Histochem. 2001, 45, 51–56. [Google Scholar] [CrossRef] [PubMed]
- Deniaud-Bouet, E.; Kervarec, N.; Michel, G.; Tonon, T.; Kloareg, B.; Herve, C. Chemical and enzymatic fractionation of cell walls from Fucales: Insights into the structure of the extracellular matrix of brown algae. Ann. Bot. 2014, 114, 1203–1216. [Google Scholar] [CrossRef] [PubMed]
- Vanegas, C.H.; Hernon, A.; Bartlett, J. Enzymatic and organic acid pretreatment of seaweed: Effect on reducing sugars production and on biogas inhibition. Int. J. Ambient Energy 2015, 36, 2–7. [Google Scholar] [CrossRef]
- Moen, E.; Horn, S.; Østgaard, K. Biological degradation of Ascophyllum nodosum. J. Appl. Phycol. 1997, 9, 347–357. [Google Scholar] [CrossRef]
- Berteau, O.; Mulloy, B. Sulfated fucans, fresh perspectives: Structures, functions, and biological properties of sulfated fucans and an overview of enzymes active toward this class of polysaccharide. Glycobiology 2003, 13, 29R–40R. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Jasso, R.M.; Mussatto, S.I.; Pastrana, L.; Aguilar, C.N.; Teixeira, J.A. Chemical composition and antioxidant activity of sulphated polysaccharides extracted from Fucus vesiculosus using different hydrothermal processes. Chem. Pap. 2014, 68, 203–209. [Google Scholar] [CrossRef]
- Robic, A.; Sassi, J.-F.; Dion, P.; Lerat, Y.; Lahaye, M. Seasonal variability of physicochemical and rheological properties of ulvan in two Ulva species (Chlorophyta) from the Brittany coast 1. J. Phycol. 2009, 45, 962–973. [Google Scholar] [CrossRef] [PubMed]
- Savithramma, N.; Linga Rao, M.; Venkateswarlu, P. Isolation and Identification of Phenolic Compounds from Boswellia ovalifoliolata Bal. & Henry and Their Free Radical Scavenger Activity. Int. J. Drug Deliv. Technol. 2014, 4, 14–21. [Google Scholar]
- Pérez, M.J.; Falqué, E.; Domínguez, H. Antimicrobial Action of Compounds from Marine Seaweed. Mar. Drugs 2016, 14, 52. [Google Scholar] [CrossRef]
- Holdt, S.; Kraan, S. Bioactive compounds in seaweed: Functional food applications and legislation. J. Appl. Phycol. 2011, 23, 543–597. [Google Scholar] [CrossRef]
- Glombitza, K.W.; Forster, M.; Farnham, W.F. Antibiotics from algae. 25. Polyhydroxyphenyl ethers from the brown alga Sargassum muticum (yendo) fensholt. Bot. Mar. 1982, 25, 449–453. [Google Scholar] [CrossRef]
- Montero, L.; Sanchez-Camargo, A.P.; Garcia-Canas, V.; Tanniou, A.; Stiger-Pouvreau, V.; Russo, M.; Rastrelli, L.; Cifuentes, A.; Herrero, M.; Ibanez, E. Anti-proliferative activity and chemical characterization by comprehensive two-dimensional liquid chromatography coupled to mass spectrometry of phlorotannins from the brown macroalga Sargassum muticum collected on North-Atlantic coasts. J. Chromatogr. A 2016, 1428, 115–125. [Google Scholar] [CrossRef] [PubMed]
- Moorthi, P.V.; Balasubramanian, C. Antimicrobial properties of marine seaweed, Sargassum muticum against human pathogens. J. Coast. Life Med. 2015, 3, 122–125. [Google Scholar] [CrossRef]
- Lordan, S.; Smyth, T.J.; Soler-Vila, A.; Stanton, C.; Ross, R.P. The α-amylase and α-glucosidase inhibitory effects of Irish seaweed extracts. Food Chem. 2013, 141, 2170–2176. [Google Scholar] [CrossRef] [PubMed]
- Dai, X.; Li, X.; Zhang, D.; Chen, Y.; Dai, L. Simultaneous enhancement of methane production and methane content in biogas from waste activated sludge and perennial ryegrass anaerobic co-digestion: The effects of pH and C/N ratio. Bioresour. Technol. 2016, 216, 323–330. [Google Scholar] [CrossRef] [PubMed]
- Tanniou, A.; Vandanjon, L.; Incera, M.; Leon, E.S.; Husa, V.; Le Grand, J.; Nicolas, J.L.; Poupart, N.; Kervarec, N.; Engelen, A.; et al. Assessment of the spatial variability of phenolic contents and associated bioactivities in the invasive alga Sargassum muticum sampled along its European range from Norway to Portugal. J. Appl. Phycol. 2014, 26, 1215–1230. [Google Scholar] [CrossRef]
- Tabassum, M.R.; Xia, A.; Murphy, J.D. Seasonal variation of chemical composition and biomethane production from the brown seaweed Ascophyllum nodosum. Bioresour. Technol. 2016, 216, 219–226. [Google Scholar] [CrossRef]
- Milledge, J.J.; Nielsen, B.V.; Harvey, P.J. The inhibition of anaerobic digestion by model phenolic compounds representative of those from Sargassum muticum. J. Appl. Phycol. 2018. [Google Scholar] [CrossRef]
- Hierholtzer, A.; Akunna, J.C. Modelling sodium inhibition on the anaerobic digestion process. Water Sci. Technol. 2012, 66, 1565–1573. [Google Scholar] [CrossRef]
- Lefebvre, O.; Moletta, R. Treatment of organic pollution in industrial saline wastewater: A literature review. Water Res. 2006, 40, 3671–3682. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Alam, M.A.; Kong, X.; Wang, Z.; Li, L.; Sun, Y.; Yuan, Z. Effect of salinity on the microbial community and performance on anaerobic digestion of marine macroalgae. J. Chem. Technol. Biotechnol. 2017, 92, 2392–2399. [Google Scholar] [CrossRef]
- Chen, Y.; Cheng, J.J.; Creamer, K.S. Inhibition of anaerobic digestion process: A review. Bioresour. Technol. 2008, 99, 4044–4064. [Google Scholar] [CrossRef]
- Chen, W.H.; Han, S.K.; Sung, S. Sodium inhibition of thermophilic methanogens. J. Environ. Eng. 2003, 129, 506–512. [Google Scholar] [CrossRef]
- Ramakrishnan, B.; Kumaraswamy, S.; Mallick, K.; Adhya, T.K.; Rao, V.R.; Sethunathan, N. Effect of various anionic species on net methane production in flooded rice soils. World J. Microbiol. Biotechnol. 1998, 14, 743–749. [Google Scholar] [CrossRef]
- El-Dessouky, H.T.; Ettouney, H.M. Fundamentals of Salt Water Desalination; Elsevier: Amsterdam, The Nertherlands, 2002. [Google Scholar]
- Nikolaison, L.; Dahl, J.; Bech, K.S.; Bruhn, A.; Rasmussen, M.B.; Bjerre, A.B.; Nielsen, H.B.; Ambus, P.; Rost, K.A.; Kadar, Z.; et al. Energy Production from Macroalgae. In Proceedings of the 20th European Biomass Conference, Milan, Italy, 18–22 June 2012. [Google Scholar]
- Adams, J.M.M.; Schmidt, A.; Gallagher, J.A. The impact of sample preparation of the macroalgae Laminaria digitata on the production of the biofuels bioethanol and biomethane. J. Appl. Phycol. 2015, 27, 985–991. [Google Scholar] [CrossRef]
- Milledge, J.; Nielsen, B.; Sadek, M.; Harvey, P. Effect of Freshwater Washing Pretreatment on Sargassum muticum as a Feedstock for Biogas Production. Energies 2018, 11, 1771. [Google Scholar] [CrossRef]
- Roberts, K.P.; Heaven, S.; Banks, C.J. Quantification of methane losses from the acclimatisation of anaerobic digestion to marine salt concentrations. Renew. Energy 2016, 86, 497–506. [Google Scholar] [CrossRef]
- Marquez, G.P.B.; Reichardt, W.T.; Azanza, R.V.; Onda, D.F.L.; Lluisma, A.O.; Montaño, M.N.E.J.W.; Valorization, B. Dominance of Hydrogenotrophic Methanogens at the Peak of Biogas Production in Thalassic Digesters. Waste Biomass Valoriz. 2015, 6, 201–207. [Google Scholar] [CrossRef]
- Jard, G.; Jackowiak, D.; Carrère, H.; Delgenes, J.P.; Torrijos, M.; Steyer, J.P.; Dumas, C. Batch and semi-continuous anaerobic digestion of Palmaria palmata: Comparison with Saccharina latissima and inhibition studies. Chem. Eng. J. 2012, 209, 513–519. [Google Scholar] [CrossRef]
- Sudmalis, D.; Millah, S.K.; Gagliano, M.C.; Butré, C.I.; Plugge, C.M.; Rijnaarts, H.H.M.; Zeeman, G.; Temmink, H. The potential of osmolytes and their precursors to alleviate osmotic stress of anaerobic granular sludge. Water Res. 2018, 147, 142–151. [Google Scholar] [CrossRef]
- Vyrides, I.; Stuckey, D.C. Compatible solute addition to biological systems treating waste/wastewater to counteract osmotic and other environmental stresses: A review. Crit. Rev. Biotechnol. 2017, 37, 865–879. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Fang, Z.; Liang, P.; Zhang, X.; Qiu, Y.; Kimura, K.; Huang, X. Anaerobic digestion performance of concentrated municipal sewage by forward osmosis membrane: Focus on the impact of salt and ammonia nitrogen. Bioresour. Technol. 2019, 276, 204–210. [Google Scholar] [CrossRef]
- Wang, X.; Lu, X.; Li, F.; Yang, G. Effects of Temperature and Carbon-Nitrogen (C/N) Ratio on the Performance of Anaerobic Co-Digestion of Dairy Manure, Chicken Manure and Rice Straw: Focusing on Ammonia Inhibition. PLoS ONE 2014, 9, e97265. [Google Scholar] [CrossRef] [PubMed]
- Puyuelo, B.; Ponsá, S.; Gea, T.; Sánchez, A. Determining C/N ratios for typical organic wastes using biodegradable fractions. Chemosphere 2011, 85, 653–659. [Google Scholar] [CrossRef] [PubMed]
- Persson, S.P.E.; Bartlett, H.D.; Branding, A.E.; Regan, R.W. Agricultural Anaerobic Digesters; Pennsylvania State University: Pennsylvania, PA, USA, 1979. [Google Scholar]
- Peu, P.; Picard, S.; Diara, A.; Girault, R.; Béline, F.; Bridoux, G.; Dabert, P. Prediction of hydrogen sulphide production during anaerobic digestion of organic substrates. Bioresour. Technol. 2012, 121, 419–424. [Google Scholar] [CrossRef] [PubMed]
- Baird, M.E.; Middleton, J.H. On relating physical limits to the carbon: Nitrogen ratio of unicellular algae and benthic plants. J. Mar. Syst. 2004, 49, 169–175. [Google Scholar] [CrossRef]
- Wernberg, T.; Thomsen, M.S.; Staehr, P.A.; Pedersen, M.F. Comparative phenology of Sargassum muticum and Halidrys siliquosa (Phaeophyceae: Fucales) in Limfjorden, Denmark. Bot. Mar. 2001, 44, 31–39. [Google Scholar] [CrossRef]
- Lapointe, B.E.; West, L.E.; Sutton, T.T.; Hu, C. Ryther revisited: Nutrient excretions by fishes enhance productivity of pelagic Sargassum in the western North Atlantic Ocean. J. Exp. Mar. Biol. Ecol. 2014, 458, 46–56. [Google Scholar] [CrossRef]
- Oyesiku, O.O.; Egunyomi, A. Identification and chemical studies of pelagic masses of Sargassum natans (Linnaeus) Gaillon and S. fluitans (Borgessen) Borgesen (brown algae), found offshore in Ondo State, Nigeria. Afr. J. Biotechnol. 2014, 13, 1188–1193. [Google Scholar] [CrossRef]
- Wang, S.; Wang, Q.; Jiang, X.M.; Han, X.X.; Ji, H.S. Compositional analysis of bio-oil derived from pyrolysis of seaweed. Energy Conv. Manag. 2013, 68, 273–280. [Google Scholar] [CrossRef]
- Jankowska, E.; Sahu, A.K.; Oleskowicz-Popiel, P. Biogas from microalgae: Review on microalgae’s cultivation, harvesting and pretreatment for anaerobic digestion. Renew. Sustain. Energy Rev. 2017, 75, 692–709. [Google Scholar] [CrossRef]
- Solé-Bundó, M.; Garfí, M.; Matamoros, V.; Ferrer, I. Co-digestion of microalgae and primary sludge: Effect on biogas production and microcontaminants removal. Sci. Total Environ. 2019. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, J.V.; Alves, M.M.; Costa, J.C. Optimization of biogas production from Sargassum sp. using a design of experiments to assess the co-digestion with glycerol and waste frying oil. Bioresour. Technol. 2015, 175, 480–485. [Google Scholar] [CrossRef] [PubMed]
- Angelidaki, I.; Karakashev, D.; Alvarado-Morales, M. Anaerobic Co-Digestion of Cast Seaweed and Organic Residues; Technical University of Denmark: Lyngby, Denmark, 2017. [Google Scholar]
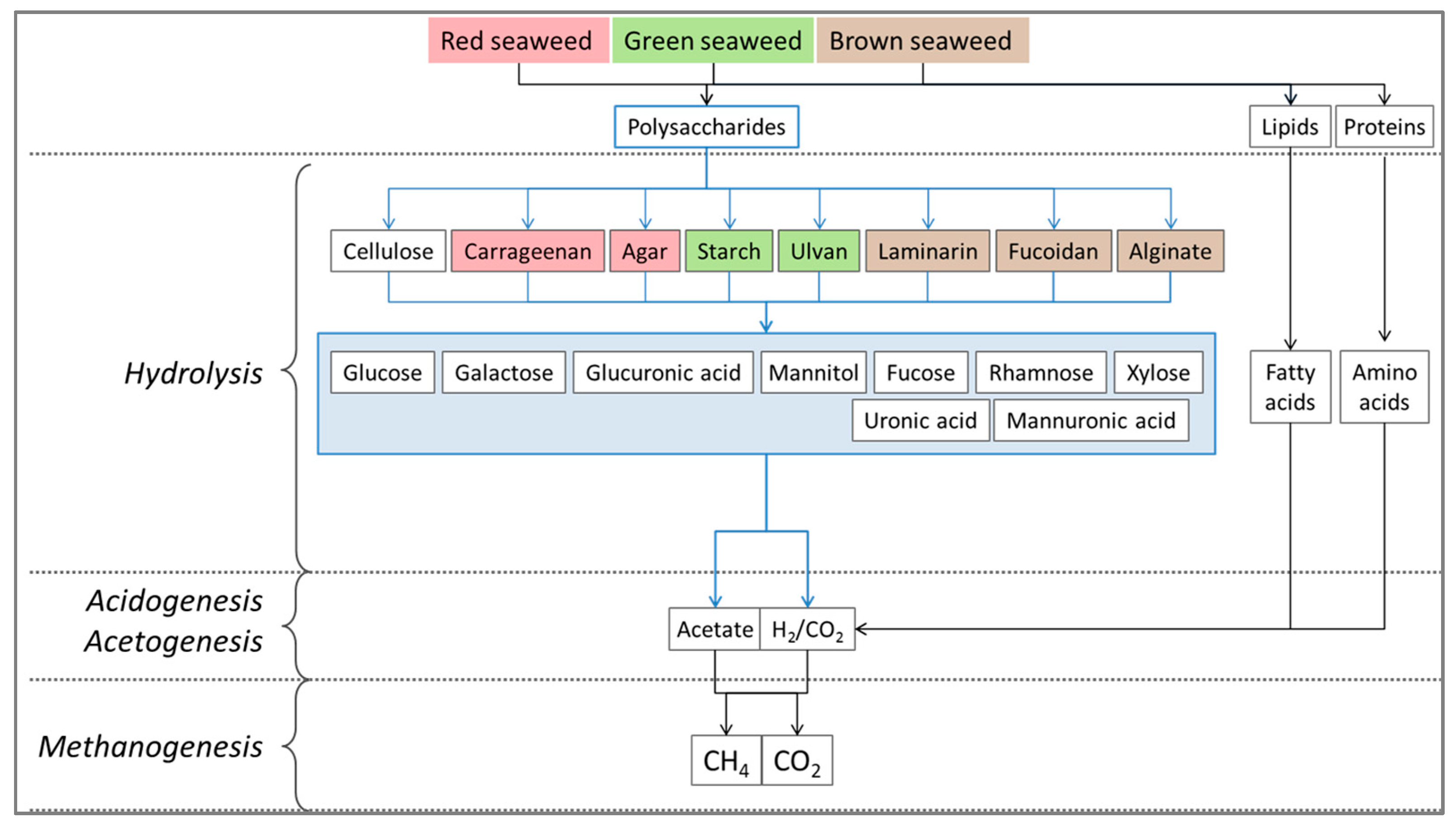
| Species | Location | Production (Wet Tonnes) 2014–2009 (% Increase) | Industry | Harvesting |
|---|---|---|---|---|
| Eucheuma spp | China, Philippines, United Rep. of Tanzania, Kiribati, Indonesia, Malaysia, Solomon Islands and Fiji Islands | 9,053,044 (2014) 2,875,547 (2009) 315% | Carrageenan production | 10–12 weeks |
| Laminaria japonica | China, Japan, the RO Korea and the Democratic People’s Republic of Korea. | 7,654,586 (2014) 4,930,705 (2009) 155% | Algin, mannitol, and iodine | 8 months |
| Porphyra spp | China, RO Korea, and Japan | 1,141,710 (2014) 1,074,750 (2009) 106% | Food (Nori) | 10 days to 5 months |
| Gracilaria spp | Asia (Indonesia, Korea Philippines, Vietnam China, Taiwan), Africa (Namibia and South Africa), America (Argentina, Brazil, Chile and Peru), | 3,751,395 (2014) 1,526,393 (2009) 246% | Agar | 30–45 days |
| Undaria pinnatifida | China, RO Korea, and Japan Small scale cultivation French Brittany coast and the Spanish Galician coast, | 2,358,597 (2014) 1,694,540 (2009) 139% | Food (Wakame) | Yearly |
| Algae | Lipids | Proteins | Carbohydrates |
|---|---|---|---|
| Green algae | |||
| Codium fragile | 1.8 | 10.9 | 32.3 |
| Enteromorpha linza | 1.8 | 31.6 | 37.4 |
| Ulva Lactuca | 6.2 | 20.6 | 54.3 |
| Red algae | |||
| Gelidium amansii | 0–3.1 | 15.6–16.3 | 61–67.3 |
| Porphyra tenera | 4.4 | 38.7 | 35.9 |
| Gracilaria verrucosa | 3.2 | 15.6 | 33.5 |
| Brown algae | |||
| Laminaria japonica | 1.8–2.4 | 9.4–14.8 | 51.9–59.7 |
| Hizikia fusiforme | 0.4–1.5 | 5.9–13.9 | 28.6–59 |
| Saccharina japonica | 0.5 | 19.9 | 44.5 |
| Sargassum fulvellum | 1.6 | 10.6 | 66 |
| Ecklonia stolonifera | 2.4 | 13.6 | 48.6 |
| Unduria pinnatifida | 1.8–2.0 | 15.9–18.3 | 40.1–52 |
| Sargassum fulvelum | 1.4 | 13 | 39.6 |
| Algae | Lipid | Protein | Carbohydrate |
|---|---|---|---|
| Spirulina platensis | 4–9 | 50–65 | 8–14 |
| Chlorella sp. | 14–22 | 51–58 | 12–17 |
| Scenedesmus sp. | 12–14 | 50–56 | 10–52 |
| Dunaliella sp. | 6–8 | 49–57 | 4–32 |
| Synechococcus sp. | 11 | 63 | 15 |
| Euglena sp. | 14–20 | 39–61 | 14–18 |
| Prymnesium sp. | 22–38 | 28–45 | 25–33 |
| Anabaena sp. | 4–7 | 48 | 25–30 |
| Chlamydomonas sp. | 14–22 | 43–56 | 2.9–17 |
| Spirulina maxima | 6–7 | 60–71 | 13–16 |
| Spirogyra | 11–21 | 6–20 | 33–64 |
| Tetraselmis | 16–45 | 52 | 15 |
| Algae | Ash | Carbon | Hydrogen | Oxygen | Nitrogen | Sulphur |
|---|---|---|---|---|---|---|
| Fucus vesiculosus1 | 22.82 | 32.88 | 4.77 | 35.63 | 2.53 | 2.44 |
| Chorda filum1 | 11.61 | 39.14 | 4.69 | 37.23 | 1.42 | 1.62 |
| Laminaria digitata1 | 25.75 | 31.59 | 4.85 | 34.16 | 0.9 | 2.44 |
| Fucus serratus1 | 23.36 | 33.5 | 4.78 | 34.44 | 2.39 | 1.31 |
| Laminaria hyperborea1 | 17.97 | 34.97 | 5.31 | 35.09 | 1.12 | 2.06 |
| Macrocyctis pyrifera1 | 38.35 | 27.3 | 4.08 | 34.8 | 2.03 | 1.89 |
| Laminaria saccharina2 | 24.2 | 31.3 | 3.7 | 36.3 | 2.4 | 0.7 |
| Sargassum muticum3 | 29.45 | 30.66 | 3.95 | 29.56 | 4.89 | 1.49 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Milledge, J.J.; Nielsen, B.V.; Maneein, S.; Harvey, P.J. A Brief Review of Anaerobic Digestion of Algae for Bioenergy. Energies 2019, 12, 1166. https://doi.org/10.3390/en12061166
Milledge JJ, Nielsen BV, Maneein S, Harvey PJ. A Brief Review of Anaerobic Digestion of Algae for Bioenergy. Energies. 2019; 12(6):1166. https://doi.org/10.3390/en12061166
Chicago/Turabian StyleMilledge, John J., Birthe V. Nielsen, Supattra Maneein, and Patricia J. Harvey. 2019. "A Brief Review of Anaerobic Digestion of Algae for Bioenergy" Energies 12, no. 6: 1166. https://doi.org/10.3390/en12061166
APA StyleMilledge, J. J., Nielsen, B. V., Maneein, S., & Harvey, P. J. (2019). A Brief Review of Anaerobic Digestion of Algae for Bioenergy. Energies, 12(6), 1166. https://doi.org/10.3390/en12061166







