Optimization of the Electrolyte Parameters and Components in Zinc Particle Fuel Cells
Abstract
1. Introduction
2. Methodology
2.1. Construction of Experimental System Platform
2.2. Construction of Zn-air Fuel Cells
2.3. Measurements and Analyses
3. Results and Discussion
3.1. Effects of Flowing Electrolyte Parameters on ZAFC Power Performance
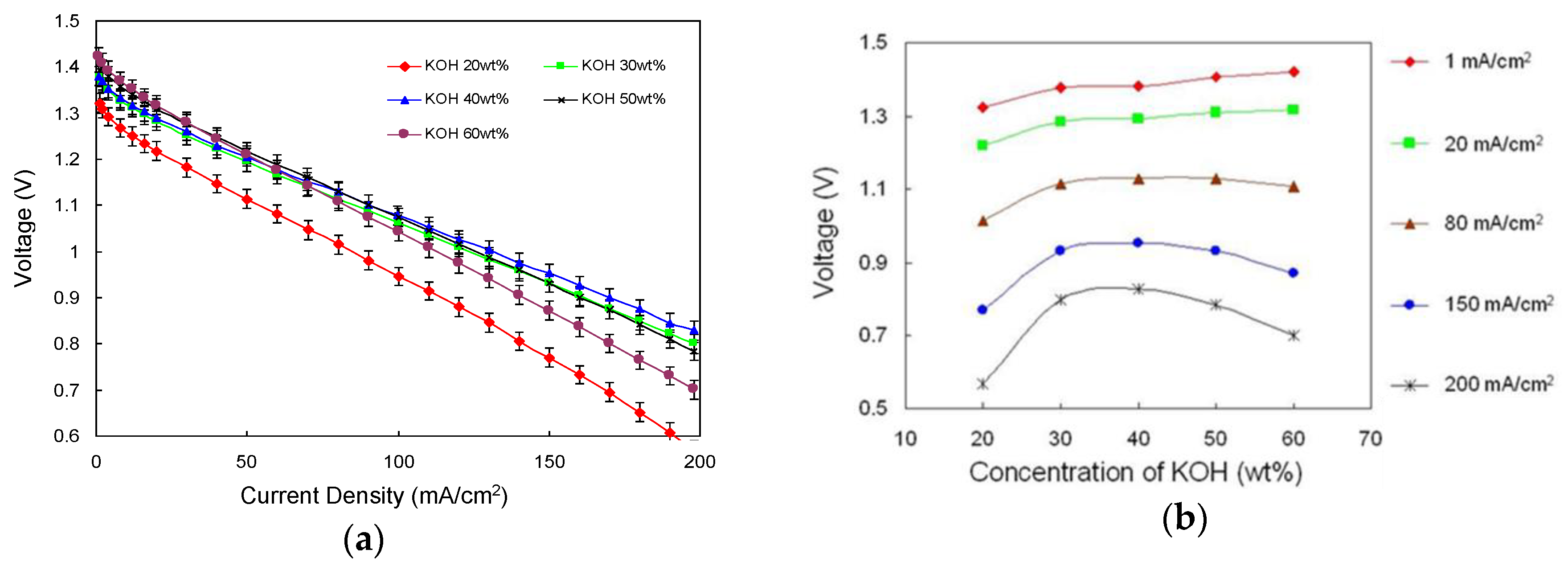
3.1.1. Concentration of Electrolyte
3.1.2. Flowing Electrolyte Temperature
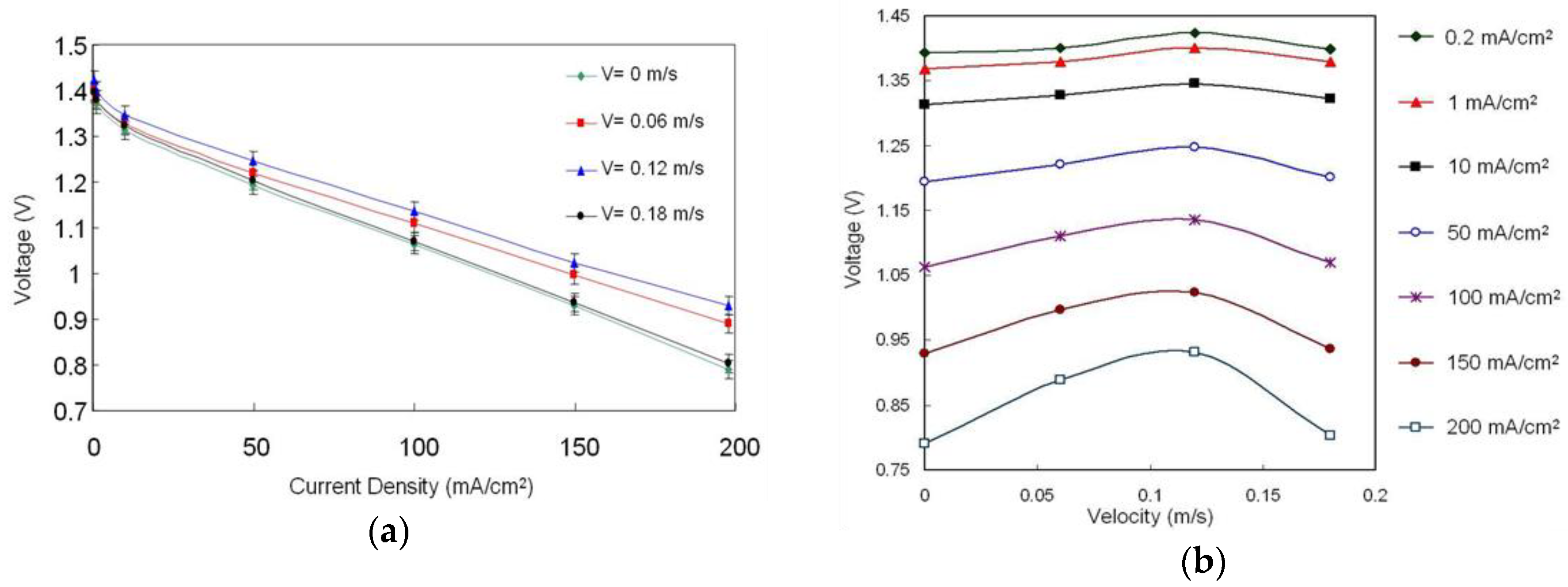
3.1.3. Influence of Electrolyte Flow Velocity
3.2. Effect of Current Collector Surface area
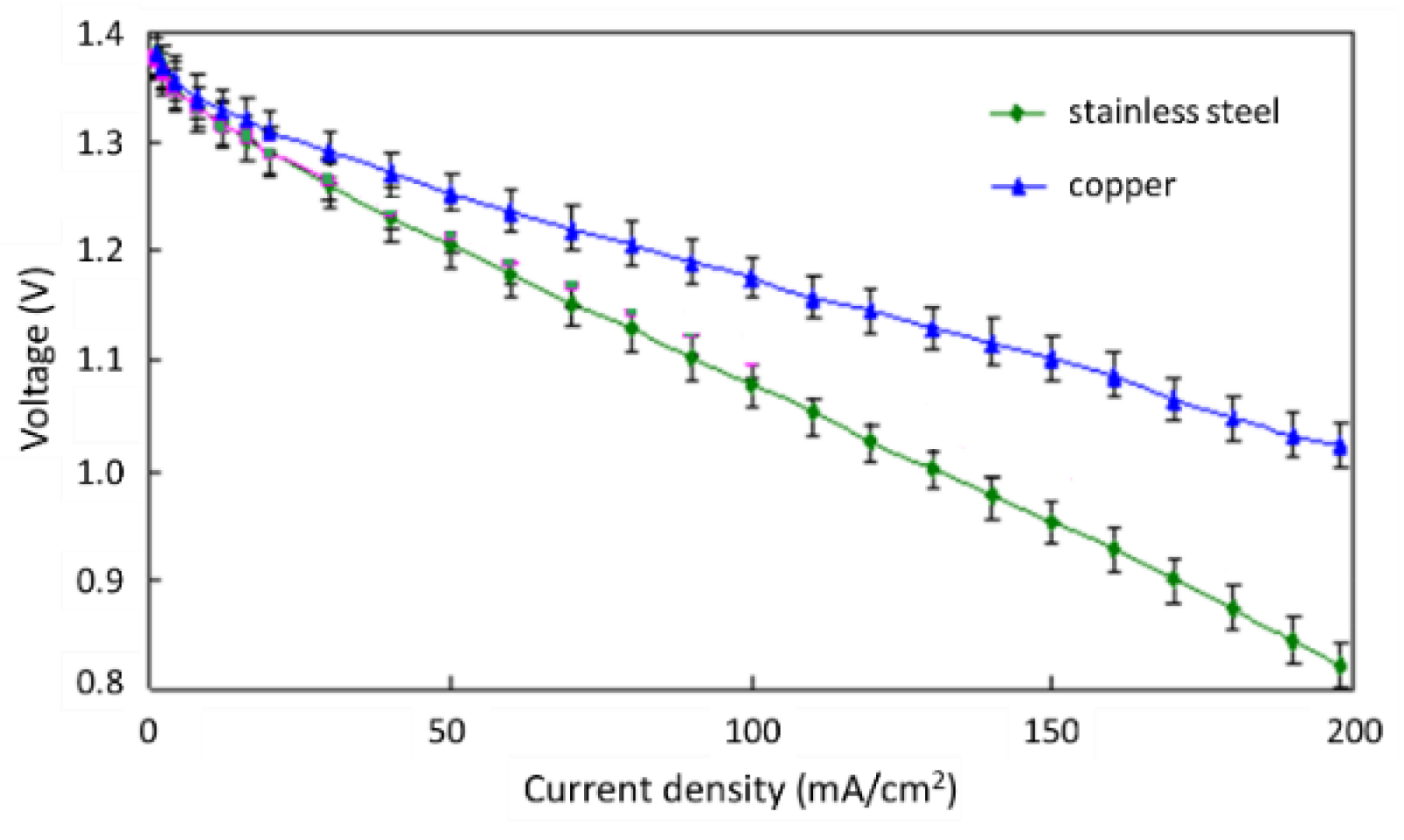
3.3. Conductor Material at an Air Electrode
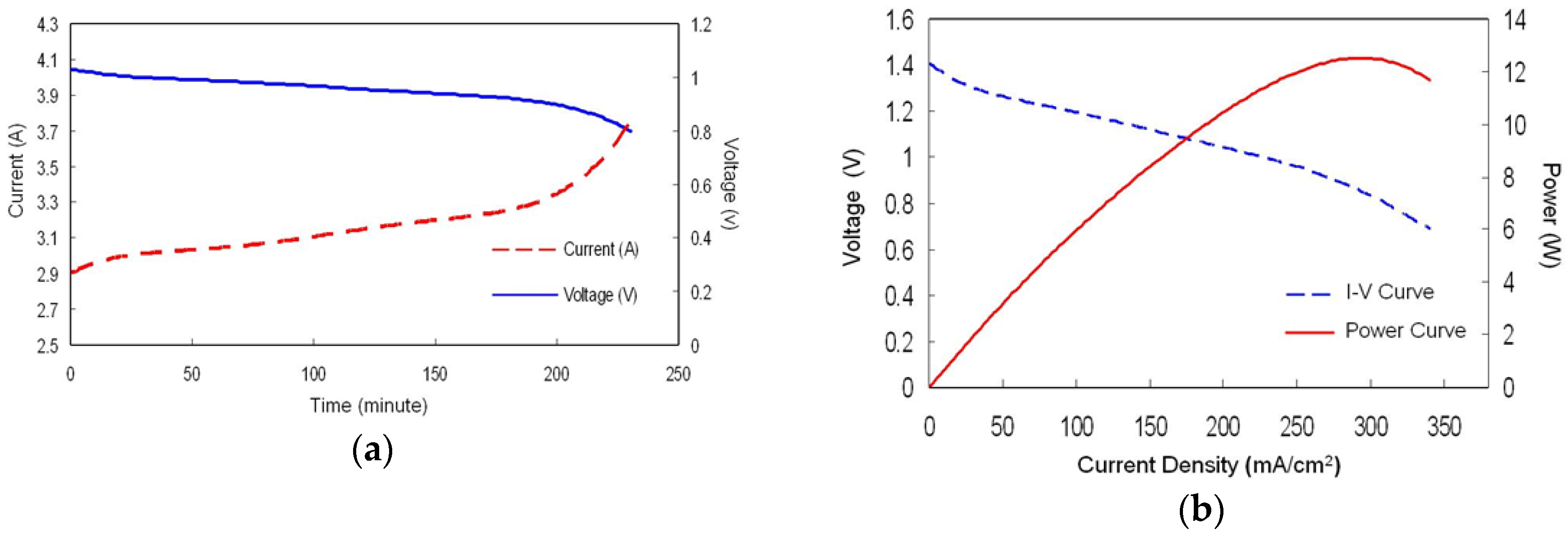
3.4. Power Density Curves
4. Conclusions
- A maximum cell voltage at 200 mA cm−2 was obtained at the KOH concentration of 40 wt%, but a further increase in the electrolyte concentration to 50 wt% and 60 wt% reduced the cell voltage. However, higher voltages can be maintained while discharging at high current densities.
- Furthermore, the increase of electrolyte temperature, resulted in an increase in the ionic conductivity. The temperature of the electrolyte increased from room temperature to 65 ℃, and the cell voltage increased obviously. The loss of H2O in electrolyte at higher temperature, aggravated the polarization concentration of the fuel cell, leading to a slow rise in voltage.
- The electrolyte flow velocity should be maintained at 0.12 m/s for an enhanced performance. The experimental results display that as the surface area and grid shape of the anode current conductor (Ni mesh) increased, more electrons were conducted and the fuel cell power production was increased. The shape and area of the collector grid should be designed to facilitate the addition of Zn particles and increase the area in a limited space.
- The air-cathode conductor material should be made of copper metal rather than carbon black, which can increase the ionic conductivity and facilitate heightened power performance of the ZAFC.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wang, C.T.; Sangeetha, T.; Yan, W.M.; Chong, W.T.; Saw, L.H.; Zhao, F.; Chang, C.T.; Wang, C.H. Application of interface material and effects of oxygen gradient on the performance of single-chamber sediment microbial fuel cells (SSMFCs). J. Environ. Sci. 2019, 75, 163–168. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.T.; Sangeetha, T.; Zhao, F.; Garg, A.; Chang, C.T.; Wang, C.H. Sludge selection on the performance of sediment microbial fuel cells. Int. J. Energy Res. 2018, 42, 4250–4255. [Google Scholar] [CrossRef]
- Oh, S.J.; Min, Y.J.; Lee, M.H.; Choi, J.H.; Kim, M.S.; Jo, N.J.; Eom, S. Design and electrochemical characteristics of single-layer cathode for flexible tubular type zinc-air fuel cells. J. Alloys Compd. 2018, 740, 895–900. [Google Scholar] [CrossRef]
- Xu, M.; Ivey, D.G.; Xie, Z.; Qu, W. Rechargeable Zn-air batteries: Progress in electrolyte development and cell configuration advancement. J. Power Sources 2015, 283, 358–371. [Google Scholar] [CrossRef]
- Zhu, B.; Huang, Y.; Fan, L.; Ma, Y.; Wang, B.; Xia, C.; Afzal, M.; Zhang, B.; Dong, W.; Wang, H.; et al. Novel fuel cell with nanocomposite functional layer designed by perovskite solar cell principle. Nano Energy 2016, 19, 156–164. [Google Scholar] [CrossRef]
- Huang, K.D.; Sangeetha, T.; Cheng, W.F.; Lin, C.; Chen, P.T. Computational fluid dynamics approach for performance prediction in a Zinc–air fuel cell. Energies 2018, 11, 2185. [Google Scholar] [CrossRef]
- Mele, C.; Bilotta, A.; Bocchetta, P.; Bozzini, B. Characterization of the particulate anode of a laboratory flow Zn–air fuel cell. J. Appl. Electrochem. 2017, 47, 877–888. [Google Scholar] [CrossRef]
- Wang, K.; Pei, P.; Wang, Y.; Liao, C.; Wang, W.; Huang, S. Advanced rechargeable zinc-air battery with parameter optimization. Appl. Energy 2018, 225, 848–856. [Google Scholar] [CrossRef]
- Wang, K.; Pei, P.; Ma, Z.; Xu, H.; Li, P.; Wang, X. Morphology control of zinc regeneration for zinc–air fuel cell and battery. J. Power Sources 2014, 271, 65–75. [Google Scholar] [CrossRef]
- Arenas, L.F.; Loh, A.; Trudgeon, D.P.; Li, X.; de León, C.P.; Walsh, F.C. The characteristics and performance of hybrid redox flow batteries with zinc negative electrodes for energy storage. Renew. Sustain. Energy Rev. 2018, 90, 992–1016. [Google Scholar] [CrossRef]
- Fu, J.; Cano, Z.P.; Park, M.G.; Yu, A.; Fowler, M.; Chen, Z. Electrically rechargeable zinc-air batteries: Progress, challenges, and perspectives. Adv. Mater. 2017, 29, 1604685. [Google Scholar] [CrossRef] [PubMed]
- Park, J.E.; Lim, M.S.; Kim, J.K.; Choi, H.J.; Sung, Y.E.; Cho, Y.H. Optimization of cell components and operating conditions in primary and rechargeable zinc–air battery. J. Ind. Eng. Chem. 2019, 69, 161–170. [Google Scholar] [CrossRef]
- Cai, X.; Lai, L.; Lin, J.; Shen, Z. Correction: Recent advances in air electrodes for Zn–air batteries: Electrocatalysis and structural design. Mater. Horiz. 2017, 4, 945–976. [Google Scholar] [CrossRef]
- An, K.; Zheng, Y.; Xu, X.; Wang, Y. Filter paper derived three-dimensional mesoporous carbon with Co3O4 loaded on surface: An excellent binder-free air-cathode for rechargeable Zinc-air battery. J. Solid State Chem. 2019, 270, 539–546. [Google Scholar] [CrossRef]
- Chen, Y.M.; Wang, C.T.; Yang, Y.C. Effect of wall boundary layer thickness on power performance of a recirculation microbial fuel cell. Energies 2018, 11, 1003. [Google Scholar] [CrossRef]
- Wang, C.T.; Huang, Y.S.; Sangeetha, T.; Yan, W.M. Assessment of recirculation batch mode operation in bufferless bio-cathode microbial fuel cells (MFCs). Appl. Energy 2018, 209, 120–126. [Google Scholar] [CrossRef]
- Lan, T.H.; Yan, W.M.; Sangeetha, T.; Ou, Y.T.; Wang, C.T.; Yang, Y.C. 2D Numerical physical model settings for three electron transfer pathways in microbial fuel cells. Sens. Mater. 2017, 29, 1055–1060. [Google Scholar] [CrossRef]
- Lan, T.H.; Wang, C.T.; Sangeetha, T.; Yang, Y.C.; Garg, A. Constructed mathematical model for nanowire electron transfer in microbial fuel cells. J. Power Sources 2018, 402, 483–488. [Google Scholar] [CrossRef]
- Zhu, A.L.; Wilkinson, D.P.; Zhang, X.; Xing, Y.; Rozhin, A.G.; Kulinich, S.A. Zinc regeneration in rechargeable zinc-air fuel cells—A review. J. Energy Storage 2016, 8, 35–50. [Google Scholar] [CrossRef]
- Mainar, A.R.; Iruin, E.; Colmenares, L.C.; Kvasha, A.; de Meatza, I.; Bengoechea, M.; Leonet, O.; Boyano, I.; Zhang, Z.; Blazquez, J.A. An overview of progress in electrolytes for secondary zinc-air batteries and other storage systems based on zinc. J. Energy Storage 2018, 15, 304–328. [Google Scholar] [CrossRef]
- Zhang, X.G. Secondary batteries–zinc systems| zinc electrodes: Overview. Encycl. Electrochem. Power Source 2009, 454–468. [Google Scholar] [CrossRef]
- Gavrilović-Wohlmuther, A.; Laskos, A.; Zelger, C.; Gollas, B.; Whitehead, A.H. Effects of electrolyte concentration, temperature, flow velocity and current density on Zn deposit morphology. J. Energy Power Eng. 2015, 9, 1019–1028. [Google Scholar] [CrossRef]
- Sapkota, P.; Kim, H. An experimental study on the performance of a zinc air fuel cell with inexpensive metal oxide catalysts and porous organic polymer separators. J. Ind. Eng. Chem. 2010, 16, 39–44. [Google Scholar] [CrossRef]
- Stamm, J.; Varzi, A.; Latz, A.; Horstmann, B. Modeling nucleation and growth of zinc oxide during discharge of primary zinc-air batteries. J. Power Sources 2017, 360, 136–149. [Google Scholar] [CrossRef]
- Pichler, B.; Weinberger, S.; Reščec, L.; Grimmer, I.; Gebetsroither, F.; Bitschnau, B.; Hacker, V. Bifunctional electrode performance for zinc-air flow cells with pulse charging. Electrochim. Acta 2017, 251, 488–497. [Google Scholar] [CrossRef]
- Pei, P.; Wang, K.; Ma, Z. Technologies for extending zinc–air battery’s cyclelife: A review. Appl. Energy 2014, 128, 315–324. [Google Scholar] [CrossRef]
- Pei, P.; Ma, Z.; Wang, K.; Wang, X.; Song, M.; Xu, H. High performance zinc air fuel cell stack. J. Power Sources 2014, 249, 13–20. [Google Scholar] [CrossRef]
- Xia, Y.; Liu, X.; Bai, Y.; Li, H.; Deng, X.; Niu, X.; Wu, X.; Zhou, D.; Lv, M.; Wang, Z.; et al. Electrical conductivity optimization in electrolyte-free fuel cells by single-component Ce0.8Sm0.2O2-δ–Li0.15Ni0.45Zn0.4 layer. RSC Adv. 2012, 2, 3828–3834. [Google Scholar] [CrossRef]
- Asghar, M.I.; Jouttijärvi, S.; Jokiranta, R.; Valtavirta, A.M.; Lund, P.D. Wide bandgap oxides for low-temperature single-layered nanocomposite fuel cell. Nano Energy 2018, 53, 391–397. [Google Scholar] [CrossRef]
- Fan, L.; Su, P.C. Layer-structured LiNi0. 8Co0. 2O2: A new triple (H+/O2−/e−) conducting cathode for low temperature proton conducting solid oxide fuel cells. J. Power Sources 2016, 306, 369–377. [Google Scholar] [CrossRef]
- Qu, S.; Song, Z.; Liu, J.; Li, Y.; Kou, Y.; Ma, C.; Han, X.; Deng, Y.; Zhao, N.; Hu, W.; et al. Electrochemical approach to prepare integrated air electrodes for highly stretchable zinc-air battery array with tunable output voltage and current for wearable electronics. Nano Energy 2017, 39, 101–110. [Google Scholar] [CrossRef]




© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sangeetha, T.; Chen, P.-T.; Cheng, W.-F.; Yan, W.-M.; Huang, K.D. Optimization of the Electrolyte Parameters and Components in Zinc Particle Fuel Cells. Energies 2019, 12, 1090. https://doi.org/10.3390/en12061090
Sangeetha T, Chen P-T, Cheng W-F, Yan W-M, Huang KD. Optimization of the Electrolyte Parameters and Components in Zinc Particle Fuel Cells. Energies. 2019; 12(6):1090. https://doi.org/10.3390/en12061090
Chicago/Turabian StyleSangeetha, Thangavel, Po-Tuan Chen, Wu-Fu Cheng, Wei-Mon Yan, and K. David Huang. 2019. "Optimization of the Electrolyte Parameters and Components in Zinc Particle Fuel Cells" Energies 12, no. 6: 1090. https://doi.org/10.3390/en12061090
APA StyleSangeetha, T., Chen, P.-T., Cheng, W.-F., Yan, W.-M., & Huang, K. D. (2019). Optimization of the Electrolyte Parameters and Components in Zinc Particle Fuel Cells. Energies, 12(6), 1090. https://doi.org/10.3390/en12061090




