Microbial Lipid Production from Corn Stover by the Oleaginous Yeast Rhodosporidium toruloides Using the PreSSLP Process
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Corn Stover Pretreatment Procedure
2.3. Strain and Inoculum Culture Condition
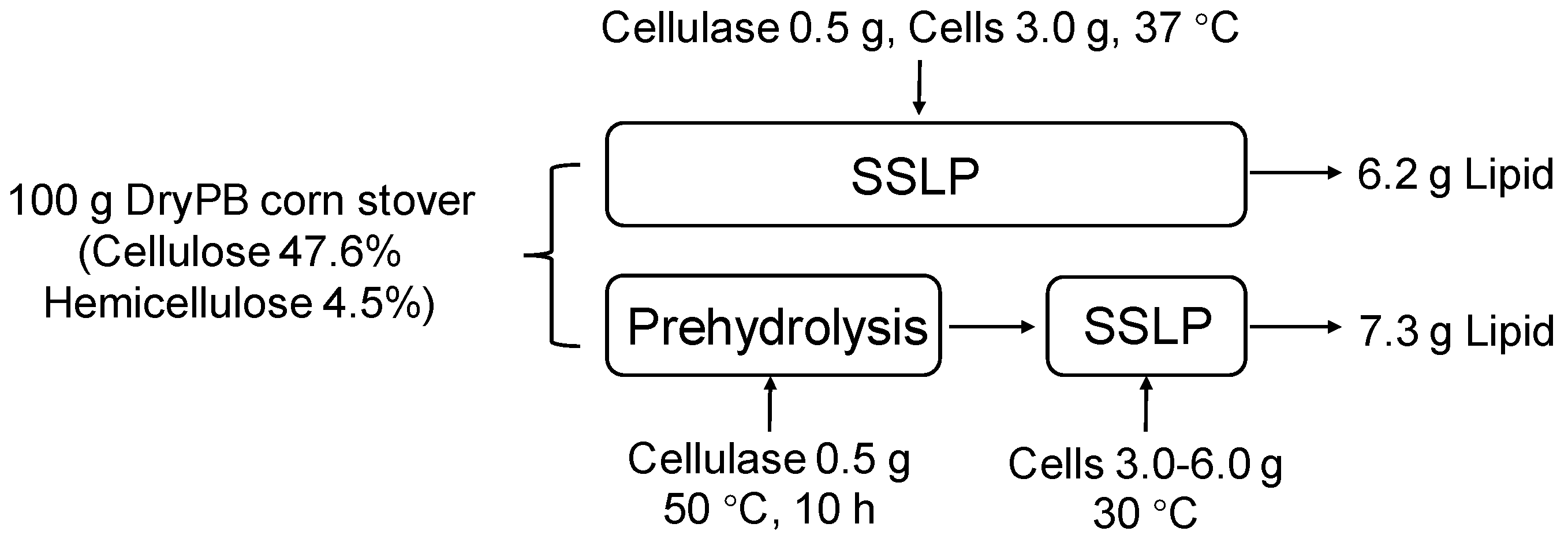
2.4. Lipid Production from DryPB Corn Stover
2.5. Analytical Methods
3. Results and Discussion
3.1. Lipid Production Using SSLP Process
3.2. Lipid Production Using PreSSLP Process
3.3. Lipid Production with Higher Solid Loadings
3.4. Fatty Acid Composition
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Kumar, D.; Singh, B.; Korstad, J. Utilization of lignocellulosic biomass by oleaginous yeast and bacteria for production of biodiesel and renewable diesel. Renew. Sustain. Energy Rev. 2017, 73, 654–671. [Google Scholar] [CrossRef]
- Jin, M.J.; Slininger, P.J.; Dien, B.S.; Waghmode, S.; Moser, B.R.; Orjuela, A.; Sousal, L.D.; Balan, V. Microbial lipid-based lignocellulosic biorefinery: Feasibility and challenges. Trends Biotechnol. 2015, 33, 43–54. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.T.; Wang, Y.P.; Liu, H.J.; Zhang, J.A. Enhanced lipid production with undetoxified corncob hydrolysate by Rhodotorula glutinis using a high cell density culture strategy. Bioresour. Technol. 2015, 180, 32–39. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.H.; Zhao, Z.B.; Bai, F.W. High-density cultivation of oleaginous yeast Rhodosporidium toruloides Y4 in fed-batch culture. Enz. Microb. Technol. 2007, 41, 312–317. [Google Scholar] [CrossRef]
- Koutinas, A.A.; Chatzifragkou, A.; Kopsahelis, N.; Papanikolaou, S.; Kookos, I.K. Design and techno-economic evaluation of microbial oil production as a renewable resource for biodiesel and oleochemical production. Fuel 2014, 116, 566–577. [Google Scholar] [CrossRef]
- Vanneste, J.; Ennaert, T.; Vanhulsel, A.; Sels, B. Unconventional pretreatment of lignocellulose with low-temperature plasma. ChemSusChem 2017, 10, 14–31. [Google Scholar] [CrossRef]
- Sun, S.N.; Sun, S.L.; Cao, X.F.; Sun, R.C. The role of pretreatment in improving the enzymatic hydrolysis of lignocellulosic materials. Bioresour. Technol. 2016, 199, 49–58. [Google Scholar] [CrossRef] [PubMed]
- Annamalai, N.; Sivakumar, N.; Oleskowicz-Popiel, P. Enhanced production of microbial lipids from waste office paper by the oleaginous yeast Cryptococcus Curvatus. Fuel 2018, 217, 420–426. [Google Scholar] [CrossRef]
- Huang, C.; Zong, M.H.; Wu, H.; Liu, Q.P. Microbial oil production from rice straw hydrolysate by Trichosporon fermentans. Bioresour. Technol. 2009, 100, 4535–4538. [Google Scholar] [CrossRef]
- Xue, Y.P.; Jin, M.J.; Orjuela, A.; Slininger, P.J.; Dien, B.S.; Dale, B.E.; Balan, V. Microbial lipid production from AFEX (TM) pretreated corn stover. RSC Adv. 2015, 5, 28725–28734. [Google Scholar] [CrossRef]
- Gong, Z.W.; Shen, H.W.; Yang, X.B.; Wang, Q.; Xie, H.B.; Zhao, Z.B.K. Lipid production from corn stover by the oleaginous yeast Cryptococcus curvatus. Biotechnol. Biofuels 2014, 7, 158. [Google Scholar] [CrossRef]
- Brar, K.K.; Sarma, A.K.; Aslam, M.; Polikarpov, I.; Chadha, B.S. Potential of oleaginous yeast Trichosporon sp., for conversion of sugarcane bagasse hydrolysate into biodiesel. Bioresour. Technol. 2017, 242, 161–168. [Google Scholar] [CrossRef]
- Yu, X.C.; Zheng, Y.B.; Dorgan, K.M.; Chen, S.L. Oil production by oleaginous yeasts using the hydrolysate from pretreatment of wheat straw with dilute sulfuric acid. Bioresour. Technol. 2011, 102, 6134–6140. [Google Scholar] [CrossRef]
- Zhang, J.A.; Zhu, Z.N.; Wang, X.F.; Wang, N.; Wang, W.; Bao, J. Biodetoxification of toxins generated from lignocellulose pretreatment using a newly isolated fungus, Amorphotheca resinae ZN1, and the consequent ethanol fermentation. Biotechnol. Biofuels 2010, 3, 26. [Google Scholar] [CrossRef]
- Zhang, J.A.; Wang, X.S.; Chu, D.Q.; He, Y.Q.; Bao, J. Dry pretreatment of lignocellulose with extremely low steam and water usage for bioethanol production. Bioresour. Technol. 2011, 102, 4480–4488. [Google Scholar] [CrossRef]
- He, Y.Q.; Zhang, J.; Bao, J. Dry dilute acid pretreatment by co-currently feeding of corn stover feedstock and dilute acid solution without impregnation. Bioresour. Technol. 2014, 158, 360–364. [Google Scholar] [CrossRef]
- He, Y.Q.; Zhang, L.P.; Zhang, J.; Bao, J. Helically agitated mixing in dry dilute acid pretreatment enhances the bioconversion of corn stover into ethanol. Biotechnol. Biofuels 2014, 7, 1. [Google Scholar] [CrossRef]
- He, Y.Q.; Zhang, J.; Bao, J. Acceleration of biodetoxification on dilute acid pretreated lignocellulose feedstock by aeration and the consequent ethanol fermentation evaluation. Biotechnol. Biofuels 2016, 9, 19. [Google Scholar] [CrossRef]
- Wang, J.; Gao, Q.Q.; Zhang, H.Z.; Bao, J. Inhibitor degradation and lipid accumulation potentials of oleaginous yeast Trichosporon cutaneum using lignocellulose feedstock. Bioresour. Technol. 2016, 218, 892–901. [Google Scholar] [CrossRef]
- Zhou, P.P.; Meng, J.; Bao, J. Fermentative production of high titer citric acid from corn stover feedstock after dry dilute acid pretreatment and biodetoxification. Bioresour. Technol. 2017, 224, 563–572. [Google Scholar] [CrossRef]
- Liu, G.; Zhang, Q.; Li, H.X.; Qureshi, A.S.; Zhang, J.; Bao, X.M.; Bao, J. Dry biorefining maximizes the potentials of simultaneous saccharification and co-fermentation for cellulosic ethanol production. Biotechnol. Bioeng. 2018, 115, 60–69. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Wang, Y.M.; Yu, Z.C.; Bao, J. Simultaneous saccharification and microbial lipid fermentation of corn stover by oleaginous yeast Trichosporon Cutaneum. Bioresour. Technol. 2012, 118, 13–18. [Google Scholar] [CrossRef]
- Gong, Z.W.; Shen, H.W.; Wang, Q.; Yang, X.B.; Xie, H.B.; Zhao, Z.B.K. Efficient conversion of biomass into lipids by using the simultaneous saccharification and enhanced lipid production process. Biotechnol. Biofuels 2013, 6, 36. [Google Scholar] [CrossRef] [PubMed]
- Ghose, T.K. Measurement of cellulase activities. Pure Appl. Chem. 1987, 59, 257–268. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- National Renewable Energy Laboratory (NREL). Determination of Structural Carbohydrates and Lignin in Biomass. Laboratory Analytical Procedure (LAP); NREL/TP-510-42618; 2012. Available online: https://www.nrel.gov/docs/gen/fy13/42618.pdf (accessed on 20 November 2017).
- Miller, G.L. Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal. Chem. 1959, 31, 426–428. [Google Scholar] [CrossRef]
- Wu, S.G.; Hu, C.M.; Jin, G.J.; Zhao, X.; Zhao, Z.K. Phosphate-limitation mediated lipid production by Rhodosporidium toruloides. Bioresour. Technol. 2010, 101, 6124–6129. [Google Scholar] [CrossRef]
- Qin, L.; Zhao, X.; Li, W.C.; Zhu, J.Q.; Liu, L.; Li, B.Z.; Yuan, Y.J. Process analysis and optimization of simultaneous saccharification and co-fermentation of ethylenediamine-pretreated corn stover for ethanol production. Biotechnol. Biofuels 2018, 11, 118. [Google Scholar] [CrossRef]
- Zhu, J.Q.; Qin, L.; Li, W.C.; Zhang, J.; Bao, J.; Huang, Y.D.; Li, B.Z.; Yuan, Y.J. Simultaneous saccharification and co-fermentation of dry diluted acid pretreated corn stover at high dry matter loading: Overcoming the inhibitors by non-tolerant yeast. Bioresour. Technol. 2015, 198, 39–46. [Google Scholar] [CrossRef]
- Paulova, L.; Patakova, P.; Rychtera, M.; Melzoch, K. High solid fed-batch SSF with delayed inoculation for improved production of bioethanol from wheat straw. Fuel 2014, 122, 294–300. [Google Scholar] [CrossRef]
- Qiu, J.W.; Tian, D.; Shen, F.; Hu, J.G.; Zeng, Y.M.; Yang, G.; Zhang, Y.Z.; Deng, S.H.; Zhang, J. Bioethanol production from wheat straw by phosphoric acid plus hydrogen peroxide (PHP) pretreatment via simultaneous saccharification and fermentation (SSF) at high solid loadings. Bioresour. Technol. 2018, 268, 355–362. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Singh, S.; Singh, O.V. Bioconversion of lignocellulosic biomass: Biochemical and molecular perspectives. J. Ind. Microbiol. Biotechnol. 2008, 35, 377–391. [Google Scholar] [CrossRef] [PubMed]
- Dong, C.Y.; Wang, Y.; Chan, K.L.; Bhatia, A.; Leu, S.Y. Temperature profiling to maximize energy yield with reduced water input in a lignocellulosic ethanol biorefinery. Appl. Energy 2018, 214, 63–72. [Google Scholar] [CrossRef]
- Rudolf, A.; Baudel, H.; Zacchi, G.; Hahn-Hagerdal, B.; Liden, G. Simultaneous saccharification and fermentation of steam-pretreated bagasse using Saccharomyces cerevisiae TMB3400 and Pichia stipitis CBS6054. Biotechnol. Bioeng. 2008, 99, 783–790. [Google Scholar] [CrossRef] [PubMed]
- Ye, G.Y.; Zeng, D.F.; Zhang, S.S.; Fan, M.S.; Zhang, H.D.; Xie, J. Ethanol production from mixtures of sugarcane bagasse and Dioscorea composita extracted residue with high solid loading. Bioresour. Technol. 2018, 257, 23–29. [Google Scholar] [CrossRef] [PubMed]
- Hodge, D.B.; Karim, M.N.; Schell, D.J.; McMillan, J.D. Soluble and insoluble solids contributions to high-solids enzymatic hydrolysis of lignocellulose. Bioresour. Technol. 2008, 99, 8940–8948. [Google Scholar] [CrossRef] [PubMed]
- Kristensen, J.B.; Felby, C.; Jorgensen, H. Yield-determining factors in high-solids enzymatic hydrolysis of lignocellulose. Biotechnol. Biofuels 2009, 2, 11. [Google Scholar] [CrossRef]
- Yang, X.B.; Jin, G.J.; Gong, Z.W.; Shen, H.W.; Bai, F.W.; Zhao, Z.K. Recycling biodiesel-derived glycerol by the oleaginous yeast Rhodosporidium toruloides Y4 through the two-stage lipid production process. Biochem. Eng. J. 2014, 91, 86–91. [Google Scholar] [CrossRef]
- Yang, X.B.; Jin, G.J.; Wang, Y.D.; Shen, H.W.; Zhao, Z.K. Lipid production on free fatty acids by oleaginous yeasts under non-growth conditions. Bioresour. Technol. 2015, 193, 557–562. [Google Scholar] [CrossRef]
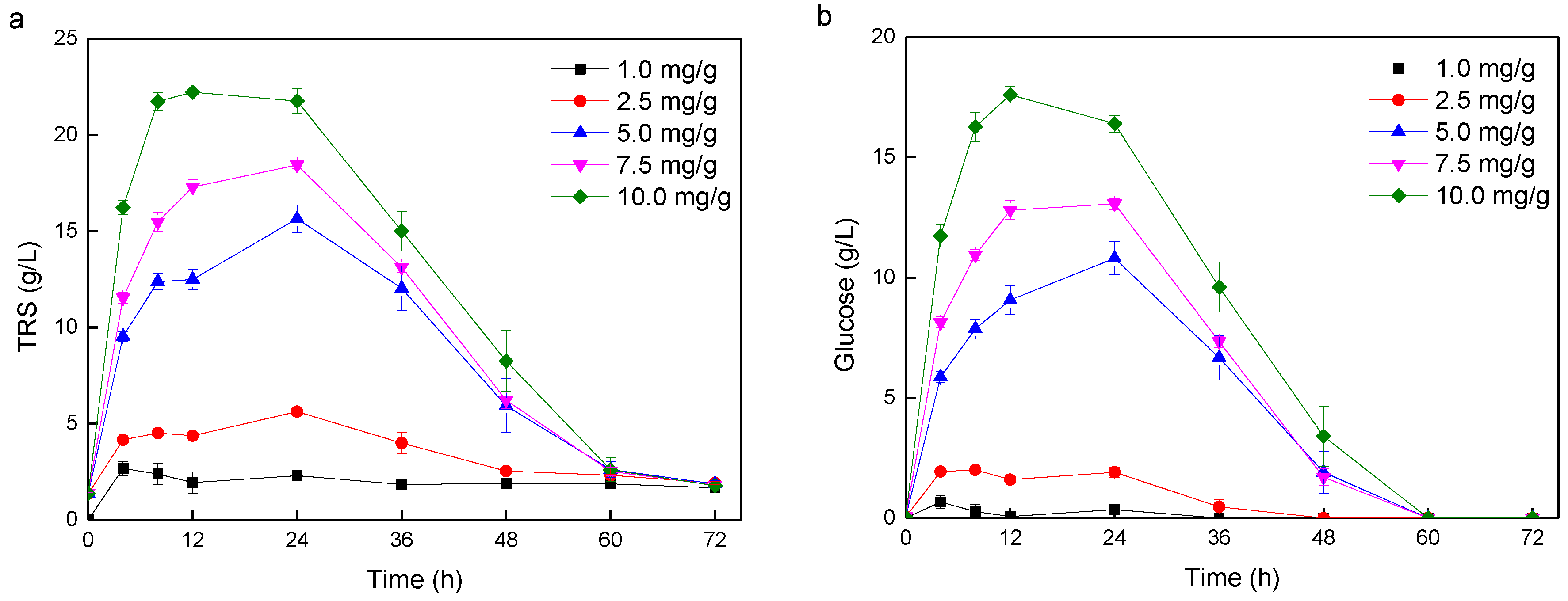
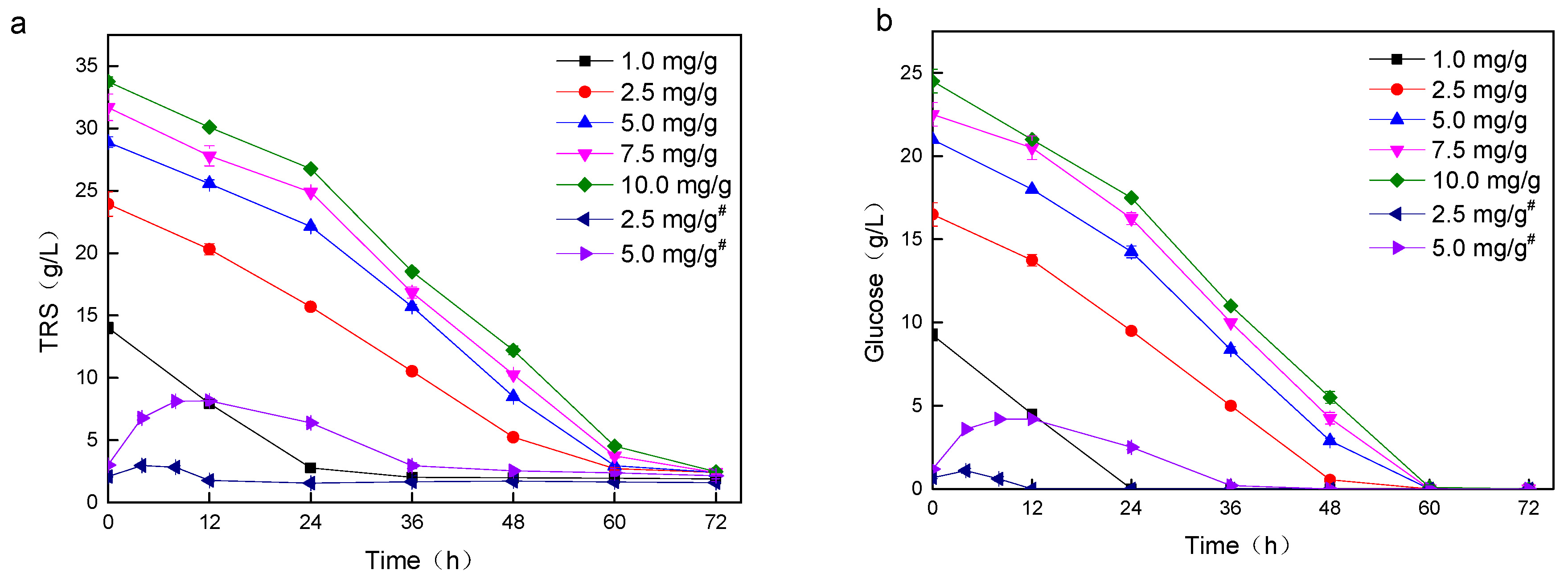
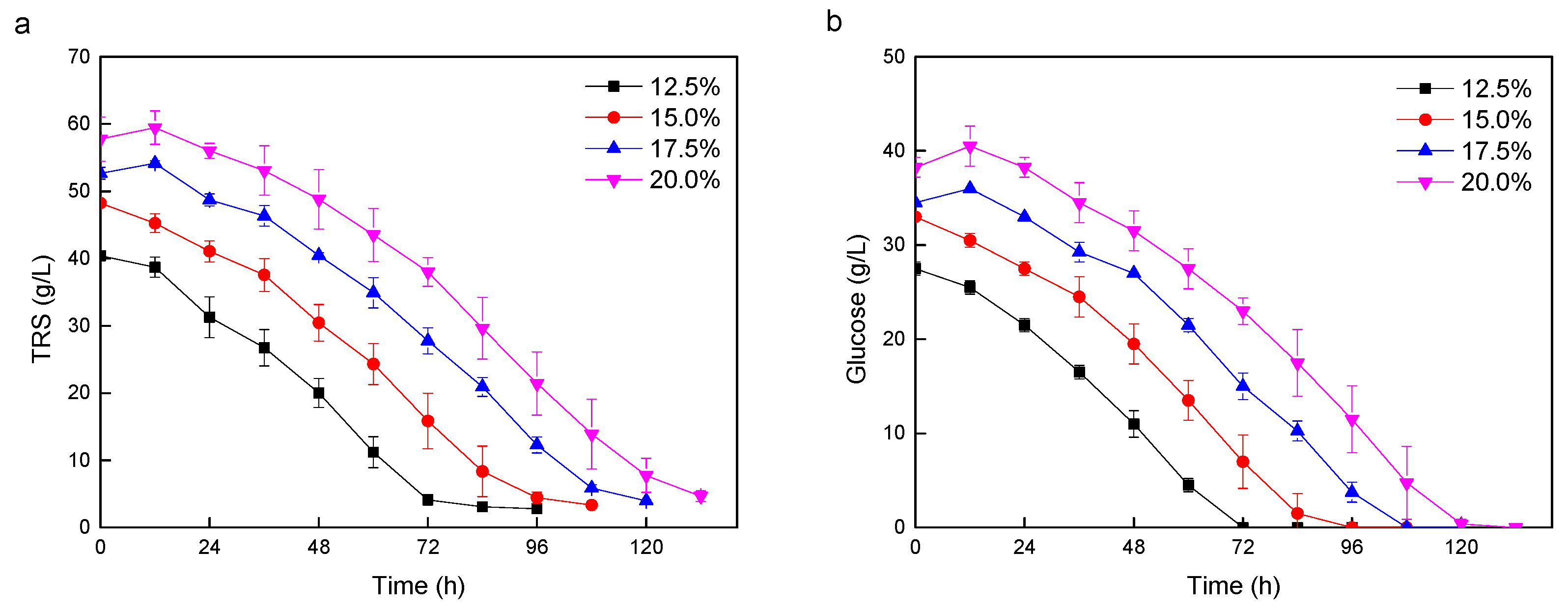
| Entry | Cellulase Dosage (mg/g) a | Total Solid Mass (g/L) | Residual TRS (g/L) | Lipid (g/L) | Lipid Yield (g/g) |
|---|---|---|---|---|---|
| 1 | 1.0 | 40.6 ± 1.4 | 1.7 ± 0.2 | 4.3 ± 0.1 | 0.043 ± 0.001 |
| 2 | 2.5 | 40.5 ± 1.2 | 1.9 ± 0.1 | 5.5 ± 0.5 | 0.055 ± 0.004 |
| 3 | 5.0 | 41.2 ± 1.1 | 1.9 ± 0.2 | 6.2 ± 0.1 | 0.062 ± 0.001 |
| 4 | 7.5 | 43.0 ± 0.2 | 1.8 ± 0.1 | 6.2 ± 0.3 | 0.061 ± 0.003 |
| 5 | 10.0 | 39.9 ± 1.1 | 1.8 ± 0.1 | 6.2 ± 0.1 | 0.062 ± 0.001 |
| Entry | Cellulase Dosage (mg/g) a | Total Solid Mass (g/L) | Residual TRS (g/L) | Lipid (g/L) | Lipid Yield (g/g) |
|---|---|---|---|---|---|
| 1 | 1.0 | 51.4 ± 1.4 | 1.9 ± 0.1 | 3.7 ± 0.2 | 0.037 ± 0.002 |
| 2 | 2.5 | 41.9 ± 2.2 | 2.4 ± 0.0 | 6.8 ± 0.3 | 0.068 ± 0.003 |
| 3 | 2.5 b | 41.0 ± 0.2 | 1.6 ± 0.0 | 4.6 ± 0.0 | 0.046 ± 0.000 |
| 4 | 5.0 | 40.4 ± 0.4 | 2.4 ± 0.0 | 7.3 ± 0.0 | 0.073 ± 0.000 |
| 5 | 5.0 b | 41.3 ± 0.3 | 2.1 ± 0.0 | 5.9 ± 0.0 | 0.059 ± 0.000 |
| 6 | 7.5 | 40.0 ± 0.4 | 2.4 ± 0.1 | 7.3 ± 0.1 | 0.073 ± 0.001 |
| 7 | 10.0 | 40.9 ± 0.5 | 2.4 ± 0.0 | 6.9 ± 0.8 | 0.069 ± 0.008 |
| Entry | Solid Loading (%) | Total Solid Mass (g/L) | Residual TRS (g/L) | Lipid (g/L) | Lipid Yield (g/g) |
|---|---|---|---|---|---|
| 1 | 10.0 | 40.4 ± 0.4 | 2.4 ± 0.0 | 7.3 ± 0.0 | 0.073 ± 0.000 |
| 2 | 12.5 | 52.2 ± 0.7 | 2.8 ± 0.1 | 10.1 ± 0.4 | 0.080 ± 0.003 |
| 3 | 15.0 | 60.3 ± 2.3 | 3.3 ± 0.5 | 11.6 ± 0.7 | 0.077 ± 0.005 |
| 4 | 17.5 | 73.9 ± 0.3 | 4.0 ± 0.1 | 12.4 ± 0.6 | 0.071 ± 0.004 |
| 5 | 20.0 | 82.9 ± 2.6 | 4.7 ± 0.8 | 12.5 ± 0.1 | 0.062 ± 0.000 |
| Strain | Pretreatment Method | Detoxification Method | Culture Mode | Solid Loading (%) | Lipid (g/L) | Lipid Yield (g/g) a | Reference |
|---|---|---|---|---|---|---|---|
| T. cutaneum CX1 | Dry acid pretreatment | Biodetoxification | SSF | 10.0 | 3.0 | 0.030 | [22] |
| 15.0 | 4.0 | 0.027 | |||||
| T. cutaneum ACCC 20271 | Dry acid pretreatment | Biodetoxification | SHF | 15.0 | 5.9 | - | [19] |
| 20.0 | 7.4 | - | |||||
| 25.0 | 8.0 | - | |||||
| L. tetrasporus NRRL Y-11562 | AFEX | No | SHF | 22.1 | 8.4 | 0.037 | [10] |
| R. toruloides AS 2.1389 | Dry acid pretreatment | Biodetoxification | SSLP | 10.0 | 6.2 | 0.062 | This study |
| R. toruloides AS 2.1389 | Dry acid pretreatment | Biodetoxification | PreSSLP | 10.0 | 7.3 | 0.073 | This study |
| 12.5 | 10.1 | 0.080 | |||||
| 15.0 | 11.6 | 0.077 | |||||
| 17.5 | 12.4 | 0.071 |
| Entry | Solid Loading (%) | Lipid (g/L) | Relative Fatty Acid Contents (%) | |||||
|---|---|---|---|---|---|---|---|---|
| Myristic Acid | Palmitic Acid | Palmitoleic Acid | Stearic Acid | Oleic Acid | Linoleic Acid | |||
| 1 | 10.0 | 7.3 ± 0.0 | 2.5 ± 0.2 | 46.0 ± 1.0 | 0.6 ± 0.0 | 15.0 ± 0.4 | 35.0 ± 0.6 | 1.0 ± 0.2 |
| 2 | 12.5 | 10.1 ± 0.4 | 2.6 ± 0.2 | 44.6 ± 1.2 | 1.0 ± 0.0 | 15.5 ± 0.9 | 36.0 ± 0.5 | 0.7 ± 0.0 |
| 3 | 15.0 | 11.6 ± 0.7 | 2.5 ± 0.6 | 41.0 ± 5.4 | 1.1 ± 0.1 | 14.9 ± 1.5 | 38.6 ± 5.6 | 1.9 ± 1.7 |
| 4 | 17.5 | 12.4 ± 0.6 | 3.6 ± 0.6 | 55.0 ± 1.7 | 0.8 ± 0.3 | 21.3 ± 3.1 | 19.3 ± 4.7 | 0.0 ± 0.0 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dai, X.; Shen, H.; Li, Q.; Rasool, K.; Wang, Q.; Yu, X.; Wang, L.; Bao, J.; Yu, D.; Zhao, Z.K. Microbial Lipid Production from Corn Stover by the Oleaginous Yeast Rhodosporidium toruloides Using the PreSSLP Process. Energies 2019, 12, 1053. https://doi.org/10.3390/en12061053
Dai X, Shen H, Li Q, Rasool K, Wang Q, Yu X, Wang L, Bao J, Yu D, Zhao ZK. Microbial Lipid Production from Corn Stover by the Oleaginous Yeast Rhodosporidium toruloides Using the PreSSLP Process. Energies. 2019; 12(6):1053. https://doi.org/10.3390/en12061053
Chicago/Turabian StyleDai, Xiaozan, Hongwei Shen, Qiang Li, Kamal Rasool, Qian Wang, Xue Yu, Lei Wang, Jie Bao, Dayu Yu, and Zongbao K. Zhao. 2019. "Microbial Lipid Production from Corn Stover by the Oleaginous Yeast Rhodosporidium toruloides Using the PreSSLP Process" Energies 12, no. 6: 1053. https://doi.org/10.3390/en12061053
APA StyleDai, X., Shen, H., Li, Q., Rasool, K., Wang, Q., Yu, X., Wang, L., Bao, J., Yu, D., & Zhao, Z. K. (2019). Microbial Lipid Production from Corn Stover by the Oleaginous Yeast Rhodosporidium toruloides Using the PreSSLP Process. Energies, 12(6), 1053. https://doi.org/10.3390/en12061053







