Rhizomucor miehei Lipase Supported on Inorganic Solids, as Biocatalyst for the Synthesis of Biofuels: Improving the Experimental Conditions by Response Surface Methodology
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Immobilization of Rhizomucor Miehei Lipase on Inorganic Supports
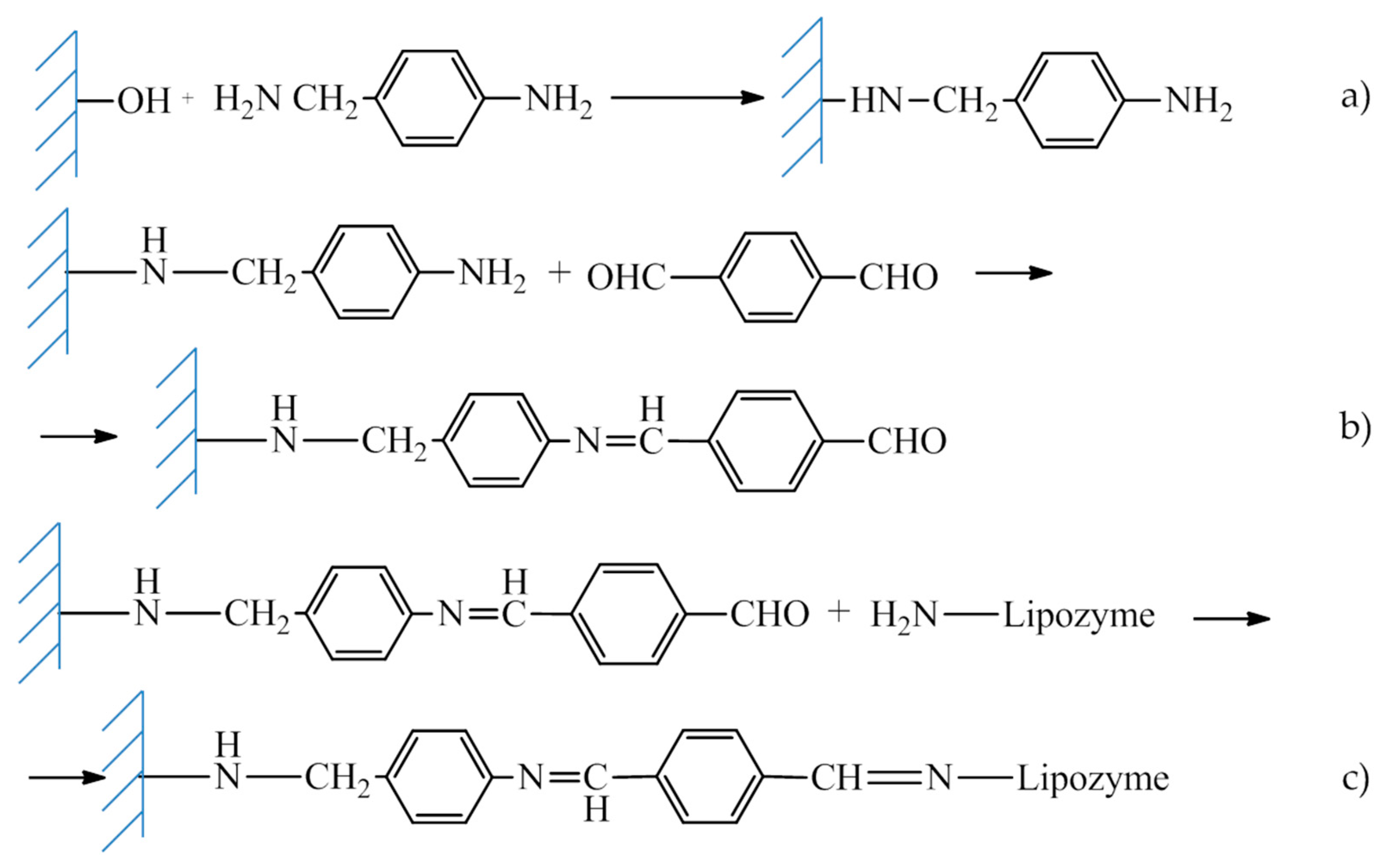
2.2.1. Immobilization of Lipozyme RM IM by Physical Adsorption
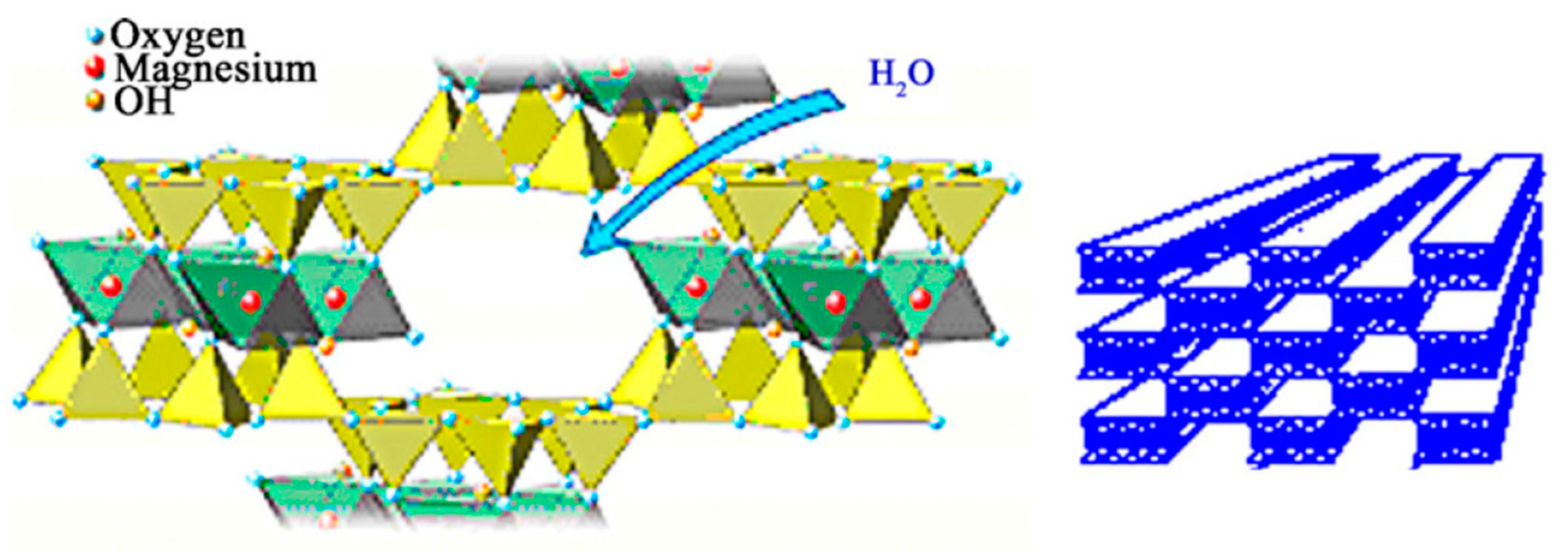
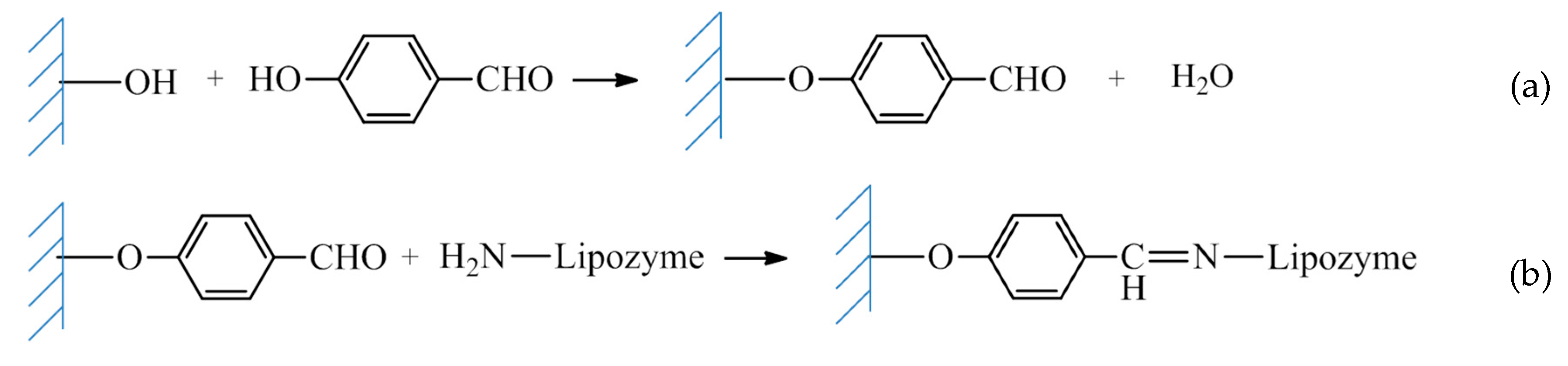
2.2.2. Covalent Immobilization of Lipozyme RM IM on Sepiolite
2.3. Ethanolisis Reactions
2.4. Analytical Method
2.5. Determination of Kinematic Viscosity
2.6. Experimental Design
2.7. Statistical Analysis
3. Results and Discussions
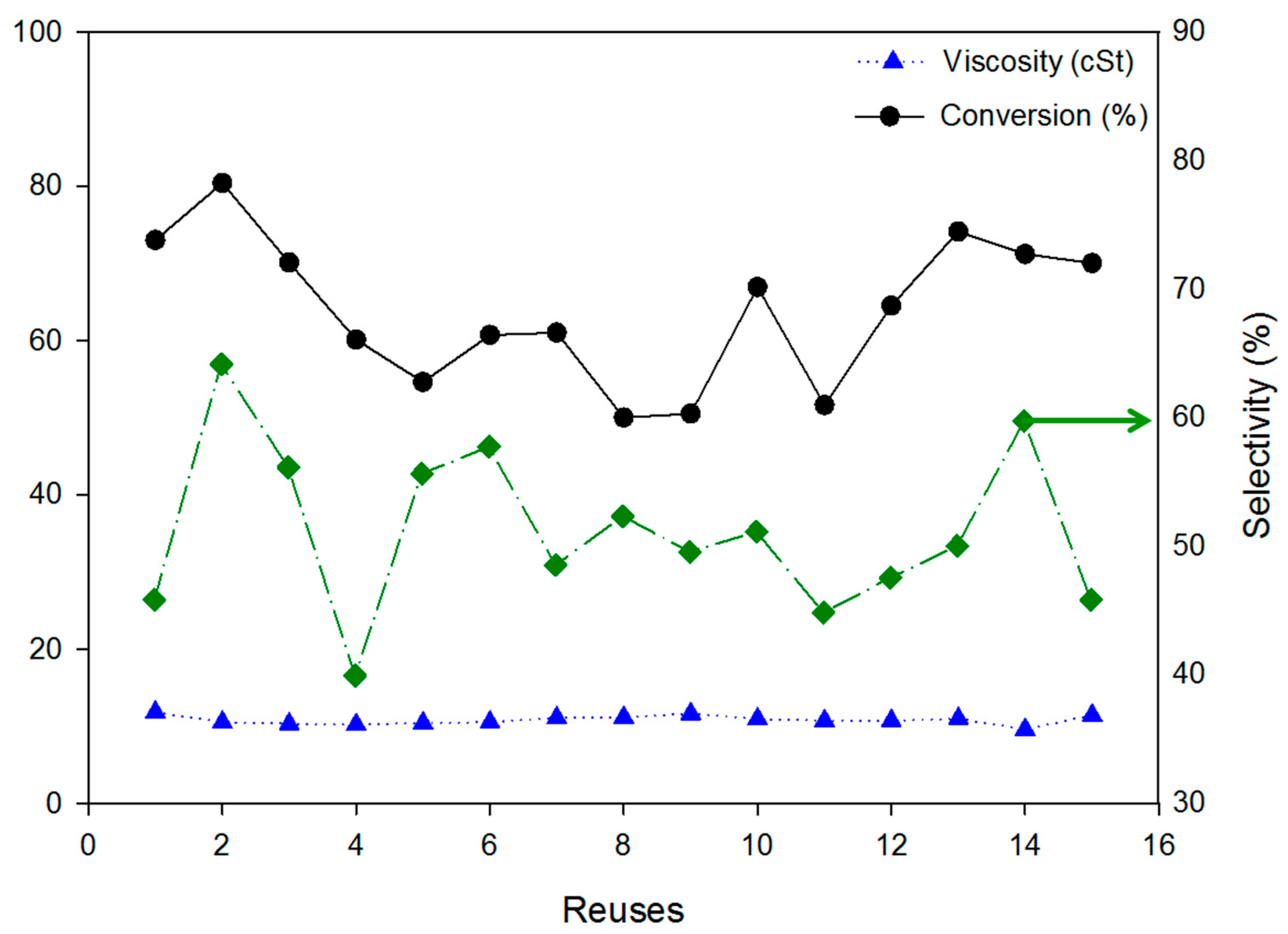
3.1. Immobilization of the Commercial Biocatalyst Lipozyme RM IM on an Inorganic Support
3.2. Analysis of Variance (ANOVA) and Optimization of the Reaction Parameters by RSM
4. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Ko, C.-H.; Chaiprapat, S.; Kim, L.-H.; Hadi, P.; Hsu, S.-C.; Leu, S.-Y. Carbon sequestration potential via energy harvesting from agricultural biomass residues in Mekong River basin, Southeast Asia. Renew. Sustain. Energy Rev. 2017, 68, 1051–1062. [Google Scholar] [CrossRef]
- Joshi, G.; Pandey, J.K.; Rana, S.; Rawat, D.S. Challenges and opportunities for the application of biofuel. Renew. Sustain. Energy Rev. 2017, 79, 850–866. [Google Scholar] [CrossRef]
- Sen, B.; Ercan, T.; Tatari, O. Does a battery-electric truck make a difference?—Life cycle emissions, costs, and externality analysis of alternative fuel-powered Class 8 heavy-duty trucks in the United States. J. Clean. Prod. 2017, 141, 110–121. [Google Scholar] [CrossRef]
- Nair, S.; Paulose, H. Emergence of green business models: The case of algae biofuel for aviation. Energy Policy 2014, 65, 175–184. [Google Scholar] [CrossRef]
- Noh, H.M.; Benito, A.; Alonso, G. Study of the current incentive rules and mechanisms to promote biofuel use in the EU and their possible application to the civil aviation sector. Transp. Res. Part D Transp. Environ. 2016, 46, 298–316. [Google Scholar] [CrossRef]
- Winnes, H.; Styhre, L.; Fridell, E. Reducing GHG emissions from ships in port areas. Res. Transp. Bus. Manag. 2015, 17, 73–82. [Google Scholar] [CrossRef]
- Russo, D.; Dassisti, M.; Lawlor, V.; Olabi, A. State of the art of biofuels from pure plant oil. Renew. Sustain. Energy Rev. 2012, 16, 4056–4070. [Google Scholar] [CrossRef]
- Collet, P.; Lardon, L.; Hélias, A.; Bricout, S.; Lombaert-Valot, I.; Perrier, B.; Lépine, O.; Steyer, J.-P.; Bernard, O. Biodiesel from microalgae–Life cycle assessment and recommendations for potential improvements. Renew. Energy 2014, 71, 525–533. [Google Scholar] [CrossRef]
- Dharma, S.; Masjuki, H.; Ong, H.C.; Sebayang, A.; Silitonga, A.; Kusumo, F.; Mahlia, T. Optimization of biodiesel production process for mixed Jatropha curcas–Ceiba pentandra biodiesel using response surface methodology. Energy Convers. Manag. 2016, 115, 178–190. [Google Scholar] [CrossRef]
- Talebian-Kiakalaieh, A.; Amin, N.A.S.; Mazaheri, H. A review on novel processes of biodiesel production from waste cooking oil. Appl. Energy 2013, 104, 683–710. [Google Scholar] [CrossRef]
- Wadumesthrige, K.; Ara, M.; Salley, S.O.; Ng, K.S. Investigation of lubricity characteristics of biodiesel in petroleum and synthetic fuel. Energy Fuels 2009, 23, 2229–2234. [Google Scholar] [CrossRef]
- Aransiola, E.; Ojumu, T.; Oyekola, O.; Madzimbamuto, T.; Ikhu-Omoregbe, D. A review of current technology for biodiesel production: State of the art. Biomass Bioenergy 2014, 61, 276–297. [Google Scholar] [CrossRef]
- Santori, G.; Di Nicola, G.; Moglie, M.; Polonara, F. A review analyzing the industrial biodiesel production practice starting from vegetable oil refining. Appl. Energy 2012, 92, 109–132. [Google Scholar] [CrossRef]
- Quispe, C.A.; Coronado, C.J.; Carvalho, J.A., Jr. Glycerol: Production, consumption, prices, characterization and new trends in combustion. Renew. Sustain. Energy Rev. 2013, 27, 475–493. [Google Scholar] [CrossRef]
- Ang, G.T.; Tan, K.T.; Lee, K.T. Recent development and economic analysis of glycerol-free processes via supercritical fluid transesterification for biodiesel production. Renew. Sustain. Energy Rev. 2014, 31, 61–70. [Google Scholar] [CrossRef]
- Okoye, P.; Hameed, B. Review on recent progress in catalytic carboxylation and acetylation of glycerol as a byproduct of biodiesel production. Renew. Sustain. Energy Rev. 2016, 53, 558–574. [Google Scholar] [CrossRef]
- Luna, D.; Calero, J.; Sancho, E.; Luna, C.; Posadillo, A.; Bautista, F.; Romero, A.; Berbel, J.; Verdugo, C. Technological challenges for the production of biodiesel in arid lands. J. Arid Environ. 2014, 102, 127–138. [Google Scholar] [CrossRef]
- Calero, J.; Luna, D.; Sancho, E.D.; Luna, C.; Bautista, F.M.; Romero, A.A.; Posadillo, A.; Berbel, J.; Verdugo-Escamilla, C. An overview on glycerol-free processes for the production of renewable liquid biofuels, applicable in diesel engines. Renew. Sustain. Energy Rev. 2015, 42, 1437–1452. [Google Scholar] [CrossRef]
- Ilham, Z.; Saka, S. Two-step supercritical dimethyl carbonate method for biodiesel production from Jatropha curcas oil. Bioresour. Technol. 2010, 101, 2735–2740. [Google Scholar] [CrossRef] [PubMed]
- Casas, A.; Ruiz, J.R.; Ramos, M.A.J.S.; Pérez, A. Effects of triacetin on biodiesel quality. Energy Fuels 2010, 24, 4481–4489. [Google Scholar] [CrossRef]
- Caballero, V.; Bautista, F.M.; Campelo, J.M.; Luna, D.; Marinas, J.M.; Romero, A.A.; Hidalgo, J.M.; Luque, R.; Macario, A.; Giordano, G. Sustainable preparation of a novel glycerol-free biofuel by using pig pancreatic lipase: Partial 1, 3-regiospecific alcoholysis of sunflower oil. Process Biochem. 2009, 44, 334–342. [Google Scholar] [CrossRef]
- Luna, D.; Bautista, F.M.; Caballero, V.; Campelo, J.M.; Marinas, J.M.; Romero, A.A. Method for Producing Biodiesel Using Porcine Pancreatic Lipase as an Enzymatic Catalyst. WO Patent 2008009772A1, 24 January 2008. [Google Scholar]
- Luna, C.; Luna, D.; Bautista, F.M.; Estevez, R.; Calero, J.; Posadillo, A.; Romero, A.A.; Sancho, E.D. Application of Enzymatic Extracts from a CALB Standard Strain as Biocatalyst within the Context of Conventional Biodiesel Production Optimization. Molecules 2017, 22, 2025. [Google Scholar] [CrossRef] [PubMed]
- Luna, C.; Sancho, E.; Luna, D.; Caballero, V.; Calero, J.; Posadillo, A.; Verdugo, C.; Bautista, F.M.; Romero, A.A. Biofuel that keeps glycerol as monoglyceride by 1, 3-selective ethanolysis with pig pancreatic lipase covalently immobilized on AlPO4 support. Energies 2013, 6, 3879–3900. [Google Scholar] [CrossRef]
- Verdugo, C.; Luna, D.; Posadillo, A.; Sancho, E.D.; Rodríguez, S.; Bautista, F.; Luque, R.; Marinas, J.M.; Romero, A.A. Production of a new second generation biodiesel with a low cost lipase derived from Thermomyces lanuginosus: Optimization by response surface methodology. Catal. Today 2011, 167, 107–112. [Google Scholar] [CrossRef]
- Calero, J.; Verdugo, C.; Luna, D.; Sancho, E.D.; Luna, C.; Posadillo, A.; Bautista, F.M.; Romero, A.A. Selective ethanolysis of sunflower oil with Lipozyme RM IM, an immobilized Rhizomucor miehei lipase, to obtain a biodiesel-like biofuel, which avoids glycerol production through the monoglyceride formation. New Biotechnol. 2014, 31, 596–601. [Google Scholar] [CrossRef] [PubMed]
- Luna, C.; Verdugo, C.; Sancho, E.D.; Luna, D.; Calero, J.; Posadillo, A.; Bautista, F.M.; Romero, A.A. A biofuel similar to biodiesel obtained by using a lipase from Rhizopus oryzae, optimized by response surface methodology. Energies 2014, 7, 3383–3399. [Google Scholar] [CrossRef]
- Bautista, F.M.; Bravo, M.C.; Campelo, J.M.; García, A.; Luna, D.; Marinas, J.M.; Romero, A.A. Covalent immobilization of porcine pancreatic lipase on amorphous AlPO4 and other inorganic supports. J. Chem. Technol. Biotechnol. 1998, 72, 249–254. [Google Scholar] [CrossRef]
- Verdugo, C.; Luque, R.; Luna, D.; Hidalgo, J.M.; Posadillo, A.; Sancho, E.D.; Rodriguez, S.; Ferreira-Dias, S.; Bautista, F.; Romero, A.A. A comprehensive study of reaction parameters in the enzymatic production of novel biofuels integrating glycerol into their composition. Bioresour. Technol. 2010, 101, 6657–6662. [Google Scholar] [CrossRef] [PubMed]
- Tan, K.T.; Lee, K.T.; Mohamed, A.R. A glycerol-free process to produce biodiesel by supercritical methyl acetate technology: An optimization study via response surface methodology. Bioresour. Technol. 2010, 101, 965–969. [Google Scholar] [CrossRef] [PubMed]




| Variables | Unit | Levels | ||
|---|---|---|---|---|
| −1 | 0 | 1 | ||
| Immobilized Lipozyme RM IM | g | 0.01 | 0.02 | 0.03 |
| Oil/ethanol ratio (v/v) | mL/mL | 12/2.9 | - | 12/3.5 |
| NaOH 10N amount | µL | 25 | 37.5 | 50 |
| Support | Viscosity (cSt) | Conversion (%) | Selectivity (%) |
|---|---|---|---|
| Unsupported Lipozyme | 7.9 | 100 | 62.4 |
| Lipo-Sep | 18.8 | - | - |
| Lipo-Silica | 11.6 | 83.0 | 31.4 |
| Lipo-1Sep/AlPO4 | 23.7 | 1 - | 1 - |
| Lipo-2Sep/AlPO4 | 16.2 | 1 - | 1 - |
| Run | Studied Variables | Output Variables | |||
|---|---|---|---|---|---|
| Amount Lipozyme RM IM (g) | Oíl/ethanol ratio (mol/mol) | Amount of 10 N NaOH (µL) | Viscosity (cSt) | Selectivity (%) | |
| 1 | 1 | −1 | 0 | 11.36 | 39.7 |
| 2 | 1 | 1 | −1 | 10.16 | 50.1 |
| 3 | −1 | 1 | 1 | 9.98 | 67.9 |
| 4 | 0 | 1 | −1 | 9.88 | 49.8 |
| 5 | −1 | −1 | 0 | 11.48 | 45.3 |
| 6 | 1 | −1 | 1 | 11.56 | 47.6 |
| 7 | −1 | 1 | −1 | 10.56 | 51.4 |
| 8 | −1 | 1 | 0 | 9.09 | 55.2 |
| 9 | 0 | −1 | −1 | 11.84 | 40.7 |
| 10 | 0 | −1 | 0 | 11.52 | 41.1 |
| 11 | 0 | 1 | 1 | 8.92 | 69.8 |
| 12 | 1 | 1 | 0 | 10.14 | 51.6 |
| 13 | −1 | −1 | 1 | 10.82 | 52.7 |
| 14 | 0 | 1 | 0 | 9.31 | 56.2 |
| 15 | 0 | −1 | 1 | 9.98 | 48.7 |
| 16 | 1 | 1 | 1 | 9.86 | 67.1 |
| 17 | 1 | −1 | −1 | 11.64 | 35.1 |
| 18 | −1 | −1 | −1 | 12.29 | 43.2 |
| Repeated experiments | |||||
| 19 | 1 | 1 | 1 | 9.94 | 58.7 |
| 20 | 0 | −1 | 1 | 9.94 | 53.1 |
| 21 | 1 | −1 | 1 | 11.09 | 44.4 |
| 22 | −1 | 1 | 1 | 10.18 | 64.5 |
| 23 | 1 | −1 | −1 | 12.21 | 34.5 |
| 24 | −1 | 1 | 0 | 10.01 | 60.5 |
| 25 | −1 | −1 | 0 | 11.52 | 45.7 |
| 26 | −1 | 1 | −1 | 10.01 | 54.7 |
| 27 | 0 | 1 | −1 | 8.89 | 56.6 |
| 28 | −1 | −1 | −1 | 11.56 | 36.8 |
| 29 | 0 | 1 | 1 | 9.53 | 61.8 |
| 30 | 0 | 1 | 0 | 9.73 | 59.7 |
| 31 | 0 | −1 | 0 | 10.44 | 44.3 |
| 32 | 1 | 1 | 0 | 10.88 | 54.3 |
| 33 | 0 | −1 | −1 | 11.26 | 33.2 |
| 34 | 1 | −1 | 0 | 11.48 | 37.4 |
| 35 | −1 | −1 | 1 | 10.26 | 50.2 |
| 36 | 1 | 1 | −1 | 10.03 | 50.1 |
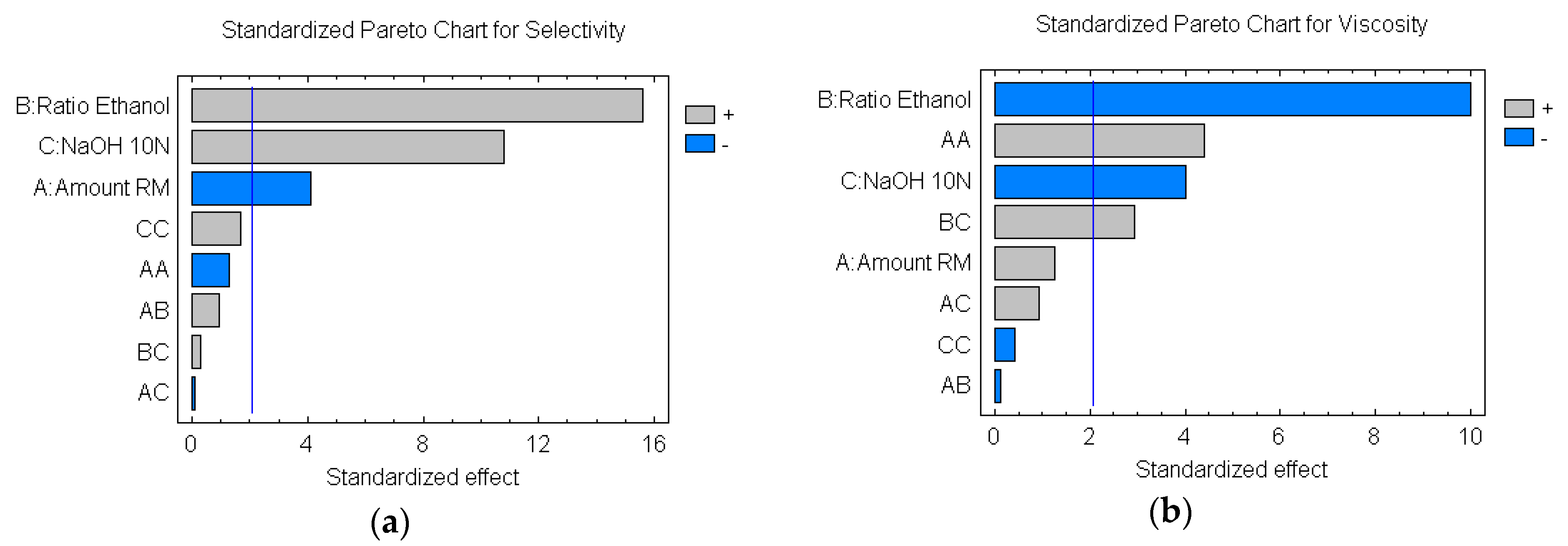
| Source | Sum of Squares | Freedom Degree | Mean Square | F-Ratio | p-Value |
|---|---|---|---|---|---|
| A: Amount Lipozyme RM | 137.76 | 1 | 137.76 | 17.05 | 0.0003 |
| B: Ratio oil/Ethanol | 1969.88 | 1 | 1969.88 | 243.81 | 0.0000 |
| C: Amount NaOH 10N | 941.254 | 1 | 941.254 | 116.50 | 0.0000 |
| AA | 13.6068 | 1 | 13.6068 | 1.68 | 0.2058 |
| AB | 6.93375 | 1 | 6.93375 | 0.86 | 0.3628 |
| AC | 0.09 | 1 | 0.09 | 0.01 | 0.9168 |
| BC | 0.63375 | 1 | 0.63375 | 0.08 | 0.7816 |
| CC | 23.0068 | 1 | 23.0068 | 2.85 | 0.1035 |
| blocks | 4.48028 | 1 | 4.48028 | 0.55 | 0.4632 |
| Total error | 210.071 | 26 | 8.07964 | ||
| Total (corr.) | 3307.72 | 35 |
| Source | Sum of Squares | Freedom Degree | Mean Square | F-Ratio | p-Value |
|---|---|---|---|---|---|
| A: Amount Lipozyme RM | 0.279504 | 1 | 0.279504 | 1.59 | 0.2189 |
| B: Ratio oil/Ethanol | 17.5701 | 1 | 17.5701 | 99.77 | 0.0000 |
| C: Amount NaOH 10N | 2.8497 | 1 | 2.8497 | 16.18 | 0.0004 |
| AA | 3.39301 | 1 | 3.39301 | 19.27 | 0.0002 |
| AB | 0.00220417 | 1 | 0.00220417 | 0.01 | 0.9118 |
| AC | 0.158006 | 1 | 0.158006 | 0.90 | 0.3523 |
| BC | 1.51504 | 1 | 1.51504 | 8.60 | 0.0069 |
| CC | 0.0325125 | 1 | 0.0325125 | 0.18 | 0.6710 |
| Blocks | 0.0568028 | 1 | 0.0568028 | 0.32 | 0.5750 |
| Total error | 4.57882 | 26 | 0.176109 | ||
| Total (corr.) | 30.4357 | 35 |
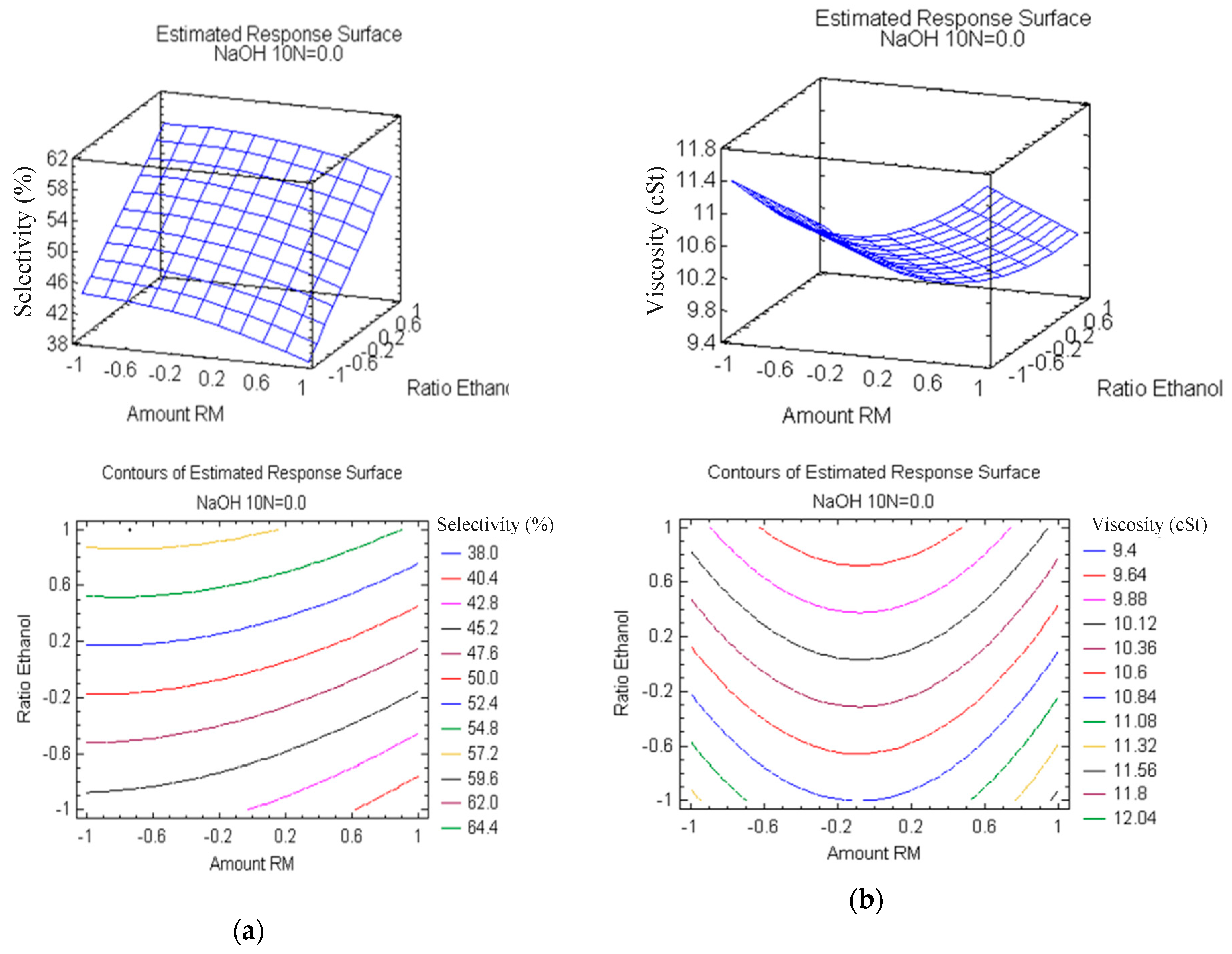
| Run | Studied Variables | Selectivity (%) | Viscosity (cSt) | ||||
|---|---|---|---|---|---|---|---|
| Amount Lipozyme RM IM (g) | Oíl/ethanol Ratio | Amount of 10 N NaOH | Exp. Value | Predicted | Exp. Value | Predicted | |
| 1 | 1 | −1 | 0 | 39.7 | 38.8375 | 11.36 | 11.6529 |
| 2 | 1 | 1 | −1 | 50.1 | 50.0528 | 10.16 | 10.1667 |
| 3 | −1 | 1 | 1 | 67.9 | 66.6194 | 9.98 | 9.7834 |
| 4 | 0 | 1 | −1 | 49.8 | 53.1403 | 9.88 | 9.51653 |
| 5 | −1 | −1 | 0 | 45.3 | 44.7042 | 11.48 | 11.4179 |
| 6 | 1 | −1 | 1 | 47.6 | 46.5583 | 11.56 | 11.0927 |
| 7 | −1 | 1 | −1 | 51.4 | 53.6194 | 10.56 | 10.1688 |
| 8 | −1 | 1 | 0 | 55.2 | 58.4236 | 9.09 | 10.0399 |
| 9 | 0 | −1 | −1 | 40.7 | 38.6708 | 11.84 | 11.4162 |
| 10 | 0 | −1 | 0 | 41.1 | 43.075 | 11.52 | 10.8842 |
| 11 | 0 | 1 | 1 | 69.8 | 65.9903 | 8.92 | 9.32986 |
| 12 | 1 | 1 | 0 | 51.6 | 54.7069 | 10.14 | 10.2365 |
| 13 | −1 | −1 | 1 | 52.7 | 52.575 | 10.82 | 10.659 |
| 14 | 0 | 1 | 0 | 56.2 | 57.8694 | 9.31 | 9.48694 |
| 15 | 0 | −1 | 1 | 48.7 | 50.8708 | 9.98 | 10.2246 |
| 16 | 1 | 1 | 1 | 67.1 | 62.7528 | 9.86 | 10.1788 |
| 17 | 1 | −1 | −1 | 35.1 | 34.5083 | 11.64 | 12.0856 |
| 18 | −1 | −1 | −1 | 43.2 | 40.225 | 12.29 | 12.0494 |
| Repeated experiments | |||||||
| 19 | 1 | 1 | 1 | 58.7 | 62.0472 | 9.94 | 10.0994 |
| 20 | 0 | −1 | 1 | 53.1 | 50.1653 | 9.94 | 10.1451 |
| 21 | 1 | −1 | 1 | 44.4 | 45.8528 | 11.09 | 11.0133 |
| 22 | −1 | 1 | 1 | 64.5 | 65.9139 | 10.18 | 9.70396 |
| 23 | 1 | −1 | −1 | 34.5 | 33.8028 | 12.21 | 12.0062 |
| 24 | −1 | 1 | 0 | 60.5 | 57.7181 | 10.01 | 9.96042 |
| 25 | −1 | −1 | 0 | 45.7 | 43.9986 | 11.52 | 11.3385 |
| 26 | −1 | 1 | −1 | 54.7 | 52.9139 | 10.01 | 10.0894 |
| 27 | 0 | 1 | −1 | 56.6 | 52.4347 | 8.89 | 9.43708 |
| 28 | −1 | −1 | −1 | 36.8 | 39.5194 | 11.56 | 11.9699 |
| 29 | 0 | 1 | 1 | 61.8 | 65.2847 | 9.53 | 9.25042 |
| 30 | 0 | 1 | 0 | 59.7 | 57.1639 | 9.73 | 9.4075 |
| 31 | 0 | −1 | 0 | 44.3 | 42.3694 | 10.44 | 10.8047 |
| 32 | 1 | 1 | 0 | 54.3 | 54.0014 | 10.88 | 10.1571 |
| 33 | 0 | −1 | −1 | 33.2 | 37.9653 | 11.26 | 11.3368 |
| 34 | 1 | −1 | 0 | 37.4 | 38.1319 | 11.48 | 11.5735 |
| 35 | −1 | −1 | 1 | 50.2 | 51.8694 | 10.26 | 10.5795 |
| 36 | 1 | 1 | −1 | 50.1 | 49.3472 | 10.03 | 10.0873 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Calero, J.; Luna, D.; Luna, C.; Bautista, F.M.; Hurtado, B.; Romero, A.A.; Posadillo, A.; Estevez, R. Rhizomucor miehei Lipase Supported on Inorganic Solids, as Biocatalyst for the Synthesis of Biofuels: Improving the Experimental Conditions by Response Surface Methodology. Energies 2019, 12, 831. https://doi.org/10.3390/en12050831
Calero J, Luna D, Luna C, Bautista FM, Hurtado B, Romero AA, Posadillo A, Estevez R. Rhizomucor miehei Lipase Supported on Inorganic Solids, as Biocatalyst for the Synthesis of Biofuels: Improving the Experimental Conditions by Response Surface Methodology. Energies. 2019; 12(5):831. https://doi.org/10.3390/en12050831
Chicago/Turabian StyleCalero, Juan, Diego Luna, Carlos Luna, Felipa M. Bautista, Beatriz Hurtado, Antonio A. Romero, Alejandro Posadillo, and Rafael Estevez. 2019. "Rhizomucor miehei Lipase Supported on Inorganic Solids, as Biocatalyst for the Synthesis of Biofuels: Improving the Experimental Conditions by Response Surface Methodology" Energies 12, no. 5: 831. https://doi.org/10.3390/en12050831
APA StyleCalero, J., Luna, D., Luna, C., Bautista, F. M., Hurtado, B., Romero, A. A., Posadillo, A., & Estevez, R. (2019). Rhizomucor miehei Lipase Supported on Inorganic Solids, as Biocatalyst for the Synthesis of Biofuels: Improving the Experimental Conditions by Response Surface Methodology. Energies, 12(5), 831. https://doi.org/10.3390/en12050831







