Enhanced CH4 Production from Corn-Stalk Pyrolysis Using Ni-5CeO2/MCM-41 as a Catalyst
Abstract
1. Introduction
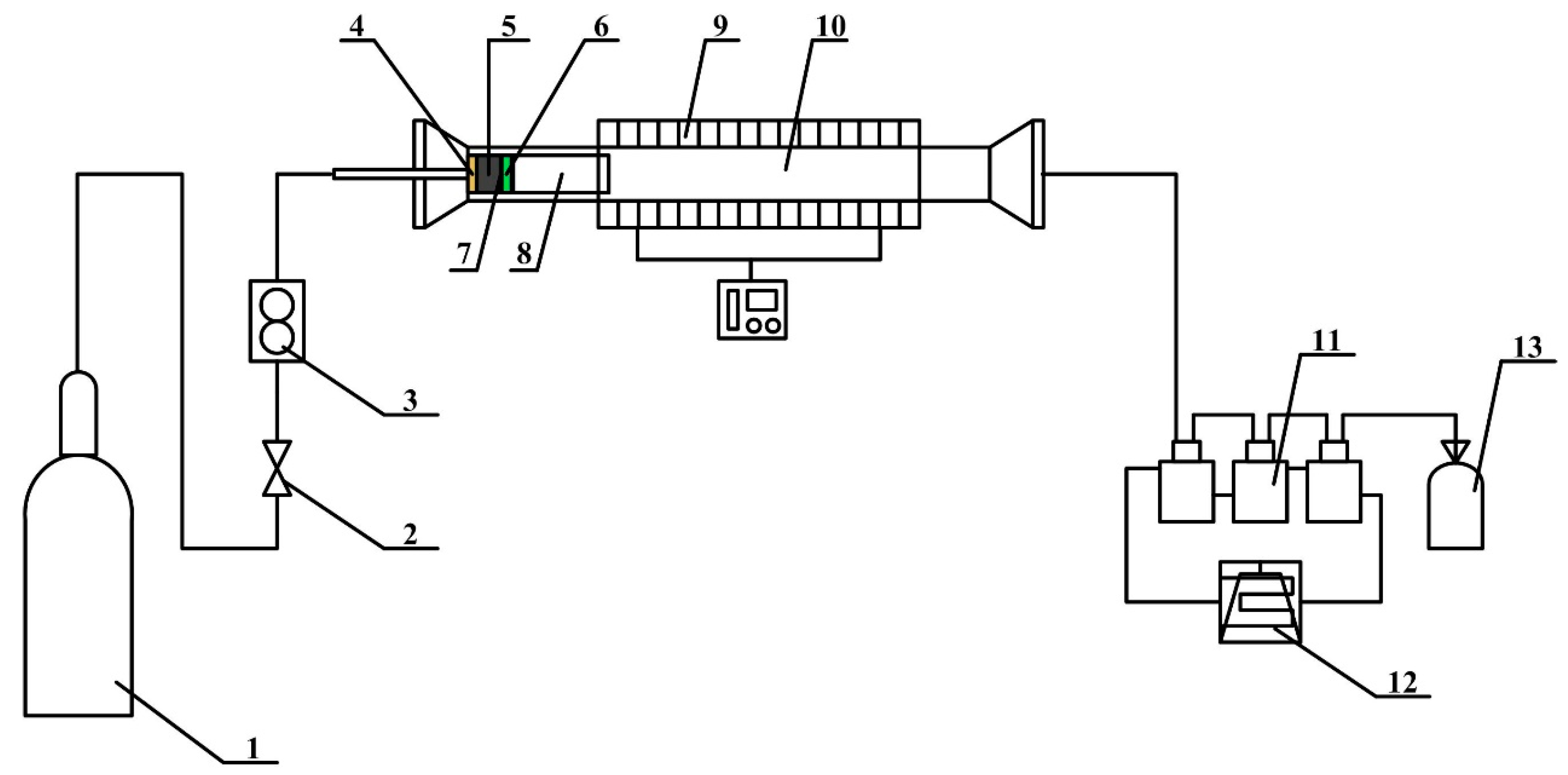
2. Materials and Methods
3. Results and Discussions
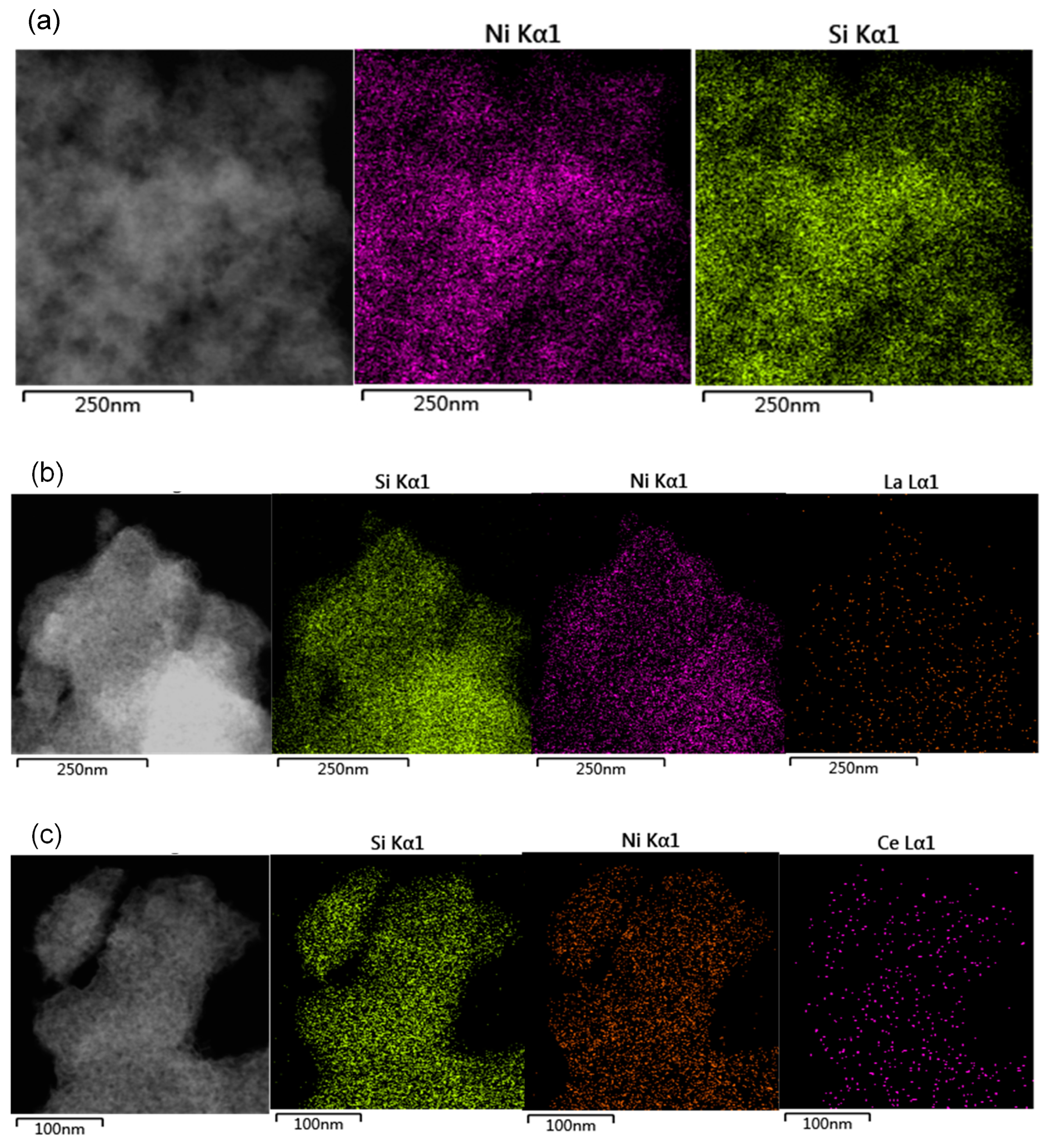
3.1. Characterization of Catalysts
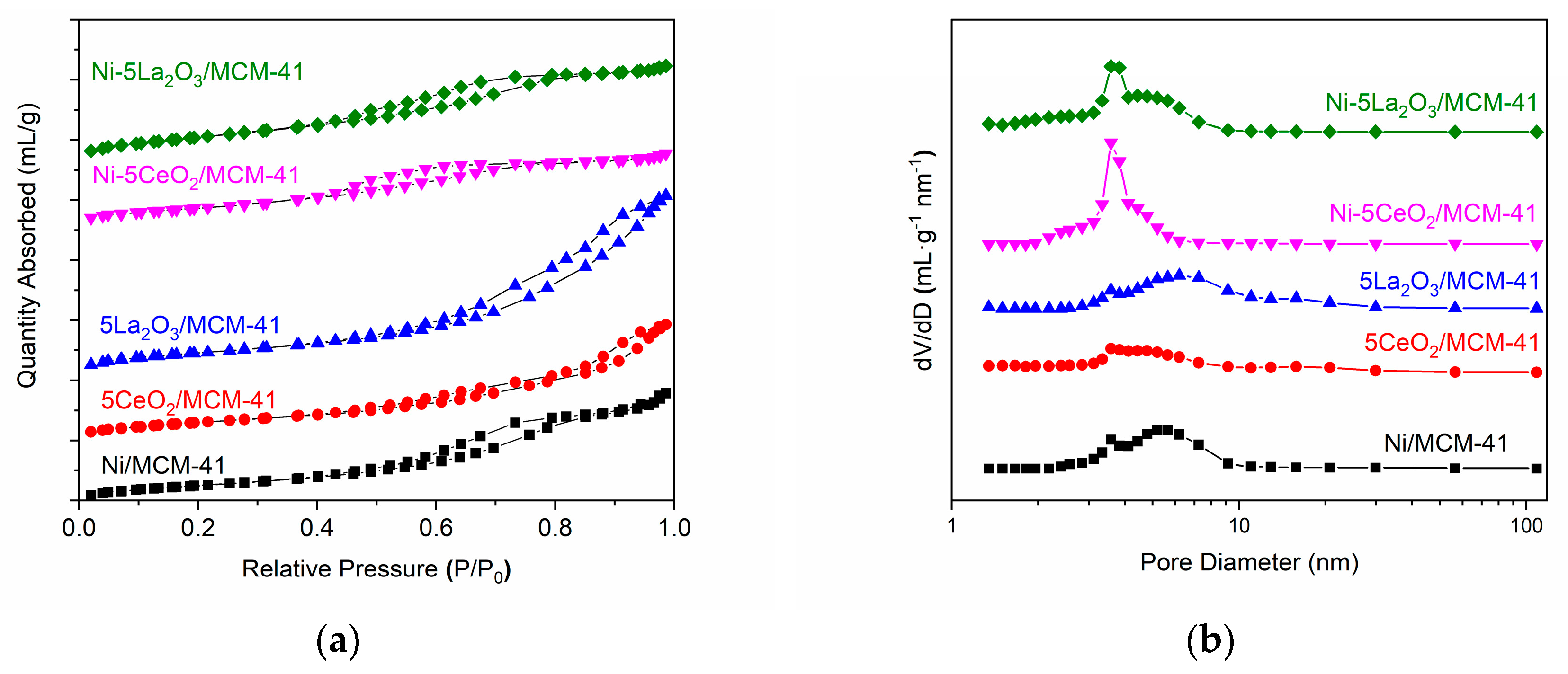
3.1.1. Textural Properties of the Catalysts
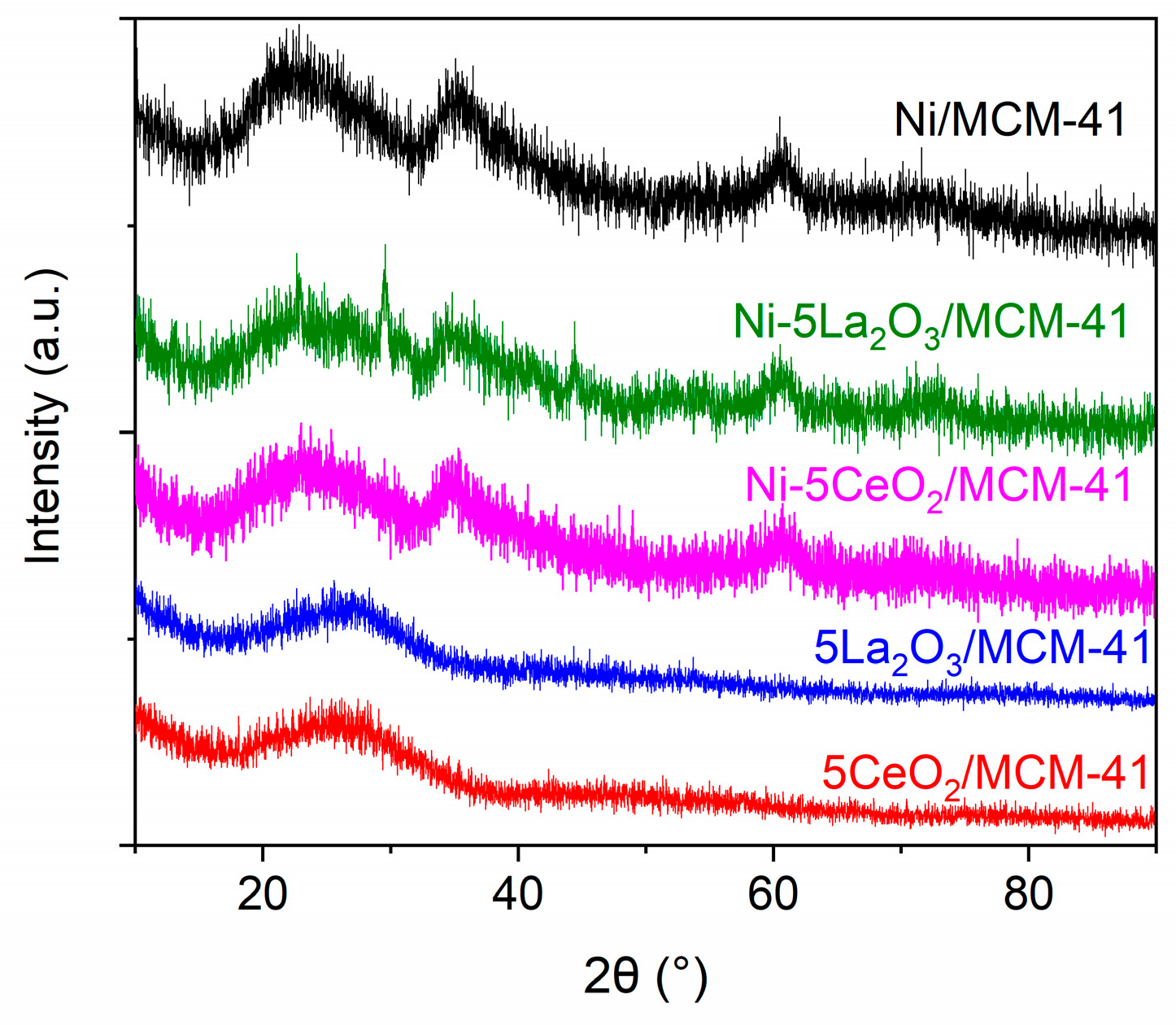
3.1.2. XRD
3.1.3. XPS
3.1.4. H2-TPR
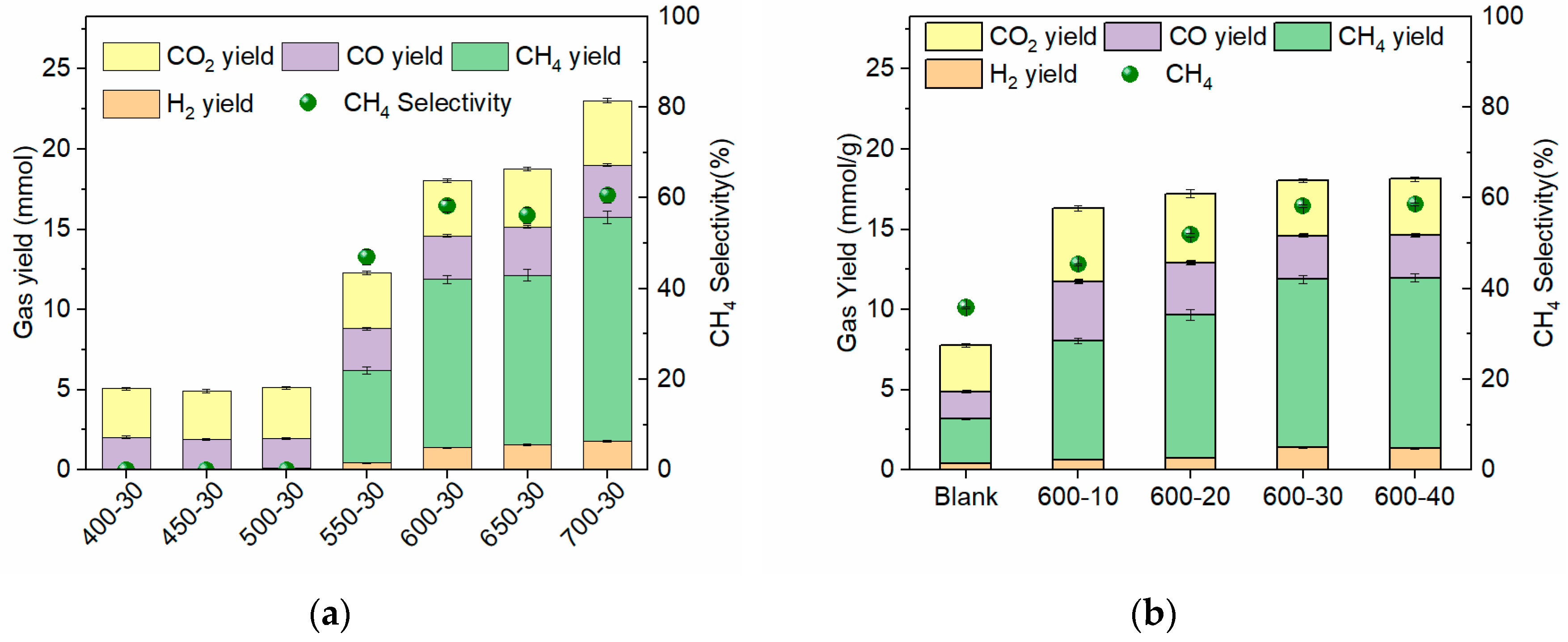
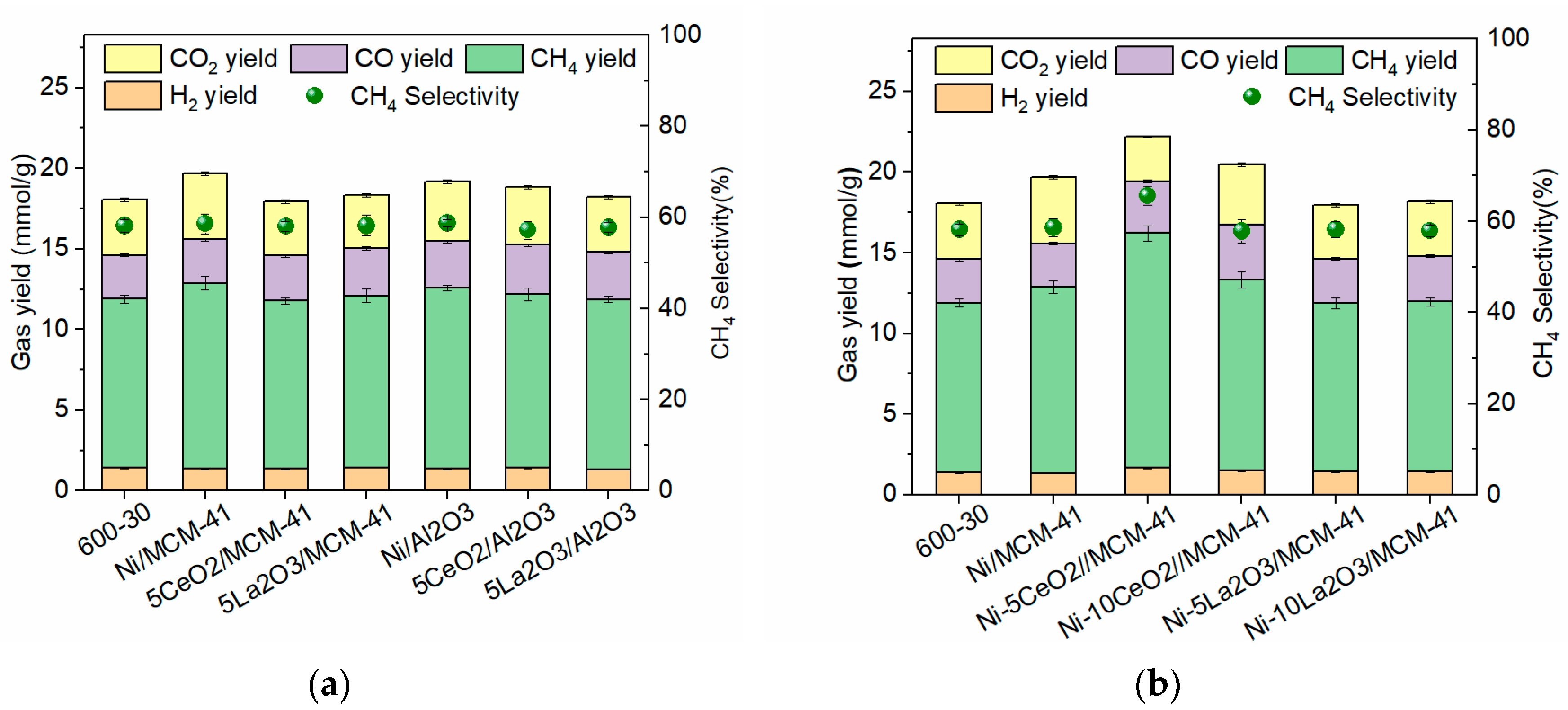
3.2. Gasification of Corn Stalk
3.2.1. Effect of Temperature and Time on the Gas Yield
3.2.2. Effect of Catalyst on the Gas Yield
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Zhang, W.; Lu, Z.; Xu, Y.; Wang, C.; Gu, Y.; Xu, H.; Streets, D.G. Black carbon emissions from biomass and coal in rural China. Atmos. Environ. 2018, 176, 158–170. [Google Scholar] [CrossRef]
- Hou, Q.; Meiting, J.U.; Weizun, L.I.; Liu, L.; Yang, Q.; Chen, Y. Research progress on biomass fractionation using ionic liquids. Chem. Ind. Eng. Prog. 2016, 35, 3022–3031. [Google Scholar] [CrossRef]
- Hou, Q.; Li, W.; Ju, M.; Liu, L.; Chen, Y.; Yang, Q.; Wang, J. Separation of polysaccharides from rice husk and wheat bran using solvent system consisting of BMIMOAc and DMI. Carbohydr. Polym. 2015, 133, 517–523. [Google Scholar] [CrossRef] [PubMed]
- Hou, Q.; Ju, M.; Li, W.; Liu, L.; Chen, Y.; Yang, Q. Pretreatment of lignocellulosic biomass with ionic liquids and ionic liquid-based solvent systems. Molecules 2017, 22, 490. [Google Scholar] [CrossRef] [PubMed]
- Hou, Q.; Li, W.; Ju, M.; Liu, L.; Chen, Y.; Yang, Q. One-pot synthesis of sulfonated graphene oxide for efficient conversion of fructose into HMF. RSC Adv. 2016, 6, 104016–104024. [Google Scholar] [CrossRef]
- Hou, Q.; Li, W.; Zhen, M.; Liu, L.; Chen, Y.; Yang, Q.; Huang, F.; Zhang, S.; Ju, M. An ionic liquid–organic solvent biphasic system for efficient production of 5-hydroxymethylfurfural from carbohydrates at high concentrations. RSC Adv. 2017, 7, 47288–47296. [Google Scholar] [CrossRef]
- Dong, C.; Chen, J.; Guan, R.; Li, X.; Xin, Y. Dual-frequency ultrasound combined with alkali pretreatment of corn stalk for enhanced biogas production. Renew. Energy 2018, 127, 444–451. [Google Scholar] [CrossRef]
- Xiong, Q.A.; Li, J.; Guo, S.; Li, G.; Zhao, J.; Fang, Y. Ash fusion characteristics during co-gasification of biomass and petroleum coke. Bioresour. Technol. 2018, 257, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Yang, W.; Fan, J.; Kan, T.; Zhang, W.; Liu, H.; Cheng, W.; Yang, H.; Wu, X.; Chen, H. Effect of sodium carboxymethyl cellulose addition on particulate matter emissions during biomass pellet combustion. Appl. Energy 2018, 230, 925–934. [Google Scholar] [CrossRef]
- Hou, Q.; Zhen, M.; Liu, L.; Chen, Y.; Huang, F.; Zhang, S.; Li, W.; Ju, M. Tin phosphate as a heterogeneous catalyst for efficient dehydration of glucose into 5-hydroxymethylfurfural in ionic liquid. Appl. Catal. B Environ. 2018, 224, 183–193. [Google Scholar] [CrossRef]
- Sikarwar, V.S.; Zhao, M.; Clough, P.; Yao, J.; Zhong, X.; Memon, M.Z.; Shah, N.; Anthony, E.; Fennell, P. An overview of advances in biomass gasification. Energy Environ. Sci. 2016, 9, 2927–3304. [Google Scholar] [CrossRef]
- Manzour, P.A. Hydrogen Production Using Catalytic Supercritical Water Gasification of Lignocellulosic Biomass. Ph.D. Thesis, University of Toronto, Toronto, ON, Canada, 2012. [Google Scholar]
- Byrd, A.J.; Pant, K.; Gupta, R.B. Hydrogen production from glycerol by reforming in supercritical water over Ru/Al2O3 catalyst. Fuel 2008, 87, 2956–2960. [Google Scholar] [CrossRef]
- Chan, F.L.; Tanksale, A. Review of recent developments in Ni-based catalysts for biomass gasification. Renew. Sustain. Energy Rev. 2014, 38, 428–438. [Google Scholar] [CrossRef]
- Wu, C.; Williams, P.T. A novel nano-Ni/SiO2 catalyst for hydrogen production from steam reforming of ethanol. Environ. Sci. Technol. 2010, 44, 5993–5998. [Google Scholar] [CrossRef] [PubMed]
- Yang, R.-X.; Chuang, K.-H.; Wey, M.-Y. Effects of nickel species on Ni/Al2O3 catalysts in carbon nanotube and hydrogen production by waste plastic gasification: Bench-and pilot-scale tests. Energy Fuels 2015, 29, 8178–8187. [Google Scholar] [CrossRef]
- Miranda, B.; Chimentão, R.; Santos, J.; Gispert-Guirado, F.; Llorca, J.; Medina, F.; Bonillo, F.L.; Sueiras, J. Conversion of glycerol over 10% Ni/γ-Al2O3 catalyst. Appl. Catal. B Environ. 2014, 147, 464–480. [Google Scholar] [CrossRef]
- Ashik, U.; Daud, W.W. Probing the differential methane decomposition behaviors of n-Ni/SiO2, n-Fe/SiO2 and n-Co/SiO2 catalysts prepared by co-precipitation cum modified Stöber method. Rsc Adv. 2015, 5, 67227–67241. [Google Scholar] [CrossRef]
- Ji, G.; Xu, X.; Yang, H.; Zhao, X.; He, X.; Zhao, M. Enhanced hydrogen production from sawdust decomposition using hybrid-functional Ni-CaO-Ca2SiO4 materials. Environ. Sci. Technol. 2017, 51, 11484–11492. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.O.; Ahn, I.Y.; Moon, S.H. Effect of CeO2-addition sequence on the performance of CeO2-modified Ni/Al2O3 catalyst in autothermal reforming of iso-octane. Korean J. Chem. Eng. 2009, 26, 1252–1258. [Google Scholar] [CrossRef]
- Sánchez-Sánchez, M.; Navarro, R.; Fierro, J. Ethanol steam reforming over Ni/MxOy–Al2O3 (M = Ce, La, Zr and Mg) catalysts: Influence of support on the hydrogen production. Int. J. Hydrogen Energy 2007, 32, 1462–1471. [Google Scholar] [CrossRef]
- Pattiya, A.; Titiloye, J.O.; Bridgwater, A.V. Fast pyrolysis of cassava rhizome in the presence of catalysts. J. Anal. Appl. Pyrolysis 2008, 81, 72–79. [Google Scholar] [CrossRef]
- Du, G.; Lim, S.; Yang, Y.; Wang, C.; Pfefferle, L.; Haller, G.L. Methanation of carbon dioxide on Ni-incorporated MCM-41 catalysts: The influence of catalyst pretreatment and study of steady-state reaction. J. Catal. 2007, 249, 370–379. [Google Scholar] [CrossRef]
- Zhang, J.; Xin, Z.; Meng, X.; Tao, M. Synthesis, characterization and properties of anti-sintering nickel incorporated MCM-41 methanation catalysts. Fuel 2013, 109, 693–701. [Google Scholar] [CrossRef]
- Corthals, S.; Van Nederkassel, J.; Geboers, J.; De Winne, H.; Van Noyen, J.; Moens, B.; Sels, B.; Jacobs, P. Influence of composition of MgAl2O4 supported NiCeO2ZrO2 catalysts on coke formation and catalyst stability for dry reforming of methane. Catal. Today 2008, 138, 28–32. [Google Scholar] [CrossRef]
- Liu, D.; Lau, R.; Borgna, A.; Yang, Y. Carbon dioxide reforming of methane to synthesis gas over Ni-MCM-41 catalysts. Appl. Catal. A Gen. 2009, 358, 110–118. [Google Scholar] [CrossRef]
- Li, D.; Zeng, L.; Li, X.; Wang, X.; Ma, H.; Assabumrungrat, S.; Gong, J. Ceria-promoted Ni/SBA-15 catalysts for ethanol steam reforming with enhanced activity and resistance to deactivation. Appl. Catal. B Environ. 2015, 176, 532–541. [Google Scholar] [CrossRef]
- Carraro, P.M.; Blanco, A.A.G.; Soria, F.A.; Lener, G.; Sapag, K.; Eimer, G.A.; Oliva, M.I. Understanding the role of nickel on the hydrogen storage capacity of Ni/MCM-41 materials. Microporous Mesoporous Mater. 2016, 231, 31–39. [Google Scholar] [CrossRef]
- Lensveld, D.J.; Gerbrand Mesu, J.; Jos van Dillen, A.; de Jong, K.P. Synthesis and characterisation of MCM-41 supported nickel oxide catalysts. Microporous Mesoporous Mater. 2001, 44–45, 401–407. [Google Scholar] [CrossRef]
- Gangwar, B.P.; Maiti, S.C.; Sharma, S. Modifying the hygroscopic property of La2O3 by Pr, Sm and Nd doping. J. Solid State Chem. 2017, 256, 109–115. [Google Scholar] [CrossRef]
- Beche, E.; Peraudeau, G.; Flaud, V.; Perarnau, D. An XPS investigation of (La2O3)1-x (CeO2)2x (ZrO2)2 compounds. Surf. Interface Anal. 2012, 44, 1045–1050. [Google Scholar] [CrossRef]
- Reddy, B.M.; Chowdhury, B.; Smirniotis, P.G. An XPS study of La2O3 and In2O3 influence on the physicochemical properties of MoO3/TiO2 catalysts. Appl. Catal. A Gen. 2001, 219, 53–60. [Google Scholar] [CrossRef]
- Cheng, D.-G.; Chong, M.; Chen, F.; Zhan, X. XPS Characterization of CeO2 Catalyst for hydrogenation of benzoic acid to benzaldehyde. Catal. Lett. 2008, 120, 82–85. [Google Scholar] [CrossRef]
- Holgado, J.P.; Alvarez, R.; Munuera, G. Study of CeO2 XPS spectra by factor analysis: Reduction of CeO2. Appl. Surf. Sci. 2000, 161, 301–315. [Google Scholar] [CrossRef]
- Yang, C.; Fan, H.; Qiu, S.; Xi, Y.; Fu, Y. Microstructure and dielectric properties of La2O3 films prepared by ion beam assistant electron-beam evaporation. J. Non Cryst. Solids 2009, 355, 33–37. [Google Scholar] [CrossRef]
- Wang, N.; Liu, J.; Gu, W.; Song, Y.; Wang, F. Toward synergy of carbon and La2O3 in their hybrid as an efficient catalyst for the oxygen reduction reaction. RSC Adv. 2016, 6, 77786–77795. [Google Scholar] [CrossRef]
- Eliche-Quesada, D.; Mérida-Robles, J.; Maireles-Torres, P.; Rodríguez-Castellón, E.; Jiménez-López, A. Hydrogenation and ring opening of tetralin on supported nickel zirconium-doped mesoporous silica catalysts. influence of the nickel precursor. Langmuir 2003, 19, 4985–4991. [Google Scholar] [CrossRef]
- Wang, X.; Zhu, L.; Liu, Y.; Wang, S. CO2 methanation on the catalyst of Ni/MCM-41 promoted with CeO2. Sci. Total Environ. 2018, 625, 686–695. [Google Scholar] [CrossRef] [PubMed]


| Catalysts | SBET (m2/g) | VPore (mL/g) | DPore (nm) |
|---|---|---|---|
| Ni/Al2O3 | 50.3 | 0.45 | 35.6 |
| 5CeO2/Al2O3 | 44.6 | 0.38 | 34.1 |
| 5La2O3/Al2O3 | 42.9 | 0.45 | 41.9 |
| Ni/MCM-41 | 276.8 | 0.59 | 8.6 |
| 5CeO2/MCM-41 | 237.8 | 0.6 | 10 |
| 5La2O3/MCM-41 | 332.1 | 0.95 | 11.4 |
| Ni-5CeO2/MCM-41 | 282.8 | 0.39 | 5.6 |
| Ni-5La2O3/MCM-41 | 407.2 | 0.53 | 5.3 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, F.; Li, W.; Hou, Q.; Ju, M. Enhanced CH4 Production from Corn-Stalk Pyrolysis Using Ni-5CeO2/MCM-41 as a Catalyst. Energies 2019, 12, 774. https://doi.org/10.3390/en12050774
Huang F, Li W, Hou Q, Ju M. Enhanced CH4 Production from Corn-Stalk Pyrolysis Using Ni-5CeO2/MCM-41 as a Catalyst. Energies. 2019; 12(5):774. https://doi.org/10.3390/en12050774
Chicago/Turabian StyleHuang, Fang, Weizun Li, Qidong Hou, and Meiting Ju. 2019. "Enhanced CH4 Production from Corn-Stalk Pyrolysis Using Ni-5CeO2/MCM-41 as a Catalyst" Energies 12, no. 5: 774. https://doi.org/10.3390/en12050774
APA StyleHuang, F., Li, W., Hou, Q., & Ju, M. (2019). Enhanced CH4 Production from Corn-Stalk Pyrolysis Using Ni-5CeO2/MCM-41 as a Catalyst. Energies, 12(5), 774. https://doi.org/10.3390/en12050774





