Assessment of Electron Transfer Mechanisms during a Long-Term Sediment Microbial Fuel Cell Operation
Abstract
1. Introduction
2. Materials and Methods
2.1. Sediment Sampling
2.2. Sediment Microbial Fuel Cell Start-up and Operation
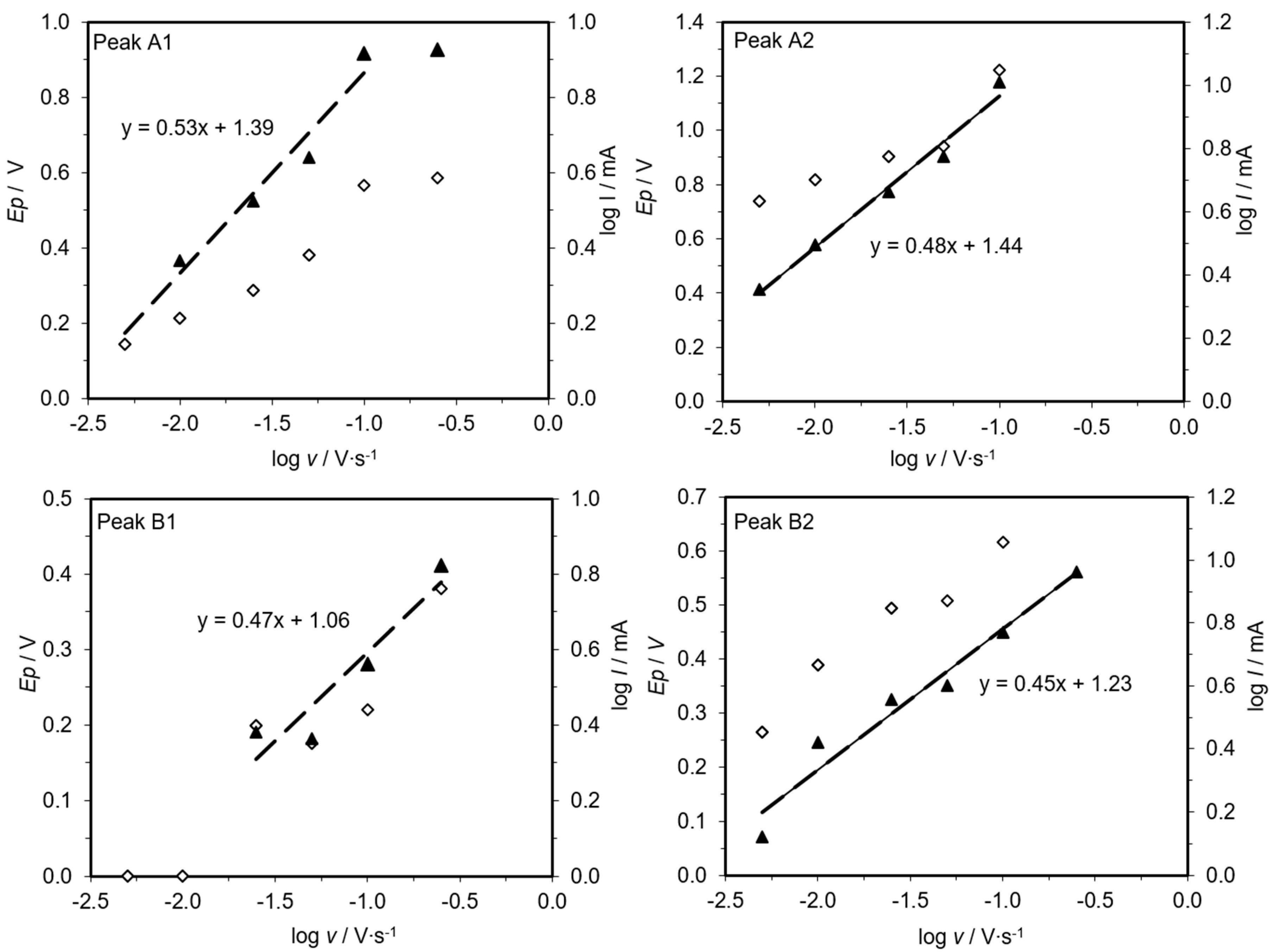
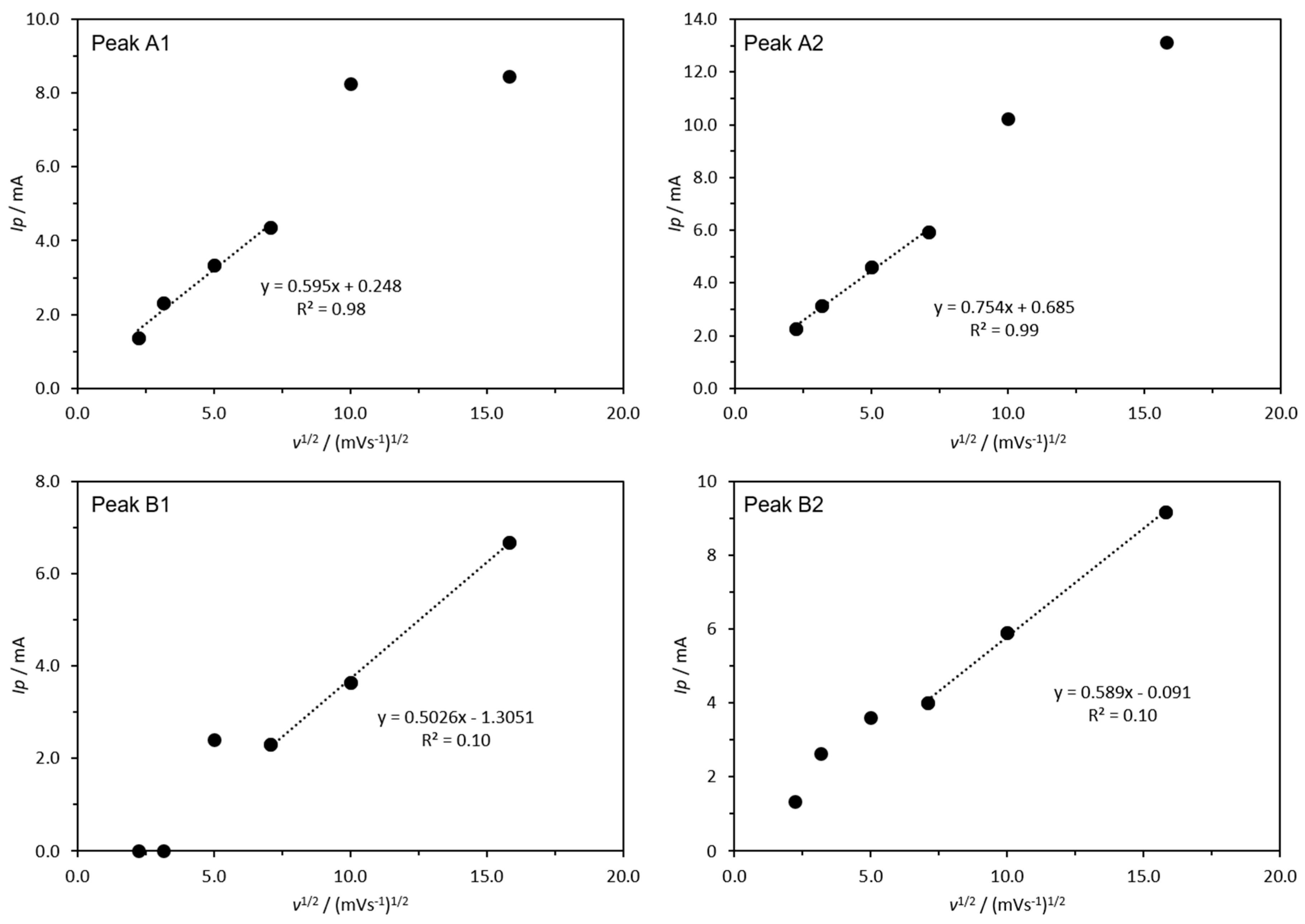
2.3. Cyclic Voltammetry
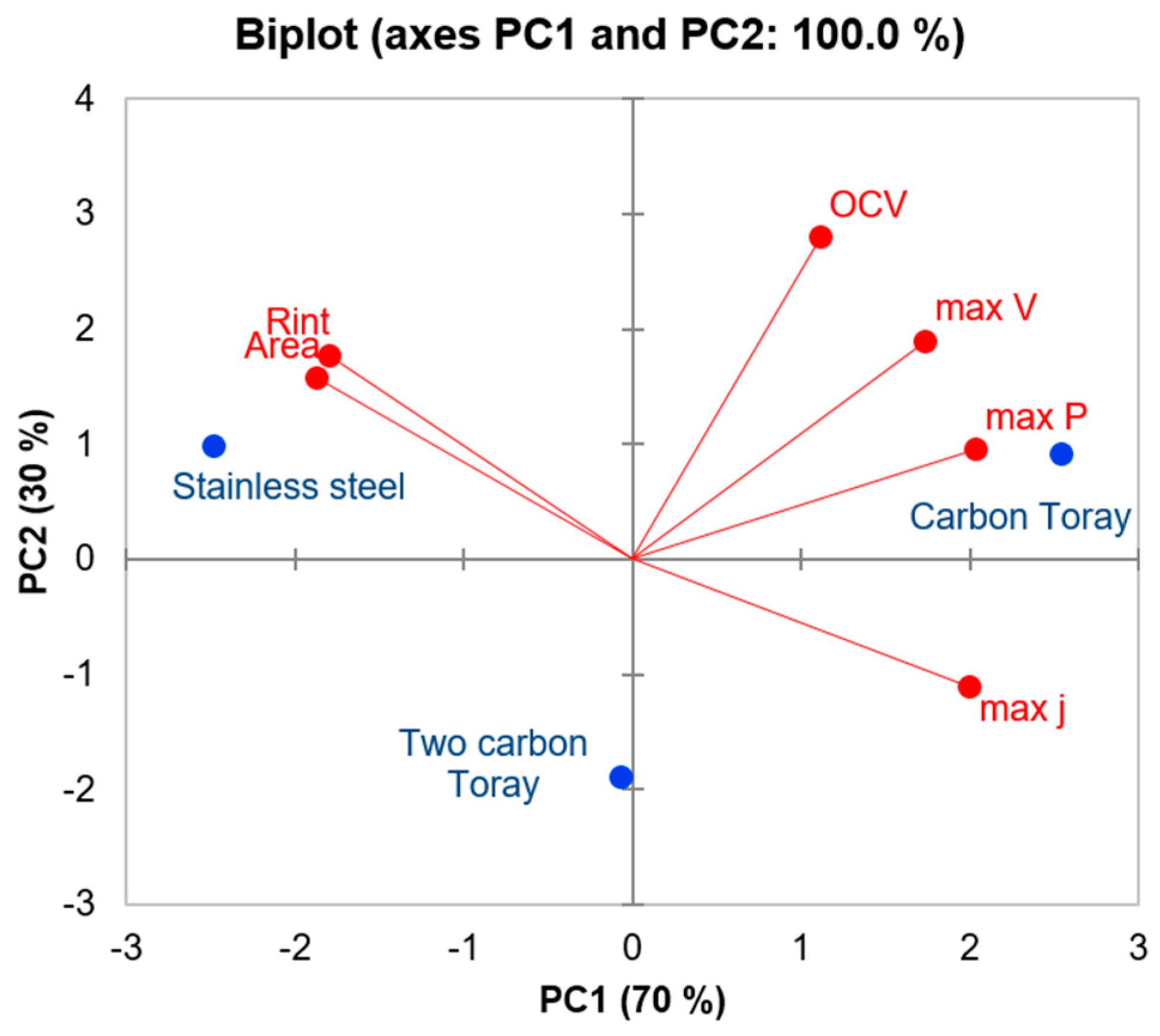
2.4. Principal Component Analysis (PCA)
3. Results
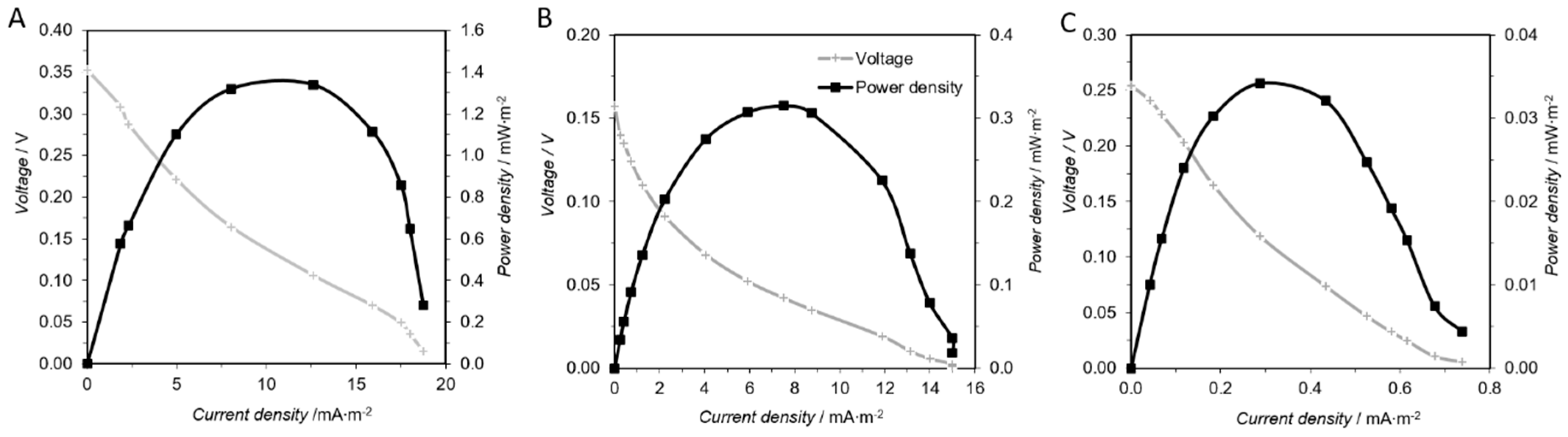
3.1. Sediment Microbial Fuel Cell Microcosm
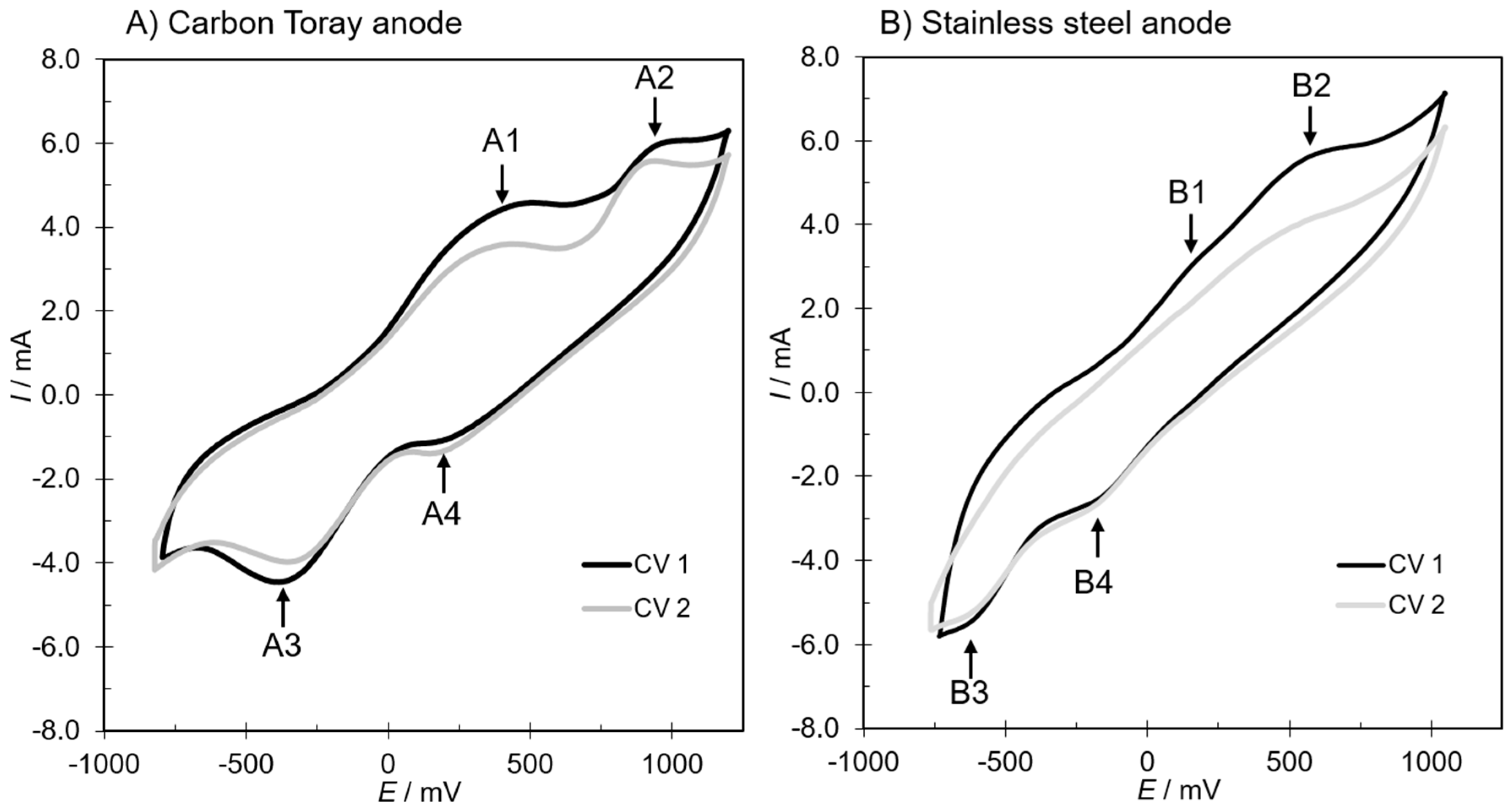
3.2. Cyclic Voltammetry Assessments
3.3. Principle Component Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Tender, L.M.; Reimers, C.E.; Iii, H.A.S.; Holmes, D.E.; Bond, D.R.; Lowy, D.A.; Pilobello, K.; Fertig, S.J.; Lovley, D.R. Harnessing microbially generated power on the seafloor. Nat. Biotechnol. 2002, 20, 821–825. [Google Scholar] [CrossRef] [PubMed]
- Holmes, D.E.; Bond, D.R.; O’Neil, R.A.; Reimers, C.E.; Tender, L.R.; Lovley, D.R. Microbial communities associated with electrodes harvesting electricity from a variety of aquatic sediments. Microb. Ecol. 2004, 48, 178–190. [Google Scholar] [CrossRef] [PubMed]
- Martins, G.; Terada, A.; Ribeiro, D.C.; Corral, A.M.; Brito, A.G.; Smets, B.F.; Nogueira, R. Structure and activity of lacustrine sediment bacteria involved in nutrient and iron cycles. FEMS Microbiol. Ecol. 2011, 77, 666–679. [Google Scholar] [CrossRef] [PubMed]
- Kwok, K.W.H.; Batley, G.E.; Wenning, R.J.; Zhu, L.; Vangheluwe, M.; Lee, S. Sediment quality guidelines: Challenges and opportunities for improving sediment management. Environ. Sci. Pollut. Res. 2014, 21, 17–27. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Tian, Y.; Qu, Y.; Qiu, Y.; Liu, J.; Feng, Y. A Pilot-scale Benthic Microbial Electrochemical System (BMES) for Enhanced Organic Removal in Sediment Restoration. Sci. Rep. 2017, 7, 39802. [Google Scholar] [CrossRef] [PubMed]
- Abbas, S.Z.; Rafatullah, M.; Ismail, N.; Syakir, M.I. A review on sediment microbial fuel cells as a new source of sustainable energy and heavy metal remediation: Mechanisms and future prospective. Int. J. Energy Res. 2017, 41, 1242–1264. [Google Scholar] [CrossRef]
- Tender, L.M.; Gray, S.A.; Groveman, E.; Lowy, D.A.; Kauffman, P.; Melhado, J.; Tyce, R.C.; Flynn, D.; Petrecca, R.; Dobarro, J. The first demonstration of a microbial fuel cell as a viable power supply: Powering a meteorological buoy. J. Power Sources 2008, 179, 571–575. [Google Scholar] [CrossRef]
- Donovan, C.; Dewan, A.; Heo, D.; Lewandowski, Z.; Beyenal, H. Sediment microbial fuel cell powering a submersible ultrasonic receiver: New approach to remote monitoring. J. Power Sources 2013, 233, 79–85. [Google Scholar] [CrossRef]
- Song, N.; Jiang, H.L. Effects of initial sediment properties on start-up times for sediment microbial fuel cells. Int. J. Hydrogen Energy 2018, 43, 10082–10093. [Google Scholar] [CrossRef]
- Song, T.-S.; Yan, Z.-S.; Zhao, Z.-W.; Jiang, H.-L. Removal of organic matter in freshwater sediment by microbial fuel cells at various external resistances. J. Chem. Technol. Biotechnol. 2010, 85, 1489–1493. [Google Scholar] [CrossRef]
- Xu, P.; Xiao, E.; Xu, D.; Li, J.; Zhang, Y.; Dai, Z.; Zhou, Q.; Wu, Z. Enhanced phosphorus reduction in simulated eutrophic water: A comparative study of submerged macrophytes, sediment microbial fuel cells, and their combination. Environ. Technol. 2018, 39, 1144–1157. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Zhao, H.; Zhao, N.; Ni, J.; Gu, X. Enhanced phosphorus flux from overlying water to sediment in a bioelectrochemical system. Bioresour. Technol. 2016, 216, 182–187. [Google Scholar] [CrossRef] [PubMed]
- Martins, G.; Peixoto, L.; Brito, A.G.; Nogueira, R. Phosphorus-iron interaction in sediments: Can an electrode minimize phosphorus release from sediments? Rev. Environ. Sci. Biotechnol. 2014, 13, 265–275. [Google Scholar] [CrossRef]
- Zhou, Y.-L.; Yang, Y.; Chen, M.; Zhao, Z.-W.; Jiang, H.-L. To improve the performance of sediment microbial fuel cell through amending colloidal iron oxyhydroxide into freshwater sediments. Bioresour. Technol. 2014, 159, 232–239. [Google Scholar] [CrossRef] [PubMed]
- He, Z.; Shao, H.; Angenent, L.T. Increased power production from a sediment microbial fuel cell with a rotating cathode. Biosens. Bioelectron. 2007, 22, 3252–3255. [Google Scholar] [CrossRef] [PubMed]
- Martins, G.; Peixoto, L.; Ribeiro, D.C.; Parpot, P.; Brito, A.G.; Nogueira, R. Towards implementation of a benthic microbial fuel cell in lake Furnas (Azores): Phylogenetic affiliation and electrochemical activity of sediment bacteria. Bioelectrochemistry 2010, 78, 67–71. [Google Scholar] [CrossRef] [PubMed]
- Song, T.; Tan, W.; Wu, X.; Zhou, C.C. Effect of graphite felt and activated carbon fiber felt on performance of freshwater sediment microbial fuel cell. J. Chem. Technol. Biotechnol. 2012, 87, 1436–1440. [Google Scholar] [CrossRef]
- Lenin Babu, M.; Venkata Mohan, S. Influence of graphite flake addition to sediment on electrogenesis in a sediment-type fuel cell. Bioresour. Technol. 2012, 110, 206–213. [Google Scholar] [CrossRef]
- Sonawane, J.M.; Patil, S.A.; Ghosh, P.C.; Adeloju, S.B. Low-cost stainless-steel wool anodes modified with polyaniline and polypyrrole for high-performance microbial fuel cells. J. Power Sources 2018, 379, 103–114. [Google Scholar] [CrossRef]
- Chen, W.; Liu, Z.; Su, G.; Fu, Y.; Zai, X.; Zhou, C.; Wang, J. Composite-modified anode by MnO2/polypyrrole in marine benthic microbial fuel cells and its electrochemical performance. Int. J. Energy Res. 2017, 41, 845–853. [Google Scholar] [CrossRef]
- Ewing, T.; Ha, P.T.; Beyenal, H. Evaluation of long-term performance of sediment microbial fuel cells and the role of natural resources. Appl. Energy 2017, 192, 490–497. [Google Scholar] [CrossRef]
- Fu, Y.B.; Liu, Z.H.; Su, G.; Zai, X.R.; Ying, M.; Yu, J. Modified Carbon Anode by MWCNTs/PANI Used in Marine Sediment Microbial Fuel Cell and its Electrochemical Performance. Fuel Cells 2016, 16, 377–383. [Google Scholar] [CrossRef]
- Fu, Y.; Xu, Q.; Zai, X.; Liu, Y.; Lu, Z. Low electrical potential anode modified with Fe/ferric oxide and its application in marine benthic microbial fuel cell with higher voltage and power output. Appl. Surf. Sci. 2014, 289, 472–477. [Google Scholar] [CrossRef]
- An, J.; Kim, B.; Nam, J.; Ng, H.Y.; Chang, I.S. Comparison in performance of sediment microbial fuel cells according to depth of embedded anode. Bioresour. Technol. 2013, 127, 138–142. [Google Scholar] [CrossRef] [PubMed]
- Martins, G.; Peixoto, L.; Teodorescu, S.; Parpot, P.; Nogueira, R.; Brito, A.G. Impact of an external electron acceptor on phosphorus mobility between water and sediments. Bioresour. Technol. 2014, 151, 419–423. [Google Scholar] [CrossRef] [PubMed]
- De Schamphelaire, L.; Cabezas, A.; Marzorati, M.; Friedrich, M.W.; Boon, N.; Verstraete, W. Microbial community analysis of anodes from sediment microbial fuel cells powered by rhizodeposits of living rice plants. Appl. Environ. Microbiol. 2010, 76, 2002–2008. [Google Scholar] [CrossRef] [PubMed]
- Martins, G.; Henriques, I.; Ribeiro, D.C.; Correia, A.; Bodelier, P.L.E.; Cruz, J.V.; Brito, A.G.; Nogueira, R. Bacterial Diversity and Geochemical Profiles in Sediments from Eutrophic Azorean Lakes. Geomicrobiol. J. 2012, 29, 704–715. [Google Scholar] [CrossRef]
- Parpot, P.; Santos, P.R.B.; Bettencourt, A.P. Electro-oxidation of d-mannose on platinum, gold and nickel electrodes in aqueous medium. J. Electroanal. Chem. 2007, 610, 154–162. [Google Scholar] [CrossRef]
- Gabriel, K.R. Biplot graphic display of matrices with application to principal component analysis. Biometrika 1971, 58, 453–467. [Google Scholar] [CrossRef]
- Lobato, J.; González del Campo, A.; Fernández, F.J.; Cañizares, P.; Rodrigo, M.A. Lagooning microbial fuel cells: A first approach by coupling electricity-producing microorganisms and algae. Appl. Energy 2013, 110, 220–226. [Google Scholar] [CrossRef]
- Elgrishi, N.; Rountree, K.J.; McCarthy, B.D.; Rountree, E.S.; Eisenhart, T.T.; Dempsey, J.L. A Practical Beginner’s Guide to Cyclic Voltammetry. J. Chem. Educ. 2018, 95, 197–206. [Google Scholar] [CrossRef]
- Ryckelynck, N.; Stecher, H.A.; Reimers, C.E. Understanding the Anodic Mechanism of a Seafloor Fuel Cell: Interactions between Geochemistry and Microbial Activity. Biogeochemistry 2005, 76, 113–139. [Google Scholar] [CrossRef]
- Mitov, M.; Bardarov, I.; Mandjukov, P.; Hubenova, Y. Chemometrical assessment of the electrical parameters obtained by long-term operating freshwater sediment microbial fuel cells. Bioelectrochemistry 2015, 106, 105–114. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Sharbrough, E.; Liu, H. Quantification of the Internal Resistance Distribution of Microbial Fuel Cells. Environ. Sci. Technol. 2008, 42, 8101–8107. [Google Scholar] [CrossRef] [PubMed]
- Song, T.-S.; Yan, Z.-S.; Zhao, Z.-W.; Jiang, H.-L. Construction and operation of freshwater sediment microbial fuel cell for electricity generation. Bioprocess Biosyst. Eng. 2011, 34, 621–627. [Google Scholar] [CrossRef] [PubMed]
- Call, D.F.; Merrill, M.D.; Logan, B.E. High surface area stainless steel brushes as cathodes in microbial electrolysis cells. Environ. Sci. Technol. 2009, 43, 2179–2183. [Google Scholar] [CrossRef]
- Logan, B.E.; Hamelers, B.; Rozendal, R.; Schröder, U.; Keller, J.; Freguia, S.; Aelterman, P.; Verstraete, W.; Rabaey, K. Microbial Fuel Cells: Methodology and Technology. Environ. Sci. Technol. 2006, 40, 5181–5192. [Google Scholar] [CrossRef]
- Pfeffer, C.; Larsen, S.; Song, J.; Dong, M.; Besenbacher, F.; Meyer, R.L.; Kjeldsen, K.U.; Schreiber, L.; Gorby, Y.A.; El-Naggar, M.Y.; et al. Filamentous bacteria transport electrons over centimetre distances. Nature 2012, 491, 218–221. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, L.P.; Risgaard-Petersen, N. Rethinking Sediment Biogeochemistry after the Discovery of Electric Currents. Ann. Rev. Mar. Sci. 2015, 7, 425–442. [Google Scholar] [CrossRef]
- Risgaard-Petersen, N.; Kristiansen, M.; Frederiksen, R.B.; Dittmer, A.L.; Bjerg, J.T.; Trojan, D.; Schreiber, L.; Damgaard, L.R.; Schramm, A.; Nielsen, L.P. Cable Bacteria in Freshwater Sediments. Appl. Environ. Microbiol. 2015, 81, 6003–6011. [Google Scholar] [CrossRef]
- Ribeiro, D.C.; Martins, G.; Nogueira, R.; Brito, A.G. Mineral cycling and pH gradient related with biological activity under transient anoxic-oxic conditions: Effect on P mobility in volcanic lake sediments. Environ. Sci. Technol. 2014, 48, 9205–9210. [Google Scholar] [CrossRef] [PubMed]
- Marzocchi, U.; Trojan, D.; Larsen, S.; Louise Meyer, R.; Peter Revsbech, N.; Schramm, A.; Peter Nielsen, L.; Risgaard-Petersen, N. Electric coupling between distant nitrate reduction and sulfide oxidation in marine sediment. ISME J. 2014, 8, 1682–1690. [Google Scholar] [CrossRef] [PubMed]
- Asensio, Y.; Montes, I.B.; Fernandez-Marchante, C.M.; Lobato, J.; Cañizares, P.; Rodrigo, M.A. Selection of cheap electrodes for two-compartment microbial fuel cells. J. Electroanal. Chem. 2017, 785, 235–240. [Google Scholar] [CrossRef]
- Sacco, N.J.; Figuerola, E.L.M.; Pataccini, G.; Bonetto, M.C.; Erijman, L.; Cortón, E. Performance of planar and cylindrical carbon electrodes at sedimentary microbial fuel cells. Bioresour. Technol. 2012, 126, 328–335. [Google Scholar] [CrossRef] [PubMed]
- Brito, A.G.; Melo, L.F. Mass transfer coefficients within anaerobic biofilms: Effects of external liquid velocity. Water Res. 1999, 33, 3673–3678. [Google Scholar] [CrossRef]
- Li, Y.; Williams, I.; Xu, Z.; Li, B.; Li, B. Energy-positive nitrogen removal using the integrated short-cut nitrification and autotrophic denitrification microbial fuel cells (MFCs). Appl. Energy 2016, 163, 352–360. [Google Scholar] [CrossRef]
- Hong, S.W.; Chang, I.S.; Choi, Y.S.; Kim, B.H.; Chung, T.H. Responses from freshwater sediment during electricity generation using microbial fuel cells. Bioprocess Biosyst. Eng. 2009, 32, 389–395. [Google Scholar] [CrossRef] [PubMed]
- Martins, G.; Ribeiro, D.C.; Pacheco, D.; Cruz, J.V.; Cunha, R.; Gonçalves, V.; Nogueira, R.; Brito, A.G. Prospective scenarios for water quality and ecological status in Lake Sete Cidades (Portugal): The integration of mathematical modelling in decision processes. Appl. Geochem. 2008, 23, 2171–2181. [Google Scholar] [CrossRef]
- Daghio, M.; Aulenta, F.; Vaiopoulou, E.; Franzetti, A.; Arends, J.B.A.; Sherry, A.; Suárez-Suárez, A.; Head, I.M.; Bestetti, G.; Rabaey, K. Electrobioremediation of oil spills. Water Res. 2017, 114, 351–370. [Google Scholar] [CrossRef] [PubMed]
- Bellagamba, M.; Cruz Viggi, C.; Ademollo, N.; Rossetti, S.; Aulenta, F. Electrolysis-driven bioremediation of crude oil-contaminated marine sediments. New Biotechnol. 2017, 38, 84–90. [Google Scholar] [CrossRef] [PubMed]
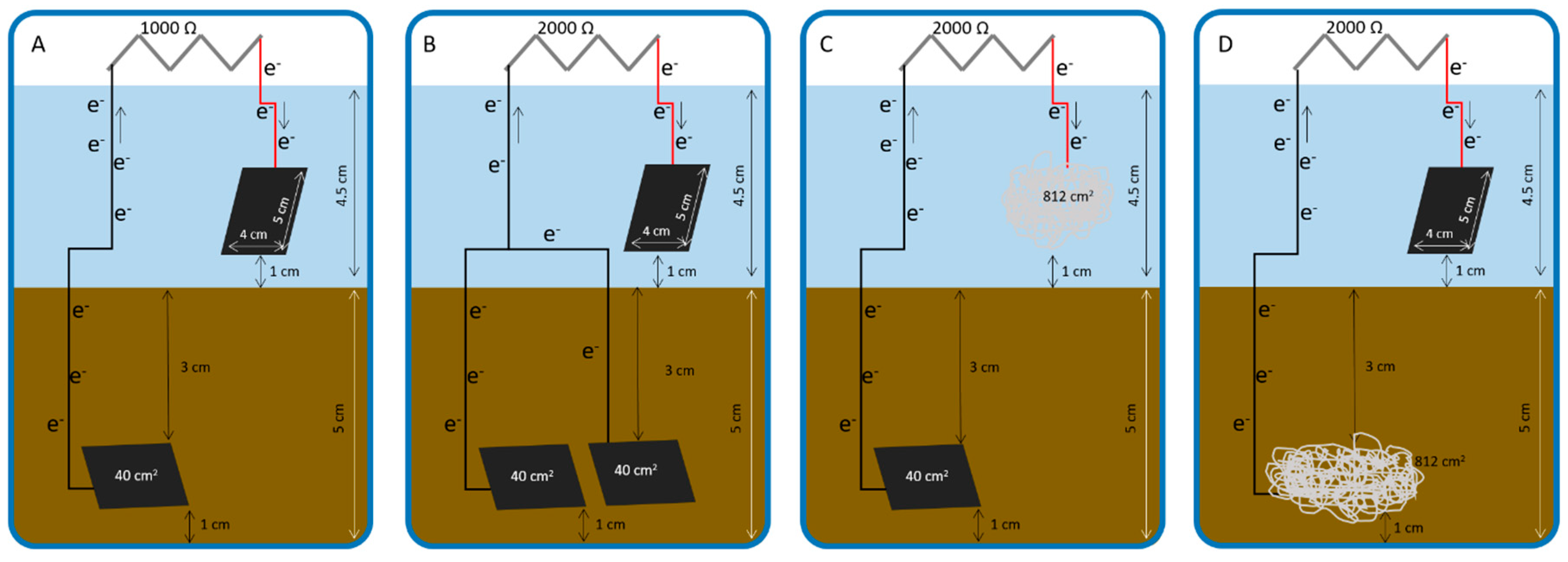
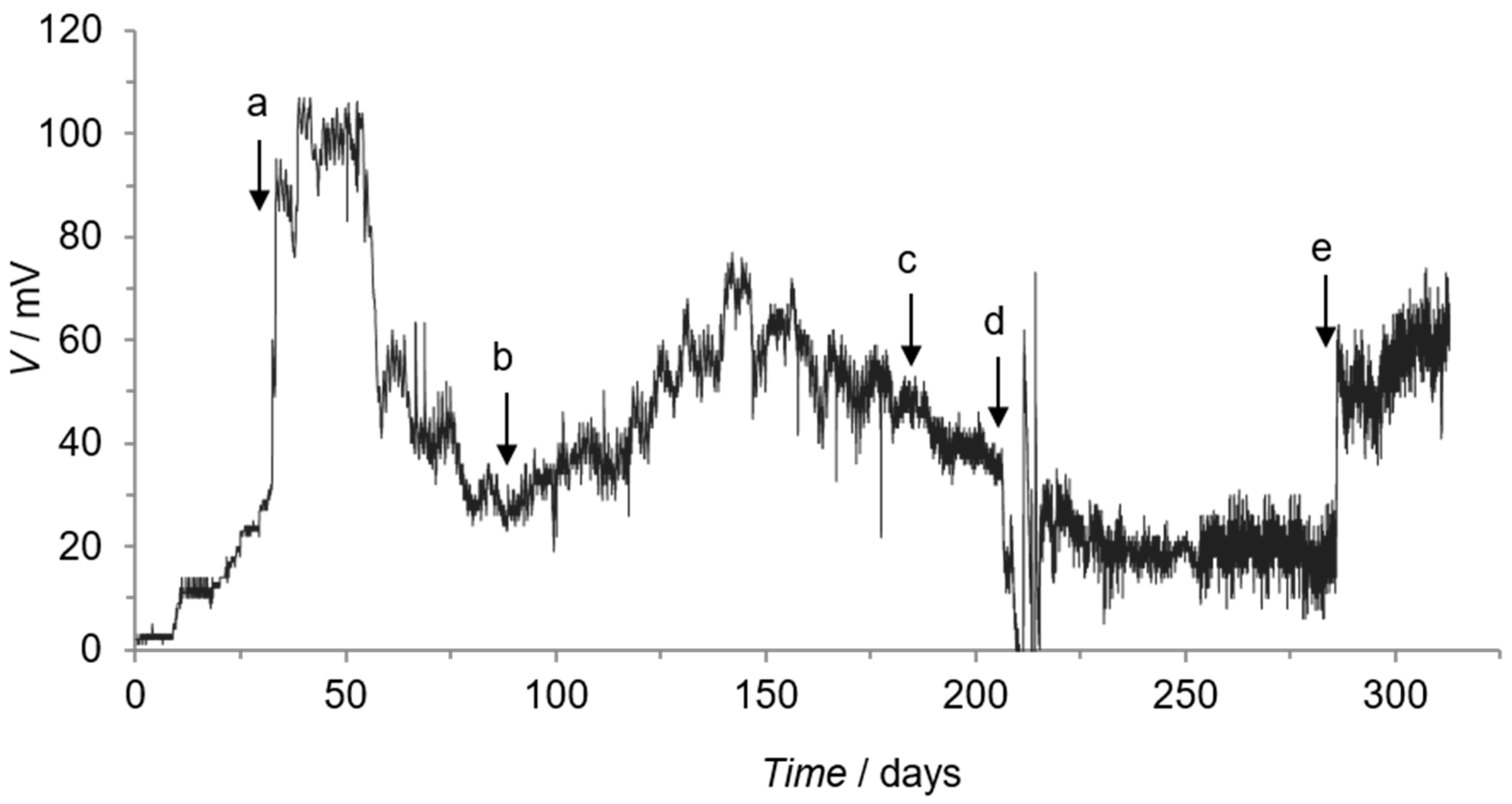
| Operation Day | Anode Material | Anode Area/cm2 | Cathode Material | Cathode Area/cm2 | External Resistor/Ω |
|---|---|---|---|---|---|
| 1 | carbon Toray | 40 | carbon Toray | 40 | 1000 |
| 33 (a) | carbon Toray | 40 | carbon Toray | 40 | 2000 |
| 88 (b) | carbon Toray | 80 * | carbon Toray | 40 | 2000 |
| 185 (c) | carbon Toray | 80 * | stainless steel | 812 | 2000 |
| 206 # (d) | carbon Toray | 40 | stainless steel | 812 | 2000 |
| 286 $ (e) | stainless steel | 812 | carbon Toray | 40 | 2000 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peixoto, L.; Parpot, P.; Martins, G. Assessment of Electron Transfer Mechanisms during a Long-Term Sediment Microbial Fuel Cell Operation. Energies 2019, 12, 481. https://doi.org/10.3390/en12030481
Peixoto L, Parpot P, Martins G. Assessment of Electron Transfer Mechanisms during a Long-Term Sediment Microbial Fuel Cell Operation. Energies. 2019; 12(3):481. https://doi.org/10.3390/en12030481
Chicago/Turabian StylePeixoto, Luciana, Pier Parpot, and Gilberto Martins. 2019. "Assessment of Electron Transfer Mechanisms during a Long-Term Sediment Microbial Fuel Cell Operation" Energies 12, no. 3: 481. https://doi.org/10.3390/en12030481
APA StylePeixoto, L., Parpot, P., & Martins, G. (2019). Assessment of Electron Transfer Mechanisms during a Long-Term Sediment Microbial Fuel Cell Operation. Energies, 12(3), 481. https://doi.org/10.3390/en12030481







