PrBaCo2O6−δ-Ce0.8Sm0.2O1.9 Composite Cathodes for Intermediate-Temperature Solid Oxide Fuel Cells: Stability and Cation Interdiffusion
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. Thermodynamic Analysis
3.2. Experimental Verification of the Results of Thermodynamic Analysis
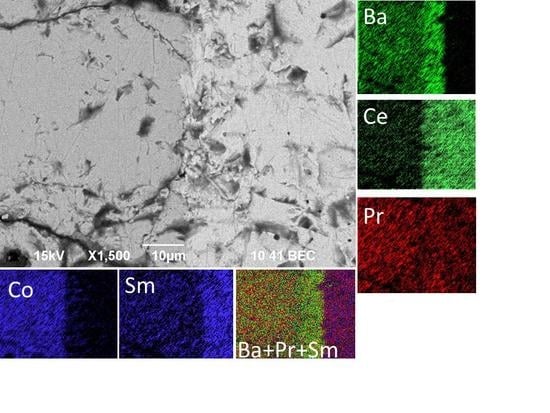
3.3. The Effect of Cation Interdiffusion and Chemical Reactivity on The Properties of PBC–SDC Composites
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Anjum, U.; Vashishtha, S.; Agarwal, M.; Tiwari, P.; Sinha, N.; Agrawal, A.; Basu, S.; Haider, M.A. Oxygen anion diffusion in double perovskite GdBaCo2O5+δ and LnBa0.5Sr0.5Co2−xFexO5+δ (Ln = Gd, Pr, Nd) electrodes. Int. J. Hydrog. Energy 2016, 41, 7631–7640. [Google Scholar] [CrossRef]
- Li, S.; Xia, T.; Li, Q.; Sun, L.; Huo, L.; Zhao, H. A-site Ba-deficiency layered perovskite EuBa1−xCo2O6−δ cathodes for intermediate-temperature solid oxide fuel cells: Electrochemical properties and oxygen reduction reaction kinetics. Int. J. Hydrog. Energy 2017, 42, 24412–24425. [Google Scholar] [CrossRef]
- Yi, K.; Sun, L.; Li, Q.; Xia, T.; Huo, L.; Zhao, H.; Li, J.; Lü, Z.; Bassat, J.-M.; Rougier, A.; et al. Effect of Nd-deficiency on electrochemical properties of NdBaCo2O6−δ cathode for intermediate-temperature solid oxide fuel cells. Int. J. Hydrog. Energy 2016, 41, 10228–10238. [Google Scholar] [CrossRef]
- Pang, S.; Wang, W.; Chen, T.; Wang, Y.; Xu, K.; Shen, X.; Xi, X.; Fan, J. The effect of potassium on the properties of PrBa1−xCo2O5+δ (x = 0.00–0.10) cathodes for intermediate-temperature solid oxide fuel cells. Int. J. Hydrog. Energy 2016, 41, 13705–13714. [Google Scholar] [CrossRef]
- Subardi, A.; Chen, C.-C.; Fu, Y.-P. Oxygen transportation, electrical conductivity and electrochemical properties of layered perovskite SmBa0.5Sr0.5Co2O5+δ. Int. J. Hydrog. Energy 2017, 42, 5284–5294. [Google Scholar] [CrossRef]
- Zhang, L.; Li, S.; Xia, T.; Sun, L.; Huo, L.; Zhao, H. Co-deficient PrBaCo2−xO6−δ perovskites as cathode materials for intermediate-temperature solid oxide fuel cells: Enhanced electrochemical performance and oxygen reduction kinetics. Int. J. Hydrog. Energy 2018, 43, 3761–3775. [Google Scholar] [CrossRef]
- Huang, X.; Feng, J.; Abdellatif, H.R.S.; Zou, J.; Zhang, G.; Ni, C. Electrochemical evaluation of double perovskite PrBaCo2−xMnxO5+δ (x = 0, 0.5, 1) as promising cathodes for IT-SOFCs. Int. J. Hydrog. Energy 2018, 43, 8962–8971. [Google Scholar] [CrossRef]
- Zhang, K.; Ge, L.; Ran, R.; Shao, Z.; Liu, S. Synthesis, characterization and evaluation of cation-ordered LnBaCo2O5+δ as materials of oxygen permeation membranes and cathodes of SOFCs. Acta Mater. 2008, 56, 4876–4889. [Google Scholar] [CrossRef]
- Li, N.; Lü, Z.; Wei, B.; Huang, X.; Chen, K.; Zhang, Y.; Su, W. Characterization of GdBaCo2O5+δ cathode for IT-SOFCs. J. Alloys Compd. 2008, 454, 274–279. [Google Scholar] [CrossRef]
- Tarancón, A.; Peña-Martínez, J.; Marrero-López, D.; Morata, A.; Ruiz-Morales, J.C.; Núñez, P. Stability, chemical compatibility and electrochemical performance of GdBaCo2O5+x layered perovskite as a cathode for intermediate temperature solid oxide fuel cells. Solid State Ion. 2008, 179, 2372–2378. [Google Scholar] [CrossRef]
- Chen, D.; Ran, R.; Shao, Z. Assessment of PrBaCo2O5+δ+Sm0.2Ce0.8O1.9 composites prepared by physical mixing as electrodes of solid oxide fuel cells. J. Power Sources 2010, 195, 7187–7195. [Google Scholar] [CrossRef]
- Kim, J.H.; Kim, Y.; Connor, P.A.; Irvine, J.T.S.; Bae, J.; Zhou, W. Structural, thermal and electrochemical properties of layered perovskite SmBaCo2O5+d, a potential cathode material for intermediate-temperature solid oxide fuel cells. J. Power Sources 2009, 194, 704–711. [Google Scholar] [CrossRef]
- Zhao, L.; He, B.; Xun, Z.; Wang, H.; Peng, R.; Meng, G.; Liu, X. Characterization and evaluation of NdBaCo2O5+δ cathode for proton-conducting solid oxide fuel cells. Int. J. Hydrog. Energy 2010, 35, 753–756. [Google Scholar] [CrossRef]
- Kim, G.; Wang, S.; Jacobson, A.J.; Reimus, L.; Brodersen, P.; Mims, C.A. Rapid oxygen ion diffusion and surface exchange kinetics in PrBaCo2O5+x with a perovskite related structure and ordered A cations. J. Mater. Chem. 2007, 17, 2500–2505. [Google Scholar] [CrossRef]
- Frison, R.; Portier, S.; Martin, M.; Conder, K. Study of oxygen tracer diffusion in PrBaCo2O5.74 by SIMS. Nucl. Instrum. Methods Phys. Res. Sect. B 2012, 273, 142–145. [Google Scholar] [CrossRef]
- Yoo, C.-Y.; Boukamp, B.A.; Bouwmeester, H.J.M. Oxygen surface exchange kinetics on PrBaCo2O5+δ. Solid State Ion. 2014, 262, 668–671. [Google Scholar] [CrossRef]
- Zhao, L.; He, B.; Lin, B.; Ding, H.; Wang, S.; Ling, Y.; Peng, R.; Meng, G.; Liu, X. High performance of proton-conducting solid oxide fuel cell with a layered PrBaCo2O5+δ cathode. J. Power Sources 2009, 194, 835–837. [Google Scholar] [CrossRef]
- Zhou, Q.; Wang, F.; Shen, Y.; He, T. Performances of LnBaCo2O5+x–Ce0.8Sm0.2O1.9 composite cathodes for intermediate-temperature solid oxide fuel cells. J. Power Sources 2010, 195, 2174–2181. [Google Scholar] [CrossRef]
- Zhao, L.; Nian, Q.; He, B.; Lin, B.; Ding, H.; Wang, S.; Peng, R.; Meng, G.; Liu, X. Novel layered perovskite oxide PrBaCuCoO5+δ as a potential cathode for intermediate-temperature solid oxide fuel cells. J. Power Sources 2010, 195, 453–456. [Google Scholar] [CrossRef]
- Zhu, C.; Liu, X.; Yi, C.; Pei, L.; Wang, D.; Yan, D.; Yao, K.; Lü, T.; Su, W. High-performance PrBaCo2O5+δ–Ce0.8Sm0.2O1.9 composite cathodes for intermediate temperature solid oxide fuel cell. J. Power Sources 2010, 195, 3504–3507. [Google Scholar] [CrossRef]
- Chen, D.; Ran, R.; Zhang, K.; Wang, J.; Shao, Z. Intermediate-temperature electrochemical performance of a polycrystalline PrBaCo2O5+δ cathode on samarium-doped ceria electrolyte. J. Power Sources 2009, 188, 96–105. [Google Scholar] [CrossRef]
- Pelosato, R.; Cordaro, G.; Stucchi, D.; Cristiani, C.; Dotelli, G. Cobalt based layered perovskites as cathode material for intermediate temperature Solid Oxide Fuel Cells: A brief review. J. Power Sources 2015, 298, 46–67. [Google Scholar] [CrossRef]
- Choi, S.; Park, S.; Shin, J.; Kim, G. The effect of calcium doping on the improvement of performance and durability in a layered perovskite cathode for intermediate-temperature solid oxide fuel cells. J. Mater. Chem. A 2015, 3, 6088–6095. [Google Scholar] [CrossRef]
- Ivanov, I.L.; Malyshkin, D.A.; Tsvetkova, N.S.; Sereda, V.V.; Kiselev, E.A.; Zuev, A.Y.; Tsvetkov, D.S. Oxygen content and thermodynamics of formation of double perovskites REBaCo2O6−δ (RE = Gd, Pr). Thermochim. Acta 2014, 578, 28–32. [Google Scholar] [CrossRef]
- Cordfunke, E.H.P.; Booij, A.S.; Huntelaar, M.E. The thermochemical properties of BaCeO3(s) and SrCeO3(s) from T = (5 to 1500) K. J. Chem. Thermodyn. 1998, 30, 437–447. [Google Scholar] [CrossRef]
- Sahu, S.K.; Tanasescu, S.; Scherrer, B.; Marinescu, C.; Navrotsky, A. Energetics of lanthanide cobalt perovskites: LnCoO3−δ (Ln = La, Nd, Sm, Gd). J. Mater. Chem. A 2015, 3, 19490–19496. [Google Scholar] [CrossRef]
- Petrov, A.N.; Cherepanov, V.A.; Zuyev, A.Yu.; Zhukovsky, V.M. Thermodynamic stability of ternary oxides in Ln-M-O (Ln = La, Pr, Nd; M = Co, Ni, Cu) systems. J. Solid State Chem. 1988, 77, 1–14. [Google Scholar] [CrossRef]
- Tsubouchi, S.; Kyômen, T.; Itoh, M.; Oguni, M. Electric, magnetic, and calorimetric properties and phase diagram of Pr1−xCaxCoO3 (0 < ~x < 0.55). Phys. Rev. B 2004, 69, 144406. [Google Scholar] [CrossRef]
- Fact-Web Suite of Interactive Programs. Available online: www.factsage.com (accessed on 25 December 2018).
- Petrov, A.N.; Zuev, A.Y.; Vylkov, A.I. Thermodynamics of point defects and mechanism of charge transfer in copper-containing lanthanum cobaltite LaCo1−xCuxO3−δ (x = 0.3). Russ. J. Phys. Chem. A 2005, 79, 220–225. [Google Scholar]
- Tsvetkov, D.S.; Sereda, V.V.; Malyshkin, D.A.; Druzhinina, A.I.; Zuev, A.Y. Thermodynamics of formation of double perovskite NdBaCo2O6−δ. J. Chem. Thermodyn. under review.
- WMO Greenhouse Gas Bulletin No. 12. Available online: https://public.wmo.int (accessed on 25 December 2018).
- Kim, J.P.; Pyo, D.W.; Magnone, E.; Park, J.H. Preparation and Oxygen Permeability of ReBaCo2O5+δ (Re = Pr, Nd, Y) Ceramic Membranes. Adv. Mater. Res. 2012, 560, 959–964. [Google Scholar] [CrossRef]
- Arulmozhi, N.; Kan, W.H.; Thangadurai, V.; Karan, K. Kinetics and thermodynamics of carbonation of a promising SOFC cathode material La0.5Ba0.5CoO3−δ (LBC). J. Mater. Chem. A 2013, 1, 15117–15127. [Google Scholar] [CrossRef]
- Zhu, L.; Wei, B.; Lü, Z.; Feng, J.; Xu, L.; Gao, H.; Zhang, Y.; Huang, X. Performance degradation of double-perovskite PrBaCo2O5+δ oxygen electrode in CO2 containing atmospheres. Appl. Surf. Sci. 2017, 416, 649–655. [Google Scholar] [CrossRef]
- Kim, D.-J. Lattice Parameters, Ionic Conductivities, and Solubility Limits in Fluorite-Structure MO2 Oxide [M = Hf4+, Zr4+, Ce4+, Th4+, U4+] Solid Solutions. J. Am. Ceram. Soc. 1989, 72, 1415–1421. [Google Scholar] [CrossRef]
- Chen, M.; Hallstedt, B.; Grundy, A.N.; Gauckler, L.J. CeO2−CoO Phase Diagram. J. Am. Ceram. Soc. 2004, 86, 1567–1570. [Google Scholar] [CrossRef]
- Etsell, T.H.; Flengas, S.N. Electrical properties of solid oxide electrolytes. Chem. Rev. 1970, 70, 339–376. [Google Scholar] [CrossRef]
- Bevan, D.J.M.; Summerville, E. Chapter 28 Mixed rare earth oxides. In Handbook on the Physics and Chemistry of Rare Earths; Gschneider, K.A., Eyring, L., Eds.; Elsevier: North Holland, Amsterdam, The Netherlands, 1979; Volume 3, pp. 401–524. [Google Scholar]
- Jiang, X.; Shi, Y.; Zhou, W.; Li, X.; Su, Z.; Pang, S.; Jiang, L. Effects of Pr3+-deficiency on structure and properties of PrBaCo2O5+δ cathode material–A comparison with Ba2+-deficiency case. J. Power Sources 2014, 272, 371–377. [Google Scholar] [CrossRef]
- Basbus, J.F.; Caneiro, A.; Suescun, L.; Lamas, D.G.; Mogni, L.V. Anomalous X-ray diffraction study of Pr-substituted BaCeO3−δ. Acta Crystallogr. Sect. B 2015, 71, 455–462. [Google Scholar] [CrossRef]
- Einstein, A. Autobiographical Notes in The Library of Living Philosophers, V.VII.; Open Court Publishing Company: La Salle, IL, USA, 1973; p. 33. [Google Scholar]
- Kuru, Y.; Bishop, S.R.; Kim, J.J.; Yildiz, B.; Tuller, H.L. Chemomechanical properties and microstructural stability of nanocrystalline Pr-doped ceria: An in situ X-ray diffraction investigation. Solid State Ion. 2011, 193, 1–4. [Google Scholar] [CrossRef]
- Pikalova, E.Y.; Maragou, V.I.; Demina, A.N.; Demin, A.K.; Tsiakaras, P.E. The effect of co-dopant addition on the properties of Ln0.2Ce0.8O2−δ (Ln=Gd, Sm, La) solid-state electrolyte. J. Power Sources 2008, 181, 199–206. [Google Scholar] [CrossRef]
- Tsvetkova, N.S.; Zuev, A.Y.; Tsvetkov, D.S. Investigation of GdBaCo2−xFexO6−δ (x = 0, 0.2)—Ce0.8Sm0.2O2 composite cathodes for intermediate temperature solid oxide fuel cells. J. Power Sources 2013, 243, 403–408. [Google Scholar] [CrossRef]
- Brett, D.J.L.; Atkinson, A.; Brandon, N.P.; Skinner, S.J. Intermediate temperature solid oxide fuel cells. Chem. Soc. Rev. 2008, 37, 1568–1578. [Google Scholar] [CrossRef]
- Lin, Y.; Ran, R.; Zhang, C.; Cai, R.; Shao, Z. Performance of PrBaCo2O5+δ as a Proton-Conducting Solid-Oxide Fuel Cell Cathode. J. Phys. Chem. A 2010, 114, 3764–3772. [Google Scholar] [CrossRef]
- Medvedev, D.A.; Lyagaeva, J.G.; Gorbova, E.V.; Demin, A.K.; Tsiakaras, P. Advanced materials for SOFC application: Strategies for the development of highly conductive and stable solid oxide proton electrolytes. Prog. Mater. Sci. 2016, 75, 38–79. [Google Scholar] [CrossRef]
- Strandbakke, R.; Cherepanov, V.A.; Zuev, A.Y.; Tsvetkov, D.S.; Argirusis, C.; Sourkouni, G.; Prünte, S.; Norby, T. Gd- and Pr-based double perovskite cobaltites as oxygen electrodes for proton ceramic fuel cells and electrolyser cells. Solid State Ion. 2015, 278, 120–132. [Google Scholar] [CrossRef]







© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsvetkov, D.; Tsvetkova, N.; Ivanov, I.; Malyshkin, D.; Sereda, V.; Zuev, A. PrBaCo2O6−δ-Ce0.8Sm0.2O1.9 Composite Cathodes for Intermediate-Temperature Solid Oxide Fuel Cells: Stability and Cation Interdiffusion. Energies 2019, 12, 417. https://doi.org/10.3390/en12030417
Tsvetkov D, Tsvetkova N, Ivanov I, Malyshkin D, Sereda V, Zuev A. PrBaCo2O6−δ-Ce0.8Sm0.2O1.9 Composite Cathodes for Intermediate-Temperature Solid Oxide Fuel Cells: Stability and Cation Interdiffusion. Energies. 2019; 12(3):417. https://doi.org/10.3390/en12030417
Chicago/Turabian StyleTsvetkov, Dmitry, Nadezhda Tsvetkova, Ivan Ivanov, Dmitry Malyshkin, Vladimir Sereda, and Andrey Zuev. 2019. "PrBaCo2O6−δ-Ce0.8Sm0.2O1.9 Composite Cathodes for Intermediate-Temperature Solid Oxide Fuel Cells: Stability and Cation Interdiffusion" Energies 12, no. 3: 417. https://doi.org/10.3390/en12030417
APA StyleTsvetkov, D., Tsvetkova, N., Ivanov, I., Malyshkin, D., Sereda, V., & Zuev, A. (2019). PrBaCo2O6−δ-Ce0.8Sm0.2O1.9 Composite Cathodes for Intermediate-Temperature Solid Oxide Fuel Cells: Stability and Cation Interdiffusion. Energies, 12(3), 417. https://doi.org/10.3390/en12030417