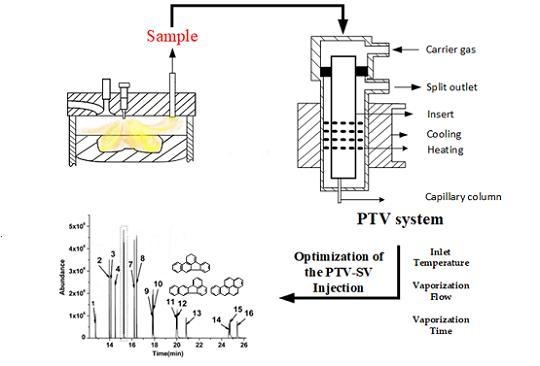
Optimization of Programmed Temperature Vaporization Injection for Determination of Polycyclic Aromatic Hydrocarbons from Diesel Combustion Process
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of the Real Sample
2.2. GC-MS Conditions
2.3. Calibration
2.4. Method Validation
3. Results and Discussion
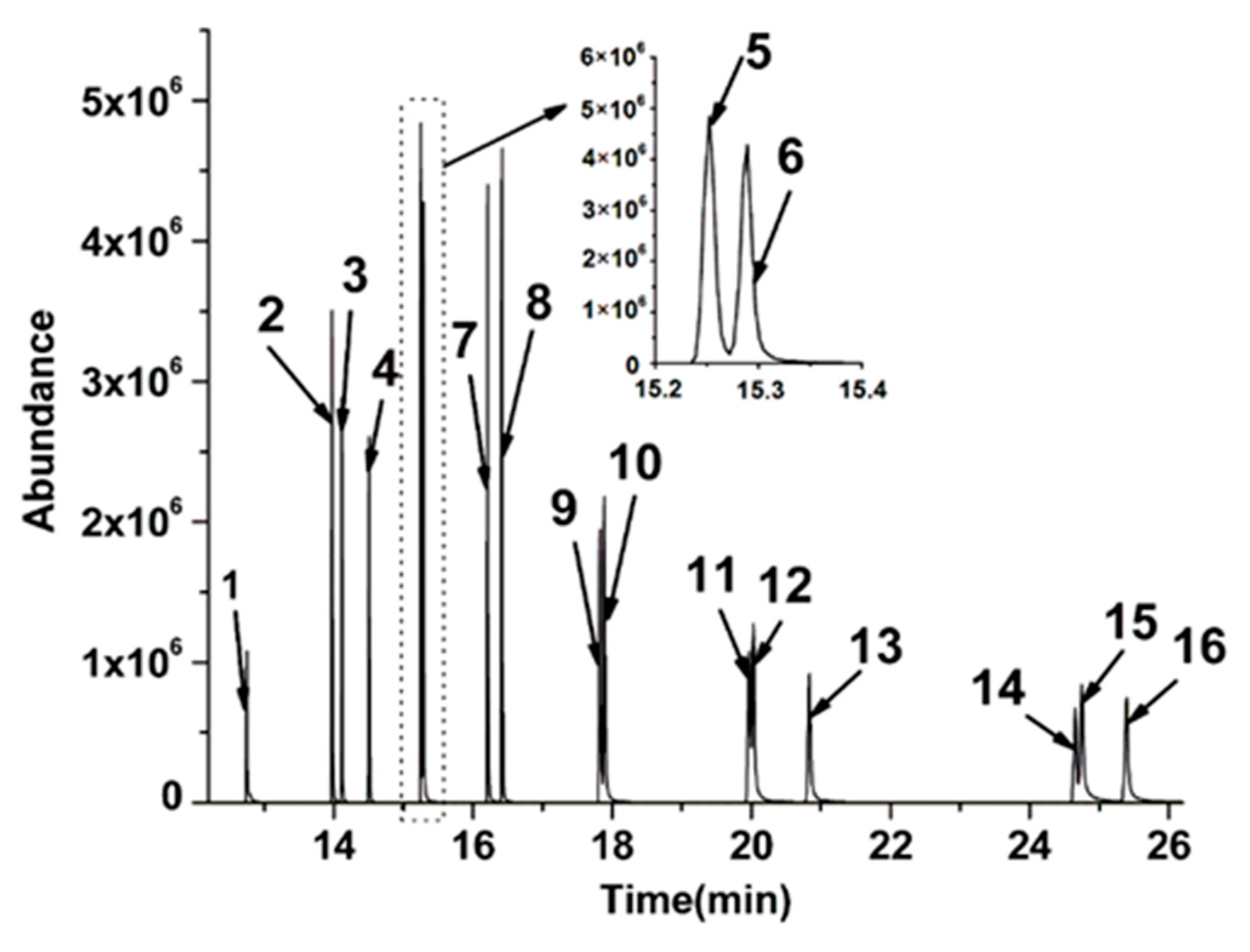
3.1. Optimization of the PTV-SV Injection
3.2. Method Validation
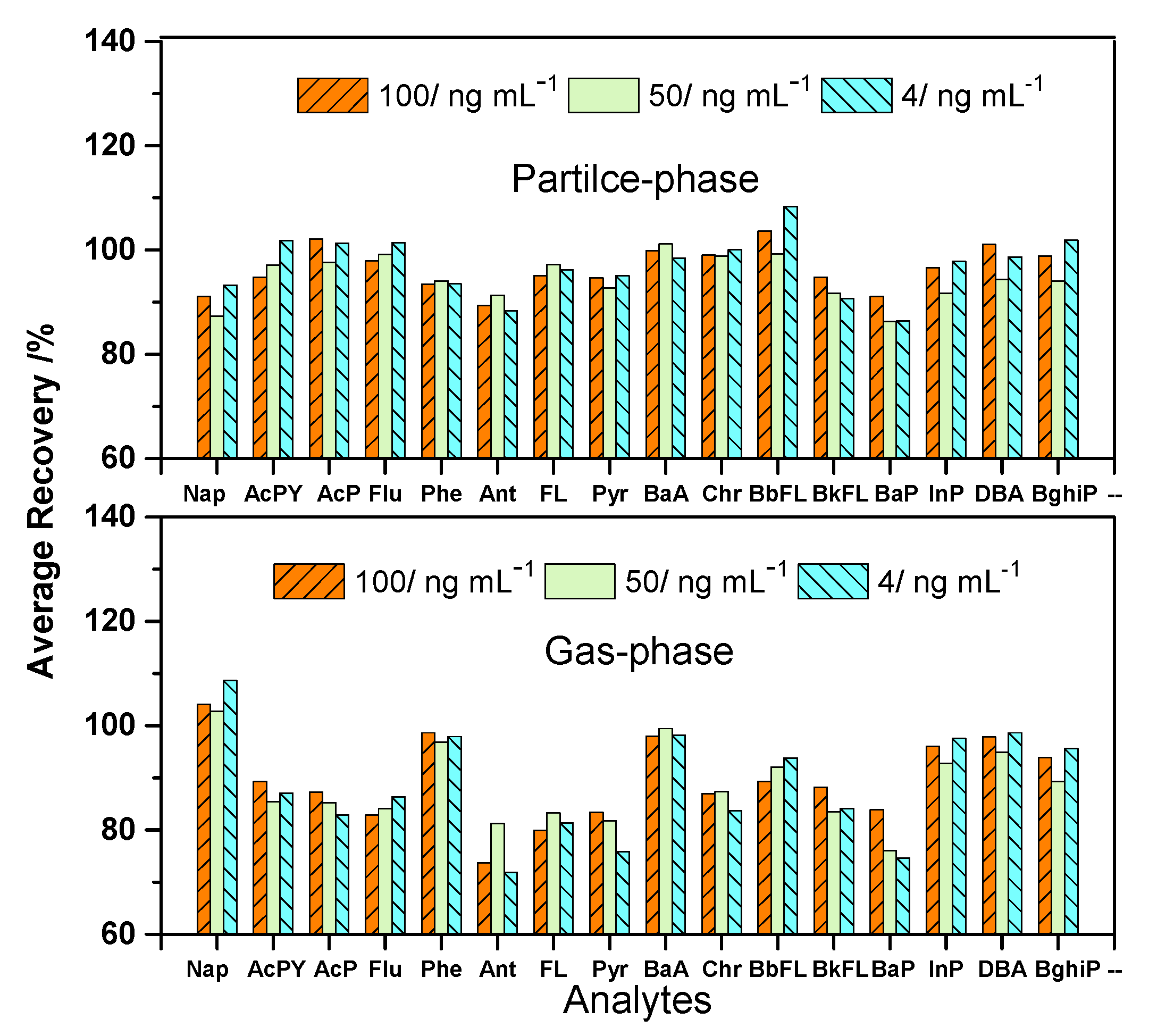
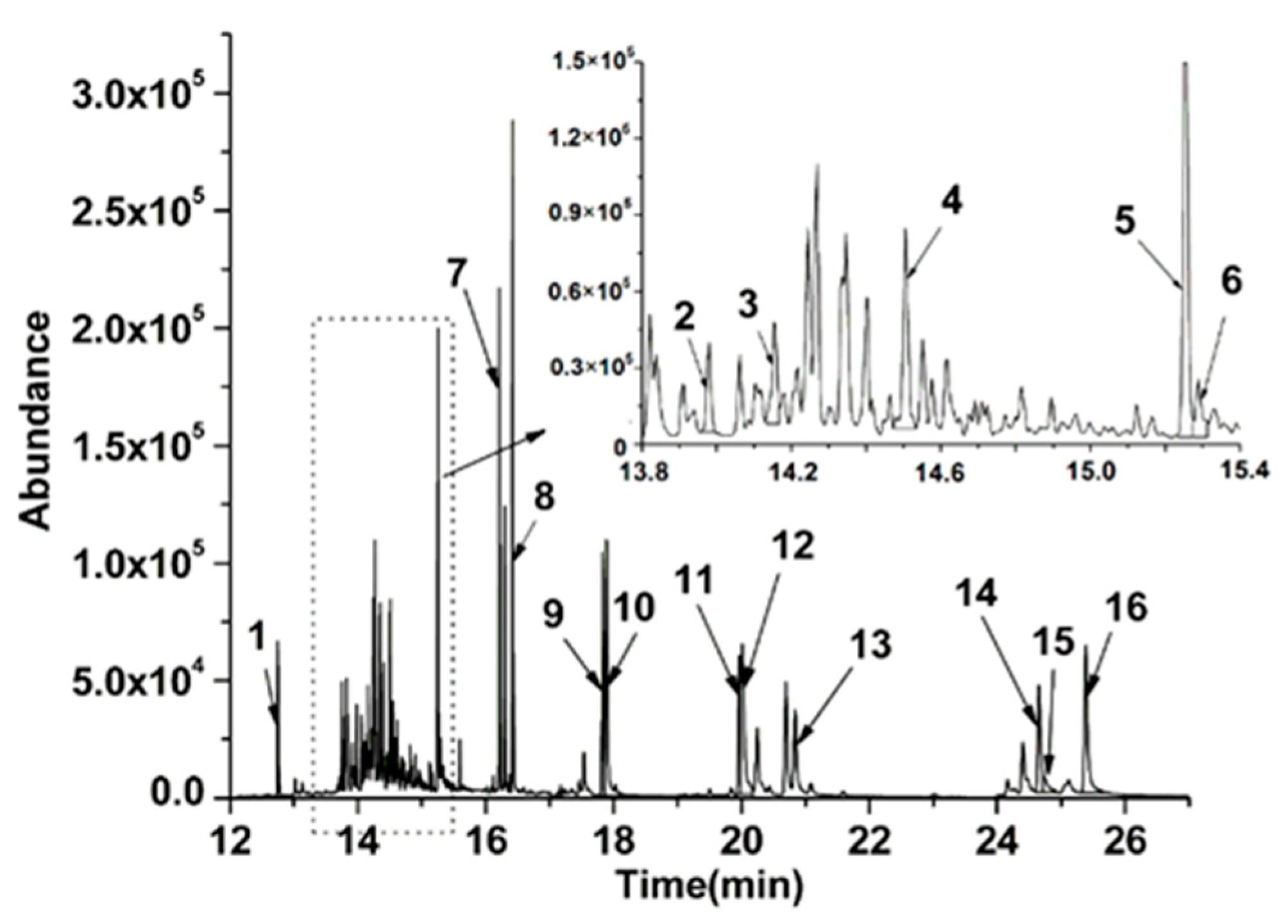
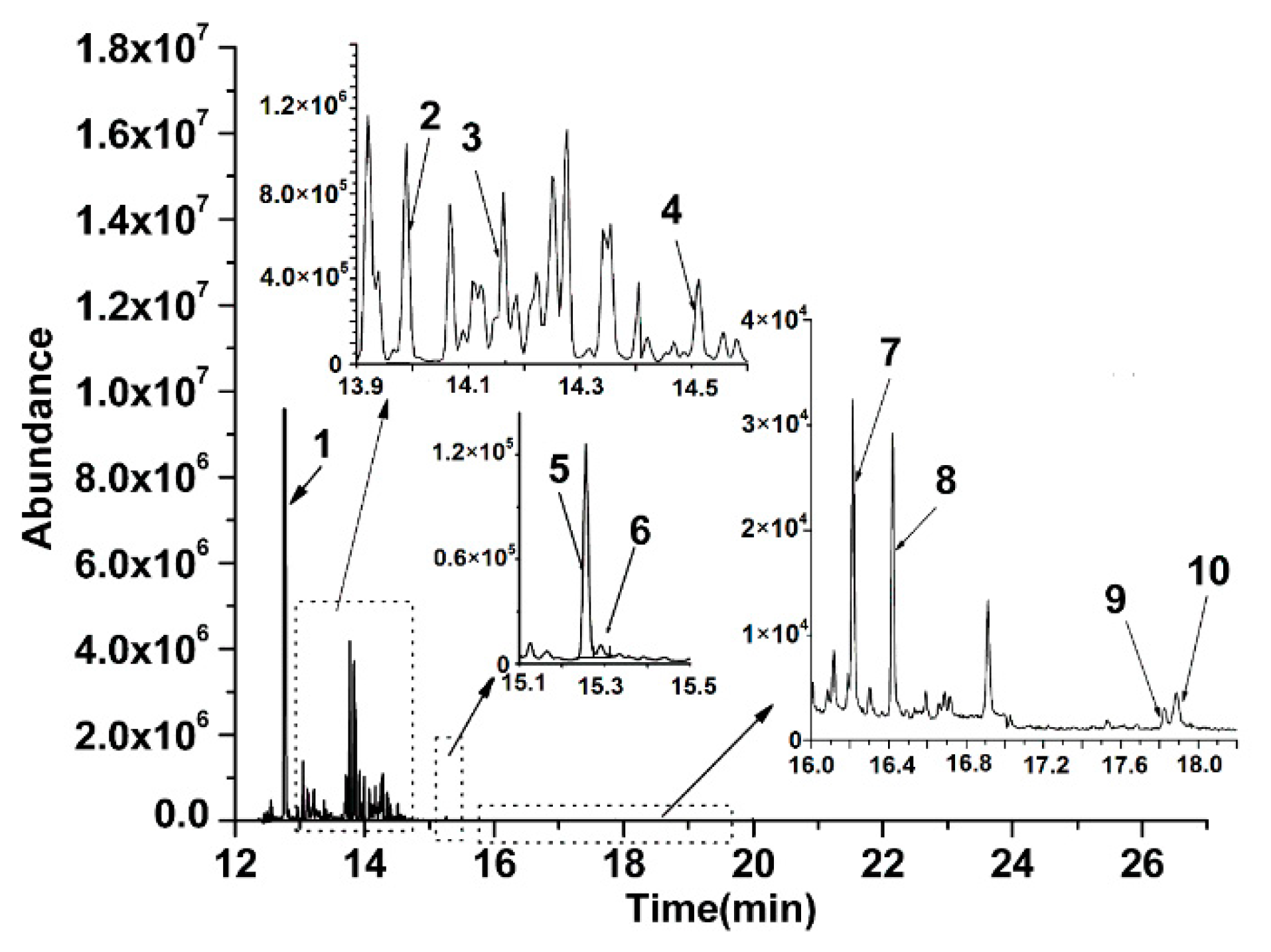
3.3. Analysis of the Real Sample
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Ballesteros, R.; Hernández, J.J.; Lyons, L.L. An experimental study of the influence of biofuel origin on particle-associated PAH emissions. Atmos. Environ. 2010, 44, 930–938. [Google Scholar] [CrossRef]
- Dobbins, R.A.; Fletcher, R.A.; Benner, B.A.; Hoeft, S. Polycyclic aromatic hydrocarbons in flames, in diesel fuels, and in diesel emissions. Combust. Flame 2006, 144, 773–781. [Google Scholar] [CrossRef]
- Yilmaz, N.; Davis, S.M. Polycyclic aromatic hydrocarbon (PAH) formation in a diesel engine fueled with diesel, biodiesel and biodiesel/n-butanol blends. Fuel 2016, 181, 729–740. [Google Scholar] [CrossRef]
- Ge, J.C.; Kim, H.Y.; Yoon, S.K.; Choi, N.J. Reducing volatile organic compound emissions from diesel engines using canola oil biodiesel fuel and blends. Fuel 2018, 218, 266–274. [Google Scholar] [CrossRef]
- Furuhata, T.; Kobayashi, Y.; Hayashida, K.; Arai, M. Behavior of PAHs and PM in a Diffusion Flame of Paraffin Fuels. Fuel 2012, 91, 16–25. [Google Scholar] [CrossRef]
- Ying, Y.Y.; Xu, C.X.; Liu, D.; Jiang, B.; Wang, P.F.; Wang, W. Nanostructure and Oxidation Reactivity of Nascent. Energies Soot Particles in Ethylene/Pentanol Flames. Energies 2017, 10, 122. [Google Scholar] [CrossRef]
- Meng, Z.M.; Li, J.S.; Fang, J.; Tan, J.; Qin, Y.; Jiang, Y.; Qin, Z.; Bai, W.L.; Liang, K. Experimental study on regeneration performance and particle emission characteristics of DPF with different inlet transition sections lengths. Fuel 2019, 262, 116487. [Google Scholar] [CrossRef]
- Meng, Z.M.; Chen, C.; Li, J.S.; Fang, J.; Tan, J.; Qin, Y.; Jiang, Y.; Qin, Z.; Bai, W.L.; Liang, K. Particle emission characteristics of DPF regeneration from DPF regeneration bench and diesel engine bench measurements. Fuel 2019, 262, 116589. [Google Scholar] [CrossRef]
- Sánchez, N.E.; Salafranca, J.; Callejas, A.; Millera, Á.; Bilbao, R.; Alzueta, M.U. Quantification of polycyclic aromatic hydrocarbons (PAHs) found in gas and particle phases from pyrolytic processes using gas chromatography–mass spectrometry (GC–MS). Fuel 2013, 107, 246–253. [Google Scholar] [CrossRef]
- Dandajeh, H.A.; Talibi, M.; Ladommatos, N.; Hellier, P. Influence of combustion characteristics and fuel composition on exhaust PAHs in a compression ignition engine. Energies 2019, 12, 2575. [Google Scholar] [CrossRef]
- Wang, X.W.; Song, C.L.; Lv, G.; Song, J.O.; Li, H.; Li, B. Evolution of in-cylinder polycyclic aromatic hydrocarbons in a diesel engine fueled with n-heptane and n-heptane/toluene. Fuel 2015, 158, 322–329. [Google Scholar] [CrossRef]
- Patel, K.; Fussell, R.J.; Goodall, D.M.; Keely, B.J. Application of programmable temperature vaporisation injection with resistive heating-gas chromatography flame photometric detection for the determination of organophosphorus pesticides. J. Sep. Sci. 2006, 29, 90–95. [Google Scholar] [CrossRef] [PubMed]
- Combs, M.T.; Noe, O. Use of a PTV injector to achieve inverse-large volume injection: Injection of volatile analytes in a semi-volatile solvent. J. Sep. Sci. 2001, 24, 291–296. [Google Scholar] [CrossRef]
- Bordajandi, L.R.; Dabrio, M.; Ulberth, F.; Emons, H. Optimisation of the GC-MS conditions for the determination of the 15 EU foodstuff priority pollycyclic aromatic hydrocarbons. J. Sep. Sci. 2008, 31, 1769–1778. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.L.; Lee, W.J.; Lee, C.F.F.; Wu, Y.P. Reduction in emissions of nitrogen oxides, particulate matter, and polycyclic aromatic hydrocarbon by adding water-containing butanol into a diesel-fueled engine generator. Fuel 2012, 93, 364–372. [Google Scholar] [CrossRef]
- Maricq, M.M. Chemical characterization of particulate emissions from diesel engines: A review. J. Aerosol Sci. 2007, 38, 1079–1118. [Google Scholar] [CrossRef]
- Poster, D.L.; Schantz, M.M.; Sander, L.C.; Wise, S.A. Analysis of polycyclic aromatic hydrocarbons (PAHs) in environmental samples: A critical review of gas chromatographic (GC) methods. Anal. Bioanal. Chem. 2006, 386, 859–881. [Google Scholar] [CrossRef]
- Hoh, E.; Mastovska, K. Large volume injection techniques in capillary gas chromatography. J. Chromatogr. A 2008, 1186, 2–15. [Google Scholar] [CrossRef]
- Pérez Pavón, J.L.; del Nogal Sánchez, M.; Fernández Laespada, M.E.; Moreno Cordero, B. Determination of aromatic and polycyclic aromatic hydrocarbons in gasoline using programmed temperature vaporization-gas chromatography–mass spectrometry. J. Chromatogr. A 2008, 1202, 196–202. [Google Scholar] [CrossRef]
- Bruno, P.; Caselli, M.; de Gennaro, G.; Tutino, M. Determination of polycyclic aromatic hydrocarbons (PAHs) in particulate matter collected with low volume samplers. Talanta 2007, 72, 1357–1361. [Google Scholar] [CrossRef]
- Crimmins, B.S.; Baker, J.E. Improved GC/MS methods for measuring hourly PAH and nitro-PAH concentrations in urban particulate matter. Atmos. Environ. 2006, 40, 6764–6779. [Google Scholar] [CrossRef]
- Drabova, L.; Pulkrabova, J.; Kalachova, K.; Tomaniova, M.; Kocourek, V.; Hajslova, J. Rapid determination of polycyclic aromatic hydrocarbons (PAHs) in tea using two-dimensional gas chromatography coupled with time of flight mass spectrometry. Talanta 2012, 100, 207–216. [Google Scholar] [CrossRef] [PubMed]
- Song, J.O.; Song, C.L.; Tao, Y.; Lv, G.; Dong, S.R. Diesel soot oxidation during the late combustion phase. Combust. Flame 2011, 158, 446–451. [Google Scholar] [CrossRef]

| Factor | Levels 1 | ||||
|---|---|---|---|---|---|
| −1 | −1/α | 0 | +1/α | +1 | |
| Inlet Temperature (°C) | 32 | 34 | 36 | 38 | 40 |
| Vaporization Flow (mL min−1) | 10 | 38 | 80 | 122 | 150 |
| Vaporization Time (min) | 0.6 | 0.89 | 1.32 | 1.75 | 2.04 |
| TA (°C) | 34 | 38 | 34 | 38 | 34 | 38 | 34 | 38 | 32 | 40 | 36 | 36 | 36 | 36 | 36 | 36 | 36 | 36 | 36 | 36 | |
| FB (mL min−1) | 38 | 38 | 122 | 122 | 38 | 38 | 122 | 122 | 80 | 80 | 10 | 150 | 80 | 80 | 80 | 80 | 80 | 80 | 80 | 80 | |
| tC (min) | 0.89 | 0.89 | 0.89 | 0.89 | 1.75 | 1.75 | 1.75 | 1.75 | 1.32 | 1.32 | 1.32 | 1.32 | 0.6 | 2.04 | 1.32 | 1.32 | 1.32 | 1.32 | 1.32 | 1.32 | |
| Analytes | m/z | Responses (chromatographic peak areas × 106) | |||||||||||||||||||
| Nap | 128 | 0.44 | 0.40 | 0.42 | 0.33 | 0.41 | 0.34 | 0.42 | 0.36 | 0.39 | 0.45 | 0.57 | 0.45 | 1.07 | 0.36 | 0.42 | 0.36 | 0.51 | 0.31 | 0.53 | 0.36 |
| AcPY | 152 | 0.81 | 0.54 | 0.46 | 0.40 | 0.57 | 0.32 | 0.35 | 0.35 | 0.5 | 0.40 | 1.06 | 0.39 | 1.51 | 0.37 | 0.51 | 0.43 | 0.49 | 0.25 | 0.51 | 0.41 |
| AcP | 153 | 0.72 | 0.48 | 0.38 | 0.32 | 0.48 | 0.26 | 0.28 | 0.27 | 0.41 | 0.30 | 0.94 | 0.30 | 1.39 | 0.29 | 0.42 | 0.35 | 0.39 | 0.21 | 0.39 | 0.33 |
| Flu | 166 | 1.08 | 0.98 | 0.89 | 0.63 | 0.86 | 0.65 | 0.61 | 0.49 | 0.77 | 0.65 | 1.20 | 0.65 | 1.82 | 0.55 | 0.79 | 0.65 | 0.81 | 0.51 | 0.83 | 0.64 |
| Phe | 178 | 1.94 | 2.02 | 1.97 | 1.42 | 1.78 | 1.59 | 1.54 | 1.23 | 1.57 | 1.66 | 1.82 | 1.59 | 3.29 | 1.33 | 1.72 | 1.37 | 1.85 | 1.29 | 1.91 | 1.42 |
| Ant | 178 | 2.38 | 2.38 | 2.42 | 1.95 | 2.27 | 1.99 | 2.05 | 1.77 | 2.05 | 2.15 | 2.21 | 2.04 | 3.71 | 1.87 | 2.24 | 1.84 | 2.31 | 1.67 | 2.38 | 1.95 |
| FL | 202 | 2.50 | 2.69 | 2.74 | 2.24 | 2.46 | 2.30 | 2.55 | 2.14 | 2.21 | 2.65 | 2.16 | 2.50 | 4.11 | 2.14 | 2.52 | 1.97 | 2.71 | 1.97 | 2.83 | 2.18 |
| Pyr | 202 | 2.71 | 2.92 | 2.92 | 2.40 | 2.66 | 2.48 | 2.71 | 2.25 | 2.39 | 2.84 | 2.35 | 2.69 | 4.42 | 2.29 | 2.69 | 2.13 | 2.92 | 2.11 | 3.03 | 2.35 |
| BaA | 228 | 1.11 | 1.33 | 1.45 | 1.19 | 1.17 | 1.15 | 1.52 | 1.29 | 1.05 | 1.55 | 0.88 | 1.39 | 2.34 | 1.17 | 1.32 | 0.89 | 1.44 | 0.97 | 1.58 | 1.08 |
| Chr | 228 | 2.33 | 2.89 | 3.07 | 2.63 | 2.63 | 2.60 | 3.21 | 2.61 | 2.36 | 3.25 | 2.09 | 3.01 | 4.28 | 2.61 | 2.64 | 1.92 | 3.09 | 2.06 | 3.22 | 2.25 |
| BbFL | 252 | 0.94 | 1.21 | 1.30 | 0.98 | 0.97 | 1.07 | 1.39 | 1.09 | 0.86 | 1.39 | 0.71 | 1.25 | 2.07 | 0.98 | 1.11 | 0.74 | 1.30 | 0.93 | 1.42 | 0.91 |
| BkFL | 252 | 2.08 | 2.40 | 2.57 | 2.17 | 2.13 | 2.10 | 2.72 | 2.33 | 1.91 | 2.71 | 1.62 | 2.48 | 3.95 | 2.21 | 2.35 | 1.66 | 2.57 | 1.81 | 2.77 | 1.95 |
| BaP | 252 | 0.89 | 1.15 | 1.23 | 1.07 | 1.00 | 1.09 | 1.31 | 1.03 | 0.94 | 1.3 | 0.75 | 1.29 | 2.05 | 1.04 | 1.02 | 0.76 | 1.21 | 0.85 | 1.49 | 0.96 |
| InP | 276 | 0.54 | 0.78 | 0.84 | 0.56 | 0.57 | 0.68 | 0.91 | 0.66 | 0.48 | 0.88 | 0.39 | 0.79 | 1.40 | 0.55 | 0.65 | 0.40 | 0.81 | 0.57 | 0.89 | 0.5 |
| DBA | 278 | 1.27 | 1.48 | 1.59 | 1.25 | 1.31 | 1.27 | 1.65 | 1.53 | 1.09 | 1.68 | 0.86 | 1.62 | 2.47 | 1.17 | 1.37 | 0.86 | 1.53 | 1.05 | 1.65 | 1.09 |
| BghiP | 276 | 1.21 | 1.54 | 1.59 | 1.27 | 1.25 | 1.34 | 1.78 | 1.31 | 1.08 | 1.51 | 0.90 | 1.54 | 2.54 | 1.25 | 1.46 | 0.88 | 1.57 | 1.06 | 1.74 | 1.14 |
| Terms | Nap | AcPY | AcP | Flu | Phe | Ant | FL | Pyr | ||||||||
| Estimate | P | Estimate | P | Estimate | P | Estimate | P | Estimate | P | Estimate | P | Estimate | P | Estimate | P | |
| Intercept | 429023 | <0.01 | 500533 | <0.01 | 358092 | <0.01 | 699564 | <0.01 | 1597255 | <0.01 | 2114094 | <0.01 | 2428690 | <0.01 | 2622292 | <0.01 |
| TA | −11370 | 0.792 | −70979 | 0.046 | −52280 | 0.022 | −65125 | 0.029 | −60225 | 0.05 | −62307 | 0.441 | −10133 | 0.916 | −13937 | 0.893 |
| FB | −20222 | 0.64 | −132899 | 0.013 | −129867 | <0.001 | −139076 | <0.001 | −113585 | 0.016 | −81749 | 0.318 | 22198 | 0.817 | 6284 | 0.951 |
| tC | −91661 | 0.053 | −185495 | 0.006 | −180795 | <0.001 | −227695 | <0.001 | −330210 | 0.001 | −304792 | 0.003 | −295329 | 0.01 | −324928 | 0.009 |
| FB×FB | −3421 | 0.935 | 49839 | 0.444 | 49961 | 0.05 | 39763 | 0.034 | -5003 | 0.199 | −21732 | 0.820 | −72658 | 0.444 | −79267 | 0.437 |
| tC×tC | 68448 | 0.124 | 127019 | 0.142 | 126211 | 0.022 | 132453 | 0.026 | 16836 | 0.614 | 213158 | 0.026 | 228880 | 0.045 | 216756 | 0.051 |
| TA×FB | −4572 | 0.914 | 43070 | 0.277 | 48427 | 0.164 | −7668 | 0.886 | −98224 | 0.047 | −58493 | 0.577 | −118389 | 0.356 | −124337 | 0.367 |
| ANOVA of Model | F | P | F | P | F | P | F | P | F | P | F | P | F | P | F | P |
| 5.94 | 0.025 | 9.78 | 0.001 | 4.84 | 0.025 | 6.07 | 0.013 | 6.00 | 0.027 | 11.20 | 0.004 | 3.89 | 0.065 | 6.25 | 0.023 | |
| Terms | BaA | Chr | BbFL | BkFL | BaP | InP | DBA | BghiP | ||||||||
| Estimate | P | Estimate | P | Estimate | P | Estimate | P | Estimate | P | Estimate | P | Estimate | P | Estimate | P | |
| Intercept | 1313721 | <0.01 | 2811816 | <0.01 | 1123014 | <0.01 | 2316300 | <0.01 | 1119073 | <0.01 | 684561 | <0.01 | 1385816 | <0.01 | 1295837 | <0.01 |
| TA | 40445 | 0.556 | 71199 | 0.505 | 45982 | 0.487 | 63193 | 0.542 | 37583 | 0.534 | 36277 | 0.477 | 50330 | 0.529 | 25631 | 0.723 |
| FB | 114236 | 0.116 | 191730 | 0.092 | 109445 | 0.116 | 185456 | 0.094 | 104720 | 0.103 | 78600 | 0.141 | 144740 | 0.09 | 123600 | 0.109 |
| tC | −140096 | 0.061 | −195245 | 0.087 | −128297 | 0.072 | −210611 | 0.062 | −118243 | 0.07 | −97184 | 0.076 | −147187 | 0.085 | −154754 | 0.052 |
| FB×FB | −87526 | 0.205 | −131722 | 0.218 | −69140 | 0.291 | −129254 | 0.214 | −61606 | 0.303 | −44688 | 0.372 | −66594 | 0.396 | -41246 | 0.56 |
| tC×tC | 131432 | 0.069 | 184427 | 0.095 | 122186 | 0.077 | 233996 | 0.037 | 123215 | 0.055 | 90884 | 0.086 | 140501 | 0.091 | 197852 | 0.016 |
| TA×FB | −85210 | 0.349 | −196820 | 0.174 | −124487 | 0.166 | −133386 | 0.332 | −99542 | 0.221 | −108560 | 0.122 | −77806 | 0.458 | −150545 | 0.132 |
| ANOVA of Model | F | P | F | P | F | P | F | P | F | P | F | P | F | P | F | P |
| 5.07 | 0.038 | 2.83 | 0.112 | 4.42 | 0.051 | 5.13 | 0.038 | 4.13 | 0.058 | 4.09 | 0.059 | 5.17 | 0.037 | 9.01 | 0.008 | |
| FactorInlet Temperature (°C) | Optimum | |||||||
|---|---|---|---|---|---|---|---|---|
| 38 | ||||||||
| Vaporization Flow (mL min−1) | 10 | |||||||
| Vaporization Time (min) | 0.6 | |||||||
| Analytes | Nap | AcPY | AcP | Flu | Phe | Ant | FL | Pyr |
| Desirability (%) | 0.60 | 0.77 | 0.89 | 0.97 | 1 | 0.91 | 0.99 | 0.85 |
| Analytes | BaA | Chr | BbFL | BkFL | BaP | InP | DBA | BghiP |
| Desirability | 0.69 | 0.88 | 0.83 | 0.87 | 0.77 | 0.89 | 0.74 | 0.97 |
| Composite Desirability | 0.85 | |||||||
| Analytes | Quantification ion (m/z) | Retention Time (min) | Analytes | Quantification ion (m/z) | Retention Time (min) |
|---|---|---|---|---|---|
| Nap | 128 | 12.75 | BaA | 228 | 17.87 |
| AcPY | 152 | 13.98 | Chr | 228 | 17.92 |
| AcP | 153 | 14.16 | BbFL | 252 | 19.96 |
| Flu | 166 | 14.51 | BkFL | 252 | 20.05 |
| Phe | 178 | 15.24 | BaP | 252 | 20.68 |
| Ant | 178 | 15.29 | InP | 276 | 24.66 |
| FL | 202 | 16.23 | DBA | 278 | 24.75 |
| Pyr | 202 | 16.41 | BghiP | 276 | 25.40 |
| NO. | Analytes | RSD (%) | NO. | Analytes | RSD (%) |
|---|---|---|---|---|---|
| 1 | Nap | 6.07 | 9 | BaA | 1.8 |
| 2 | AcPY | 2.85 | 10 | Chr | 1.22 |
| 3 | AcP | 2.99 | 11 | BbFL | 1.35 |
| 4 | Flu | 2.08 | 12 | BkFL | 1.64 |
| 5 | Phe | 1.34 | 13 | BaP | 2.11 |
| 6 | Ant | 1.22 | 14 | InP | 1.93 |
| 7 | FL | 1.19 | 15 | DBA | 3.77 |
| 8 | Pyr | 1.12 | 16 | BghiP | 3.89 |
| PTV-SV-GC-MS-SIM Method | Splitless-GC-MS-SIM Method | ||||||
|---|---|---|---|---|---|---|---|
| Analytes | Regression Equation y = ax + b | Coefficient of Determination (R2) | Linear Range (ng mL−1) | LOD (ng mL−1) | LOQ (ng mL−1) | LOD (ng mL−1) | LOQ (ng mL−1 ) |
| Nap | y = 27455340x − 288480 | 0.9949 | 0.5–300 | 0.082 | 0.272 | 0.592 | 1.974 |
| AcPY | y = 47113309x − 617805 | 0.9923 | 0.5–300 | 0.038 | 0.126 | 0.344 | 1.148 |
| AcP | y = 39117183x − 355452 | 0.9957 | 0.5–300 | 0.049 | 0.162 | 1.322 | 4.405 |
| Flu | y = 37767418x − 335743 | 0.9975 | 0.5–300 | 0.072 | 0.242 | 1.948 | 6.494 |
| Phe | y = 60532000x − 696491 | 0.9964 | 0.5–300 | 0.023 | 0.076 | 0.596 | 1.988 |
| Ant | y = 61253701x − 357054 | 0.9987 | 0.5–300 | 0.038 | 0.126 | 1.020 | 3.401 |
| FL | y = 64609682x − 547300 | 0.9976 | 0.5–300 | 0.016 | 0.055 | 0.256 | 0.853 |
| Pyr | y = 68056260x − 518969 | 0.998 | 0.5–300 | 0.032 | 0.106 | 0.587 | 1.957 |
| BaA | y = 47673273x − 822250 | 0.9925 | 0.5–300 | 0.067 | 0.223 | 1.035 | 3.448 |
| Chr | y = 57229694x − 250860 | 0.9998 | 0.5–300 | 0.066 | 0.221 | 0.959 | 3.195 |
| BbFL | y = 40717605x − 783554 | 0.9895 | 0.5–300 | 0.375 | 1.25 | 1.676 | 5.587 |
| BkFL | y = 59435860x + 444687 | 0.9993 | 0.5–300 | 0.326 | 1.087 | 1.255 | 4.184 |
| BaP | y = 45868925x − 778709 | 0.9929 | 0.5–300 | 0.288 | 0.962 | 5.882 | 19.608 |
| InP | y = 31717730x − 741731 | 0.9812 | 0.5–300 | 0.263 | 0.877 | 7.160 | 23.866 |
| DBA | y = 63091354x − 1133627 | 0.9926 | 0.5–300 | 0.313 | 1.042 | 11.811 | 39.370 |
| BghiP | y = 50702721x − 742759 | 0.9964 | 0.5–300 | 0.183 | 0.61 | 10.830 | 36.101 |
| Analytes | Sample (ng mL−1) | |||
|---|---|---|---|---|
| Particle-phase | RSD (%) | Gas-phase | RSD (%) | |
| Nap | 19.77 | 4.33 | 156.71 | 3.55 |
| AcPY | 5.11 | 5.77 | 23.42 | 4.84 |
| AcP | 5.36 | 3.57 | 21.55 | 5.54 |
| Flu | 11.18 | 11.47 | 15.31 | 1.48 |
| Phe | 18.29 | 12.29 | 5.79 | 2.79 |
| Ant | 2.46 | 13.44 | 0.58 | 12.76 |
| FL | 16.66 | 3.27 | 0.97 | 9.77 |
| Pyr | 21.38 | 4.13 | 0.74 | 8.98 |
| BaA | 23.58 | 12.06 | 0.23 | 13.41 |
| Chr | 21.42 | 11.73 | 0.41 | 10.97 |
| BbFL | 22.14 | 8.03 | - | - |
| BkFL | 18.98 | 11.53 | - | - |
| BaP | 16.57 | 10.12 | - | - |
| InP | 27.13 | 5.39 | - | - |
| DBA | 3.15 | 12.74 | - | - |
| BghiP | 28.53 | 3.72 | - | - |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qiao, Y.; Lyu, G.; Song, C.; Liang, X.; Zhang, H.; Dong, D. Optimization of Programmed Temperature Vaporization Injection for Determination of Polycyclic Aromatic Hydrocarbons from Diesel Combustion Process. Energies 2019, 12, 4791. https://doi.org/10.3390/en12244791
Qiao Y, Lyu G, Song C, Liang X, Zhang H, Dong D. Optimization of Programmed Temperature Vaporization Injection for Determination of Polycyclic Aromatic Hydrocarbons from Diesel Combustion Process. Energies. 2019; 12(24):4791. https://doi.org/10.3390/en12244791
Chicago/Turabian StyleQiao, Yuehan, Gang Lyu, Chonglin Song, Xingyu Liang, Huawei Zhang, and Dong Dong. 2019. "Optimization of Programmed Temperature Vaporization Injection for Determination of Polycyclic Aromatic Hydrocarbons from Diesel Combustion Process" Energies 12, no. 24: 4791. https://doi.org/10.3390/en12244791
APA StyleQiao, Y., Lyu, G., Song, C., Liang, X., Zhang, H., & Dong, D. (2019). Optimization of Programmed Temperature Vaporization Injection for Determination of Polycyclic Aromatic Hydrocarbons from Diesel Combustion Process. Energies, 12(24), 4791. https://doi.org/10.3390/en12244791