Overcharge Cycling Effect on the Surface Layers and Crystalline Structure of LiFePO4 Cathodes of Li-Ion Batteries
Abstract
1. Introduction
2. Materials and Methods
- (1)
- Voltage range: 2.5–5.0 V, current: 0.25 C (set 1, average loading of active material 7.8 mg cm−2).
- (2)
- Voltage range: 2.5–5.0 V, current: 1 C (set 2, average loading of active material 7.8 mg cm−2).
- (3)
- (Voltage range: 2.5–6.0 V, current: 1 C (set 3, average loading of active material 8.5 mg cm−2).
- (4)
- Voltage range: 2.5–4.0 V, current: 0.25 C (Reference set, average loading of active material 5.8 mg cm−2).
3. Results and Discussion
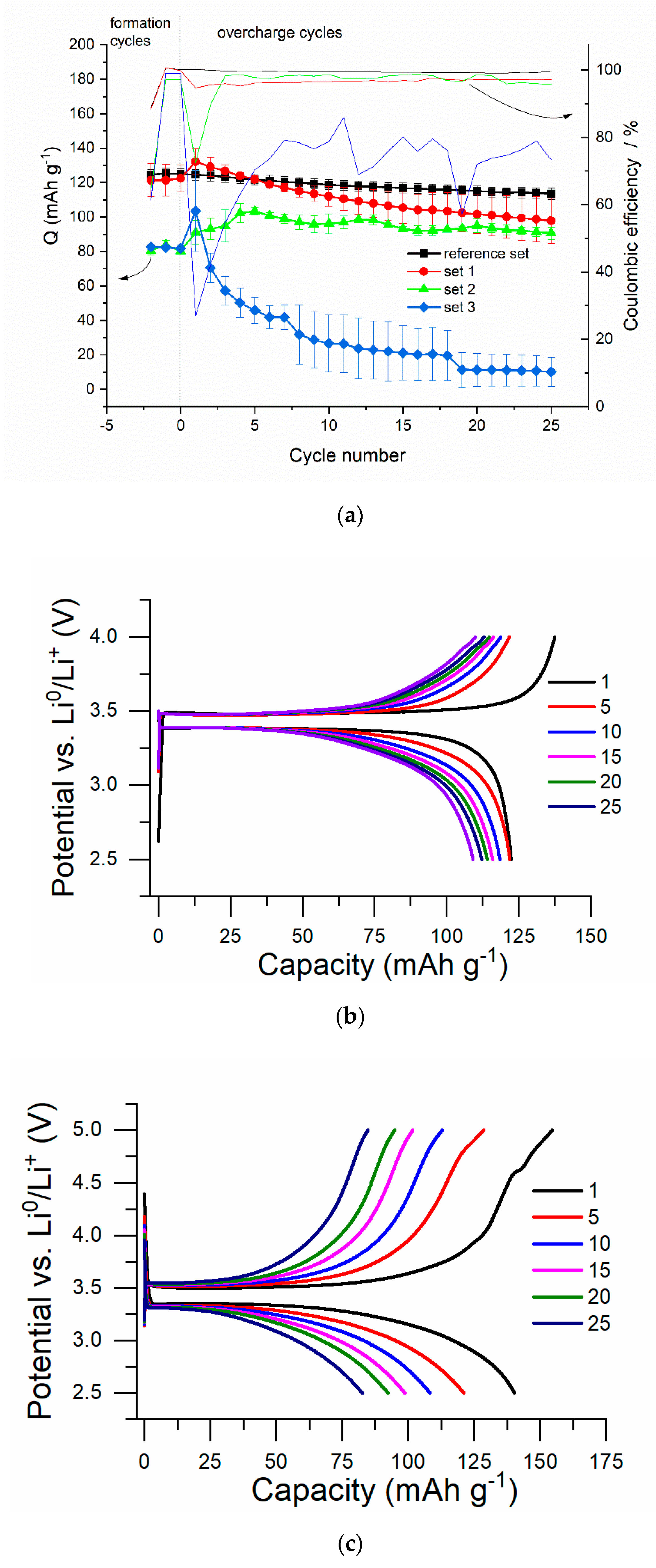
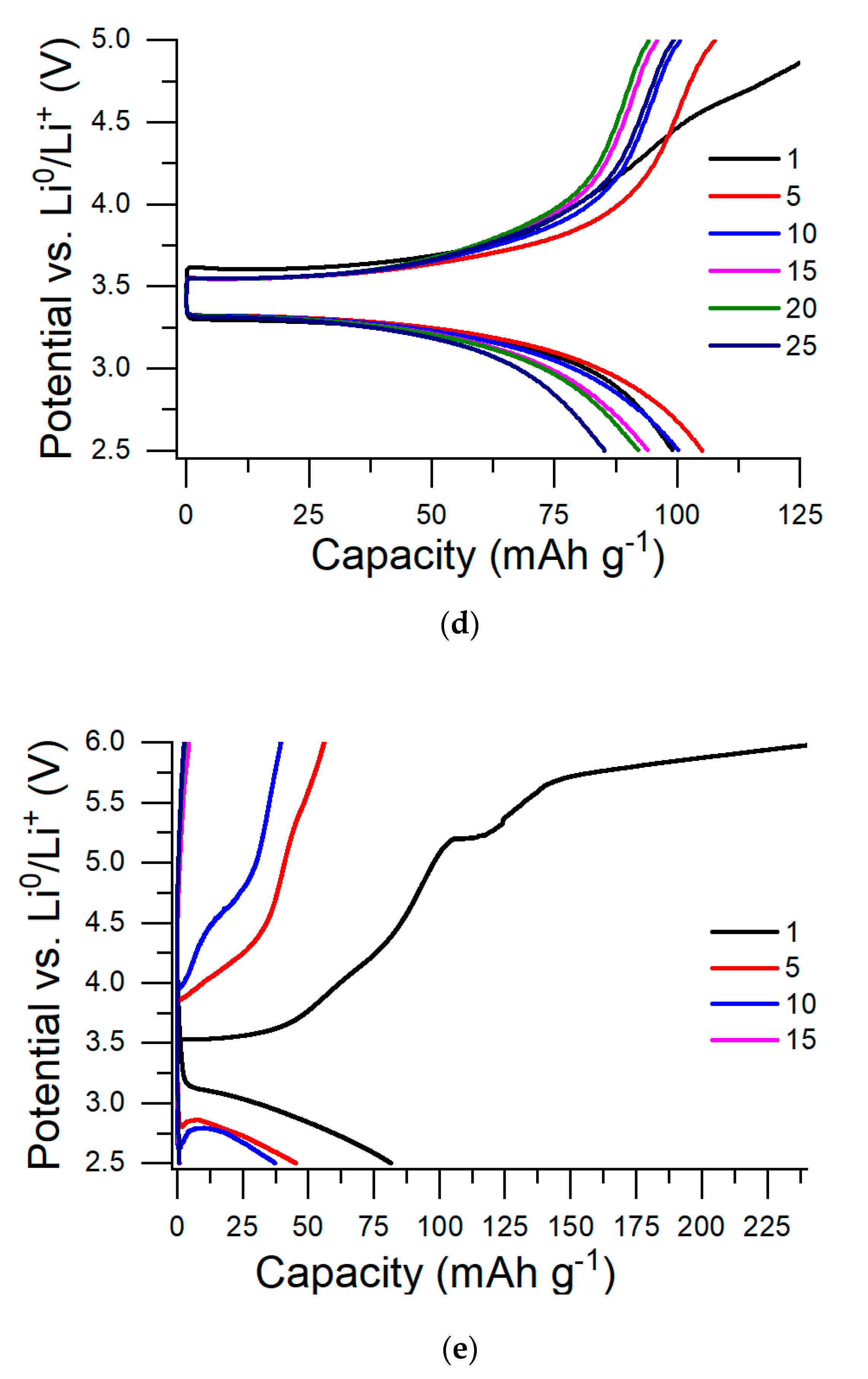
3.1. Results of Overcharging
3.2. XPS Measurements
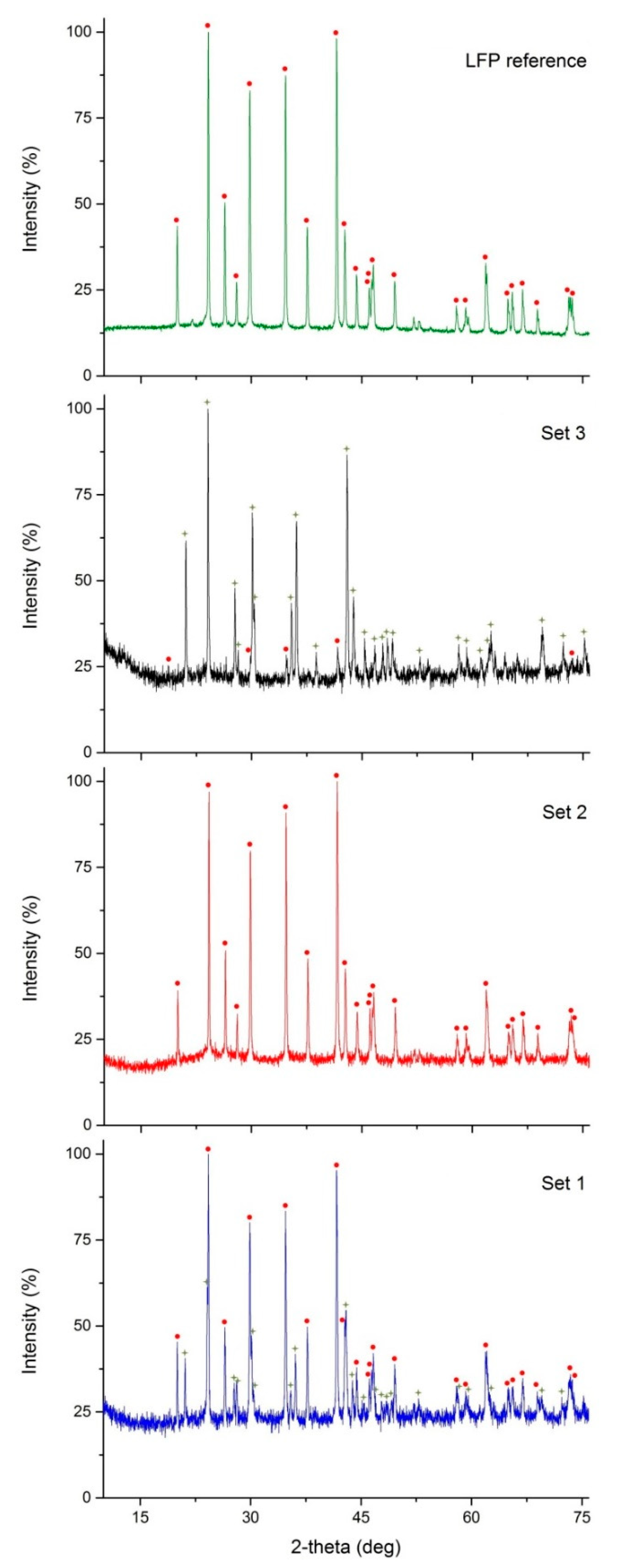
3.3. XRD Measurements
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Simon, P.; Gogotsi, Y.; Dunn, B. Where Do Batteries End and Supercapacitors Begin? Science 2014, 343, 1210–1211. [Google Scholar] [CrossRef]
- Béguin, F.; Presser, V.; Balducci, A.; Frackowiak, E. Carbons and Electrolytes for Advanced Supercapacitors. Adv. Mater. 2014, 26, 2219–2251. [Google Scholar] [CrossRef]
- Frackowiak, E. Carbon materials for supercapacitor application. Phys. Chem. Chem. Phys. 2007, 9, 1774–1785. [Google Scholar] [CrossRef]
- Sun, Y.; Zheng, G.; Seh, Z.W.; Liu, N.; Wang, S.; Sun, J.; Lee, H.R.; Cui, Y. Graphite-Encapsulated Li-Metal Hybrid Anodes for High-Capacity Li Batteries. Chem 2016, 1, 287–297. [Google Scholar] [CrossRef]
- Sun, Y.; Lee, H.-W.; Seh, Z.W.; Zheng, G.; Sun, J.; Li, Y.; Cui, Y. Lithium Sulfide/Metal Nanocomposite as a High-Capacity Cathode Prelithiation Material. Adv. Energy Mater. 2016, 6. [Google Scholar] [CrossRef]
- Sun, Y.; Lee, H.-W.; Zheng, G.; Seh, Z.W.; Sun, J.; Li, Y.; Cui, Y. In Situ Chemical Synthesis of Lithium Fluoride/Metal Nanocomposite for High Capacity Prelithiation of Cathodes. Nano Lett. 2016, 16, 1497–1501. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Lee, H.-W.; Seh, Z.W.; Liu, N.; Sun, J.; Li, Y.; Cui, Y. High-capacity battery cathode prelithiation to offset initial lithium loss. Nat. Energy 2016, 1, 15008. [Google Scholar] [CrossRef]
- Jeżowski, P.; Fic, K.; Crosnier, O.; Brousse, T.; Béguin, F. Lithium rhenium(vii) oxide as a novel material for graphite pre-lithiation in high performance lithium-ion capacitors. J. Mater. Chem. A 2016, 4, 12609–12615. [Google Scholar] [CrossRef]
- Jezowski, P.; Crosnier, O.; deunf, E.; Poizot, P.; Beguin, F.; Brousse, T. Safe and recyclable lithium-ion capacitors using sacrificial organic lithium salt. Nat. Mater. 2018, 17. [Google Scholar] [CrossRef] [PubMed]
- Schulz, N.; Hausbrand, R.; Wittich, C.; Dimesso, L.; Jaegermann, W. XPS-Surface Analysis of SEI Layers on Li-Ion Cathodes: Part II. SEI-Composition and Formation inside Composite Electrodes. J. Electrochem. Soc. 2018, 165, A833–A846. [Google Scholar] [CrossRef]
- Peled, E.; Menkin, S. Review - SEI: Past, present and future. J. Electrochem. Soc. 2017, 164, A1703–A1719. [Google Scholar] [CrossRef]
- Xu, F.; He, H.; Liu, Y.; Dun, C.; Ren, Y.; Liu, Q.; Wang, M.; Xie, J. Failure Investigation of LiFePO 4 Cells under Overcharge Conditions. J. Electrochem. Soc. 2012, 159, A678–A687. [Google Scholar] [CrossRef]
- Ouyang, D.; Liu, J.; Chen, M.; Wang, J. Investigation into the fire hazards of lithium-ion batteries under overcharging. Appl. Sci. 2017, 7, 1314. [Google Scholar] [CrossRef]
- Du Pasquier, A.; Plitz, I.; Menocal, S.; Amatucci, G. A comparative study of Li-ion battery, supercapacitor and nonaqueous asymmetric hybrid devices for automotive applications. J. Power Sources 2003, 115, 171–178. [Google Scholar] [CrossRef]
- Kikkawa, J.; Terada, S.; Gunji, A.; Nagai, T.; Kurashima, K.; Kimoto, K. Chemical states of overcharged LiCoO2 particle surfaces and interiors observed using electron energy-loss spectroscopy. J. Phys. Chem. C 2015, 119, 15823–15830. [Google Scholar] [CrossRef]
- Yang, Z.; Yang, W.; Evans, D.G.; Li, G.; Zhao, Y. Enhanced overcharge behavior and thermal stability of commercial LiCoO2 by coating with a novel material. Electrochem. commun. 2008, 10, 1136–1139. [Google Scholar] [CrossRef]
- Dahn, J.; Fuller, E.; Obrovac, M.; Vonsacken, U. Thermal stability of LixCoO2, LixNiO2 and λ-MnO2 and consequences for the safety of Li-ion cells. Solid State Ionics 1994, 69, 265–270. [Google Scholar] [CrossRef]
- Castro, L.; Dedryvère, R.; El Khalifi, M.; Lippens, P.-E.; Bréger, J.; Tessier, C.; Gonbeau, D. The Spin-Polarized Electronic Structure of LiFePO4 and FePO4 Evidenced by in-Lab XPS. J. Phys. Chem. C 2010, 114, 17995–18000. [Google Scholar] [CrossRef]
- Xiong, W.; Hu, Q.; Liu, S. A novel and accurate analytical method based on X-ray photoelectron spectroscopy for the quantitative detection of the lithium content in LiFePO4. Anal. Methods 2014, 6, 5708–5711. [Google Scholar] [CrossRef]
- Chagnes, A.; Światowska, J. Electrolyte and Solid-Electrolyte Interphase Layer in Lithium-Ion Batteries; IntechOpen: London, UK, 2012; ISBN 978-953-51-0077-5. [Google Scholar]
- Dedryvère, R.; Maccario, M.; Croguennec, L.; Le Cras, F.; Claude, D.; Gonbeau, D. X-Ray Photoelectron Spectroscopy Investigations of Carbon-Coated LixFePO4 Materials. Chem. Mater. 2008, 20, 7164–7170. [Google Scholar] [CrossRef]
- Bloom, I.; Dietz Rago, N.; Sheng, Y.; Li, J.; Wood, D.L.; Steele, L.A.; Lamb, J.; Spangler, S.; Grosso, C.; Fenton, K. Effect of overcharge on lithium-ion cells: Silicon/graphite anodes. J. Power Sources 2019, 432, 73–81. [Google Scholar] [CrossRef]
- Bareño, J.; Dietz Rago, N.; Dogan, F.; Graczyk, D.G.; Tsai, Y.; Naik, S.R.; Han, S.-D.; Lee, E.; Du, Z.; Sheng, Y.; et al. Effect of overcharge on Li(Ni0.5Mn0.3Co0.2)O2/graphite lithium ion cells with poly(vinylidene fluoride) binder. III — Chemical changes in the cathode. J. Power Sources 2018, 385, 165–171. [Google Scholar] [CrossRef]
- Bloom, I.; Bareño, J.; Dietz Rago, N.; Dogan, F.; Graczyk, D.G.; Tsai, Y.; Naik, S.R.; Han, S.-D.; Lee, E.; Du, Z.; et al. Effect of overcharge on Li(Ni0.5Mn0.3Co0.2)O2 cathodes: NMP-soluble binder. II — Chemical changes in the anode. J. Power Sources 2018, 385, 156–164. [Google Scholar] [CrossRef]
- Dietz Rago, N.; Bareño, J.; Li, J.; Du, Z.; Wood, D.L.; Steele, L.A.; Lamb, J.; Spangler, S.; Grosso, C.; Fenton, K.; et al. Effect of overcharge on Li(Ni0.5Mn0.3Co0.2)O2/Graphite lithium ion cells with poly(vinylidene fluoride) binder. I - Microstructural changes in the anode. J. Power Sources 2018, 385, 148–155. [Google Scholar] [CrossRef]
- Grosvenor, A.P.; Kobe, B.A.; Biesinger, M.; Mcintyre, N. Investigation of Multiplet Splitting of Fe 2p XPS Spectra and Bonding in Iron Compounds. Surf. Interface Anal. 2004, 36, 1564–1574. [Google Scholar] [CrossRef]
- Wang, Y. Iron(III) phosphate (FePO4) by XPS. Surf. Sci. Spectra 2002, 9. [Google Scholar] [CrossRef]
- Anishchenko, D.V.; Levin, O.V.; Malev, V.V. Quasi-equilibrium voltammetric curves of polaron-conducting polymer films. Electrochim. Acta 2016, 188, 480–489. [Google Scholar] [CrossRef]
- Ellis, B.L.; Makahnouk, W.R.M.; Makimura, Y.; Toghill, K.; Nazar, L.F. A multifunctional 3.5 V iron-based phosphate cathode for rechargeable batteries. Nat. Mater. 2007, 6, 749–753. [Google Scholar] [CrossRef]
- Chen, G.; Richardson, T.J. Thermal instability of Olivine-type LiMnPO4 cathodes. J. Power Sources 2010, 195, 1221–1224. [Google Scholar] [CrossRef]
- Yuan, L.-X.; Wang, Z.-H.; Zhang, W.-X.; Hu, X.-L.; Chen, J.-T.; Huang, Y.-H.; Goodenough, J.B. Development and challenges of LiFePO4 cathode material for lithium-ion batteries. Energy Environ. Sci. 2011, 4, 269–284. [Google Scholar] [CrossRef]
- Julien, M.C.; Mauger, A.; Zaghib, K.; Groult, H. Comparative Issues of Cathode Materials for Li-Ion Batteries. Inorganics 2014, 2. [Google Scholar] [CrossRef]
- Hu, G.; Xie, X.; Peng, Z.; Du, K.; Gan, Z.; Xu, L.; Wang, Y.; Cao, Y. Novel synthesis of FePO4·2H2O nanoparticles as a precursor of LiFePO4/C cathode material for lithium ion batteries by microreaction technology. Solid State Ionics 2019, 340, 115014. [Google Scholar] [CrossRef]
- Golubkov, A.W.; Scheikl, S.; Planteu, R.; Voitic, G.; Wiltsche, H.; Stangl, C.; Fauler, G.; Thaler, A.; Hacker, V. Thermal runaway of commercial 18650 Li-ion batteries with LFP and NCA cathodes—Impact of state of charge and overcharge. RSC Adv. 2015, 5, 57171–57186. [Google Scholar] [CrossRef]
- Larsson, F.; Mellander, B.-E. Abuse by External Heating, Overcharge and Short Circuiting of Commercial Lithium-Ion Battery Cells. J. Electrochem. Soc. 2014, 161, A1611–A1617. [Google Scholar] [CrossRef]
- Liu, Y.; Xie, J. Failure Study of Commercial LiFePO4 Cells in Overcharge Conditions Using Electrochemical Impedance Spectroscopy. J. Electrochem. Soc. 2015, 162, A2208–A2217. [Google Scholar] [CrossRef]
- Cui, W.; He, Y.-B.; Tang, Z.-Y.; Yang, Q.-H.; Xu, Q.; Su, F.-Y.; Ma, L. Improvement of overcharge performance using Li4Ti5O12 as negative electrode for LiFePO4 power battery. J. Solid State Electrochem. 2012, 16, 265–271. [Google Scholar] [CrossRef]
- Li, J.; Dong, S.; Wang, C.; Hu, Z.; Zhang, Z.; Zhang, H.; Cui, G. A study on the interfacial stability of the cathode/polycarbonate interface: implication of overcharge and transition metal redox. J. Mater. Chem. A 2018, 6, 11846–11852. [Google Scholar] [CrossRef]
- Nazri, G.-A.; Pistoia, G. Lithium Batteries: Science and Technology; Springer: Berlin, Germany, 2009. [Google Scholar]
- Rybachuk, M.; Bell, J.M. The effect of sp2 fraction and bonding disorder on micro-mechanical and electronic properties of a-C:H films. Thin Solid Films 2007, 515, 7855–7860. [Google Scholar] [CrossRef][Green Version]
- Fedorková, A.; Oriňáková, R.; Oriňák, A.; Kupková, M.; Wiemhöfer, H.-D.; Audinot, J.N.; Guillot, J. Electrochemical and XPS study of LiFePO4 cathode nanocomposite with PPy/PEG conductive network. Solid State Sci. 2012, 14, 1238–1243. [Google Scholar] [CrossRef]
- Saravanan, K.; Reddy, M. V; Balaya, P.; Gong, H.; Chowdari, B.V.R.; Vittal, J.J. Storage performance of LiFePO4 nanoplates. J. Mater. Chem. 2009, 19, 605–610. [Google Scholar] [CrossRef]
- Muruganantham, R.; Sivakumar, M.; Subadevi, R. Synthesis and electrochemical characterization of olivine-type lithium iron phosphate cathode materials via different techniques. Ionics 2016, 22, 1557–1565. [Google Scholar] [CrossRef]
- Bhuvaneswari, M.S.; Bramnik, N.N.; Ensling, D.; Ehrenberg, H.; Jaegermann, W. Synthesis and characterization of Carbon Nano Fiber/LiFePO4 composites for Li-ion batteries. J. Power Sources 2008, 180, 553–560. [Google Scholar] [CrossRef]
- McIntyre, N.S.; Zetaruk, D.G. X-ray photoelectron spectroscopic studies of iron oxides. Anal. Chem. 1977, 49, 1521–1529. [Google Scholar] [CrossRef]
- Barbaux, Y.; Dekiouk, M.; Le Maguer, D.; Gengembre, L.; Huchette, D.; Grimblot, J. Bulk and surface analysis of a Fe-P-O oxydehydrogenation catalyst. Appl. Catal. A Gen. 1992, 90, 51–60. [Google Scholar] [CrossRef]
- Allen, G.C.; Harris, S.J.; Jutson, J.A.; Dyke, J.M. A study of a number of mixed transition metal oxide spinels using X-ray photoelectron spectroscopy. Appl. Surf. Sci. 1989, 37, 111–134. [Google Scholar] [CrossRef]

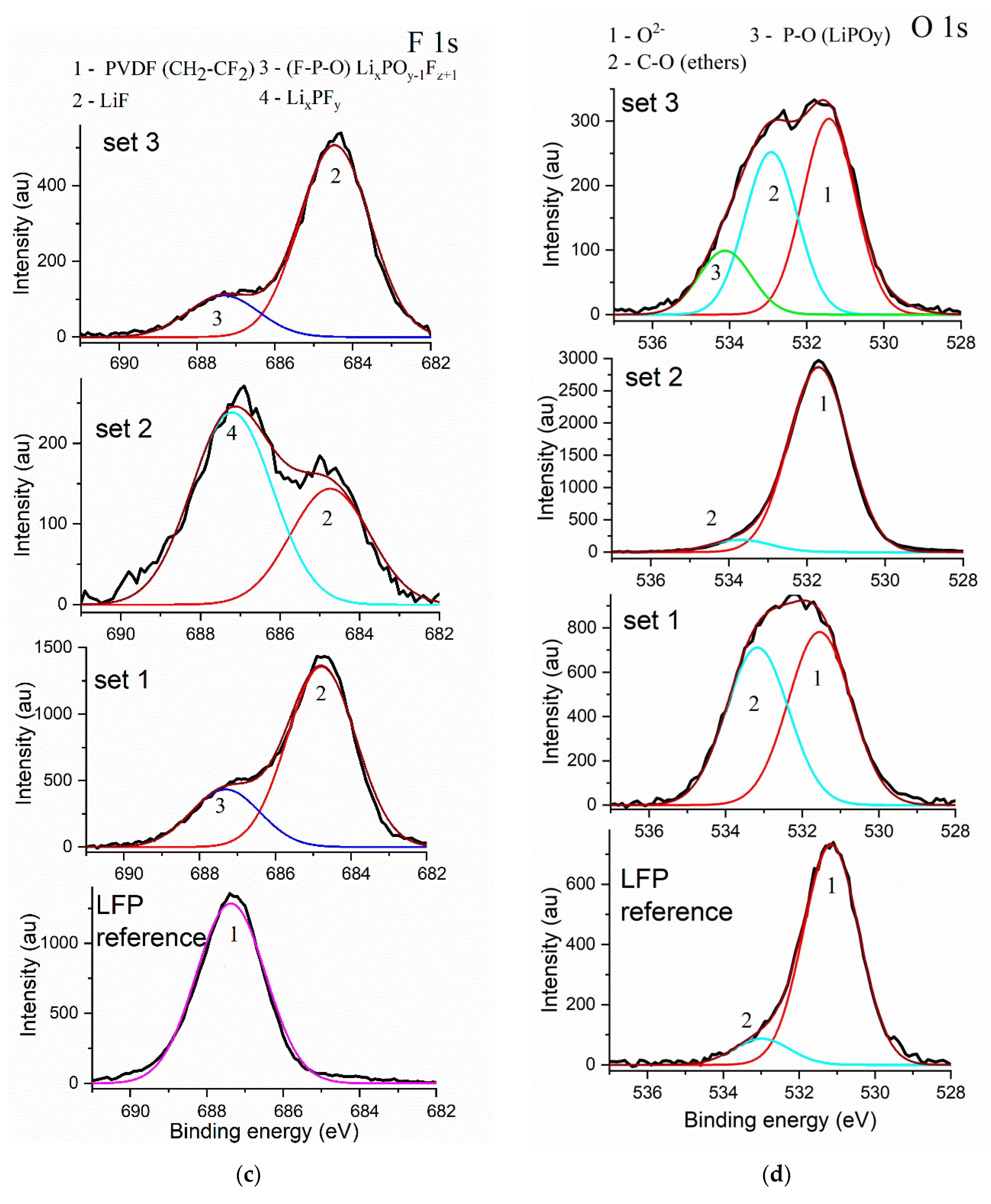

| Element | LFP Reference | Set 1 (LFP Overcharged by 0.25 C Current to 5.0 V) | Set 2 (LFP Overcharged by 1 C Current to 5.0 V) | Set 3 (LFP Overcharged by 1 C Current to 6.0 V) |
|---|---|---|---|---|
| C | Carbon black > PVDF | PVDF > Carbon black > ethers | Carbon black > PVDF > ethers | Carbon black > PVDF > ethers |
| P | LiFePO4 >> surface contamination | LiFePO4 > LixPOy−1Fz+1 | LiFePO4 > LixPFy | LiFePO4 > LixPOy−1Fz+1 |
| F | PVDF | LiF > LixPOy−1Fz+1 | LixPFy > LiF | LiF > LixPOy−1Fz+1 |
| O | LiFePO4 >> ethers | LiFePO4 > ethers | LiFePO4 >> ethers | LiFePO4 > ethers > LiPOy |
| Li + Fe | LiFePO4 > FePO4 | Fe2O3 > LiFePO4 | Fe2O3 > LiFePO4 > FePO4 | LiFePO4 ≥ FePO4 ≥ FeF3 |
| Fe | LiFePO4 | LiFePO4 | LiFePO4 | LiFePO4>Fe3O4 |
| Phase | LFP Reference | Set 1 | Set 2 | Set 3 |
|---|---|---|---|---|
| LiFePO4 | 100.0 | 74.6 (6) | 100.0 | 9.2 (12) |
| FePO4 | - | 25.4 (6) | - | 90.8 (12) |
| Rp (%) | 3.38 | 4.57 | 4.13 | 4.85 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Beletskii, E.V.; Alekseeva, E.V.; Spiridonova, D.V.; Yankin, A.N.; Levin, O.V. Overcharge Cycling Effect on the Surface Layers and Crystalline Structure of LiFePO4 Cathodes of Li-Ion Batteries. Energies 2019, 12, 4652. https://doi.org/10.3390/en12244652
Beletskii EV, Alekseeva EV, Spiridonova DV, Yankin AN, Levin OV. Overcharge Cycling Effect on the Surface Layers and Crystalline Structure of LiFePO4 Cathodes of Li-Ion Batteries. Energies. 2019; 12(24):4652. https://doi.org/10.3390/en12244652
Chicago/Turabian StyleBeletskii, Evgenii V., Elena V. Alekseeva, Dar’ya V. Spiridonova, Andrei N. Yankin, and Oleg V. Levin. 2019. "Overcharge Cycling Effect on the Surface Layers and Crystalline Structure of LiFePO4 Cathodes of Li-Ion Batteries" Energies 12, no. 24: 4652. https://doi.org/10.3390/en12244652
APA StyleBeletskii, E. V., Alekseeva, E. V., Spiridonova, D. V., Yankin, A. N., & Levin, O. V. (2019). Overcharge Cycling Effect on the Surface Layers and Crystalline Structure of LiFePO4 Cathodes of Li-Ion Batteries. Energies, 12(24), 4652. https://doi.org/10.3390/en12244652






