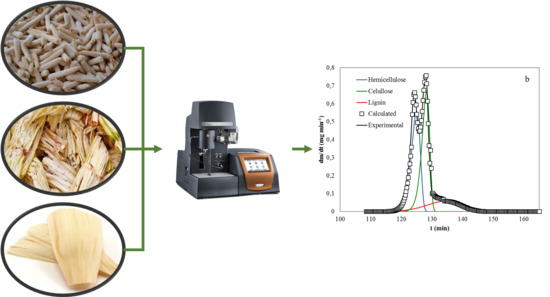
Kinetic Study of Corn and Sugarcane Waste Oxidative Pyrolysis
Abstract
1. Introduction
2. Materials and Methods
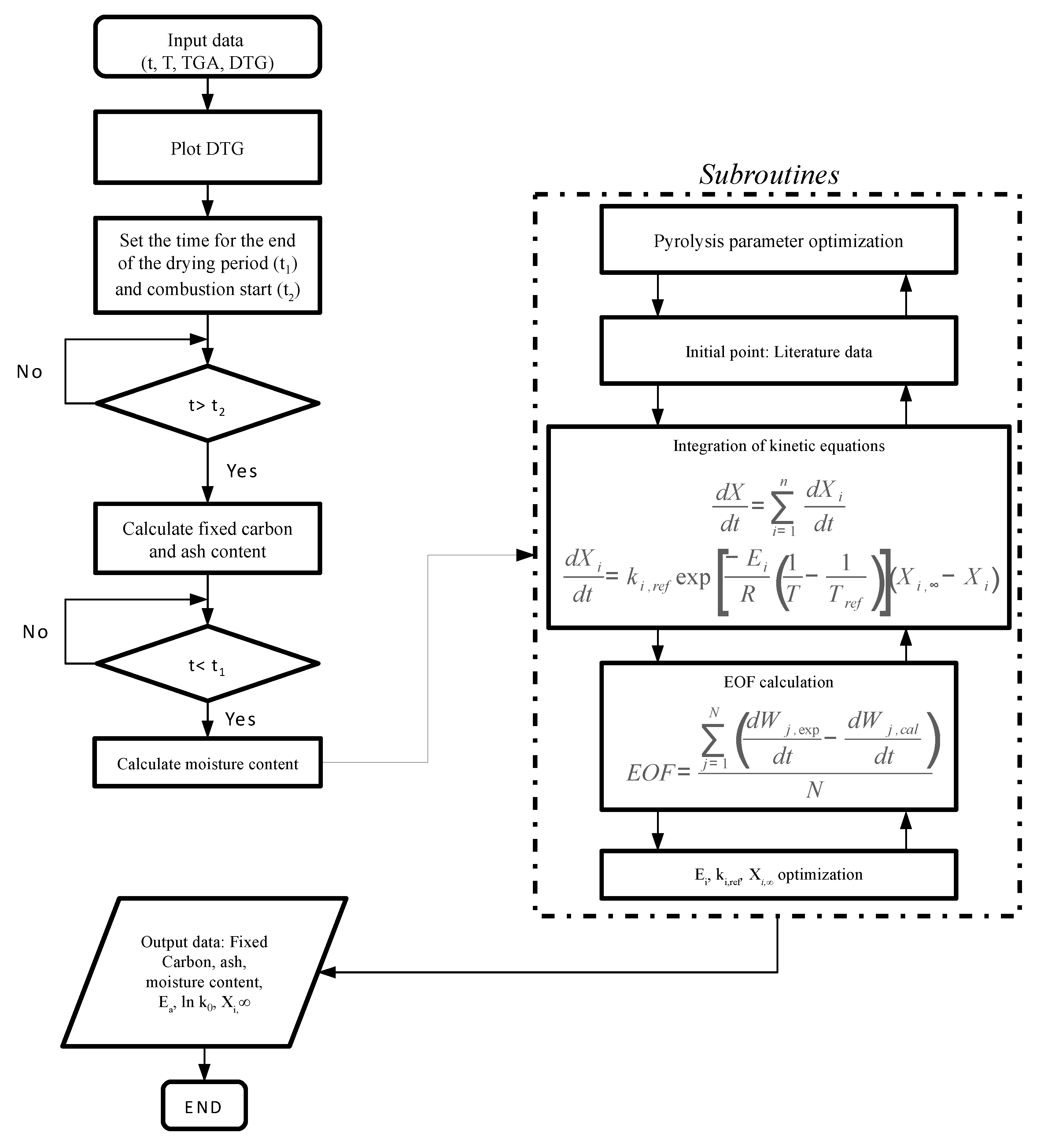
3. Kinetic Model
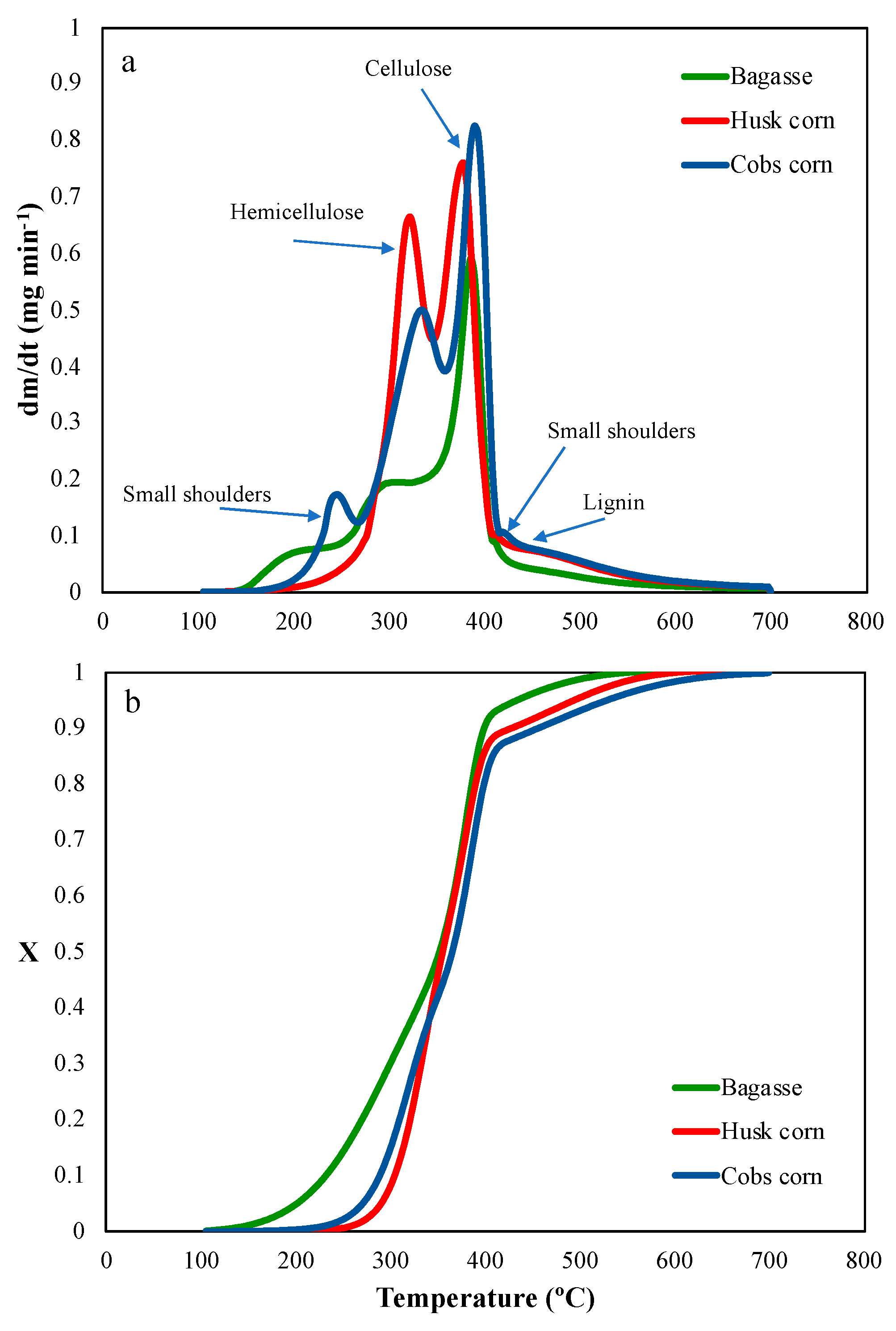
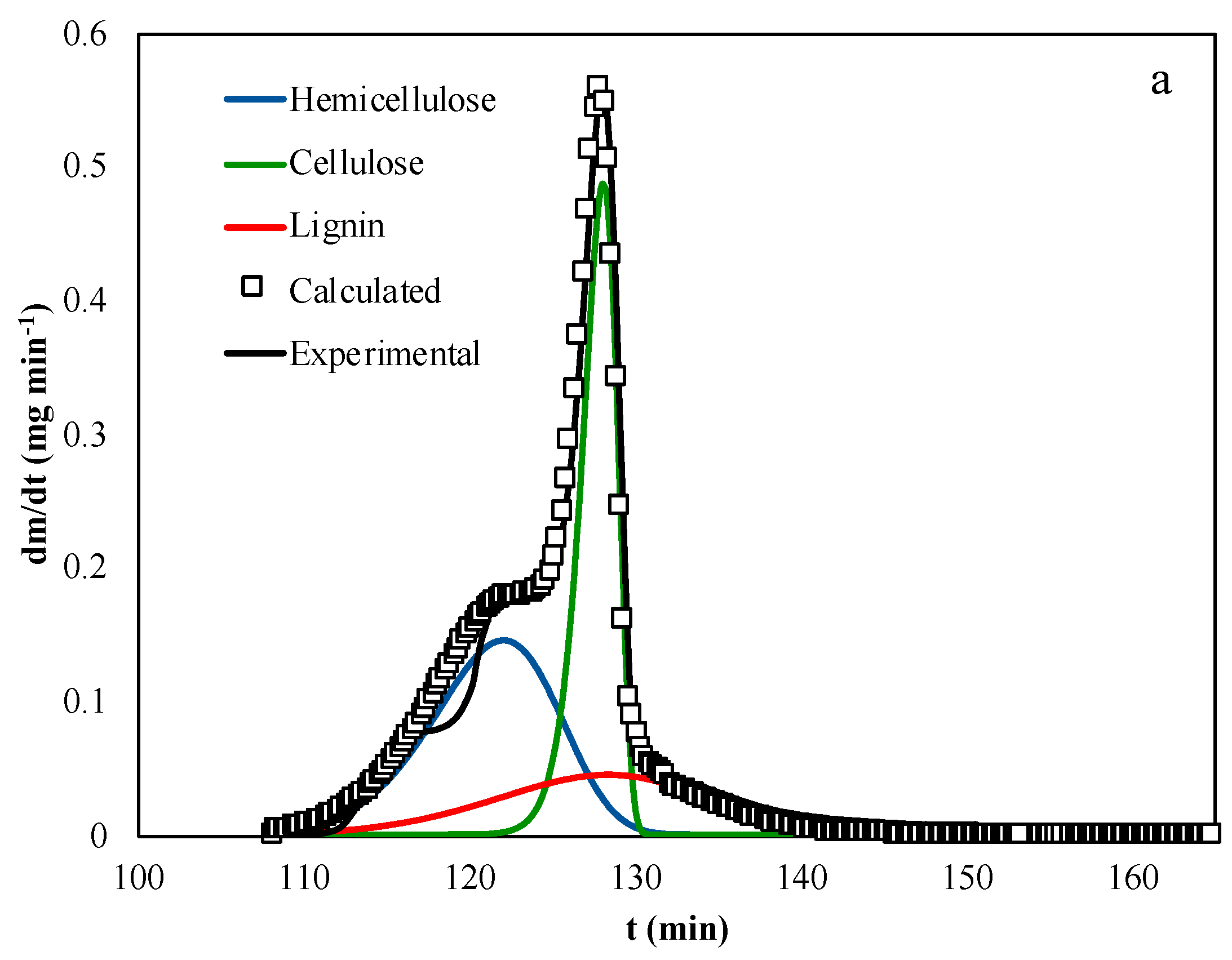
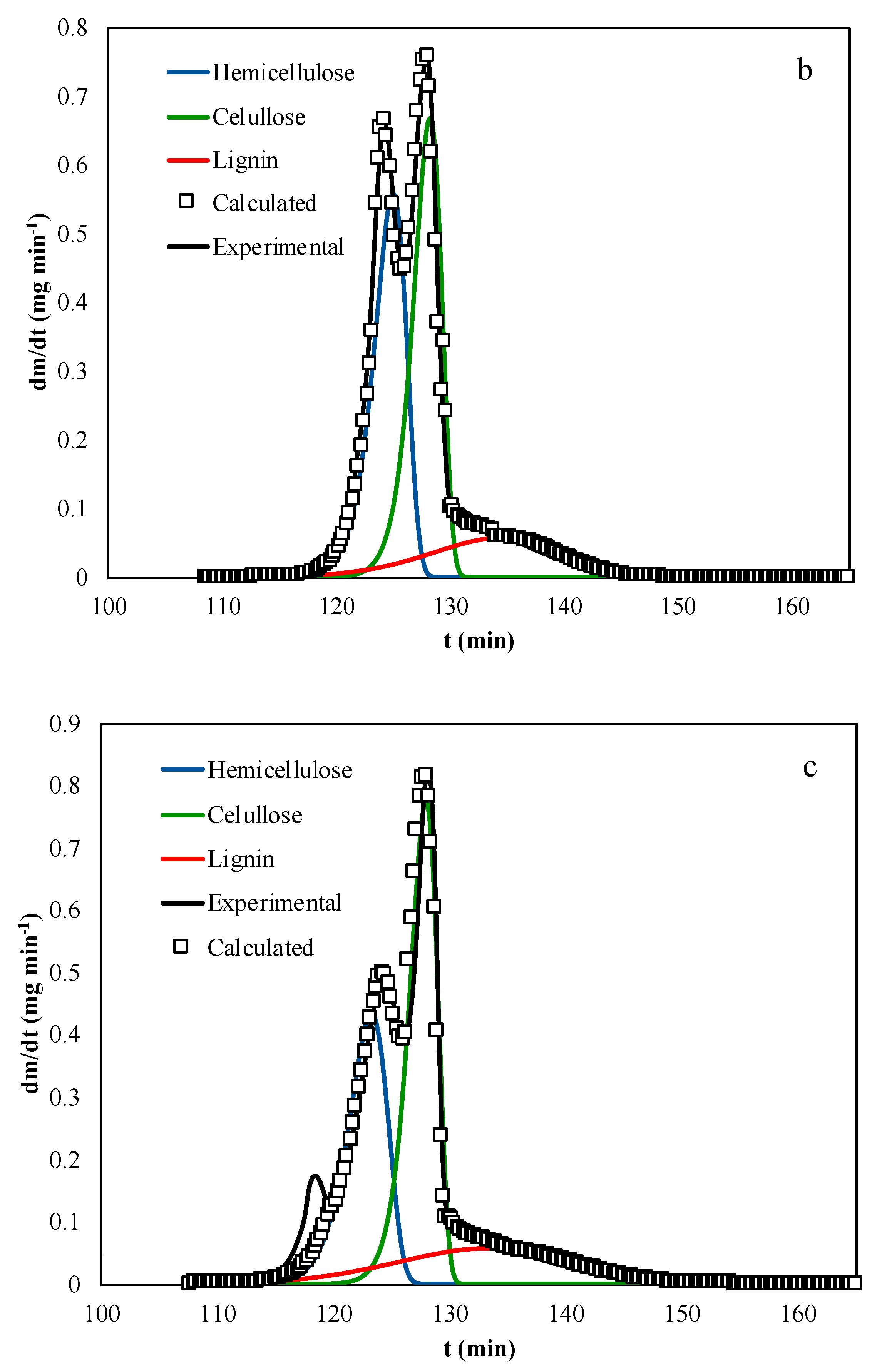
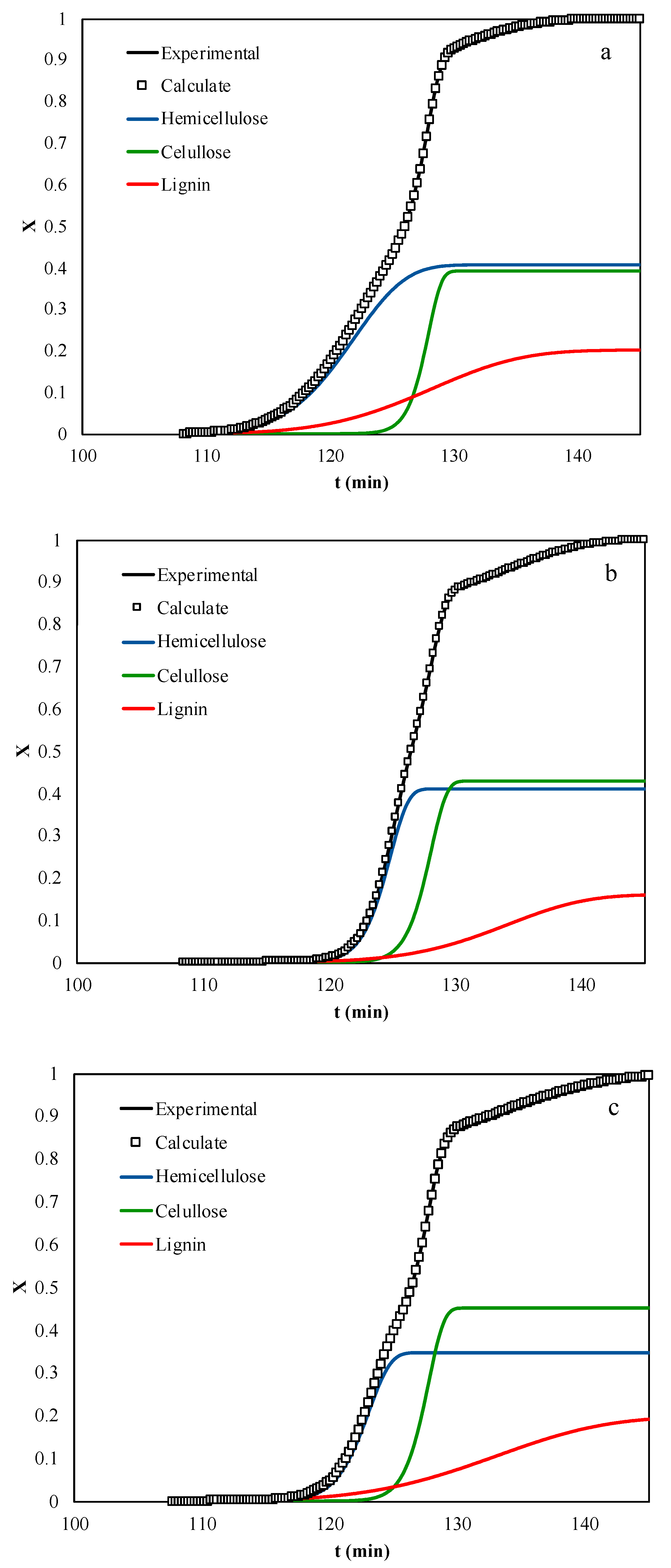
4. Results and Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Nomenclature
| EOF | objective function to be optimized |
| DTG | derivate thermogravimetric |
| ci | mass concentration of i component (wt.%) |
| Ei | activation energy of i constituent (kJ mol−1) |
| ki | kinetic constant for the weight loss of each i constituent (s−1) |
| ki,ref | number of experimental data |
| N | number of experimental data |
| t | time (min) |
| R | gas constant |
| T | temperature (°C) |
| W, W0, W∞ | weight of biomass sample at t time, at the beginning of pyrolysis and at the end of pyrolysis, respectively (mg) |
| Wi, W0,i,W∞,i | weight of i constituent in the sample at t time, at the beginning and at the end (mg) |
| X | conversion of the biomass sample by mass unit of pyrolysable mass |
| Xi, X∞,i | conversion of i constituent at t time and at the end |
References
- Ashter, S.A. 2—Biomass and its sources. In Technology and Applications of Polymers Derived from Biomass; Plastics Design Library; William Andrew Publishing: Norwich, NY, USA, 2018; pp. 11–36. ISBN 978-0-323-51115-5. [Google Scholar]
- Proskurina, S.; Heinimö, J.; Schipfer, F.; Vakkilainen, E. Biomass for industrial applications: The role of torrefaction. Renew. Energy 2017, 111, 265–274. [Google Scholar] [CrossRef]
- UNEP. Towards Sustainable Production and Use of Resources: Assessing Biofuels; UNEP: Paris, France, 2009. [Google Scholar]
- Shuba, E.S.; Kifle, D. Microalgae to biofuels: ‘Promising’ alternative and renewable energy, review. Renew. Sustain. Energy Rev. 2018, 81, 743–755. [Google Scholar] [CrossRef]
- Azizi, K.; Keshavarz Moraveji, M.; Abedini Najafabadi, H. A review on bio-fuel production from microalgal biomass by using pyrolysis method. Renew. Sustain. Energy Rev. 2018, 82, 3046–3059. [Google Scholar] [CrossRef]
- Vassilev, S.V.; Vassileva, C.G. Composition, properties and challenges of algae biomass for biofuel application: An overview. Fuel 2016, 181, 1–33. [Google Scholar] [CrossRef]
- Patel, M.; Kumar, A. Production of renewable diesel through the hydroprocessing of lignocellulosic biomass-derived bio-oil: A review. Renew. Sustain. Energy Rev. 2016, 58, 1293–1307. [Google Scholar] [CrossRef]
- Guo, M.; Song, W.; Buhain, J. Bioenergy and biofuels: History, status, and perspective. Renew. Sustain. Energy Rev. 2015, 42, 712–725. [Google Scholar] [CrossRef]
- Montingelli, M.E.; Tedesco, S.; Olabi, A.G. Biogas production from algal biomass: A review. Renew. Sustain. Energy Rev. 2015, 43, 961–972. [Google Scholar] [CrossRef]
- Amutio, M.; Lopez, G.; Aguado, R.; Artetxe, M.; Bilbao, J.; Olazar, M. Kinetic study of lignocellulosic biomass oxidative pyrolysis. Fuel 2012, 95, 305–311. [Google Scholar] [CrossRef]
- Nizami, A.S.; Rehan, M.; Waqas, M.; Naqvi, M.; Ouda, O.K.M.; Shahzad, K.; Miandad, R.; Khan, M.Z.; Syamsiro, M.; Ismail, I.M.I.; et al. Waste biorefineries: Enabling circular economies in developing countries. Bioresour. Technol. 2017, 241, 1101–1117. [Google Scholar] [CrossRef]
- Dahiya, S.; Kumar, A.N.; Shanthi Sravan, J.; Chatterjee, S.; Sarkar, O.; Mohan, S.V. Food waste biorefinery: Sustainable strategy for circular bioeconomy. Bioresour. Technol. 2018, 248, 2–12. [Google Scholar] [CrossRef]
- Williams, C.L.; Dahiya, A.; Porter, P. Chapter 1—Introduction to Bioenergy. In Bioenergy; Academic Press: Boston, MA, USA, 2015; pp. 5–36. [Google Scholar]
- Amutio, M.; Lopez, G.; Aguado, R.; Bilbao, J.; Olazar, M. Biomass Oxidative Flash Pyrolysis: Autothermal Operation, Yields and Product Properties. Energy Fuels 2012, 26, 1353–1362. [Google Scholar] [CrossRef]
- Aguado, R.; Olazar, M.; Barona, A.; Bilbao, J. Char-formation kinetics in the pyrolysis of sawdust in a conical spouted bed reactor. J. Chem. Technol. Biotechnol. 2000, 75, 583–588. [Google Scholar] [CrossRef]
- Aguado, R.; Olazar, M.; Jose, M.J.S.; Aguirre, G.; Bilbao, J. Pyrolysis of sawdust in a conical spouted bed reactor. Yields and product composition. Ind. Eng. Chem. Res. 2000, 39, 1925–1933. [Google Scholar] [CrossRef]
- Bridgwater, A.V. Review of fast pyrolysis of biomass and product upgrading. Biomass Bioenergy 2012, 38, 68–94. [Google Scholar] [CrossRef]
- Schönnenbeck, C.; Maryandyshev, P.; Trouvé, G.; Brillard, A.; Lyubov, V.; Brilhac, J.-F. Combustion of hydrolysis lignin in a drop tube furnace and subsequent gaseous and particulate emissions. Bioresour. Technol. 2019, 288, 121498. [Google Scholar] [CrossRef]
- Aguado, R.; Saldarriaga, J.F.; Atxutegi, A.; Bilbao, J.; Olazar, M. Influence of the kinetic scheme and heat balance on the modelling of biomass combustion in a conical spouted bed. Energy. 2019, 175, 758–767. [Google Scholar] [CrossRef]
- Erkiaga, A.; Lopez, G.; Barbarias, I.; Artetxe, M.; Amutio, M.; Bilbao, J.; Olazar, M. HDPE pyrolysis-steam reforming in a tandem spouted bed-fixed bed reactor for H2 production. J. Anal. Appl. Pyrolysis 2015, 116, 34–41. [Google Scholar] [CrossRef]
- Erkiaga, A.; Lopez, G.; Amutio, M.; Bilbao, J.; Olazar, M. Influence of operating conditions on the steam gasification of biomass in a conical spouted bed reactor. Chem. Eng. J. 2014, 237, 259–267. [Google Scholar] [CrossRef]
- Arabiourrutia, M.; Lopez, G.; Elordi, G.; Olazar, M.; Aguado, R.; Bilbao, J. Product distribution obtained in the pyrolysis of tyres in a conical spouted bed reactor. Chem. Eng. Sci. 2007, 62, 5271–5275. [Google Scholar] [CrossRef]
- Amutio, M.; Lopez, G.; Alvarez, J.; Moreira, R.; Duarte, G.; Nunes, J.; Olazar, M.; Bilbao, J. Flash pyrolysis of forestry residues from the Portuguese Central Inland Region within the framework of the BioREFINA-Ter project. Bioresour. Technol. 2013, 129, 512–518. [Google Scholar] [CrossRef]
- Attard, T.M.; McElroy, C.R.; Rezende, C.A.; Polikarpov, I.; Clark, J.H.; Hunt, A.J. Sugarcane waste as a valuable source of lipophilic molecules. Ind. Crops Prod. 2015, 76, 95–103. [Google Scholar] [CrossRef]
- Baudel, H.M.; Zaror, C.; de Abreu, C.A.M. Improving the value of sugarcane bagasse wastes via integrated chemical production systems: An environmentally friendly approach. Ind. Crops Prod. 2005, 21, 309–315. [Google Scholar] [CrossRef]
- Paul Guin, J.; Bhardwaj, Y.K.; Varshney, L. Radiation grafting: A voyage from bio-waste corn husk to an efficient thermostable adsorbent. Carbohydr. Polym. 2018, 183, 151–164. [Google Scholar] [CrossRef]
- El-Torki, A.M.M.; Mostafa, H.Y.; El-Masry, A.M.M.; Mahdi, M.E. Purification of heavy metals from industrail waste water using grafted corn husk pulp ion exchanger. Int. J. Adv. Res. 2015, 23, 936–949. [Google Scholar]
- Abdel-Shafy, H.I.; Mansour, M.S.M. Solid waste issue: Sources, composition, disposal, recycling, and valorization. Egypt. J. Pet. 2018, 27, 1275–1290. [Google Scholar] [CrossRef]
- Amutio, M.; Lopez, G.; Alvarez, J.; Moreira, R.; Duarte, G.; Nunes, J.; Olazar, M.; Bilbao, J. Pyrolysis kinetics of forestry residues from the Portuguese Central Inland Region. Chem. Eng. Res. Des. 2013, 91, 2682–2690. [Google Scholar] [CrossRef]
- Di Blasi, C. Modeling chemical and physical processes of wood and biomass pyrolysis. Prog. Energy Combust. Sci. 2008, 34, 47–90. [Google Scholar] [CrossRef]
- White, J.E.; Catallo, W.J.; Legendre, B.L. Biomass pyrolysis kinetics: A comparative critical review with relevant agricultural residue case studies. J. Anal. Appl. Pyrolysis 2011, 91, 1–33. [Google Scholar] [CrossRef]
- Saldarriaga, J.F.; Aguado, R.; Pablos, A.; Amutio, M.; Olazar, M.; Bilbao, J. Fast characterization of biomass fuels by thermogravimetric analysis (TGA). Fuel 2015, 140, 744–751. [Google Scholar] [CrossRef]
- Essenhigh, R.H.; Misra, M.K.; Shaw, D.W. Ignition of coal particles: A review. Combust. Flame 1989, 77, 3–30. [Google Scholar] [CrossRef]
- Annamalai, K.; Ryan, W. Interactive processes in gasification and combustion—II. Isolated carbon, coal and porous char particles. Prog. Energy Combust. Sci. 1993, 19, 383–446. [Google Scholar] [CrossRef]
- Gurgel Veras, C.A.; Saastamoinen, J.; Carvalho Jr, J.A.; Aho, M. Overlapping of the devolatilization and char combustion stages in the burning of coal particles. Combust. Flame 1999, 116, 567–579. [Google Scholar] [CrossRef]
- Senneca, O.; Chirone, R.; Salatino, P. A Thermogravimetric Study of Nonfossil Solid Fuels. 2. Oxidative Pyrolysis and Char Combustion. Energy Fuels 2002, 16, 661–668. [Google Scholar] [CrossRef]
- Saldarriaga, J.F.; Patiño, J.L.; Lizarazo, M.J. Kinetic Study of Spiny Retamo (Ulex Eurioaeus L.) Waste Oxidative Pyrolysis. Chem. Eng. Trans. 2018, 70, 1249–1254. [Google Scholar]
- Mamleev, V.; Bourbigot, S.; Yvon, J. Kinetic analysis of the thermal decomposition of cellulose: The change of the rate limitation. J. Anal. Appl. Pyrolysis 2007, 80, 141–150. [Google Scholar] [CrossRef]
- Lin, Y.C.; Cho, J.; Tompsett, G.A.; Westmoreland, P.R.; Huber, G.W. Kinetics and Mechanism of Cellulose Pyrolysis. J. Phys. Chem. C 2009, 113, 20097–20107. [Google Scholar] [CrossRef]
- Chen, W.-H.; Kuo, P.-C. Isothermal torrefaction kinetics of hemicellulose, cellulose, lignin and xylan using thermogravimetric analysis. Energy 2011, 36, 6451–6460. [Google Scholar] [CrossRef]
- Castro, A.; Soares, D.; Vilarinho, C.; Castro, F. Kinetics of thermal de-chlorination of PVC under pyrolytic conditions. Waste Manag. 2012, 32, 847–851. [Google Scholar] [CrossRef]
- Hayhurst, A.N. The kinetics of the pyrolysis or devolatilisation of sewage sludge and other solid fuels. Combust. Flame 2013, 160, 138–144. [Google Scholar] [CrossRef]
- Lopez, G.; Aguado, R.; Olazar, M.; Arabiourrutia, M.; Bilbao, J. Kinetics of scrap tyre pyrolysis under vacuum conditions. Waste Manag. 2009, 29, 2649–2655. [Google Scholar] [CrossRef]
- Cordero, T.; Rodríguez-Maroto, J.M.; Rodríguez-Mirasol, J.; Rodríguez, J.J. On the kinetics of thermal decomposition of wood and wood components. Thermochim. Acta 1990, 164, 135–144. [Google Scholar] [CrossRef]
- Mészáros, E.; Jakab, E.; Várhegyi, G. TG/MS, Py-GC/MS and THM-GC/MS study of the composition and thermal behavior of extractive components of Robinia pseudoacacia. J. Anal. Appl. Pyrolysis 2007, 79, 61–70. [Google Scholar] [CrossRef]
- Nassar, M.M.; MacKay, G.D. Mechanism of thermal decomposition of lignin. Wood Fiber Sci. 1984, 16, 441–453. [Google Scholar]
- Sebio-Puñal, T.; Naya, S.; López-Beceiro, J.; Tarrío-Saavedra, J.; Artiaga, R. Thermogravimetric analysis of wood, holocellulose, and lignin from five wood species. J. Therm. Anal. Calorim. 2012, 109, 1163–1167. [Google Scholar] [CrossRef]
- Conesa, J.A.; Marcilla, A.; Caballero, J.A.; Font, R. Comments on the validity and utility of the different methods for kinetic analysis of thermogravimetric data. J. Anal. Appl. Pyrolysis 2001, 58–59, 617–633. [Google Scholar] [CrossRef]
- Caballero, J.A.; Conesa, J.A.; Font, R.; Marcilla, A. Pyrolysis kinetics of almond shells and olive stones considering their organic fractions. J. Anal. Appl. Pyrolysis 1997, 42, 159–175. [Google Scholar] [CrossRef]
- Várhegyi, G.; Grønli, M.G.; Di Blasi, C. Effects of Sample Origin, Extraction, and Hot-Water Washing on the Devolatilization Kinetics of Chestnut Wood. Ind. Eng. Chem. Res. 2004, 43, 2356–2367. [Google Scholar] [CrossRef]
- Sun, L.; Chen, J.Y.; Negulescu, I.I.; Moore, M.A.; Collier, B.J. Kinetics modeling of dynamic pyrolysis of bagasse fibers. Bioresour. Technol. 2011, 102, 1951–1958. [Google Scholar] [CrossRef]
- Zanchetta, A.; dos Santos, A.C.F.; Ximenes, E.; da Costa Carreira Nunes, C.; Boscolo, M.; Gomes, E.; Ladisch, M.R. Temperature dependent cellulase adsorption on lignin from sugarcane bagasse. Bioresour. Technol. 2018, 252, 143–149. [Google Scholar] [CrossRef]
- Kapoor, K.; Garg, N.; Diwan, R.K.; Varshney, L.; Tyagi, A.K. Study the effect of gamma radiation pretreatment of sugarcane bagasse on its physcio-chemical morphological and structural properties. Radiat. Phys. Chem. 2017, 141, 190–195. [Google Scholar] [CrossRef]
- Manyà, J.J.; Velo, E.; Puigjaner, L. Kinetics of Biomass Pyrolysis: A Reformulated Three-Parallel-Reactions Model. Ind. Eng. Chem. Res. 2003, 42, 434–441. [Google Scholar] [CrossRef]
- Conesa, J.A.; Domene, A. Biomasses pyrolysis and combustion kinetics through n-th order parallel reactions. Thermochim. Acta 2011, 523, 176–181. [Google Scholar] [CrossRef][Green Version]
- Barneto, A.G.; Carmona, J.A.; Alfonso, J.E.M.; Alcaide, L.J. Use of autocatalytic kinetics to obtain composition of lignocellulosic materials. Bioresour. Technol. 2009, 100, 3963–3973. [Google Scholar] [CrossRef] [PubMed]
| Properties | C1 | C2 | C3 |
|---|---|---|---|
| Ultimate Analysis (%wt) | |||
| Carbon | 48.64 | 46.82 | 46.51 |
| Hydrogen | 5.87 | 5.74 | 5.68 |
| Nitrogen | 0.16 | 0.66 | 0.47 |
| Oxygen | 42.82 | 41.36 | 44.13 |
| Proximate Analysis (%wt) | |||
| Moisture | 5.72 | 6.37 | 7.18 |
| Volatile matter | 74.43 | 71.72 | 73.42 |
| Fixed carbon | 16.08 | 20.70 | 20.04 |
| Ash | 5.01 | 2.74 | 0.92 |
| HHV (MJ kg−1) | 17.92 | 17.35 | 16.92 |
| Biomass | Kinetic Parameters | Hemicellulose | Cellulose | Lignin |
|---|---|---|---|---|
| Bagasse (C1) | Log k0 (s−1) | 4.14 | 39.70 | 0.50 |
| E (kJ mol−1) | 45.16 | 237.06 | 35.23 | |
| Content (wt.%) | 30.81 | 29.52 | 15.70 | |
| Husk corn (C2) | Log k0 (s−1) | 26.04 | 32.69 | 2.99 |
| E (kJ mol−1) | 148.77 | 199.37 | 52.30 | |
| Content (wt.%) | 30.54 | 32.04 | 12.51 | |
| Cob corn (C3) | Log k0 (s−1) | 16.09 | 31.83 | 0.42 |
| E (kJ mol−1) | 100.00 | 194.00 | 36.93 | |
| Content (wt.%) | 26.84 | 34.86 | 15.84 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodríguez, F.; Cruz, Y.; Estiati, I.; Saldarriaga, J.F. Kinetic Study of Corn and Sugarcane Waste Oxidative Pyrolysis. Energies 2019, 12, 4594. https://doi.org/10.3390/en12234594
Rodríguez F, Cruz Y, Estiati I, Saldarriaga JF. Kinetic Study of Corn and Sugarcane Waste Oxidative Pyrolysis. Energies. 2019; 12(23):4594. https://doi.org/10.3390/en12234594
Chicago/Turabian StyleRodríguez, Francisco, Yuby Cruz, Idoia Estiati, and Juan F. Saldarriaga. 2019. "Kinetic Study of Corn and Sugarcane Waste Oxidative Pyrolysis" Energies 12, no. 23: 4594. https://doi.org/10.3390/en12234594
APA StyleRodríguez, F., Cruz, Y., Estiati, I., & Saldarriaga, J. F. (2019). Kinetic Study of Corn and Sugarcane Waste Oxidative Pyrolysis. Energies, 12(23), 4594. https://doi.org/10.3390/en12234594