1. Introduction
The environmental impact due to global warming has reaching a point from which there is no return. According to researchers the main culprit of global warming are the greenhouse gases, especially carbon dioxide, released by energy consumption for human activities [
1]. One of the ways to solve this problem is by using renewable energy sources for energy generation, and many countries have successfully implemented renewable energy policies [
2]. Some countries have successfully implemented solar energy as an electricity source to support daily life activities [
3,
4], however, the problem with solar energy sources is that it is only available for certain periods and therefore energy storage devices must be used. The only storage devices commercially available at this moment are batteries, which have very limited capacity and therefore scientists are attempting to find new energy storage materials [
5,
6]. Like some other countries Malaysia and Indonesia have successfully implemented biodiesel energy policies to power their transport sector [
7,
8,
9]. However, this policy has been claimed by some researchers to create conflicts with food production and destroy the tropical rain forest. Therefore, Indonesia is trying to encourage the use of cleaner energy sources such as geothermal energy for electricity generation to replace fossil fuels.
Since 1970, Indonesia has undertaken geothermal explorations in order to discover and develop high-temperature geothermal energy sources [
10]. Indonesia contains 13% of the total world’s volcanoes, of which 80% are estimated to possess geothermal energy production potential [
11]. Those volcanoes are spread over 312 locations [
12] with an average estimated geothermal potential of around 28.617 MWe which is equal to 40% of the total world’s geothermal potential [
13]. Nevertheless, only 4.7% of them have been explored and exploited for electrical energy resources, providing a capacity of 1340 MWe from 10 geothermal wells [
12]. They are: Darajat (260 MWe), Dieng (60 MWe), Kamojang (200 MWe), Salak Mountain (377 MWe), Sibayak (12 MWe), PLTP Wayang Windu (227 MWe), Lahendong (87 MWe), Ulu Belu-Sumatera Selatan (110 MWe), Ulumbu-Flores (5 MWe) and Mataloko (2.5 MWe). Apart from the stated above, there are an additional 440 MWe of geothermal energy under construction in Sarulla and Lumut Balai. However, the geothermal energy capacity that is under exploitation is considered small compared to Indonesia’s short-medium term development target of geothermal electrical capacity for 2025, which is around 6000 MWe, equal to 5% of the national energy demand [
12].
A geothermal system location can be exploited if the exploration results data are previously determined. Geothermal exploration and modeling cover three components, which are geological, geophysical and geochemical studies. The main objective of geochemical research is to estimate the temperature that can be obtained from the geothermal system. A high-temperature geothermal system can provide a high geothermal energy capacity. To obtain such information, it is necessary to carry out fluid-geochemistry analysis, which includes hydro-geochemical, gas-geochemical and isotope analyses. Apart from geothermal temperature, the geochemical study also informs the origin of the geothermal system and geochemical processes that affect the fluid’s temperature. Recently, geochemical studies on geothermal systems have been many conducted in various sites around the world, including Kangding in the eastern Himalayas [
14]; the Icelandic high-temperature geothermal areas [
15]; the western sector of the Sabatini Volcanic District and the Tolfa Mountains (Italy) [
16]; the Xining basin on the northeastern Tibetan Plateau [
17]; Mapamyum in Western Tibet, China [
18]; the Tang-Bijar oilfield springs in the Zagros region of Iran [
19]; southern Saint Lucia, in the Lesser Antilles island arc [
20]; the Southern Sula graben in Honduras [
21]; and the Nevşehir (Kozakli) area in Central Turkey [
22].
Almost half of the geothermal potential in Indonesia is found on the island of Sumatra, one which is in Seulawah Agam in Aceh Province. Seulawah Agam’s geothermal site has several manifestations, that are
Ie-Su’um, Ie-Brôuk, Ie-Jue and the Van-Heutz crater in the north zone, and the
Aleu Ie Su’um,
Alue Ie Masam,
Alue PU and
Alue Teungku manifestations in the south zone. Some studies have been done in this area. Geophysical studies of geothermal resource imagery [
23] and deep and shallow structures of geothermal sites [
24] have been done in the Seulawah Agam area. Preliminary geochemical studies have also done at three manifestations of the North Zone, which are the
Ie Su’um [
25],
Ie Ju [
26] and
Ie-Brôuk [
27] ones. Estimated depth temperature, type of geothermal water, chemical composition and fluid equilibrium of the three manifestations is predicted with hydro-geothermometry in calculating cations and anions analyzed by atomic absorption spectrophotometry (AAS) and UV-Vis spectrophotometry.
Geothermal potential determination is a complex process. There are many hydrogeochemical processes that affect the fluid emigrating from the source to the surface. This causes the unreliableness of reservoir temperature predictions based on only a single geothermometry method. Thus, hydro-geothermometry, gas-geothermometry, and isotopic methods (if available) should be applied together. Besides, the chemical identification needs to be accurate using a sensitive instrument. Especially for atomic analysis, spectra identification in a plasma gives more precise and reliable results [
28,
29]. The accuracy of the measurement determines the geothermometry calculations, hence determining the estimated temperature of the manifestation reservoir.
Refractory compound formation always deserves attention in elemental analysis. The sample may contain constituents that form a refractory (heat-stable) compound with the analyte element of interest, and is avoided by addition of a chemical competitor or use of very high temperatures such as in inductively coupled plasma (ICP). ICP uses a 3–4× higher temperature, is 100–1000× more sensitive and has a 2–3× higher linear range than flame spectrometry. In nature there are many metal oxides. The low temperatures of flame atomic spectrometry sometimes cause the spectrum to be the result of metal oxide molecules, not the desired atomic line spectrum. This is very disturbing for the accuracy.
In this study, a geochemical exploration has been conducted on some geothermal areas in the north zone of Seulawah Agam mountain, which are the Ie-Seu’um, Ie-Brôuk, Ie-Jue, and the Van Heutz-crater manifestations. The study includes in-situ, liquid geothermal, gas geothermal, and isotope analysis. Ions in the liquid and gas were analyzed with ICP-OES, ion-chromatography, UV-Vis spectrophotometry and alkalimetry titration. Isotope analysis was carried out by laser spectroscopic analysis. Several geothermometry methods were applied to obtain geochemical data, including hydro-geothermometry, gas-geothermometry, and stable isotope plots to provide information on the types, chemical composition and liquid equilibrium, reservoir temperature predictions and the origin of the geothermal fluid.
2. Study Area
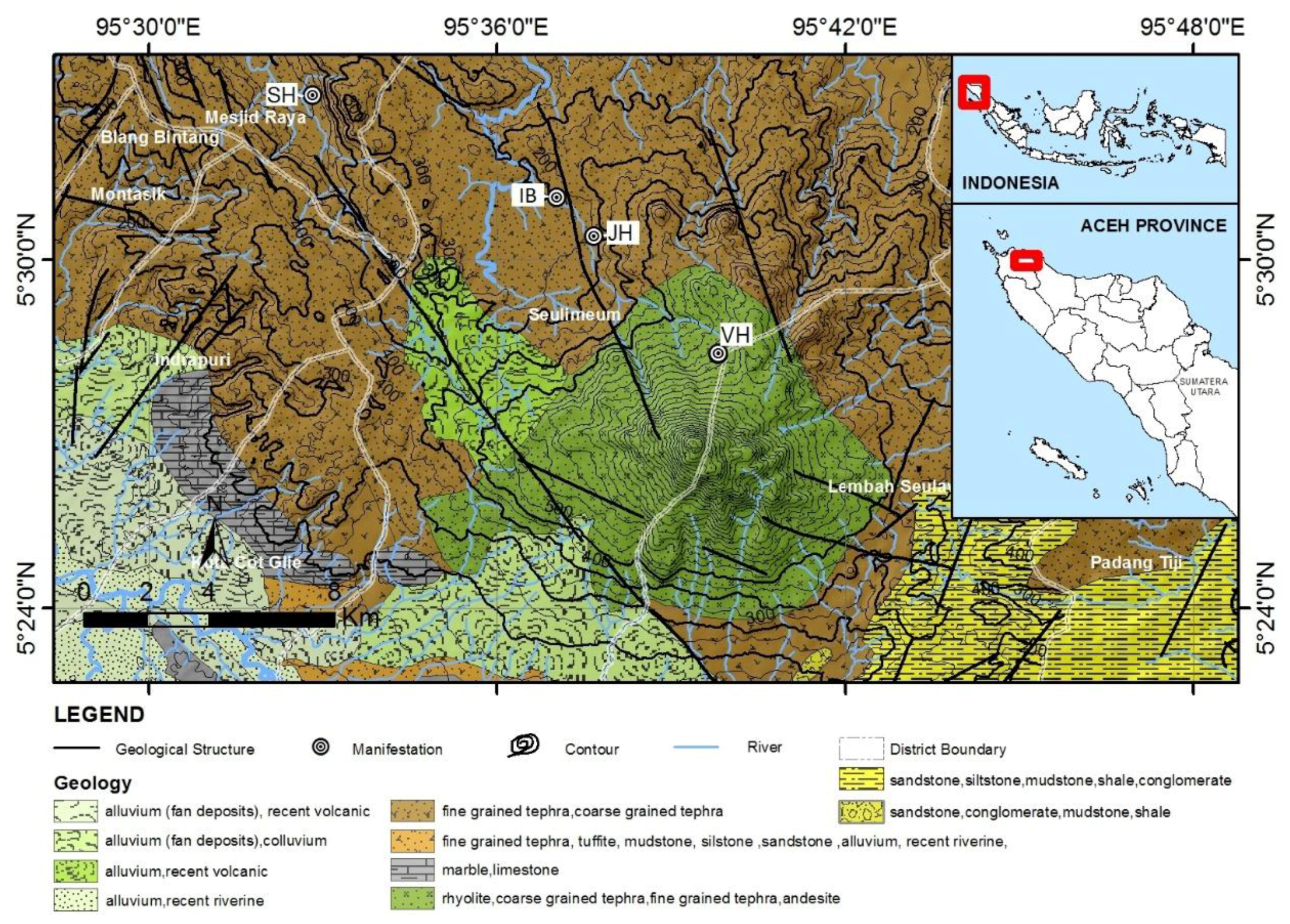
This study covers the area in the north zone of Selawah Agam mountain (
Figure 1). At this location, there are four geothermal system manifestations, which are
Ie-Seu’um,
Ie-Brôuk, Ie-Jue, and the Van-Heutz crater manifestations.
Sampling points were established at each geothermal system manifestation, which were SH1, SH2, and SH3 in Ie-Seu’um; IB1 and IB2 in Ie-Brôuk; JH1, JH2, JH3 and JH4 in Ie-Jue; and VH1, VH2, and VH3 in the Van-Heutz crater. Based on the physical observation of the condition of the north zone in the Ie-Seu’um manifestation, it appears to have clear spring water. It is found that the water discharge at SH2 is bigger than at SH1 and SH3. The surface characteristic is observed to have sediment on the brown color rocks that resembles silica sinter. In the Ie-Jue manifestation, the hot spring is found to be muddy and full of gases. Meanwhile in the Ie-Brôuk manifestation, a moderate hot spring is found to be around the puddle and have a few air bubbles, where several aquatic species, such as leeches, are found. In the Van-Heutz crater manifestation the water output appears to have high turbidity and acidic pH. The manifestation is located in a crater, thus being a source of solfatara output gas with a light yellow color that is found around the manifestation.
3. Materials and Methods
3.1. Field Observation Equipment
The observation equipment (general equipment) used, which included latex gloves, waterproof markers, compass, notebooks, camera, trekking poles, sulphur detector, boots, and a first-aid kit, follows Nicholson [
30].
3.2. Water Sampling and Analysis
Water sample collection was carried out at the four manifestations of the north zone: the Ie-Seu’um, Ie-Jue, Ie-Brôuk and Van-Heutz crater manifestations. The coordinates and elevation data of each sampling point was marked by GPS (62S, Garmin, Olathe, KS, USA). Water samples were collected and put in polyethylene (PE) bottles. The bottles are then placed in a cooling box and transported to the laboratory for cation, anion, SiO2, and isotope (δD dan δ18O) analysis.
During the placement of the water samples into the bottles, 6 N HNO3 was added to the samples for cation analysis until the pH reached <2 (acidified). On the other hand, for anion analysis, nothing is added to the samples (non-acidified). Samples for SiO2 analysis were diluted in deionized water to prevent the precipitation of silica. Before the analysis, water samples were filtered with Whatmann 0.45 μm filter paper.
An in-situ analysis is done at the hot spring sampling locations. The analysis includes the determination of water surface temperature using a thermometer (Fisher Scientific Traceable, Fisher Scientific, Hampton, NH, USA), acidity level using a pH meter (Schott Instruments, Xylem Analytics, Mainz, Germany), conductivity using a conductometer (Schott Instrument) and Total Dissolved Solids (TDS) using a TDS meter (Hanna, Hanna Instruments, Woonsocket, RI, USA). Each determination is repeated five times to obtain average values and uncertainty values (standard deviations).
Cation analysis (K, Na, Mg, Ca, Li, B) was carried out by Inductively Coupled Plasma-Optical Emission Spectrometry (ICP-OES, iCAP 7400 instrument, Thermo Fisher Scientific, Waltham, MA, USA) in a wavelength range from 166 to 847 nm with argon as combustion gas. Anion analysis (SO
4, F, Cl, NO
3) was carried out by ion chromatography (IC Plus 883, Metrohm, Herisau, Switzerland) using a Metrosep A Supp 5–150/4.0 column, eluent composition 1 mmol L
−1 NaHCO
3 + 3.2 mmol L
−1 Na
2CO
3 dissolved in aquabidest and acetone (980 mL aquabidest and 20 mL acetone), 0.700 mL min
−1 flow rate and 9.63 MPa pressure. SiO
2 analysis is carried out with a UV-Vis spectrophotometer (Genesys 10S, Thermo Scientific) employing a standard method [
31]. HCO
3- ion analysis is conducted by the alkalimetry titration method. Isotope (δD and δ
18O) analysis is carried out by laser spectroscopic analysis (LGR DT−100 Liquid Water Stable Isotope Analyzer, Los Gatos Research, Inc., San Jose, CA, USA). The liquid samples are measured according to the calibration standard and control standard. The obtained isotope ratio is correlated with the Vienna Standard Mean Ocean Water (VSMOW) international standard plot, with analysis precision standard under 0.6‰ for δD and 0.1 ‰ for δ
18O [
32].
3.3. Gas Sampling and Analysis
Gas (also known as fumarole gas) samples were collected from fumarole sources at the
Ie-Jue manifestation location. Gas was collected in Giggenbach flasks using the standard gas sampling technique method as explained by Giggenbach and Goguel [
33]. An evacuated Giggenbach bottle containing 100 mL NaOH 4 N and a little CdCl
2 indicator was used. First the hose that connects the gas source and Giggenbach bottle was checked for leaks to avoid any contamination by outside air. The valve of the Giggenback bottle was opened to let the gas enter the bottle while shaking to accelerate the gas dissolution process in the NaOH/CdCl
2 solution. The process is ended by closing the valve when the vacuum in the Giggenbach bottle is about to run out. The collected gas sample is then transported to the laboratory for analysis. The analysis of the gaseous samples is conducted using two methods: a gas chromatography method for unreactive gases (H
2, Ar, N
2, and CH
4) using a GC-TCD (HP−5890 system company, Agilent Technologies, Santa Clara, CA, USA) equipped with a Porapak column, and a titration method for reactive gases (CO
2, NH
3 and H
2S).
3.4. Geothermometry Applications
3.4.1. Hydro-Geothermometry
The depth temperature is calculated by the Na-K geothermometer equation [
34,
35,
36,
37,
38,
39] and Na-K-Ca geothermometry [
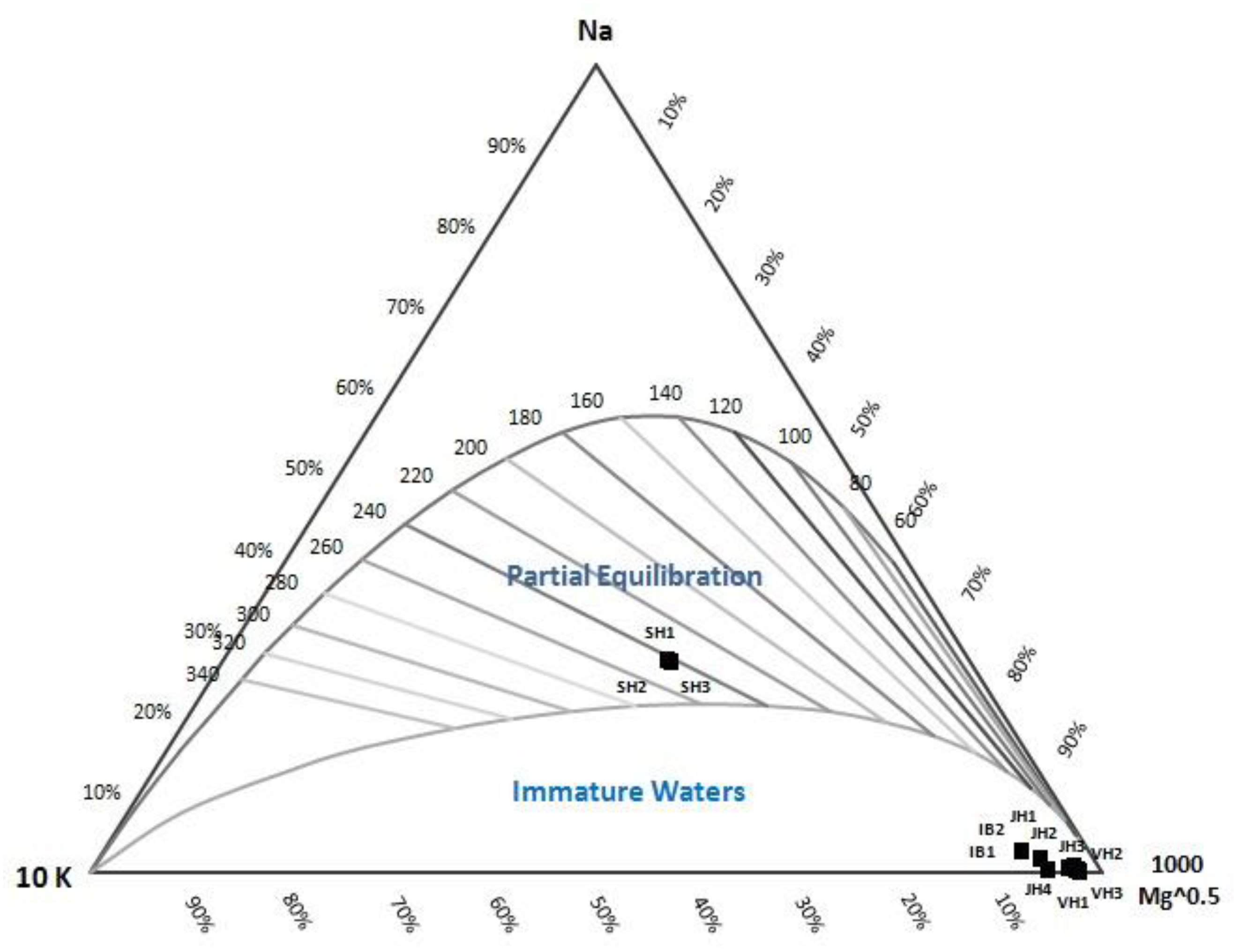
40]. The dissolved chemical equilibrium is determined by a Na-K-Mg triangle diagram [
34]. Dominant cation-anion content is estimated by a Piper diagram [
41]. The geothermal water type is determined by a Cl-SO
4-HCO
3 triangle diagram [
42]. The equations of each geothermometry technique used are presented in
Table 1.
3.4.2. Gas Geothermometry
The depth temperature is estimated by the gas geothermometry equations shown in
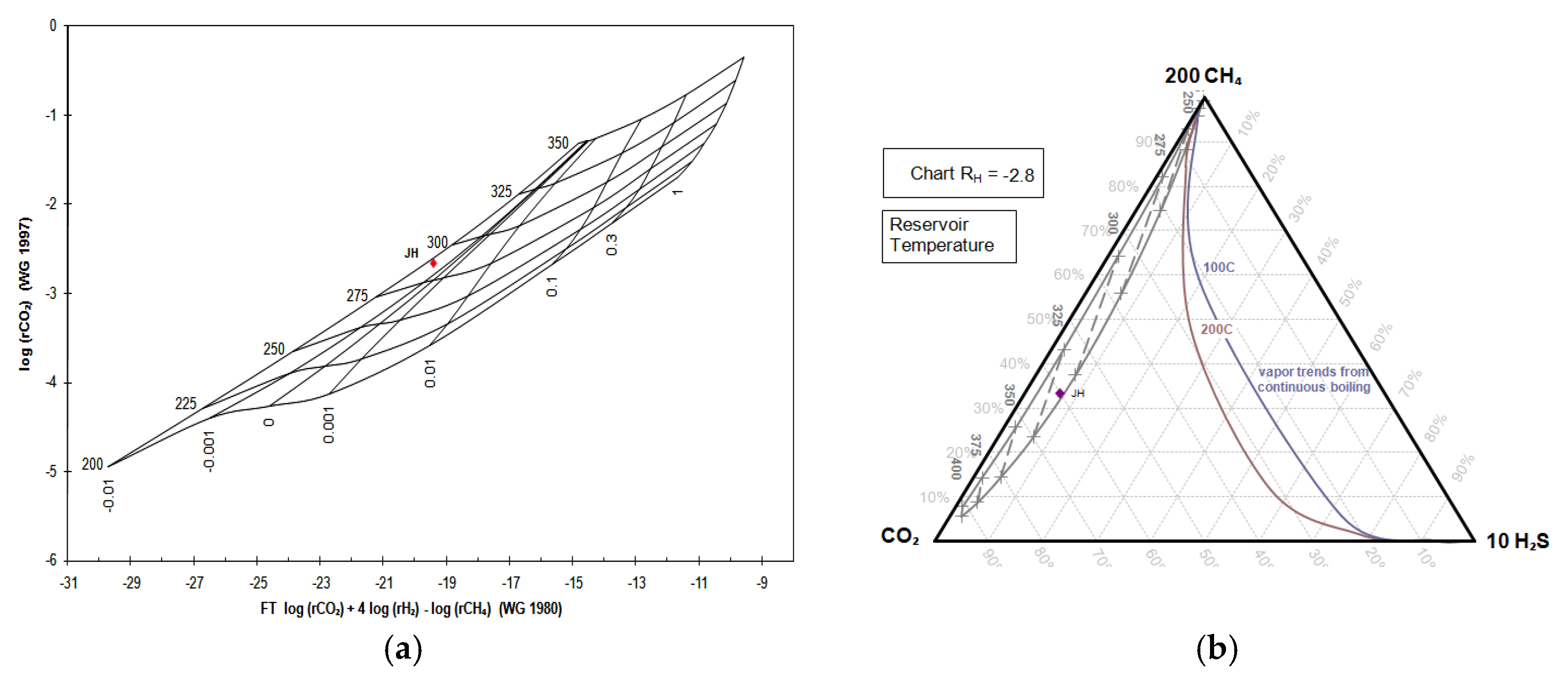
Table 2. The depth temperature is also estimated by a Grid Fischer-Tropsch CO
2 (FT-CO
2) diagram [
42] and CH
4-CO
2-H
2S diagram grid [
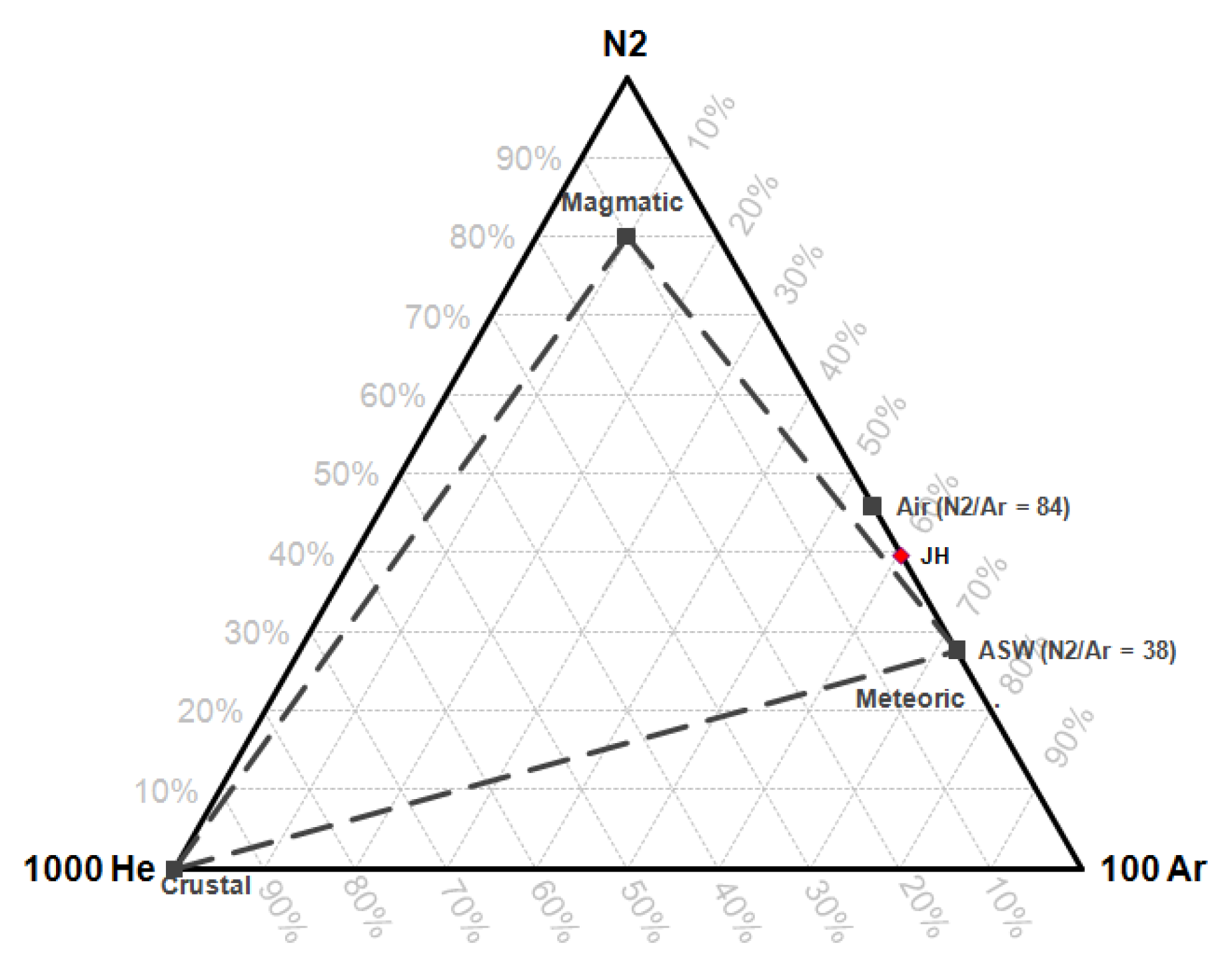
43]. The origin of the gas fluid is estimated by a N
2-He-Ar triangle diagram [
34].
3.5. Uncertainty of Measurement
All data analysis was carried out using Spreadsheet Version 3 of Powell Geoscience Ltd. (3 September 2012) by Powell and Cumming (Microsoft Corporation, Redmond, WA, USA). Uncertainty of the concentration measurements of each parameter is presented in standard deviation values and several statistical function data using the LINEST method in Microsoft Excel (Microsoft Corporation, Redmond, WA, USA) The calculation results obtained the statistical function data including slope (
m), standard deviation of slope (
Sm), intercept (
b), standard deviation of intercept (
Sb), determination coefficient (
R), standard deviation of regression (
Sr) and standard deviation of the concentration (
SC) [
45]. The equations used in the calculation of standard deviations and statistic functions are presented in
Table 3.
5. Conclusions
This study concerns the four geothermal system manifestations (Ie-Seu’um, Ie-Brouk, Ie-Jue, and Van-Heutz crater) of the Selawah Agam volcano, located in northwestern Sumatra, Indonesia. The analysis of the Cl-HCO3-SO4 triangle diagram and Piper diagram suggests that the water type and dominant chemical composition of the fluids of each manifestation are as follows: (1) chloride and Na-K-Cl for Ie-Seu’um, (2) bicarbonate and Na-Ca-HCO3 for Ie-Brouk and (3) sulphate and Ca-SO4 for Ie-Jue and the Van-Heutz crater.
The analysis of the Na-K-Mg triangle diagram suggests that the fluid equilibrium of Ie-Brôuk, Ie-Jue, and Van-Heutz crater manifestations presents an immature water state. Only the fluid from the Ie-Seu’um manifestation is in a mature water state.
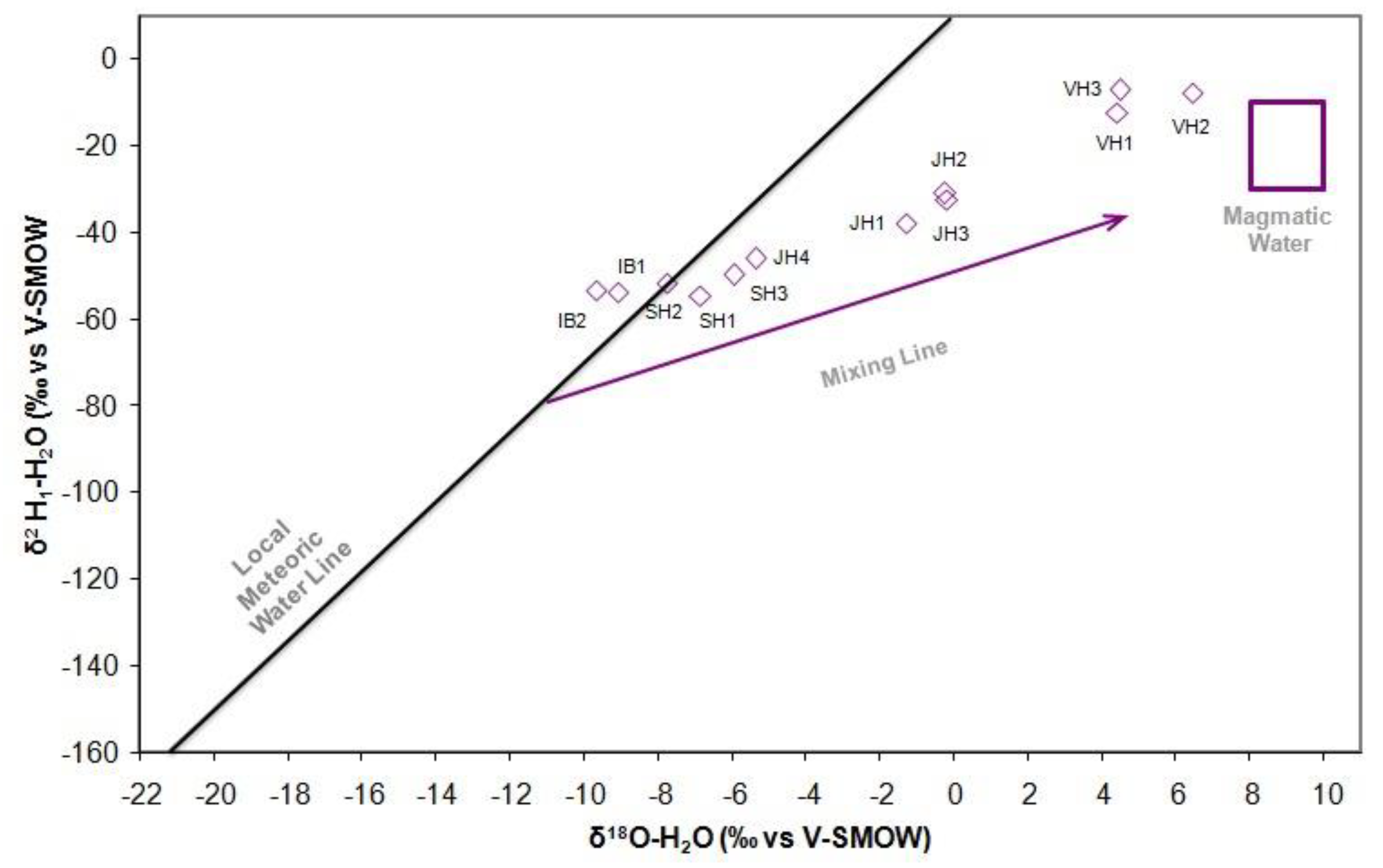
The analysis of the isotope ratio in the stable isotope plot showed that the water of Ie-Seu’um, Ie-Jue, Ie-Brôuk, and Ie-Jue are originated from meteoric water. While the Van-Heutz crater manifestation is originated from magmatic water.
The Giggenbach Na-K hydrothermal geothermometer [
34] indicates that the
Ie-Seu’um and
Ie-Brôuk manifestations have average reservoir temperatures of 241.9 ± 0.3 °C a d 321.95 ± 13.4 °C, respectively, wheras the Fournier Na-K hydrothermal geothermometer [
35] indicates that the
Ie-Jue and Van-Heutz crater manifestations have average reservoir temperatures of 472.4 ± 91.4 °C and 439.3 ± 95.3 °C, respectively.
Gas geothermometry indicates that the Ie-Jue fumarole manifestation has a reservoir temperature of 201.6–312.7 °C. The analysis of the FT-CO2 Cross-Plot and CH4-CO2-H2S triangle diagram on the fumarole manifestation shows the reservoir temperature to be 275–325 °C. The analysis of the N2-He-Ar triangle diagram suggests that the Ie-Jue fumarole gas is of meteoric water origin.
Based on the reservoir temperatures, that can be estimated well using gas geothermometry and fluid geothermometry, the geothermal manifestations of the north zone of Seulawah Agam mountain are considered to constitute a high-temperature geothermal system (high enthalpy) in accordance with Hochstein, which is indicated by the average measured temperature > 225 °C. This condition suggests that the geothermal area is suitable for power plant development. In accordance with Kasbani [
48] regarding the estimation of the energy potential for different geothermal types in Indonesia, the estimated capacity of the power plant production based on this temperature is above 100 MW and is estimated to have a reservoir with a system type dominated by steam and water or a mixture of both.