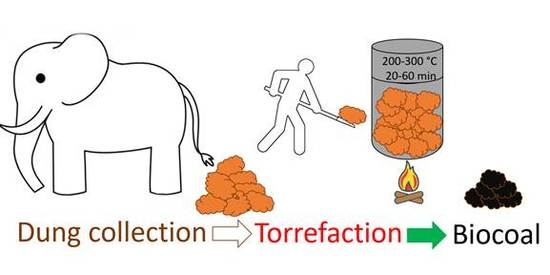
Waste to Carbon: Biocoal from Elephant Dung as New Cooking Fuel
Abstract
1. Introduction
- Whether torrefaction can be used as a method of preliminary valorization of elephant dung;
- Whether the duration of the torrefaction process at a given temperature affects the dung conversion rate (e.g., mass loss, energy densification, and improved fuel properties);
- Whether energy consumption is needed for the torrefaction of elephant dung.
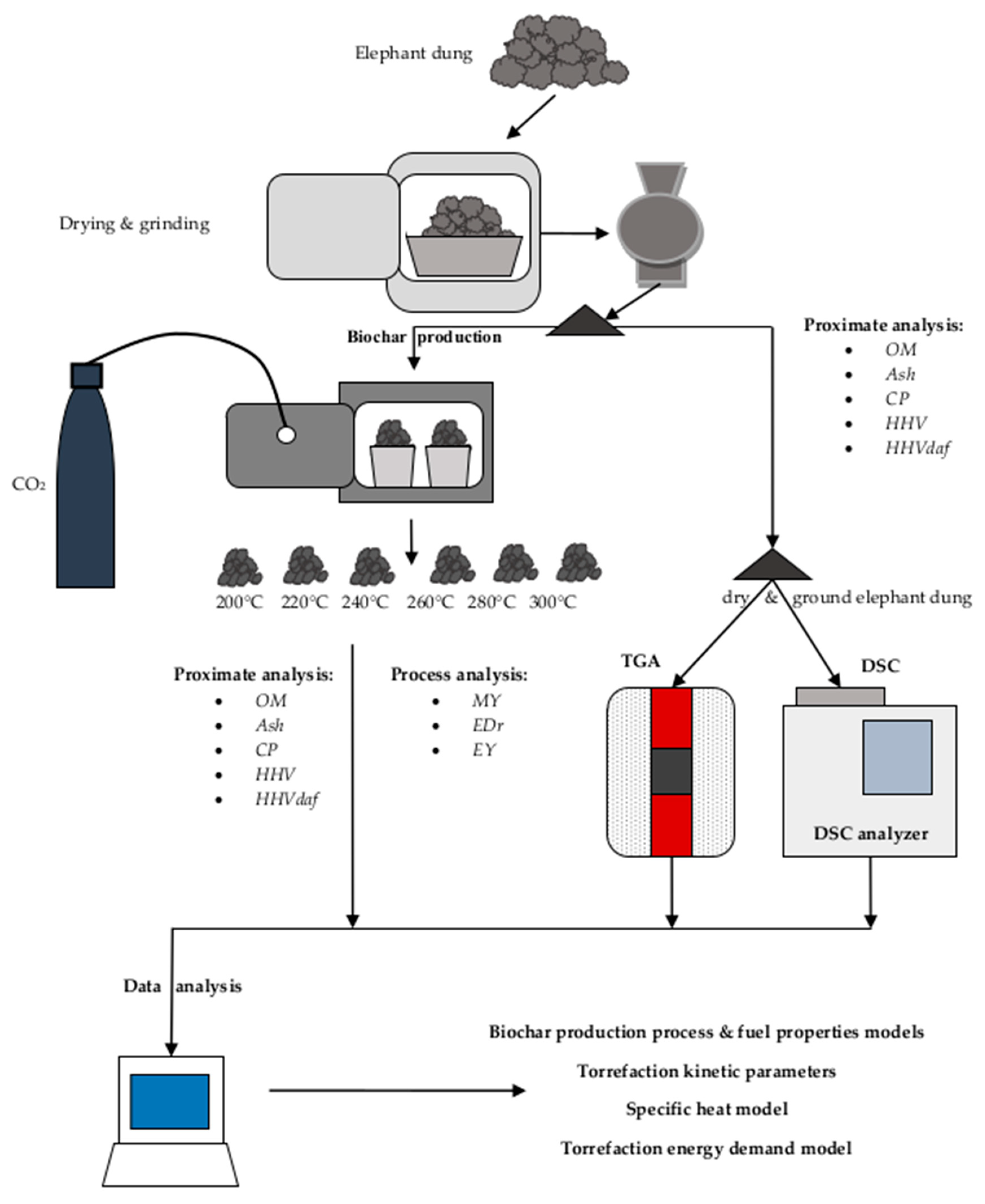
2. Materials and Methods
2.1. Feedstock
2.2. Biocoal Production Method via Torrefaction
2.3. Proximate Analysis of Raw and Torrefied Elephant Dung
- Moisture content (MC) by means of a laboratory dryer (WAMED, model KBC-65W, Warsaw, Poland) at temperature 105 °C, time 24 h, in accordance with the PN-EN 14346:2011 standard [23],
- Organic matter content (OM) by means of a laboratory dryer (WAMED, model KBC-65W, Warsaw, Poland) at temperature 550 °C, time 4 h, in accordance with the PN-EN 15169:2011 standard [24],
- ash and combustible parts (CP) by means of a laboratory dryer (WAMED, model KBC-65W, Warsaw, Poland) at temperature 815 °C, time 4 h in accordance with the PN-Z-15008-04:1993 standard [25],
- High heating value (HHV) by means of the IKA C2000 Basic calorimeter, at 17–25 °C, 30 bar pressure in accordance with the PN-G-04513:1981 standard [26].
2.4. Thermogravimetric Analysis (TGA) of Elephant Dung
2.5. Differential Scanning Calorimetry (DSC) of Raw Elephant Dung
2.6. Data-Processing Calculation Methods
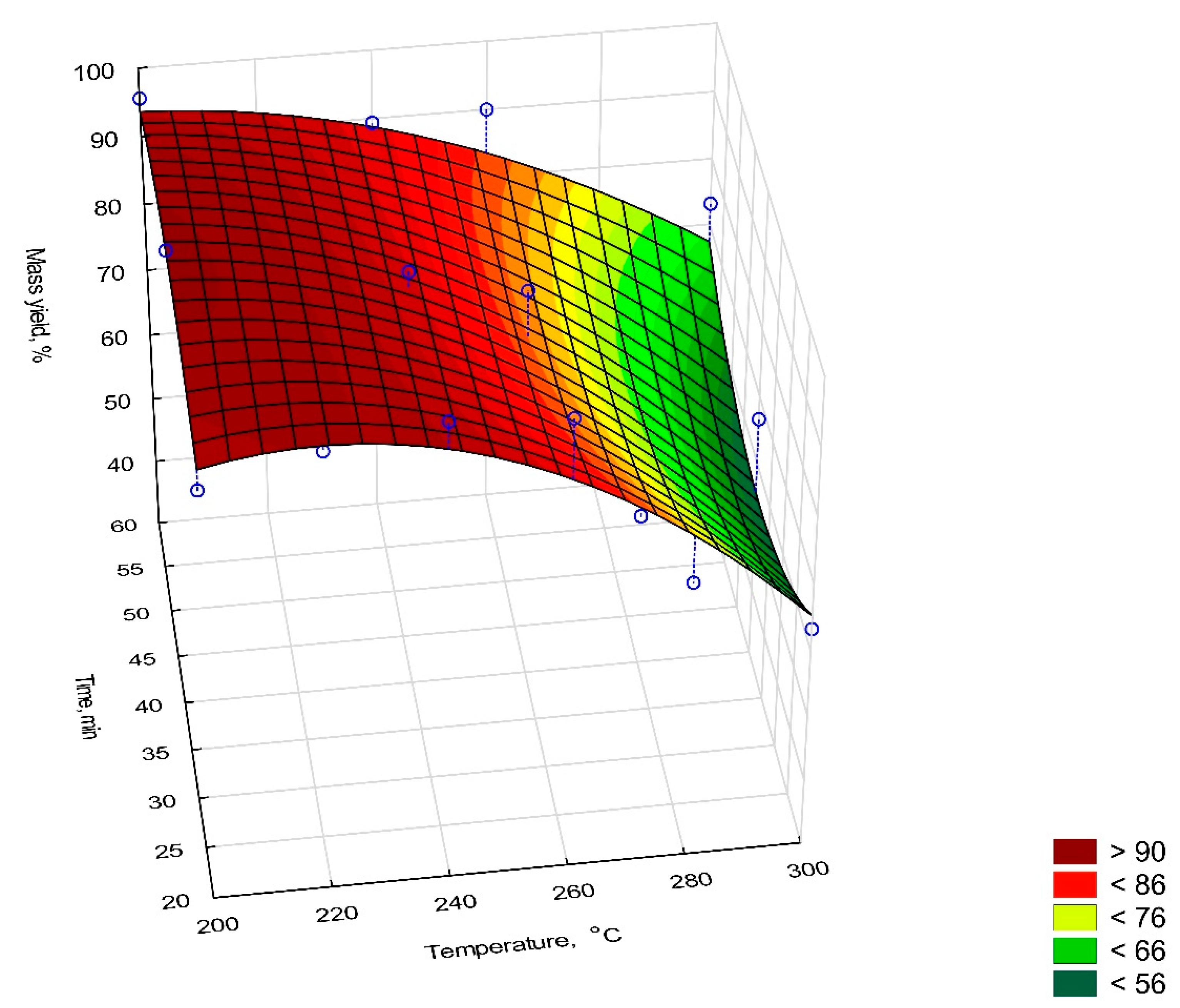
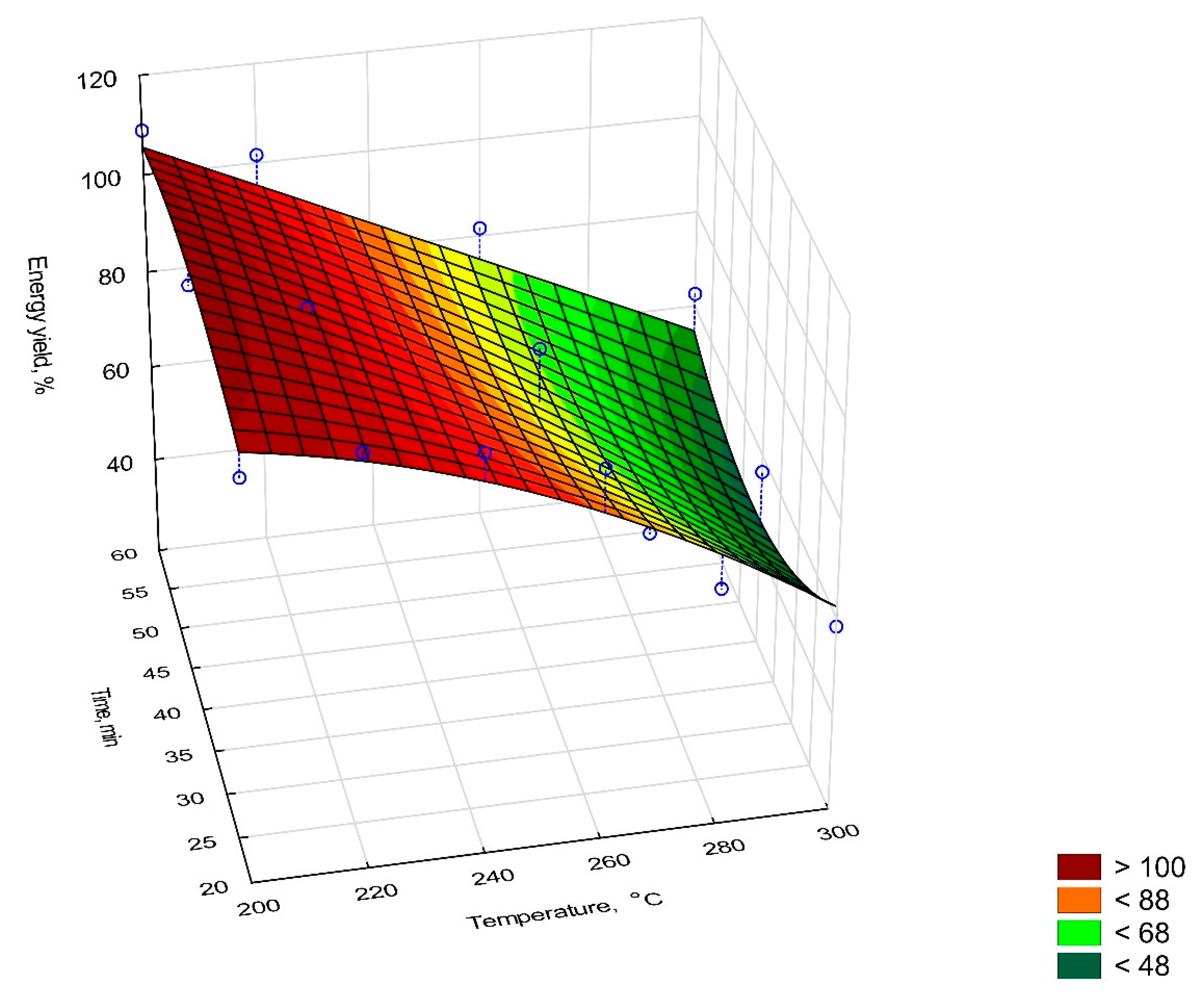
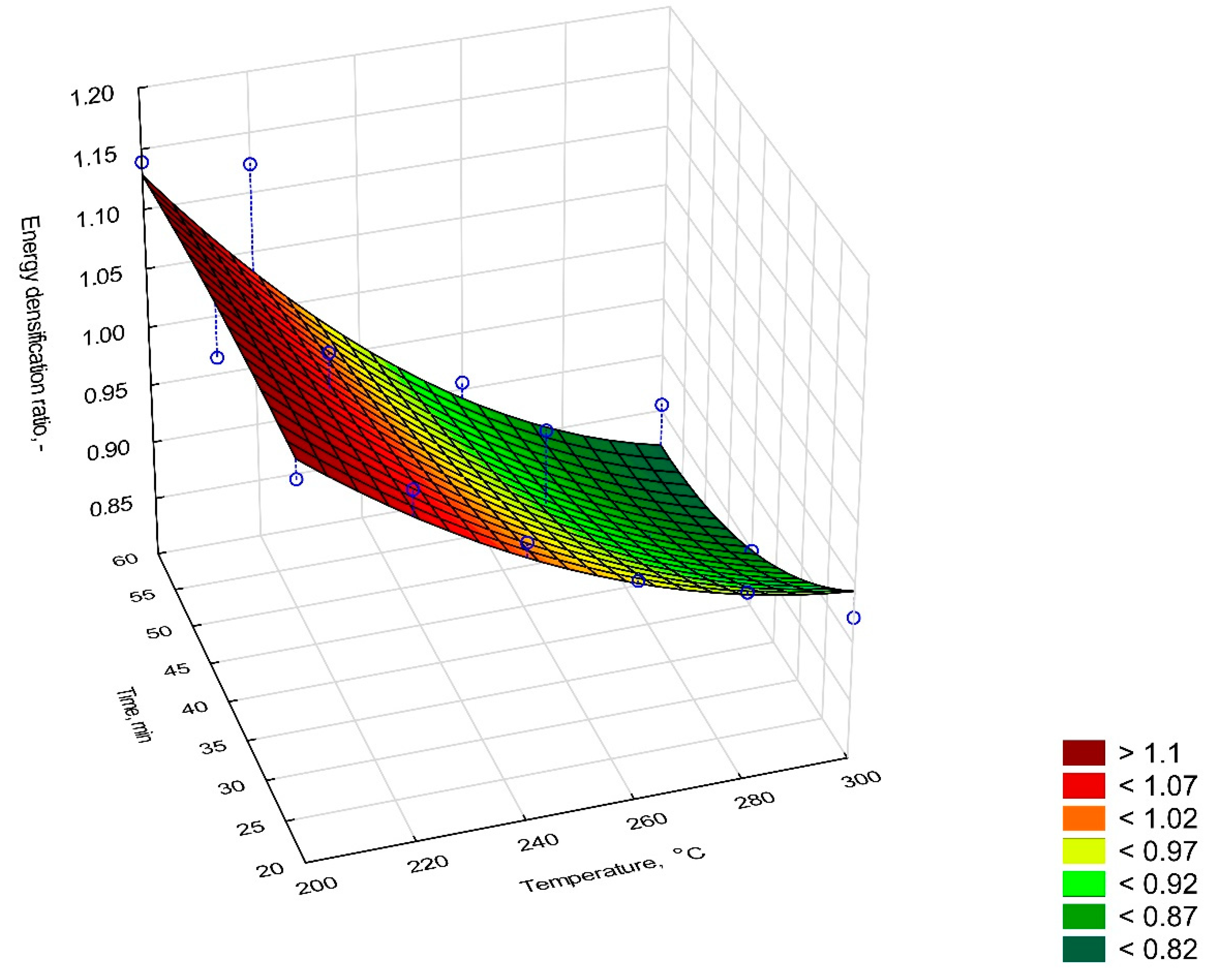
2.6.1. Mass Yield, Energy Densification Ratio, and Energy Yield
- MY—mass yield, %
- ma—the mass of dry elephant dung before torrefaction, g,
- mb—the mass of dry biocoal after torrefaction, g.
- EDr—energy densification ratio, -,
- HHVb—the high heating value of biocoal, J·g−1,
- HHVa—the high heating value of raw elephant dung, MJ·kg−1.
- EY—nergy yield, %,
- MY—mass yield, %
- EDr—energy densification ratio, -,
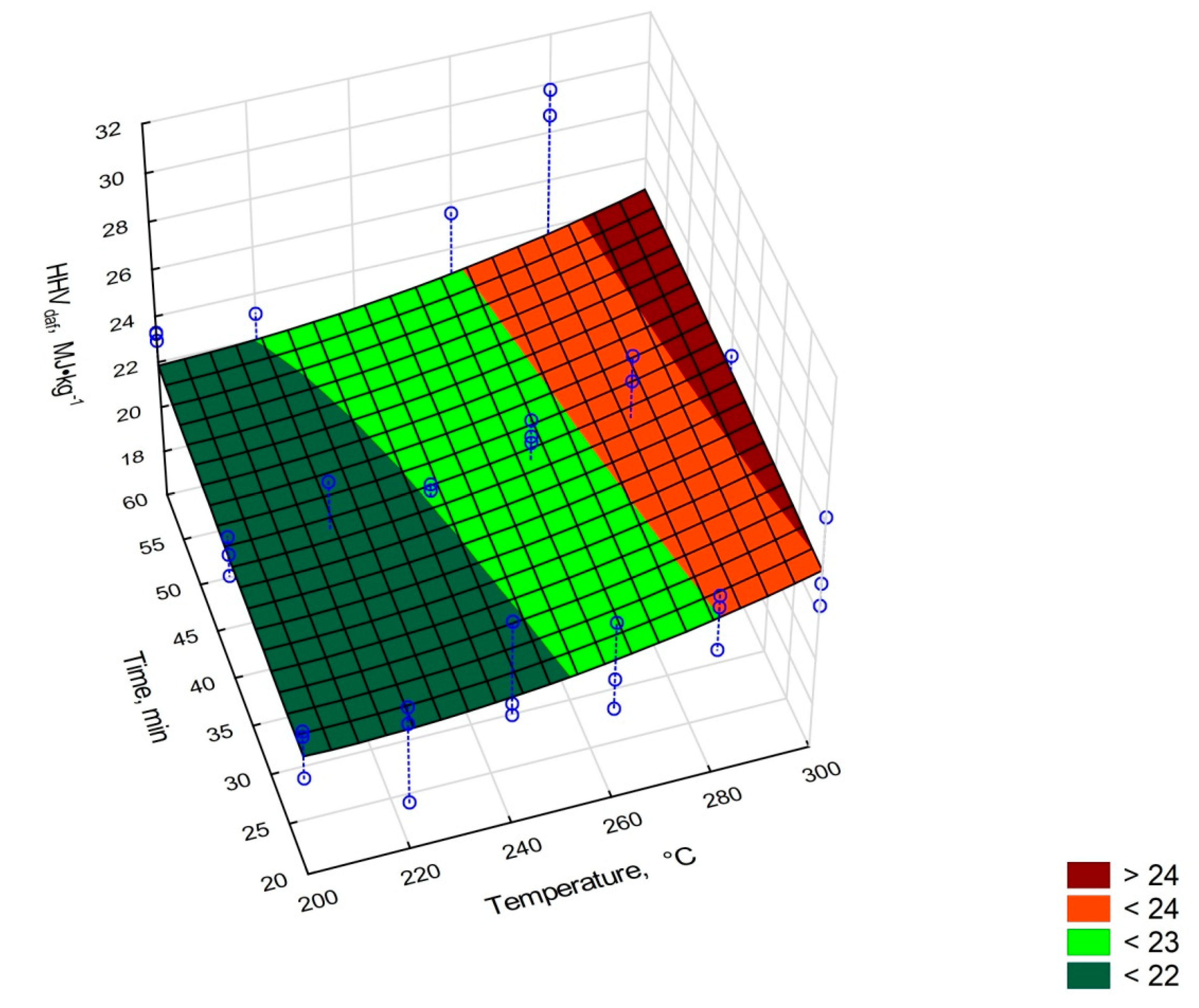
- HHVdaf—high heating value on dry and ash-free base, MJ·kg−1,
- HHV—high heating value, MJ·kg−1,
- Mf—dry mass of fuel, kg,
- Mash—the mass of ash in fuel, kg.
2.6.2. Calculation of Kinetics Parameters (Reaction Rate and Activation Energy)
- ms—mass after time t, g,
- mo—initial mass, g,
- k—the reaction rate constant, s−1,
- t—time, s.
- Ea—activation energy, J∙mol−1,
- a—the coefficient from Equation (6), K,
- R—gas constant, J∙mol−1∙K−1.
2.6.3. Calculation of Energy Demand for Torrefaction of Elephant Dung
- Q—the total amount of heat needed to heat and evaporate water, J,
- m—the mass of water in the sample, g,
- ΔT—the temperature difference between ambient temperature (20 °C) and boiling point (100 °C), under normal pressure conditions, °C,
- cp—specific heat of water, 4.2 J·(g·°C)−1,
- co—the heat of water evaporation, 2257 J·g−1.
2.6.4. Modeling of Torrefaction Process and Biocoal Fuel Properties
- f(T,t)—the property (T, t, & combinations) being analyzed,
- a1—intercept,
- a2–a7—regression coefficient,
- T—process temperature, °C,
- t—process time, min.
- SH—specific heat of elephant dung as a function of temperature, J·(kg·°C)−1,
- a1—intercept,
- a2–a9—regression coefficient,
- T—torrefaction temperature, °C.
2.6.5. Statistical Analysis
3. Results
3.1. Result of the Torrefaction Process
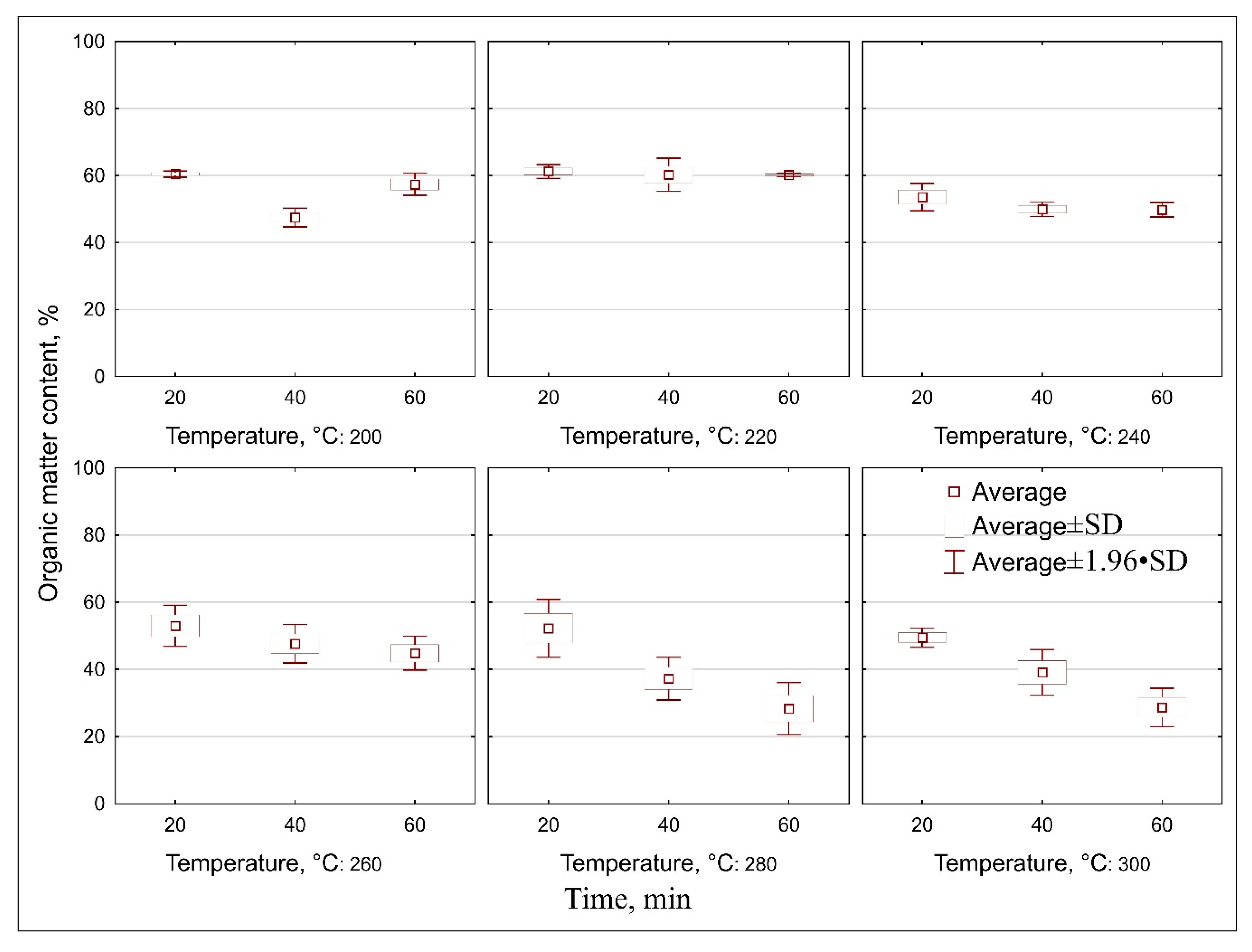
3.2. Result of Proximate Analysis of Raw and Torrefied Elephant Dung
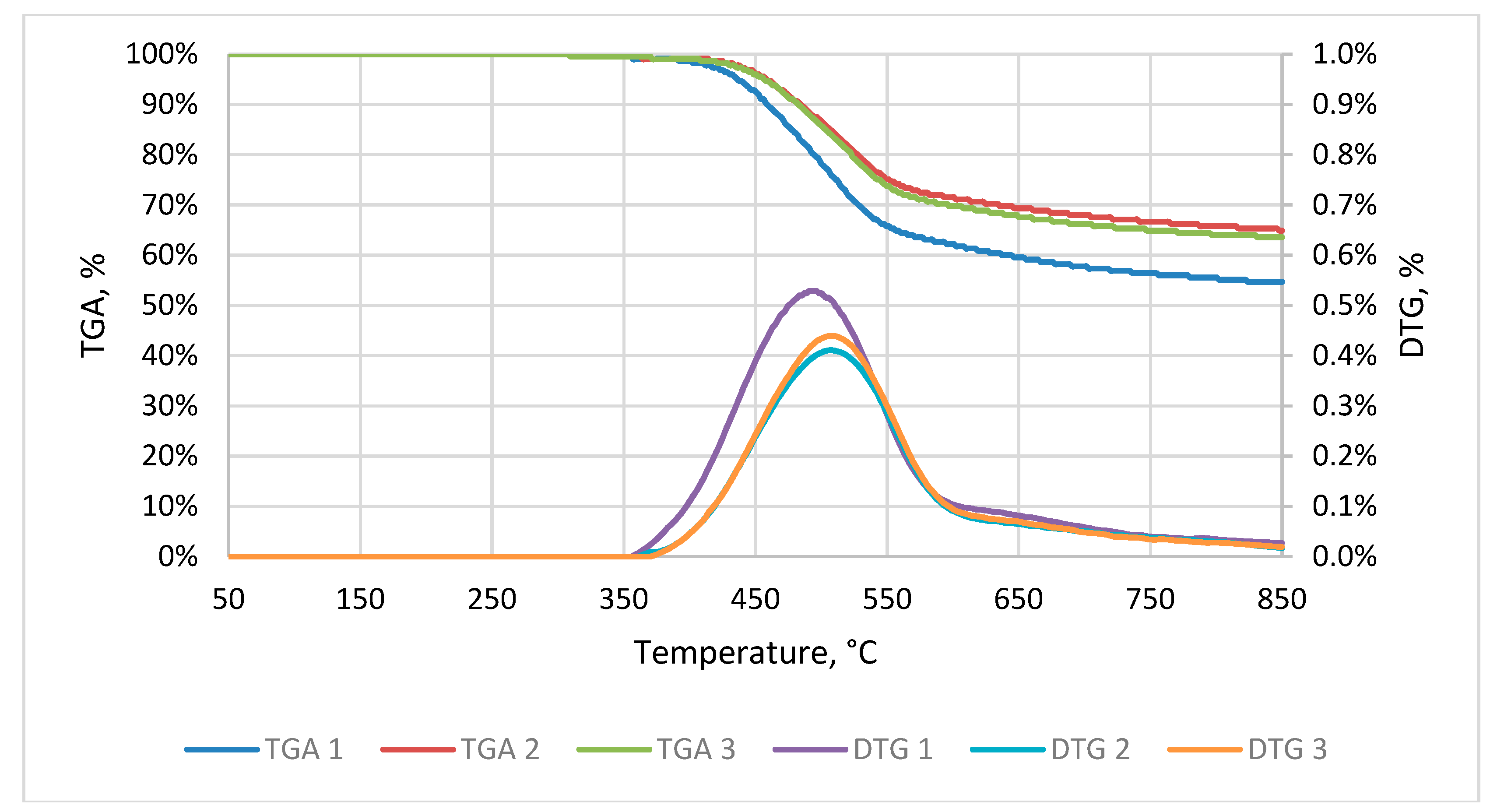
3.3. Result of the Thermogravimetric Analysis (TGA) of Elephant Dung
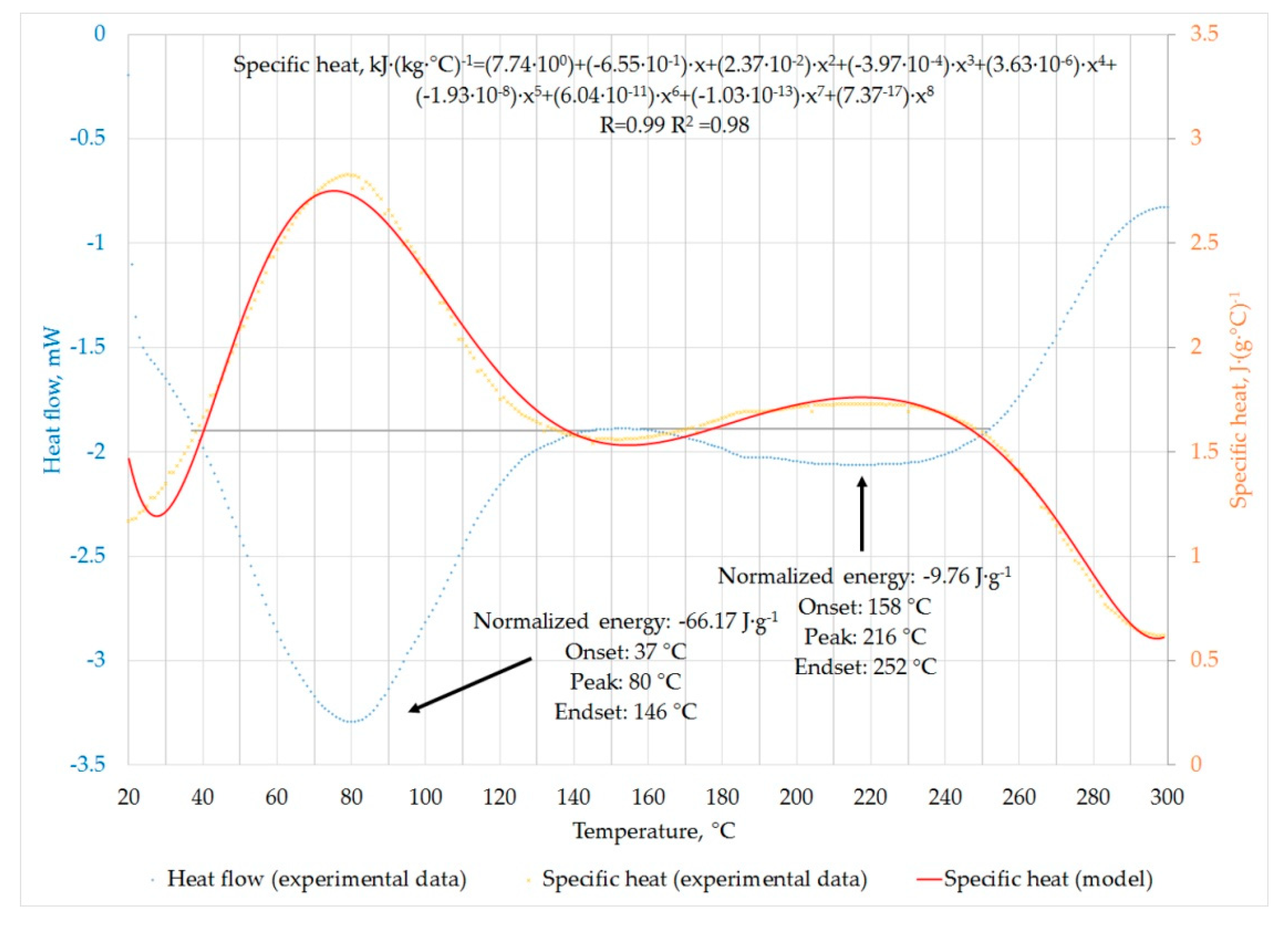
3.4. Differential Scanning Calorimetry (DSC) of Elephant Dung
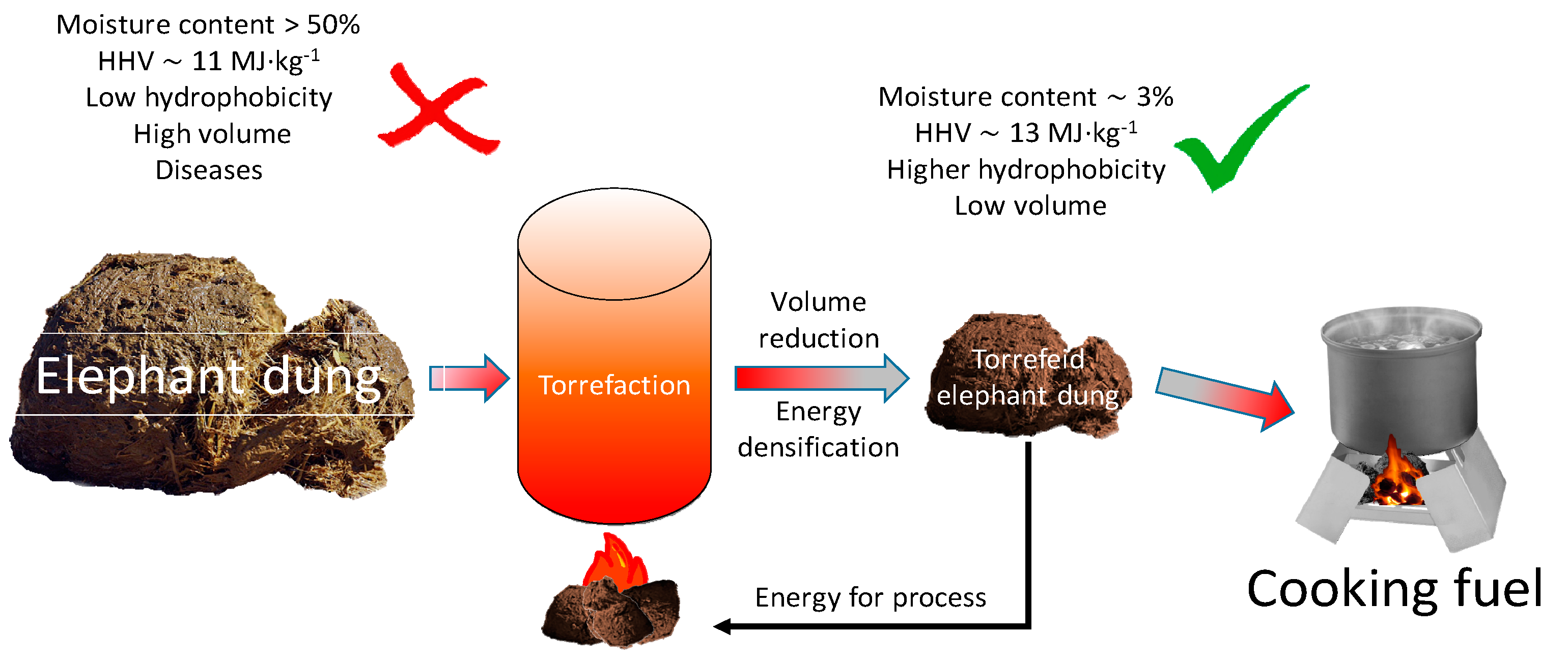
4. Discussion
4.1. The Impact of Technological Parameters on the Efficiency of the Process
4.2. Proximate Analyses of Elephant Dung and Biocoals
4.3. Thermogravimetric Analysis of Raw Material and Kinetic Parameters of Torrefaction
4.4. Differential Scanning Calorimetry of Raw Material
5. Conclusions
- Torrefaction improves the higher heating value of elephant dung. The torrefied elephant dung has an HHV = 13 MJ·kg−1 compared to the HHV = 11.41 MJ·kg−1 for unprocessed dung.
- Minimal process controls appear to be needed, and thus, scaling the torrefaction up to larger batches of dung is feasible, but due to lack of data, these options need more tests on a technical scale. Biocoals with similar quality are obtained for 200 °C to 260 °C range regardless of the duration of the process (20 to 60 min).
- The recommended temperature of the torrefaction for elephant dung is 200 °C, due to the lack of significant improvements in fuel properties with increasing process temperature.
- The activation energy for torrefaction of elephant dung at 200~300 °C was 17.7 J·mol−1, and the reaction rate constant increased from 1.16 × 10−5·s−1 to 2.73 × 10−5·s−1.
- The total energy needed to heat the dry elephant dung from 20 °C to 300 °C was approximately 485 kJ·kg−1 (obtained in laboratory conditions), and 484.81 kJ∙kg−1 (obtained from calculations) after the mass loss during the process is factored in. The total energy demand for drying and torrefaction was the total amount of energy for processing (heating, moisture evaporation, and torrefaction) was 1760.30 kJ·kg−1.
Author Contributions
Funding
Conflicts of Interest
Appendix A
| Sample | Moisture, % | Organic Matter Content, % | Ash, % | HHV, MJ·kg−1 | HHVdaf, MJ·kg−1 | |
|---|---|---|---|---|---|---|
| Elephant dung | 49.19 ± 5.84 | 48.90 ± 5.79 | 50.81 ± 5.84 | 11.41 ± 1.34 | 23.18 ± 2.39 | |
| 200 °C | 20 min | 3.33 ± 0.08 | 60.44 ± 0.46 | 39.37 ± 0.44 | 12.75 ± 0.58 | 21.75 ± 1.15 |
| 40 min | 1.14 ± 0.02 | 47.50 ± 1.42 | 52.40 ± 1.42 | 10.14 ± 0.51 | 21.56 ± 0.87 | |
| 60 min | 2.35 ± 0.08 | 57.35 ± 1.69 | 42.51 ± 1.70 | 13.00 ± 0.31 | 23.16 ± 0.19 | |
| 220 °C | 20 min | 2.11 ± 0.15 | 61.23 ± 1.04 | 38.65 ± 1.05 | 12.47 ± 1.31 | 20.77 ± 2.30 |
| 40 min | 2.15 ± 0.05 | 60.22 ± 2.52 | 39.77 ± 2.50 | 12.34 ± 1.01 | 21.00 ± 2.48 | |
| 60 min | 1.90 ± 0.06 | 60.21 ± 0.27 | 39.76 ± 0.24 | 12.82 ± 0.72 | 21.70 ± 1.24 | |
| 240 °C | 20 min | 2.11 ± 0.12 | 53.57 ± 2.09 | 46.50 ± 2.01 | 11.80 ± 1.56 | 22.48 ± 2.24 |
| 40 min | 1.03 ± 0.04 | 49.91 ± 1.12 | 50.03 ± 1.08 | 10.74 ± 0.79 | 21.71 ± 1.27 | |
| 60 min | 0.96 ± 0.05 | 49.79 ± 1.11 | 50.11 ± 1.13 | 9.51 ± 0.50 | 19.24 ± 0.59 | |
| 260 °C | 20 min | 3.20 ± 0.06 | 52.96 ± 3.14 | 47.52 ± 3.30 | 11.39 ± 0.33 | 21.79 ±1.93 |
| 40 min | 0.88 ± 0.10 | 47.63 ± 2.92 | 52.24 ± 2.97 | 11.25 ± 0.50 | 23.77 ± 0.51 | |
| 60 min | 1.07 ± 0.04 | 44.82 ± 2.58 | 55.07 ± 2.62 | 10.34 ± 0.24 | 23.33 ± 1.93 | |
| 280 °C | 20 min | 2.23 ± 0.15 | 52.21 ± 4.41 | 47.60 ± 4.45 | 11.80 ± 1.45 | 23.00 ± 1.25 |
| 40 min | 2.85 ± 0.26 | 37.23 ± 3.26 | 62.59 ± 3.31 | 8.66 ± 1.22 | 23.87 ± 2.92 | |
| 60 min | 1.64 ± 0.26 | 28.26 ± 3.97 | 71.48 ± 3.99 | 7.54 ± 0.32 | 27.20 ± 3.57 | |
| 300 °C | 20 min | 2.61 ± 0.25 | 49.47 ± 1.47 | 50.21 ± 1.53 | 11.64 ± 1.02 | 24.01 ± 1.99 |
| 40 min | 1.99 ± 0.26 | 39.09 ± 3.47 | 60.89 ± 3.46 | 9.05 ± 0.32 | 23.69 ± 1.25 | |
| 60 min | 1.24 ± 0.14 | 28.66 ± 2.92 | 71.25 ± 2.92 | 6.49 ± 0.71 | 22.86 ± 0.79 | |
| Sample | Mass Yield, % | Energy Yield, % | Energy Densification Ratio, % | |
|---|---|---|---|---|
| 200 °C | 20 min | 91.42 | 102.11 | 1.12 |
| 40 min | 98.65 | 107.78 | 1.09 | |
| 60 min | 95.59 | 108.91 | 1.13 | |
| 220 °C | 20 min | 95.43 | 104.25 | 1.09 |
| 40 min | 93.16 | 100.74 | 1.08 | |
| 60 min | 90.43 | 101.62 | 1.12 | |
| 240 °C | 20 min | 98.12 | 101.43 | 1.03 |
| 40 min | 92.78 | 87.33 | 0.94 | |
| 60 min | 89.36 | 74.46 | 0.83 | |
| 260 °C | 20 min | 97.07 | 95.66 | 0.99 |
| 40 min | 88.63 | 87.34 | 0.99 | |
| 60 min | 90.01 | 81.55 | 0.90 | |
| 280 °C | 20 min | 71.83 | 68.89 | 0.96 |
| 40 min | 53.21 | 46.29 | 0.87 | |
| 60 min | 63.33 | 51.59 | 0.81 | |
| 300 °C | 20 min | 63.28 | 58.28 | 0.92 |
| 40 min | 66.58 | 56.50 | 0.85 | |
| 60 min | 73.18 | 62.58 | 0.86 | |



| Tukey Test for OM; a Bold Font Signifies Statistically Significant Difference (p < 0.05) | 200 | 200 | 200 | 220 | 220 | 220 | 240 | 240 | 240 | 260 | 260 | 260 | 280 | 280 | 280 | 300 | 300 | 300 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 20 | 40 | 60 | 20 | 40 | 60 | 20 | 40 | 60 | 20 | 40 | 60 | 20 | 40 | 60 | 20 | 40 | 60 | ||
| 200 | 20 | 0.00 | 0.98 | 1.00 | 1.00 | 1.00 | 0.12 | 0.00 | 0.00 | 0.06 | 0.00 | 0.00 | 0.03 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | |
| 200 | 40 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.27 | 1.00 | 1.00 | 0.43 | 1.00 | 1.00 | 0.68 | 0.00 | 0.00 | 1.00 | 0.02 | 0.00 | |
| 200 | 60 | 0.98 | 0.00 | 0.90 | 0.99 | 0.99 | 0.91 | 0.07 | 0.06 | 0.77 | 0.00 | 0.00 | 0.53 | 0.00 | 0.00 | 0.04 | 0.00 | 0.00 | |
| 220 | 20 | 1.00 | 0.00 | 0.90 | 1.00 | 1.00 | 0.05 | 0.00 | 0.00 | 0.02 | 0.00 | 0.00 | 0.01 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | |
| 220 | 40 | 1.00 | 0.00 | 0.99 | 1.00 | 1.00 | 0.15 | 0.00 | 0.00 | 0.08 | 0.00 | 0.00 | 0.03 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | |
| 220 | 60 | 1.00 | 0.00 | 0.99 | 1.00 | 1.00 | 0.16 | 0.00 | 0.00 | 0.08 | 0.00 | 0.00 | 0.03 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | |
| 240 | 20 | 0.12 | 0.27 | 0.91 | 0.05 | 0.15 | 0.16 | 0.93 | 0.91 | 1.00 | 0.30 | 0.01 | 1.00 | 0.00 | 0.00 | 0.85 | 0.00 | 0.00 | |
| 240 | 40 | 0.00 | 1.00 | 0.07 | 0.00 | 0.00 | 0.00 | 0.93 | 1.00 | 0.99 | 1.00 | 0.55 | 1.00 | 0.00 | 0.00 | 1.00 | 0.00 | 0.00 | |
| 240 | 60 | 0.00 | 1.00 | 0.06 | 0.00 | 0.00 | 0.00 | 0.91 | 1.00 | 0.98 | 1.00 | 0.59 | 1.00 | 0.00 | 0.00 | 1.00 | 0.00 | 0.00 | |
| 260 | 20 | 0.06 | 0.43 | 0.77 | 0.02 | 0.08 | 0.08 | 1.00 | 0.99 | 0.98 | 0.47 | 0.03 | 1.00 | 0.00 | 0.00 | 0.95 | 0.00 | 0.00 | |
| 260 | 40 | 0.00 | 1.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.30 | 1.00 | 1.00 | 0.47 | 0.99 | 0.72 | 0.00 | 0.00 | 1.00 | 0.02 | 0.00 | |
| 260 | 60 | 0.00 | 1.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.01 | 0.55 | 0.59 | 0.03 | 0.99 | 0.07 | 0.06 | 0.00 | 0.70 | 0.35 | 0.00 | |
| 280 | 20 | 0.03 | 0.68 | 0.53 | 0.01 | 0.03 | 0.03 | 1.00 | 1.00 | 1.00 | 1.00 | 0.72 | 0.07 | 0.00 | 0.00 | 1.00 | 0.00 | 0.00 | |
| 280 | 40 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.06 | 0.00 | 0.01 | 0.00 | 1.00 | 0.02 | |
| 280 | 60 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.01 | 0.00 | 0.00 | 1.00 | |
| 300 | 20 | 0.00 | 1.00 | 0.04 | 0.00 | 0.00 | 0.00 | 0.85 | 1.00 | 1.00 | 0.95 | 1.00 | 0.70 | 1.00 | 0.00 | 0.00 | 0.00 | 0.00 | |
| 300 | 40 | 0.00 | 0.02 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.02 | 0.35 | 0.00 | 1.00 | 0.00 | 0.00 | 0.00 | |
| 300 | 60 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.02 | 1.00 | 0.00 | 0.00 | |
| Tukey Test for Ash Content; a Bold Font Signifies Statistically Significant Difference (p < 0.05) | 200 | 200 | 200 | 220 | 220 | 220 | 240 | 240 | 240 | 260 | 260 | 260 | 280 | 280 | 280 | 300 | 300 | 300 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 20 | 40 | 60 | 20 | 40 | 60 | 20 | 40 | 60 | 20 | 40 | 60 | 20 | 40 | 60 | 20 | 40 | 60 | ||
| 200 | 20 | 0.00 | 0.98 | 1.00 | 1.00 | 1.00 | 0.10 | 0.00 | 0.00 | 0.03 | 0.00 | 0.00 | 0.03 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | |
| 200 | 40 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.32 | 1.00 | 1.00 | 0.64 | 1.00 | 1.00 | 0.66 | 0.00 | 0.00 | 1.00 | 0.02 | 0.00 | |
| 200 | 60 | 0.98 | 0.00 | 0.91 | 1.00 | 1.00 | 0.88 | 0.06 | 0.06 | 0.60 | 0.00 | 0.00 | 0.57 | 0.00 | 0.00 | 0.05 | 0.00 | 0.00 | |
| 220 | 20 | 1.00 | 0.00 | 0.91 | 1.00 | 1.00 | 0.04 | 0.00 | 0.00 | 0.01 | 0.00 | 0.00 | 0.01 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | |
| 220 | 40 | 1.00 | 0.00 | 1.00 | 1.00 | 1.00 | 0.15 | 0.00 | 0.00 | 0.05 | 0.00 | 0.00 | 0.05 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | |
| 220 | 60 | 1.00 | 0.00 | 1.00 | 1.00 | 1.00 | 0.15 | 0.00 | 0.00 | 0.05 | 0.00 | 0.00 | 0.04 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | |
| 240 | 20 | 0.10 | 0.32 | 0.88 | 0.04 | 0.15 | 0.15 | 0.95 | 0.94 | 1.00 | 0.37 | 0.02 | 1.00 | 0.00 | 0.00 | 0.93 | 0.00 | 0.00 | |
| 240 | 40 | 0.00 | 1.00 | 0.06 | 0.00 | 0.00 | 0.00 | 0.95 | 1.00 | 1.00 | 1.00 | 0.59 | 1.00 | 0.00 | 0.00 | 1.00 | 0.00 | 0.00 | |
| 240 | 60 | 0.00 | 1.00 | 0.06 | 0.00 | 0.00 | 0.00 | 0.94 | 1.00 | 1.00 | 1.00 | 0.61 | 1.00 | 0.00 | 0.00 | 1.00 | 0.00 | 0.00 | |
| 260 | 20 | 0.03 | 0.64 | 0.60 | 0.01 | 0.05 | 0.05 | 1.00 | 1.00 | 1.00 | 0.69 | 0.06 | 1.00 | 0.00 | 0.00 | 1.00 | 0.00 | 0.00 | |
| 260 | 40 | 0.00 | 1.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.37 | 1.00 | 1.00 | 0.69 | 0.99 | 0.71 | 0.00 | 0.00 | 1.00 | 0.02 | 0.00 | |
| 260 | 60 | 0.00 | 1.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.02 | 0.59 | 0.61 | 0.06 | 0.99 | 0.07 | 0.06 | 0.00 | 0.65 | 0.35 | 0.00 | |
| 280 | 20 | 0.03 | 0.66 | 0.57 | 0.01 | 0.05 | 0.04 | 1.00 | 1.00 | 1.00 | 1.00 | 0.71 | 0.07 | 0.00 | 0.00 | 1.00 | 0.00 | 0.00 | |
| 280 | 40 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.06 | 0.00 | 0.01 | 0.00 | 1.00 | 0.02 | |
| 280 | 60 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.01 | 0.00 | 0.00 | 1.00 | |
| 300 | 20 | 0.00 | 1.00 | 0.05 | 0.00 | 0.00 | 0.00 | 0.93 | 1.00 | 1.00 | 1.00 | 1.00 | 0.65 | 1.00 | 0.00 | 0.00 | 0.00 | 0.00 | |
| 300 | 40 | 0.00 | 0.02 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.02 | 0.35 | 0.00 | 1.00 | 0.00 | 0.00 | 0.00 | |
| 300 | 60 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.02 | 1.00 | 0.00 | 0.00 | |
| Tukey Test for CP; a Bold Font Signifies Statistically Significant Difference (p < 0.05) | 200 | 200 | 200 | 220 | 220 | 220 | 240 | 240 | 240 | 260 | 260 | 260 | 280 | 280 | 280 | 300 | 300 | 300 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 20 | 40 | 60 | 20 | 40 | 60 | 20 | 40 | 60 | 20 | 40 | 60 | 20 | 40 | 60 | 20 | 40 | 60 | ||
| 200 | 20 | 0.00 | 0.97 | 1.00 | 1.00 | 1.00 | 0.07 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.02 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | |
| 200 | 40 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.25 | 1.00 | 1.00 | 0.00 | 1.00 | 0.99 | 0.58 | 0.00 | 0.00 | 1.00 | 0.01 | 0.00 | |
| 200 | 60 | 0.97 | 0.00 | 0.87 | 0.99 | 0.99 | 0.84 | 0.04 | 0.04 | 0.00 | 0.00 | 0.00 | 0.49 | 0.00 | 0.00 | 0.03 | 0.00 | 0.00 | |
| 220 | 20 | 1.00 | 0.00 | 0.87 | 1.00 | 1.00 | 0.03 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.01 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | |
| 220 | 40 | 1.00 | 0.00 | 0.99 | 1.00 | 1.00 | 0.11 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.03 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | |
| 220 | 60 | 1.00 | 0.00 | 0.99 | 1.00 | 1.00 | 0.11 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.03 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | |
| 240 | 20 | 0.07 | 0.25 | 0.84 | 0.03 | 0.11 | 0.11 | 0.93 | 0.92 | 0.00 | 0.29 | 0.01 | 1.00 | 0.00 | 0.00 | 0.90 | 0.00 | 0.00 | |
| 240 | 40 | 0.00 | 1.00 | 0.04 | 0.00 | 0.00 | 0.00 | 0.93 | 1.00 | 0.00 | 1.00 | 0.51 | 1.00 | 0.00 | 0.00 | 1.00 | 0.00 | 0.00 | |
| 240 | 60 | 0.00 | 1.00 | 0.04 | 0.00 | 0.00 | 0.00 | 0.92 | 1.00 | 0.00 | 1.00 | 0.53 | 1.00 | 0.00 | 0.00 | 1.00 | 0.00 | 0.00 | |
| 260 | 20 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | |
| 260 | 40 | 0.00 | 1.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.29 | 1.00 | 1.00 | 0.00 | 0.99 | 0.64 | 0.00 | 0.00 | 1.00 | 0.01 | 0.00 | |
| 260 | 60 | 0.00 | 0.99 | 0.00 | 0.00 | 0.00 | 0.00 | 0.01 | 0.51 | 0.53 | 0.00 | 0.99 | 0.04 | 0.04 | 0.00 | 0.57 | 0.27 | 0.00 | |
| 280 | 20 | 0.02 | 0.58 | 0.49 | 0.01 | 0.03 | 0.03 | 1.00 | 1.00 | 1.00 | 0.00 | 0.64 | 0.04 | 0.00 | 0.00 | 1.00 | 0.00 | 0.00 | |
| 280 | 40 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.04 | 0.00 | 0.01 | 0.00 | 1.00 | 0.01 | |
| 280 | 60 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.01 | 0.00 | 0.00 | 1.00 | |
| 300 | 20 | 0.00 | 1.00 | 0.03 | 0.00 | 0.00 | 0.00 | 0.90 | 1.00 | 1.00 | 0.00 | 1.00 | 0.57 | 1.00 | 0.00 | 0.00 | 0.00 | 0.00 | |
| 300 | 40 | 0.00 | 0.01 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.01 | 0.27 | 0.00 | 1.00 | 0.00 | 0.00 | 0.00 | |
| 300 | 60 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.01 | 1.00 | 0.00 | 0.00 | |
| Tukey Test for HHV; a Bold Font Signifies Statistically Significant Difference (p < 0.05) | 200 | 200 | 200 | 220 | 220 | 220 | 240 | 240 | 240 | 260 | 260 | 260 | 280 | 280 | 280 | 300 | 300 | 300 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 20 | 40 | 60 | 20 | 40 | 60 | 20 | 40 | 60 | 20 | 40 | 60 | 20 | 40 | 60 | 20 | 40 | 60 | ||
| 200 | 20 | 0.05 | 1.00 | 1.00 | 1.00 | 1.00 | 0.99 | 0.31 | 0.00 | 0.87 | 0.76 | 0.10 | 0.99 | 0.00 | 0.00 | 0.97 | 0.00 | 0.00 | |
| 200 | 40 | 0.05 | 0.02 | 0.13 | 0.18 | 0.04 | 0.62 | 1.00 | 1.00 | 0.93 | 0.97 | 1.00 | 0.62 | 0.78 | 0.05 | 0.77 | 0.98 | 0.00 | |
| 200 | 60 | 1.00 | 0.02 | 1.00 | 1.00 | 1.00 | 0.95 | 0.15 | 0.00 | 0.66 | 0.52 | 0.04 | 0.95 | 0.00 | 0.00 | 0.87 | 0.00 | 0.00 | |
| 220 | 20 | 1.00 | 0.13 | 1.00 | 1.00 | 1.00 | 1.00 | 0.55 | 0.01 | 0.98 | 0.94 | 0.22 | 1.00 | 0.00 | 0.00 | 1.00 | 0.00 | 0.00 | |
| 220 | 40 | 1.00 | 0.18 | 1.00 | 1.00 | 1.00 | 1.00 | 0.68 | 0.02 | 0.99 | 0.98 | 0.31 | 1.00 | 0.00 | 0.00 | 1.00 | 0.00 | 0.00 | |
| 220 | 60 | 1.00 | 0.04 | 1.00 | 1.00 | 1.00 | 0.99 | 0.25 | 0.00 | 0.82 | 0.69 | 0.07 | 0.99 | 0.00 | 0.00 | 0.95 | 0.00 | 0.00 | |
| 240 | 20 | 0.99 | 0.62 | 0.95 | 1.00 | 1.00 | 0.99 | 0.98 | 0.14 | 1.00 | 1.00 | 0.80 | 1.00 | 0.01 | 0.00 | 1.00 | 0.03 | 0.00 | |
| 240 | 40 | 0.31 | 1.00 | 0.15 | 0.55 | 0.68 | 0.25 | 0.98 | 0.94 | 1.00 | 1.00 | 1.00 | 0.98 | 0.25 | 0.01 | 1.00 | 0.59 | 0.00 | |
| 240 | 60 | 0.00 | 1.00 | 0.00 | 0.01 | 0.02 | 0.00 | 0.14 | 0.94 | 0.41 | 0.54 | 1.00 | 0.14 | 1.00 | 0.33 | 0.22 | 1.00 | 0.01 | |
| 260 | 20 | 0.87 | 0.93 | 0.66 | 0.98 | 0.99 | 0.82 | 1.00 | 1.00 | 0.41 | 1.00 | 0.98 | 1.00 | 0.03 | 0.00 | 1.00 | 0.12 | 0.00 | |
| 260 | 40 | 0.76 | 0.97 | 0.52 | 0.94 | 0.98 | 0.69 | 1.00 | 1.00 | 0.54 | 1.00 | 1.00 | 1.00 | 0.05 | 0.00 | 1.00 | 0.18 | 0.00 | |
| 260 | 60 | 0.10 | 1.00 | 0.04 | 0.22 | 0.31 | 0.07 | 0.80 | 1.00 | 1.00 | 0.98 | 1.00 | 0.79 | 0.60 | 0.02 | 0.90 | 0.91 | 0.00 | |
| 280 | 20 | 0.99 | 0.62 | 0.95 | 1.00 | 1.00 | 0.99 | 1.00 | 0.98 | 0.14 | 1.00 | 1.00 | 0.79 | 0.01 | 0.00 | 1.00 | 0.03 | 0.00 | |
| 280 | 40 | 0.00 | 0.78 | 0.00 | 0.00 | 0.00 | 0.00 | 0.01 | 0.25 | 1.00 | 0.03 | 0.05 | 0.60 | 0.01 | 0.97 | 0.01 | 1.00 | 0.20 | |
| 280 | 60 | 0.00 | 0.05 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.01 | 0.33 | 0.00 | 0.00 | 0.02 | 0.00 | 0.97 | 0.00 | 0.75 | 0.98 | |
| 300 | 20 | 0.97 | 0.77 | 0.87 | 1.00 | 1.00 | 0.95 | 1.00 | 1.00 | 0.22 | 1.00 | 1.00 | 0.90 | 1.00 | 0.01 | 0.00 | 0.05 | 0.00 | |
| 300 | 40 | 0.00 | 0.98 | 0.00 | 0.00 | 0.00 | 0.00 | 0.03 | 0.59 | 1.00 | 0.12 | 0.18 | 0.91 | 0.03 | 1.00 | 0.75 | 0.05 | 0.06 | |
| 300 | 60 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.01 | 0.00 | 0.00 | 0.00 | 0.00 | 0.20 | 0.98 | 0.00 | 0.06 | |
| Intercept/Coefficient | Value of Intercept/Coefficient | Standard Error | p | Lower Limit of Confidence | Upper Limit of Confidence |
|---|---|---|---|---|---|
| a1 | 7.74 × 100 | 2.64 × 10−1 | 0.00 | 6.59 × 100 | 7.62 × 100 |
| a2 | −6.55 × 10−1 | 2.37 × 10−2 | 0.00 | −6.55 × 10−1 | −5.62 × 10−1 |
| a3 | 2.37 × 10−2 | 8.21 × 10−4 | 0.00 | 2.09 × 10−2 | 2.41 × 10−2 |
| a4 | −3.97 × 10−4 | 1.45 × 10−5 | 0.00 | −4.11 × 10−4 | −3.54 × 10−4 |
| a5 | 3.63 × 10−6 | 0.00 × 100 | 0.00 | 3.53 × 10−6 | 3.53 × 10−6 |
| a6 | −1.93 × 10−8 | 0.00 × 100 | 0.00 | −1.90 × 10−8 | −1.90 × 10−8 |
| a7 | 6.04 × 10−11 | 0.00 × 100 | 0.00 | 5.97 × 10−11 | 5.97 × 10−11 |
| a8 | −1.03 × 10−13 | 0.00 × 100 | 0.00 | −1.02 × 10−13 | −1.02 × 10−13 |
| a9 | 7.37 × 10−17 | 0.00 × 100 | 0.00 | 7.37 × 10−17 | 7.37 × 10−17 |
| Type of Pellet | Ash, % | HHV, MJ·kg−1 | HHVdaf *, MJ·kg−1 |
|---|---|---|---|
| Pine sawdust | 0.66 | 19.52 | 19.65 |
| Wheat straw | 7.27 | 17.57 | 18.95 |
| Corn settlements | 1.27 | 18.80 | 19.04 |
| Agricultural residues | 8.27 | 18.13 | 19.76 |
References
- Brown, J.L.; Paris, S.; Prado-Oviedo, N.A.; Meehan, C.L.; Hogan, J.N.; Morfeld, K.A.; Carlstead, K. Reproductive Health Assessment of Female Elephants in North American Zoos and Association of Husbandry Practices with Reproductive Dysfunction in African Elephants (Loxodonta africana). PLoS ONE 2016, 11, e0145673. [Google Scholar] [CrossRef] [PubMed]
- Farah, N.; Amna, M.; Naila, Y.; Ishtiaq, R. Processing of Elephant Dung and its Utilization as a Raw Material for Making Exotic Paper. Res. J. Chem. Sci. 2014, 4, 94–103. [Google Scholar]
- Sannigrahi, A.K. Beneficial Utilization Of Elephant Dung Through Vermicomposting. Int. J. Recent Sci. Res. 2015, 6, 4814–4817. [Google Scholar]
- Elephants for Africa. Available online: http://www.elephantsforafrica.org/elephant-facts/ (accessed on 18 August 2019).
- Schröder, J.J.; Scholefield, D.; Cabral, F.; Hofman, G. The effects of nutrient losses from agriculture on ground and surface water quality: The position of science in developing indicators for regulation. Environ. Sci. Policy 2004, 7, 15–23. [Google Scholar] [CrossRef]
- Pérez-Godínez, E.A.; Lagunes-Zarate, J.; Corona-Hernández, J.; Barajas-Aceves, M. Growth and reproductive potential of Eisenia foetida (Sav) on various zoo animal dungs after two methods of pre-composting followed by vermicomposting. Waste Manag. 2017, 64, 67–78. [Google Scholar] [CrossRef]
- Zhou, S.; Liang, H.; Han, L.; Huang, G.; Yang, Z. The influence of manure feedstock, slow pyrolysis, and hydrothermal temperature on manure thermochemical and combustion properties. Waste Manag. 2019, 88, 85–95. [Google Scholar] [CrossRef]
- Qambrani, N.A.; Rahman, M.M.; Won, S.; Shim, S.; Ra, C. Biochar properties and eco-friendly applications for climate change mitigation, waste management, and wastewater treatment: A review. Renew. Sustain. Energy Rev. 2017, 79, 255–273. [Google Scholar] [CrossRef]
- Aira, M.; Monroy, F.; Domínguez, J.; Mato, S. How earthworm density affects microbial biomas and activity in pig manure. Eur. J. Soil Biol. 2002, 38, 7–10. [Google Scholar] [CrossRef]
- Sukasem, N.; Khanthi, K.; Prayoonkham, S. Biomethane Recovery from Fresh and Dry Water Hyacinth Anaerobic Co-Digestion with Pig Dung, Elephant Dung and Bat Dung with Different Alkali Pretreatments. Energy Procedia 2017, 138, 294–300. [Google Scholar] [CrossRef]
- Klasson, K.T.; Nghiem, N. Energy Production from Zoo Animal Wastes; Oak Ridge National Laboratory: Oak Ridge, TN, USA, 2003; Volume 3, pp. 1–8. [Google Scholar] [CrossRef]
- Fangkum, A.; Reungsang, A. Simultaneous saccharification and fermentation of cellulose for bio-hydrogen production by anaerobic mixed cultures in elephant dung. Int. J. Hydrogen Energy 2014, 39, 9028–9035. [Google Scholar] [CrossRef]
- Fangkum, A.; Reungsang, A. Biohydrogen production from mixed xylose/arabinose at thermophilic temperature by anaerobic mixed cultures in elephant dung. Int. J. Hydrogen Energy 2011, 36, 13928–13938. [Google Scholar] [CrossRef]
- Moosophin, K.; Phachan, N.; Apiraksakorn, J. Screening of cellulolytic clostridia from animal dung and compost for direct butanol production from cellulosic materials. Curr. Opin. Biotechnol. 2013, 24, 48–143. [Google Scholar] [CrossRef]
- Li, J.; Xiao, F.; Zhang, L.; Amirkhanian, S.N. Life cycle assessment and life cycle cost analysis of recycled solid waste materials in highway pavement: A review. J. Clean. Prod. 2019, 233, 1182–1206. [Google Scholar] [CrossRef]
- Jia, J.; Shu, L.; Zang, G.; Xu, L.; Abudula, A.; Ge, K. Energy analysis of a co-gasification of woody biomass and animal manure, solid oxide fuel cells and micro gas turbine hybrid system. Energy 2018, 149, 750–761. [Google Scholar] [CrossRef]
- Das, K.; Hiloidhari, M.; Baruah, D.C.; Nonhebel, S. Impact of Time Expenditure on Household Preferences for Cooking Fuels. Energy 2018, 151, 309–316. [Google Scholar] [CrossRef]
- International Monetary Fund Report for Selected Countries and Subjects. Available online: https://data.worldbank.org/indicator/SP.POP.TOTL?name_desc=false (accessed on 23 August 2019).
- Ji, C.; Cheng, K.; Nayak, D.; Pan, G. Environmental and economic assessment of crop residue competitive utilization for biochar, briquette fuel and combined heat and power generation. J. Clean. Prod. 2018, 192, 916–923. [Google Scholar] [CrossRef]
- Kamara, J.; Galukande, M.; Maeda, F.; Luboga, S.; Renzaho, A. Understanding the Challenges of Improving Sanitation and Hygiene Outcomes in a Community Based Intervention: A Cross-Sectional Study in Rural Tanzania. Int. J. Environ. Res. Public Health 2017, 14, 602. [Google Scholar] [CrossRef]
- Białowiec, A.; Micuda, M.; Szumny, A.; Łyczko, J.; Koziel, J.A. Waste to Carbon: Influence of Structural Modification on VOC Emission Kinetics from Stored Carbonized Refuse-Derived Fuel. Sustainability 2019, 11, 935. [Google Scholar] [CrossRef]
- Stępień, P.; Białowiec, A. Kinetic Parameters of Torrefaction Process of Alternative Fuel Produced From Municipal Solid Waste and Characteristic of Carbonized Refuse Derived Fuel. Detritus 2018, 3, 75–83. [Google Scholar] [CrossRef]
- Waste Characteristics. Calculation of Dry Mass on the Basis of Dry Residue or Water Content. Polish standard PN-EN 14346:2011. Available online: https://infostore.saiglobal.com/enau/Standards/PN-EN-14346-2011-932471_SAIG_PKN_PKN_2197939/ (accessed on 23 August 2019).
- Waste Characteristics. Content of Organic Matter. Polish Standard PN-EN 15169:2011. Available online: http://sklep.pkn.pl/pn-en-15169-2011p.html (accessed on 23 August 2019).
- Municipal Solid Waste. Combustible and Non-Combustible Content. Polish Standard PN-Z-15008-04:1993. Available online: http://sklep.pkn.pl/pn-z-15008-04-1993p.html (accessed on 23 August 2019).
- Solid Fuels. Determination of the Higher Heating Value and the Lower Heating Value. Polish Standard PN-G-04513:1981. Available online: http://sklep.pkn.pl/pn-g-04513-1981p.html (accessed on 23 August 2019).
- Chin, K.L.; H’ng, P.S.; Go, W.Z.; Wong, W.Z.; Lim, T.W.; Maminski, M.; Paridah, M.T.; Luqman, A.C. Optimization of torrefaction conditions for high energy density solid biofuel from oil palm biomass and fast growing species available in Malaysia. Ind. Crops Prod. 2013, 49, 768–774. [Google Scholar] [CrossRef]
- Pulka, J.; Wiśniewski, D.; Gołaszewski, J.; Białowiec, A. Is the biochar produced from sewage sludge a good quality solid fuel? Arch. Environ. Prot. 2016, 42, 125–134. [Google Scholar] [CrossRef]
- Peleg, M.; Normand, M.D.; Corradini, M.G. The Arrhenius Equation Revisited. Crit. Rev. Food Sci. Nutr. 2012, 52, 830–851. [Google Scholar] [CrossRef] [PubMed]
- Stępień, P.; Serowik, M.; Koziel, J.A.; Białowiec, A. Waste to carbon energy demand model and data based on the TGA and DSC analysis of individual MSW components. Data 2019, 4, 53. [Google Scholar] [CrossRef]
- Stępień, P.; Mysior, M.; Białowiec, A. Technical and technological problems and potential application waste torrefaction. In Innovations in Waste Management—Selected Issues; Manczarski, P., Ed.; Wydawnictwo Uniwersytetu Przyrodniczego: Wrocław, Poland, 2018; Volume 1, pp. 59–78. Available online: https://www.researchgate.net/publication/325367684_Innowacje_w_gospodarce_odpadami_Zagadnienia_wybrane (accessed on 23 August 2019).
- Świechowski, K.; Liszewski, M.; Bąbelewski, P.; Koziel, J.A.; Białowiec, A. Oxytree Pruned Biomass Torrefaction: Mathematical Models of the Influence of Temperature and Residence Time on Fuel Properties Improvement. Materials 2019, 12, 2228. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Harris, S.; Anandhi, A.; Chen, G. Predicting biochar properties and functions based on feedstock and pyrolysis temperature: A review and data syntheses. J. Clean. Prod. 2019, 215, 890–902. [Google Scholar] [CrossRef]
- Kim, D.; Lee, K.; Park, K.Y. Upgrading the characteristics of biochar from cellulose, lignin, and xylan for solid biofuel production from biomass by hydrothermal carbonization. J. Ind. Eng. Chem. 2016, 42, 95–100. [Google Scholar] [CrossRef]
- Gascó, G.; Paz-Ferreiro, J.; Álvarez, M.L.; Saa, A.; Méndez, A. Biochars and hydrochars prepared by pyrolysis and hydrothermal carbonisation of pig manure. Waste Manag. 2018, 79, 395–403. [Google Scholar] [CrossRef]
- Van der Stelt, M.J.C. Chemistry and Reaction Kinetics of Biowaste Torrezfaction; Technische Universiteit Eindhoven: Eindhoven, The Netherlands, 2011. [Google Scholar] [CrossRef]
- Pahla, G.; Mamvura, T.A.; Ntuli, F.; Muzenda, E. Energy densification of animal waste lignocellulose biomass and raw biomass. S. Afr. J. Chem. Eng. 2017, 24, 168–175. [Google Scholar] [CrossRef]
- Higgins, M.J.; Adams, G.; Chen, Y.-C.; Erdal, Z.; Forbes, R.H.; Glindemann, D.; Hargreaves, J.R.; McEwen, D.; Murthy, S.N.; Novak, J.T.; et al. Role of protein, amino acids, and enzyme activity on odor production from anaerobically digested and dewatered biosolids. Water Environ. Res. 2008, 80, 127–135. [Google Scholar] [CrossRef]
- Lehmann, J.; Joseph, S. Biochar for Environmental Management: Science and Technology; Earthscan: London, UK, 2009; ISBN 184407658X. [Google Scholar]
- Ro, K.S.; Libra, J.A.; Bae, S.; Berge, N.D.; Flora, J.R.V.; Pecenka, R. Combustion Behavior of Animal-Manure-Based Hydrochar and Pyrochar. ACS Sustain. Chem. Eng. 2019, 7, 470–478. [Google Scholar] [CrossRef]
- Avellone, E.A.; Baumeister, T.; Saunders, H. Marks Standard Handbook for Mechanical Engineers; McGraw-Hill Education: New York, NY, USA, 2008; pp. 101–105. [Google Scholar]
- Oshita, K.; Toda, S.; Takaoka, M.; Kanda, H.; Fujimori, T.; Matsukawa, K.; Fujiwara, T. Solid fuel production from cattle manure by dewatering using liquefied dimethyl ether. Fuel 2015, 159, 7–14. [Google Scholar] [CrossRef]
- Dębska, A.; Koziołek, S.; Bieniek, J.; Białowiec, A. Potencjał produkcji biogazu z odpadów we wrocławskim Ogrodzie Zoologicznym. Annu. Set Environ. Prot. 2016, 18, 337–351. [Google Scholar]
- Matthiessen, M.K.; Larney, F.J.; Selinger, L.B.; Olson, A.F. Influence of Loss-on-Ignition Temperature and Heating Time on Ash Content of Compost and Manure. Commun. Soil Sci. Plant Anal. 2005, 36, 2561–2573. [Google Scholar] [CrossRef]
- Świechowski, K.; Koziel, J.A.; Liszewski, M.; Bąbelewski, P.; Białowiec, A. Fuel Properties of Torrefied Biomass from Pruning of Oxytree. Data 2019, 4, 55. [Google Scholar] [CrossRef]
- Dudek, M.; Świechowski, K.; Manczarski, P.; Koziel, J.A.; Białowiec, A. The Effect of Biochar Addition on the Biogas Production Kinetics from the Anaerobic Digestion of Brewers’ Spent Grain. Energies 2019, 12, 1518. [Google Scholar] [CrossRef]
- Gautam, S.; Edwards, R.; Yadav, A.; Weltman, R.; Pillarsetti, A.; Arora, N.K.; Smith, K.R. Probe-based measurements of moisture in dung fuel for emissions measurements. Energy Sustain. Dev. 2016, 35, 1–6. [Google Scholar] [CrossRef]
- Lang, Q.; Guo, Y.; Zheng, Q.; Liu, Z.; Gai, C. Co-hydrothermal carbonization of lignocellulosic biomass and swine manure: Hydrochar properties and heavy metal transformation behavior. Bioresour. Technol. 2018, 266, 242–248. [Google Scholar] [CrossRef]
- Rousset, P.; Aguiar, C.; Labbé, N.; Commandré, J.-M. Enhancing the combustible properties of bamboo by torrefaction. Bioresour. Technol. 2011, 102, 8225–8231. [Google Scholar] [CrossRef]
- Bach, Q.-V.; Skreiberg, Ø. Upgrading biomass fuels via wet torrefaction: A review and comparison with dry torrefaction. Renew. Sustain. Energy Rev. 2016, 54, 665–677. [Google Scholar] [CrossRef]
- Dyjakon, A.; Noszczyk, T. The Influence of Freezing Temperature Storage on the Mechanical Durability of Commercial Pellets from Biomass. Energies 2019, 12, 2627. [Google Scholar] [CrossRef]
- Lacey, J.A.; Aston, J.E.; Westover, T.L.; Cherry, R.S.; Thompson, D.N. Removal of introduced inorganic content from chipped forest residues via air classification. Fuel 2015, 160, 265–273. [Google Scholar] [CrossRef]
- Várhegyi, G.; Bobály, B.; Jakab, E.; Chen, H. Thermogravimetric study of biomass pyrolysis kinetics. A distributed activation energy model with prediction tests. Energy Fuels 2011, 25, 24–32. [Google Scholar] [CrossRef]
- Bach, V.; Tran, K.-Q. Dry and wet torrefaction of woody biomass—A comparative study on combustion kinetics. Energy Proceedia 2015, 75, 150–155. [Google Scholar] [CrossRef]
- Soria-Verdugo, A.; Goos, E.; García-Hernando, N. Effect of the number of TGA curves employed on the biomass pyrolysis kinetics results obtained using the Distributed Activation Energy Model. Fuel Process. Technol. 2015, 134, 360–371. [Google Scholar] [CrossRef]
- Pulka, J.; Manczarski, P.; Koziel, J.A.; Białowiec, A. Torrefaction of Sewage Sludge: Kinetics and Fuel Properties of Biochars. Energies 2019, 12, 565. [Google Scholar] [CrossRef]
- Syguła, E.; Koziel, J.A.; Białowiec, A. Proof-of-Concept of Spent Mushrooms Compost Torrefaction—Studying the Process Kinetics and the Influence of Temperature and Duration on the Calorific Value of the Produced Biocoal. Energies 2019, 12, 3060. [Google Scholar] [CrossRef]
- Basak, S.; Samanta, K.K. Thermal behaviour and the cone calorimetric analysis of the jute fabric treated in different pH condition. J. Therm. Anal. Calorim. 2019, 135, 3095–3105. [Google Scholar] [CrossRef]
- Wang, J.; Wang, G.; Zhang, M.; Chen, M.; Li, D.; Min, F.; Chen, M.; Zhang, S.; Ren, Z.; Yan, Y. A comparative study of thermolysis characteristics and kinetics of seaweeds and fir wood. Process Biochem. 2006, 41, 1883–1886. [Google Scholar] [CrossRef]
- Said, N.; Abdel daiem, M.M.; García-Maraver, A.; Zamorano, M. Reduction of Ash Sintering Precursor Components in Rice Straw by Water Washing. BioResources 2014, 9, 6756–6764. [Google Scholar] [CrossRef]
- Burhenne, L.; Messmer, J.; Aicher, T.; Laborie, M.-P. The effect of the biomass components lignin, cellulose and hemicellulose on TGA and fixed bed pyrolysis. J. Anal. Appl. Pyrolysis 2013, 101, 177–184. [Google Scholar] [CrossRef]




| Intercept/Coefficient | Value of Intercept/Coefficient | Standard Error | p | Lower Limit of Confidence | Upper Limit of Confidence |
|---|---|---|---|---|---|
| a1 | −2.58 × 102 | 2.18 × 102 | 0.00 | −7.38 × 102 | 2.22 × 102 |
| a2 | 2.72 × 100 | 1.44 × 100 | 0.00 | −4.52 × 10−-1 | 5.89 × 100 |
| a3 | −5.12 × 10−3 | 2.39 × 10−3 | 0.00 | −1.04 × 10−2 | 1.41 × 10−4 |
| a4 | 6.91 × 100 | 6.19 × 100 | 0.00 | −6.71 × 100 | 2.05 × 101 |
| a5 | −4.22 × 10−2 | 4.01 × 10−2 | 0.00 | −1.30 × 10−1 | 4.61 × 10−2 |
| a6 | −3.01 × 10−2 | 2.45 × 10−2 | 0.00 | −8.40 × 10−2 | 2.38 × 10−2 |
| a7 | 7.70 × 10−7 | 0.00 × 100 | 0.00 | 7.70 × 10−7 | 7.70 × 10−7 |
| Intercept/Coefficient | Value of Intercept/Coefficient | Standard Error | p | Lower Limit of Confidence | Upper Limit of Confidence |
|---|---|---|---|---|---|
| a1 | −1.19 × 102 | 2.48 × 102 | 0.00 | −6.65 × 102 | 4.27 × 102 |
| a2 | 1.69 × 100 | 1.64 × 100 | 0.00 | −1.91 × 100 | 5.30 × 100 |
| a3 | −3.17 × 10−3 | 2.72 × 10−3 | 0.00 | −9.16 × 10−3 | 2.82 × 10−3 |
| a4 | 8.26 × 100 | 7.04 × 100 | 0.00 | −7.23 × 100 | 2.38 × 101 |
| a5 | −4.74 × 10−2 | 4.56 × 10−2 | 0.00 | −1.48 × 10−1 | 5.30 × 10−2 |
| a6 | −3.65 × 10−2 | 2.79 × 10−2 | 0.00 | −9.78 × 10−2 | 2.48 × 10−2 |
| a7 | 8.73 × 10−7 | 0.00 × 100 | 0.00 | 8.73 × 10−7 | 8.73 × 10−7 |
| Intercept/Coefficient | Value of Intercept/Coefficient | Standard Error | p | Lower Limit of Confidence | Upper Limit of Confidence |
|---|---|---|---|---|---|
| a1 | 1.99 × 100 | 1.38 × 100 | 0.00 | −1.05 × 100 | 5.04 × 100 |
| a2 | −7.21 × 10−3 | 9.14 × 10−3 | 0.00 | −2.73 × 10−2 | 1.29 × 10−2 |
| a3 | 1.41 × 10−5 | 1.52 × 10−5 | 0.00 | −1.93 × 10−5 | 4.75 × 10−5 |
| a4 | 2.23 × 10−2 | 3.93 × 10−2 | 0.00 | −6.41 × 10−2 | 1.09 × 10−1 |
| a5 | −9.11 × 10−5 | 2.54 × 10−4 | 0.00 | −6.51 × 10−4 | 4.69 × 10−4 |
| a6 | −1.06 × 10−4 | 1.55 × 104 | 0.00 | −4.48 × 10−4 | 2.36 × 10−4 |
| a7 | 1.90 × 10−9 | 0.00 × 100 | 0.00 | 1.90 × 10−9 | 1.90 × 10−9 |
| Intercept/Coefficient | Value of Intercept/Coefficient | Standard Error | p | Lower Limit of Confidence | Upper Limit of Confidence |
|---|---|---|---|---|---|
| a1 | 9.74 × 101 | 6.33 × 101 | 0.00 | −3.00 × 101 | 2.25 × 102 |
| a2 | 1.24 × 10−2 | 4.18 × 10−1 | 0.00 | −8.29 × 10−1 | 8.54 × 10−1 |
| a3 | −4.95 × 10−4 | 6.95 × 10−4 | 0.00 | −1.89 × 10−3 | 9.03 × 10−4 |
| a4 | −3.25 × 100 | 1.80 × 100 | 0.00 | −6.87 × 100 | 3.62 × 10−1 |
| a5 | 3.20 × 10−2 | 1.16 × 10−2 | 0.00 | 8.53 × 103 | 5.54 × 10−2 |
| a6 | 9.62 × 10−3 | 7.11 × 10−3 | 0.00 | −4.69 × 10−3 | 2.39 × 10−2 |
| a7 | −3.85 × 10−7 | 0.00 × 100 | 0.00 | −3.85 × 10−7 | −3.85 × 107 |
| Intercept/Coefficient | Value of Intercept/Coefficient | Standard Error | p | Lower Limit of Confidence | Upper Limit of Confidence |
|---|---|---|---|---|---|
| a1 | −2.82 × 100 | 6.34 × 101 | 0.00 | −1.30 × 102 | 1.25 × 102 |
| a2 | 2.76 × 10−2 | 4.19 × 10−1 | 0.00 | −8.15 × 10−1 | 8.70 × 10−1 |
| a3 | 4.22 × 10−4 | 6.95 × 10−4 | 0.00 | −9.76 × 10−4 | 1.82 × 10−3 |
| a4 | 3.32 × 100 | 1.80 × 100 | 0.00 | −3.01 × 10−1 | 6.94 × 100 |
| a5 | −3.24 × 10−2 | 1.17 × 10−2 | 0.00 | −5.58 × 10−2 | −8.93 × 10−3 |
| a6 | −9.87 × 10−3 | 7.12 × 10−3 | 0.00 | −2.42 × 10−2 | 4.44 × 10−3 |
| a7 | 3.91 × 10−7 | 0.00 × 100 | 0.00 | 3.91 × 10−7 | 3.91 × 10−7 |
| Intercept/Coefficient | Value of Intercept/Coefficient | Standard Error | p | Lower Limit of Confidence | Upper Limit of Confidence |
|---|---|---|---|---|---|
| a1 | −1.19 × 102 | 1.14 × 102 | 0.00 | −3.48 × 102 | 1.11 × 102 |
| a2 | 1.61 × 100 | 7.54 × 10−1 | 0.00 | 9.02 × 10−2 | 3.12 × 100 |
| a3 | −3.10 × 10−3 | 1.25 × 10−3 | 0.00 | −5.62 × 10−3 | −5.79 × 10−4 |
| a4 | −2.38 × 10−1 | 3.24 × 100 | 0.00 | −6.75 × 100 | 6.28 × 100 |
| a5 | 1.73 × 10−2 | 2.10 × 10−2 | 0.00 | −2.49 × 10−2 | 5.95 × 10−2 |
| a6 | −5.28 × 10−3 | 1.28 × 10−2 | 0.00 | −3.11 × 10−2 | 2.05 × 10−2 |
| a7 | −4.29 × 10−8 | 0.00 × 100 | 0.00 | −4.29 × 10−8 | −4.29 × 10−9 |
| Intercept/Coefficient | Value of Intercept/Coefficient | Standard Error | p | Lower Limit of Confidence | Upper Limit of Confidence |
|---|---|---|---|---|---|
| a1 | 2.25 × 101 | 1.50 × 101 | 0.00 | −7.67 × 100 | 5.27 × 101 |
| a2 | −3.04 × 10−2 | 9.92 × 10−2 | 0.00 | −2.30 × 10−1 | 1.69 × 10−1 |
| a3 | −2.45 × 10−6 | 1.65 × 10−4 | 0.00 | −3.34 × 10−4 | 3.29 × 10−4 |
| a4 | −6.30 × 10−1 | 4.26 × 10−1 | 0.00 | −1.49 × 100 | 2.27 × 10−1 |
| a5 | 6.68 × 10−3 | 2.76 × 10−3 | 0.00 | 1.12 × 10−3 | 1.22 × 10−2 |
| a6 | 1.84 × 10−3 | 1.69 × 10−3 | 0.00 | −1.55 × 10−3 | 5.23 × 10−3 |
| a7 | −8.13 × 10−8 | 0.00 × 100 | 0.00 | −8.13 × 10−8 | −8.13 × 10−8 |
| Intercept/Coefficient | Value of Intercept/Coefficient | Standard Error | p | Lower Limit of Confidence | Upper Limit of Confidence |
|---|---|---|---|---|---|
| a1 | 3.54 × 101 | 2.99 × 101 | 0.00 | −2.48 × 101 | 9.56 × 101 |
| a2 | −1.28 × 10−1 | 1.98 × 10−1 | 0.00 | −5.25 × 10−1 | 2.70 × 10−1 |
| a3 | 2.91 × 10−4 | 3.28 × 10−4 | 0.00 | −3.69 × 10−4 | 9.52 × 10−4 |
| a4 | −1.04 × 10−1 | 8.49 × 10−1 | 0.00 | −1.81 × 100 | 1.60 × 100 |
| a5 | 6.68 × 10−4 | 5.50 × 10−3 | 0.00 | −1.04 × 10−2 | 1.17 × 10−2 |
| a6 | 4.75 × 10−4 | 3.36 × 10−3 | 0.00 | −6.29 × 10−3 | 7.23 × 10−3 |
| a7 | −1.03 × 10−8 | 0.00 × 100 | 0.00 | −1.03 × 10−8 | −1.03 × 10−8 |
| T, °C | T−1, °C−1 | k, s−1 | ln(k), s−1 | Ea, J·mol−1 |
|---|---|---|---|---|
| 200 | 2.11 × 10−3 | 1.16 × 10−5 a | −11.40 | 17,700 |
| 220 | 2.03 × 10−3 | 1.24 × 10−5 a | −11.30 | |
| 240 | 1.95 × 10−3 | 1.49 × 10−5 a | −11.10 | |
| 260 | 1.88 × 10−3 | 1.50 × 10−5 a | −11.10 | |
| 280 | 1.81 × 10−3 | 1.92 × 10−5 a,b | −10.90 | |
| 300 | 1.75 × 10−3 | 2.73 × 10−5 b | −10.50 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stępień, P.; Świechowski, K.; Hnat, M.; Kugler, S.; Stegenta-Dąbrowska, S.; Koziel, J.A.; Manczarski, P.; Białowiec, A. Waste to Carbon: Biocoal from Elephant Dung as New Cooking Fuel. Energies 2019, 12, 4344. https://doi.org/10.3390/en12224344
Stępień P, Świechowski K, Hnat M, Kugler S, Stegenta-Dąbrowska S, Koziel JA, Manczarski P, Białowiec A. Waste to Carbon: Biocoal from Elephant Dung as New Cooking Fuel. Energies. 2019; 12(22):4344. https://doi.org/10.3390/en12224344
Chicago/Turabian StyleStępień, Paweł, Kacper Świechowski, Martyna Hnat, Szymon Kugler, Sylwia Stegenta-Dąbrowska, Jacek A. Koziel, Piotr Manczarski, and Andrzej Białowiec. 2019. "Waste to Carbon: Biocoal from Elephant Dung as New Cooking Fuel" Energies 12, no. 22: 4344. https://doi.org/10.3390/en12224344
APA StyleStępień, P., Świechowski, K., Hnat, M., Kugler, S., Stegenta-Dąbrowska, S., Koziel, J. A., Manczarski, P., & Białowiec, A. (2019). Waste to Carbon: Biocoal from Elephant Dung as New Cooking Fuel. Energies, 12(22), 4344. https://doi.org/10.3390/en12224344