A Study on the Use of Water as a Medium for the Thermal Inactivation of Endogenous Lipase in Oil of Palm Fruit
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
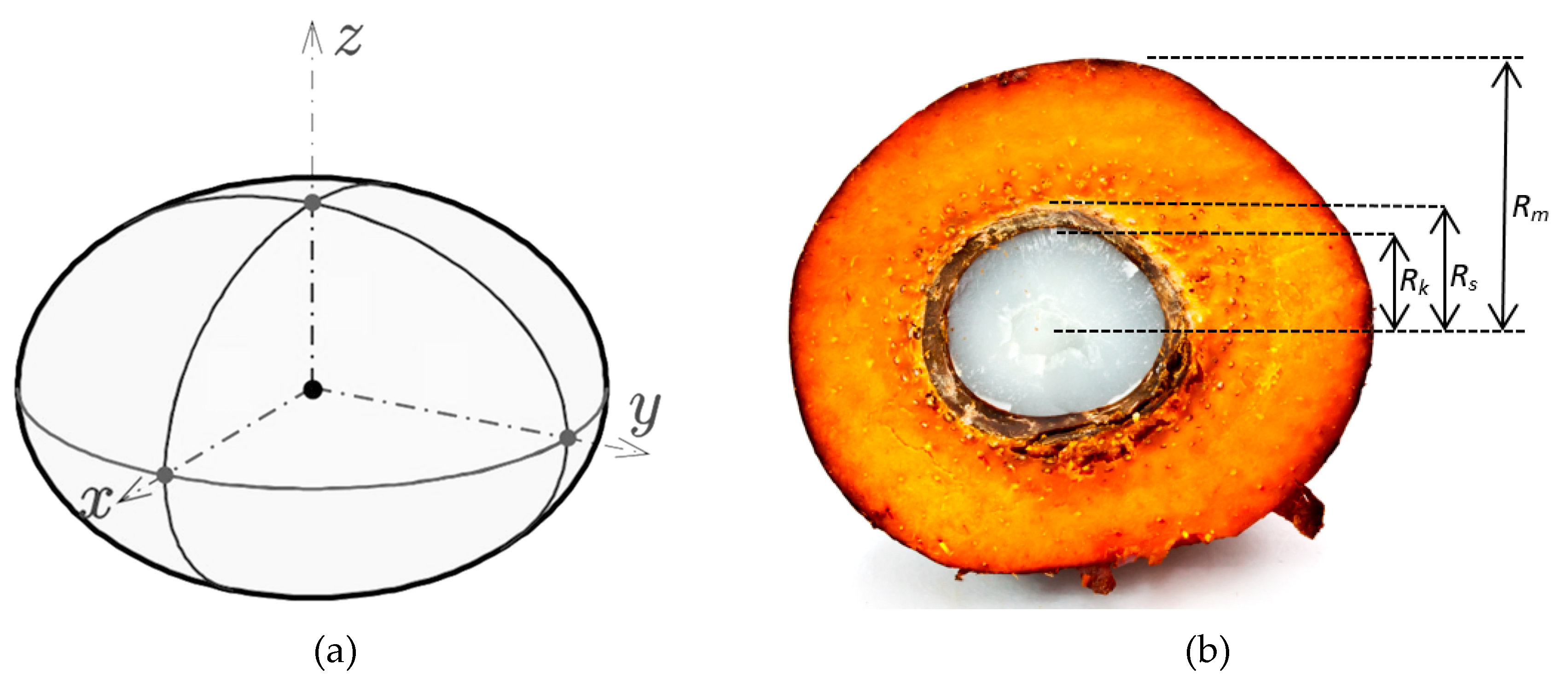
2.2. Determination of OPF Dimension
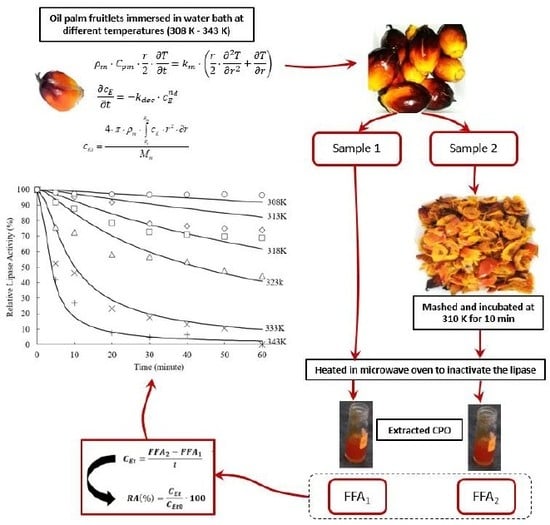
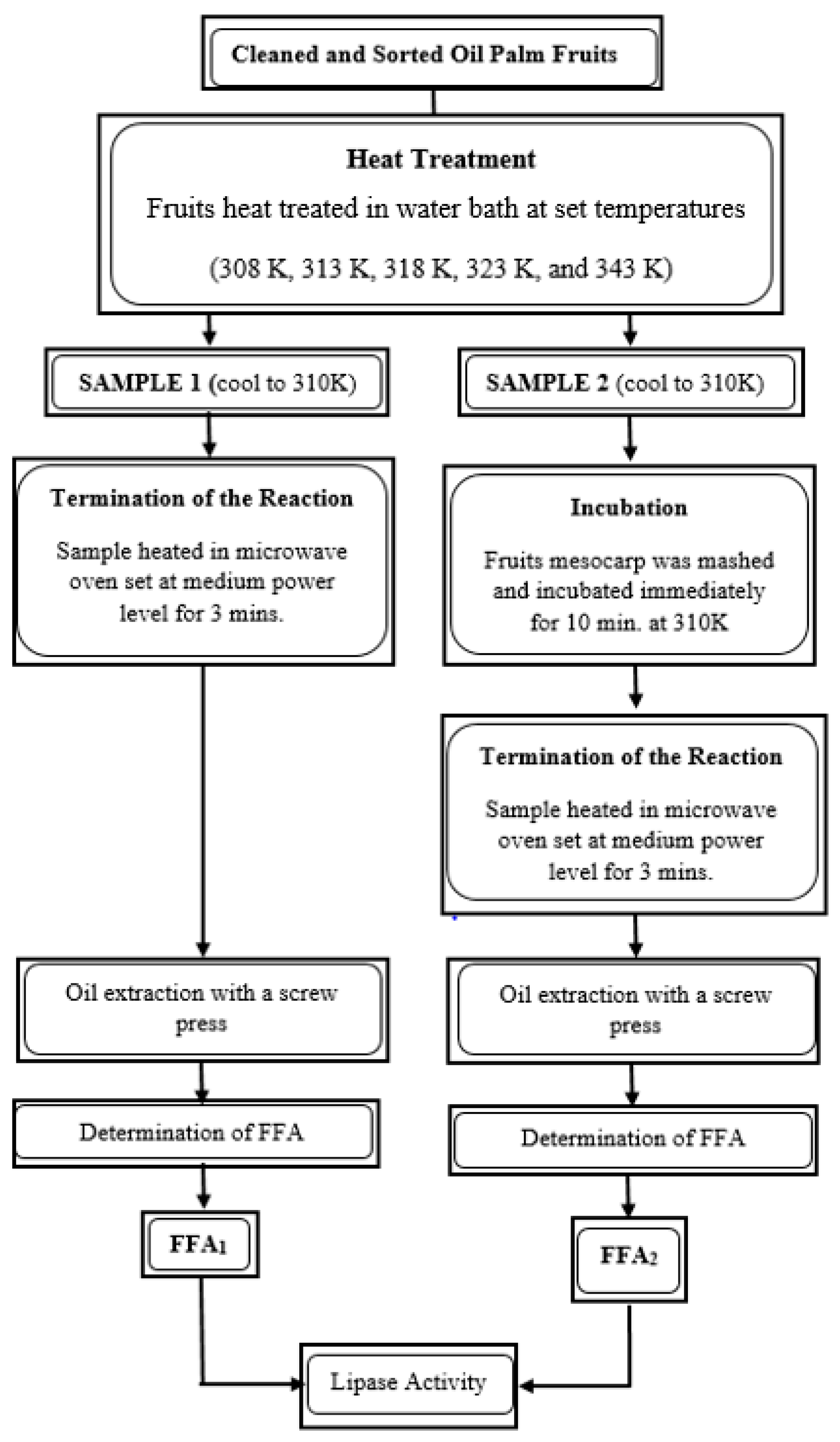
2.3. Heat Treatment of Fresh Palm Fruit
2.4. In Vivo Lipase Assay
2.5. Determination of FFA Content
2.6. Histological Light Microscopy
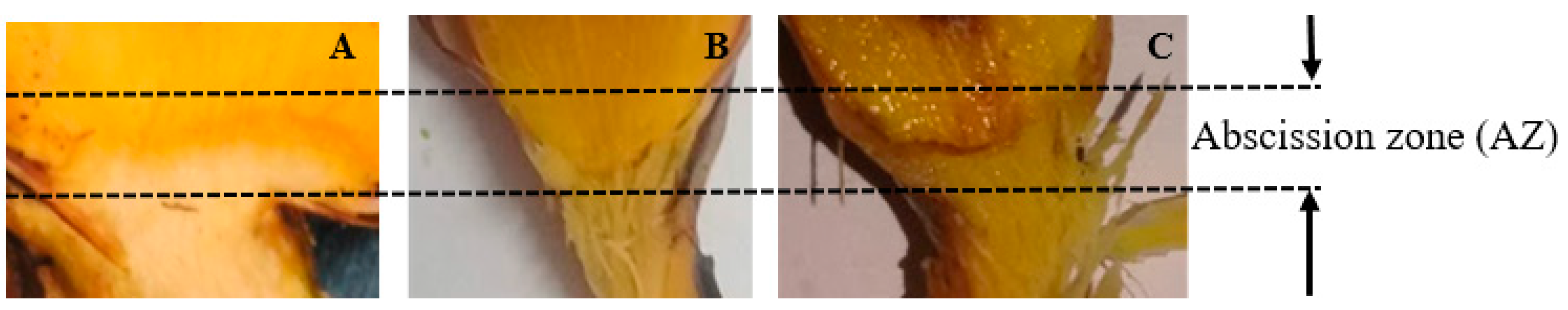
2.7. Abscission Zone Preparation
3. Mathematical Modeling of the Heat Inactivation of Endogenous Lipase in Palm Fruit
3.1. Heat Transfer
3.2. Inactivation Kinetics
3.3. Model Performance and Sensitivity Analysis
4. Results and Discussion
4.1. Experimental Results
4.1.1. Geometrical Dimension of OPF
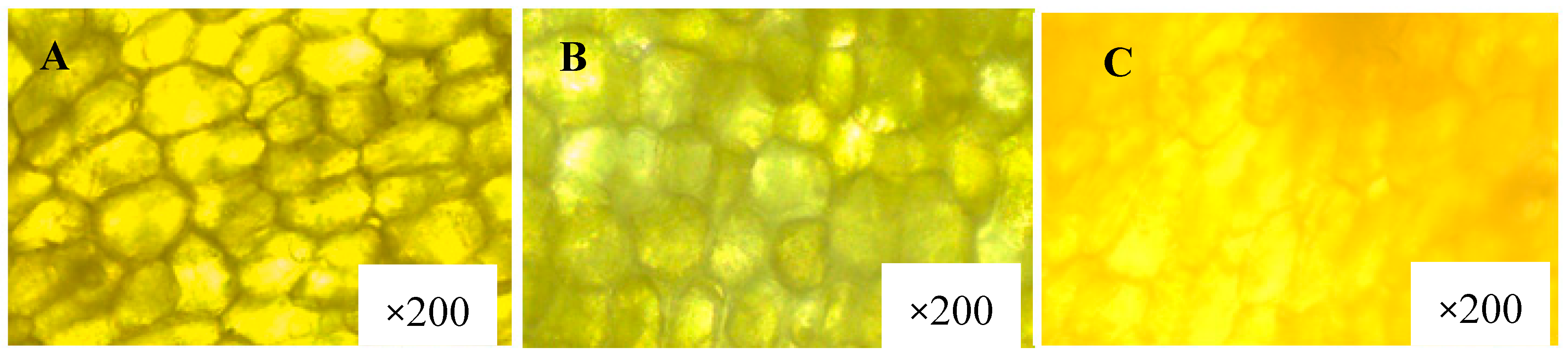
4.1.2. Effect of Hot Water Treatment on the Integrity of Oil Cell/Globule Membrane
4.1.3. Effect of Hot Water Treatment on Abscission of OPF
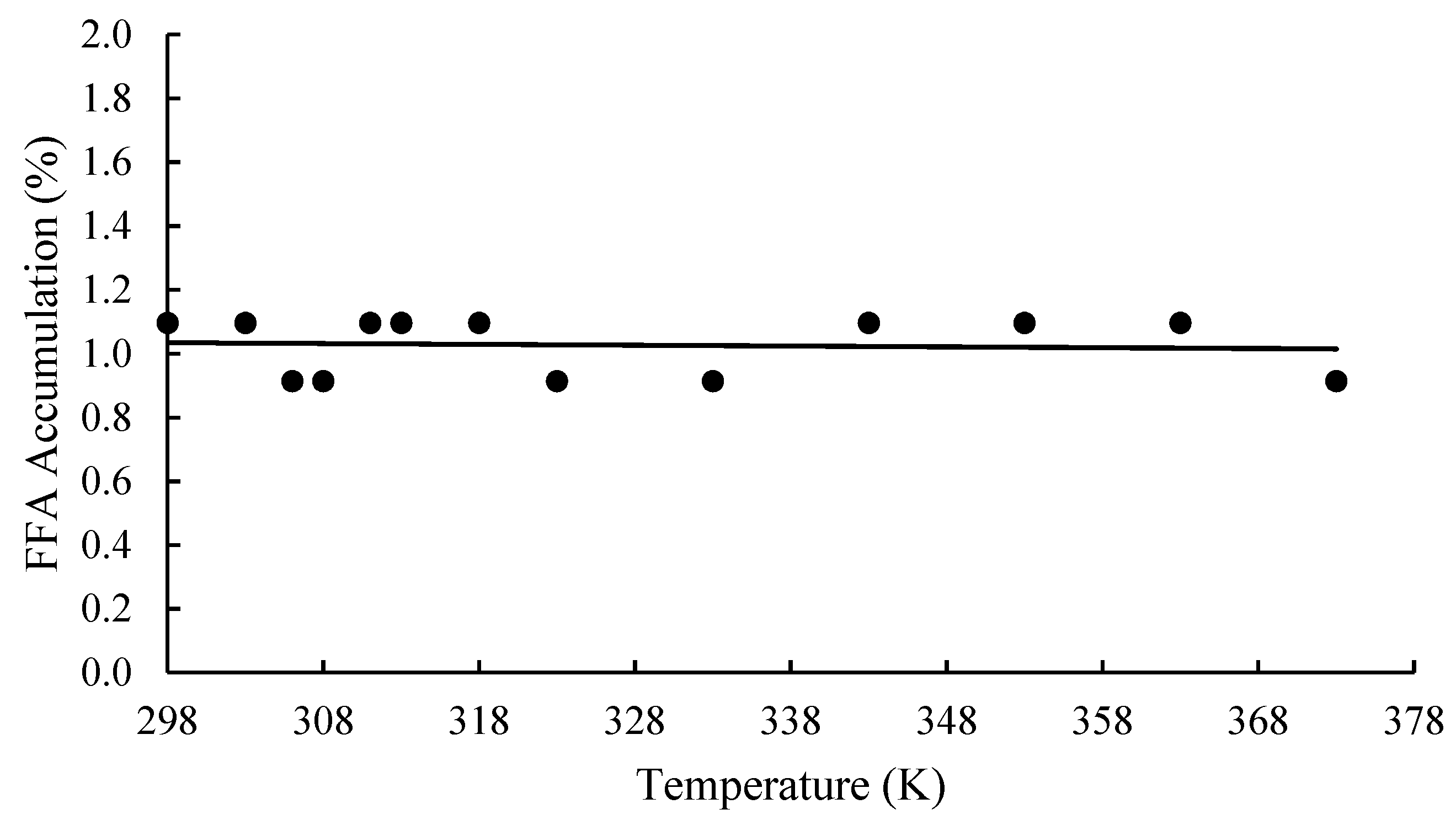
4.1.4. Effects of Heat Treatment on FFA Accumulation in OPF
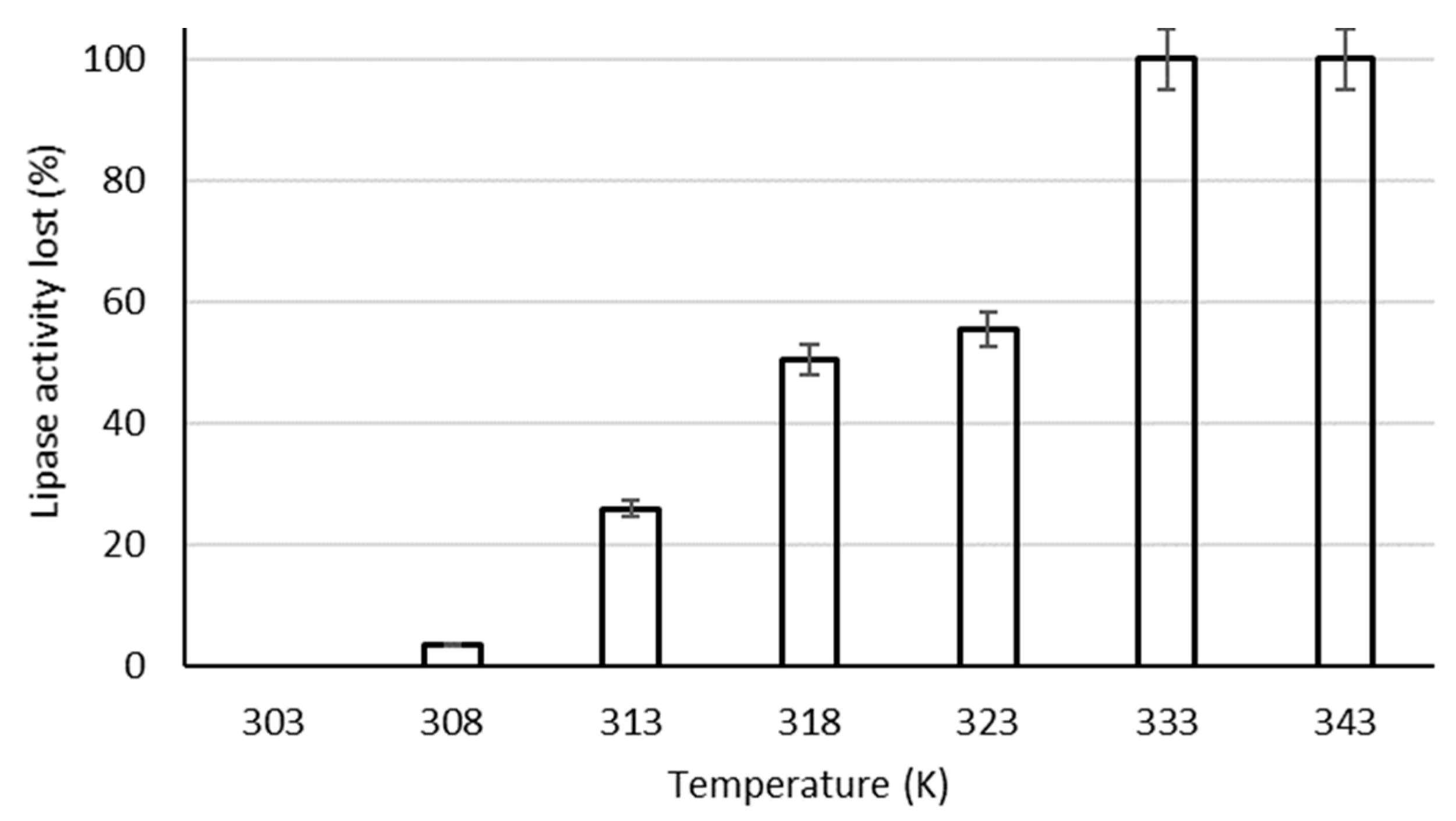
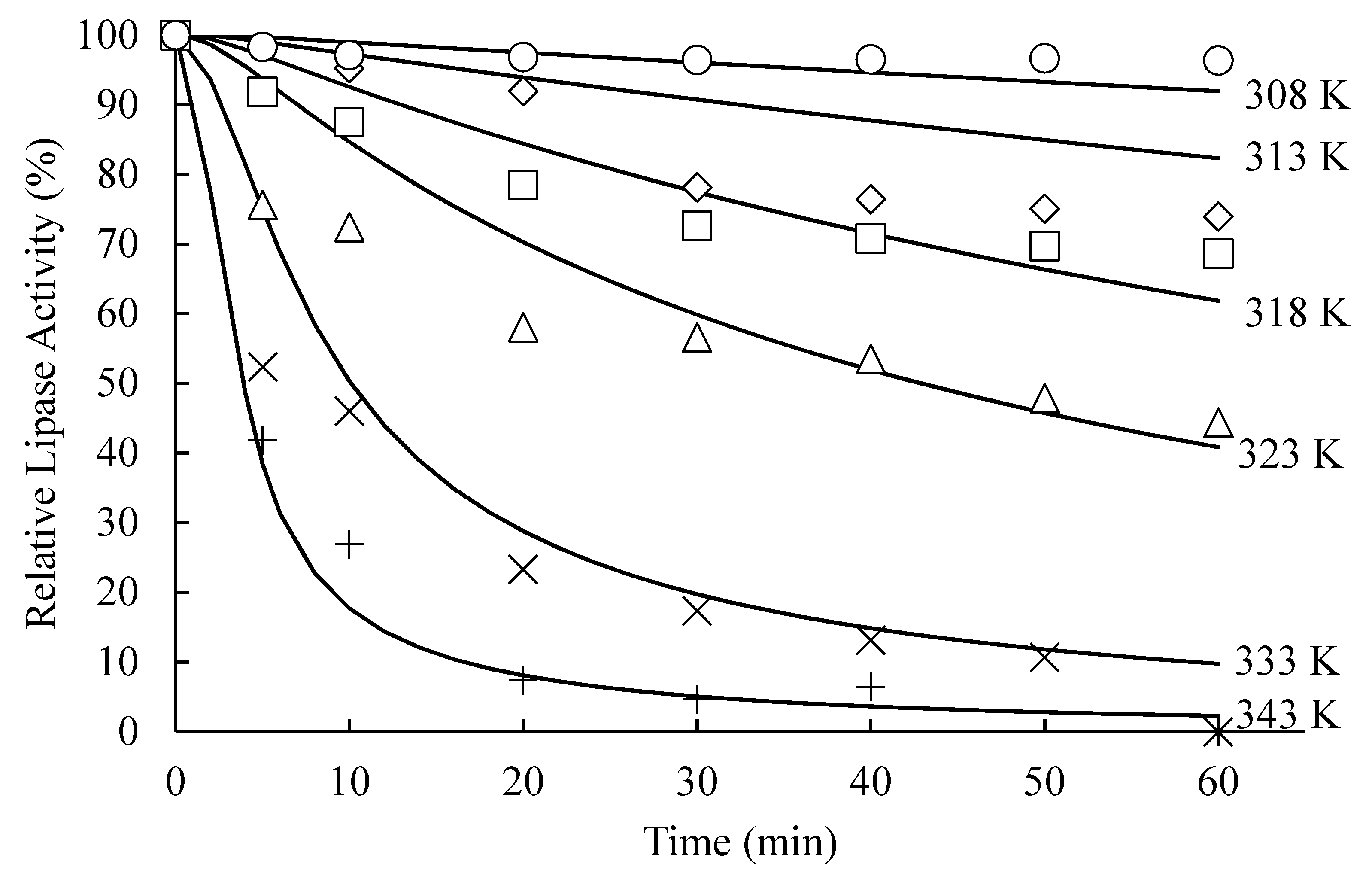
4.1.5. Effects of Heat Treatment on in Vivo Activity of the Residual Lipase
4.2. Simulation Results
4.2.1. Parameter Estimation and Model Performance
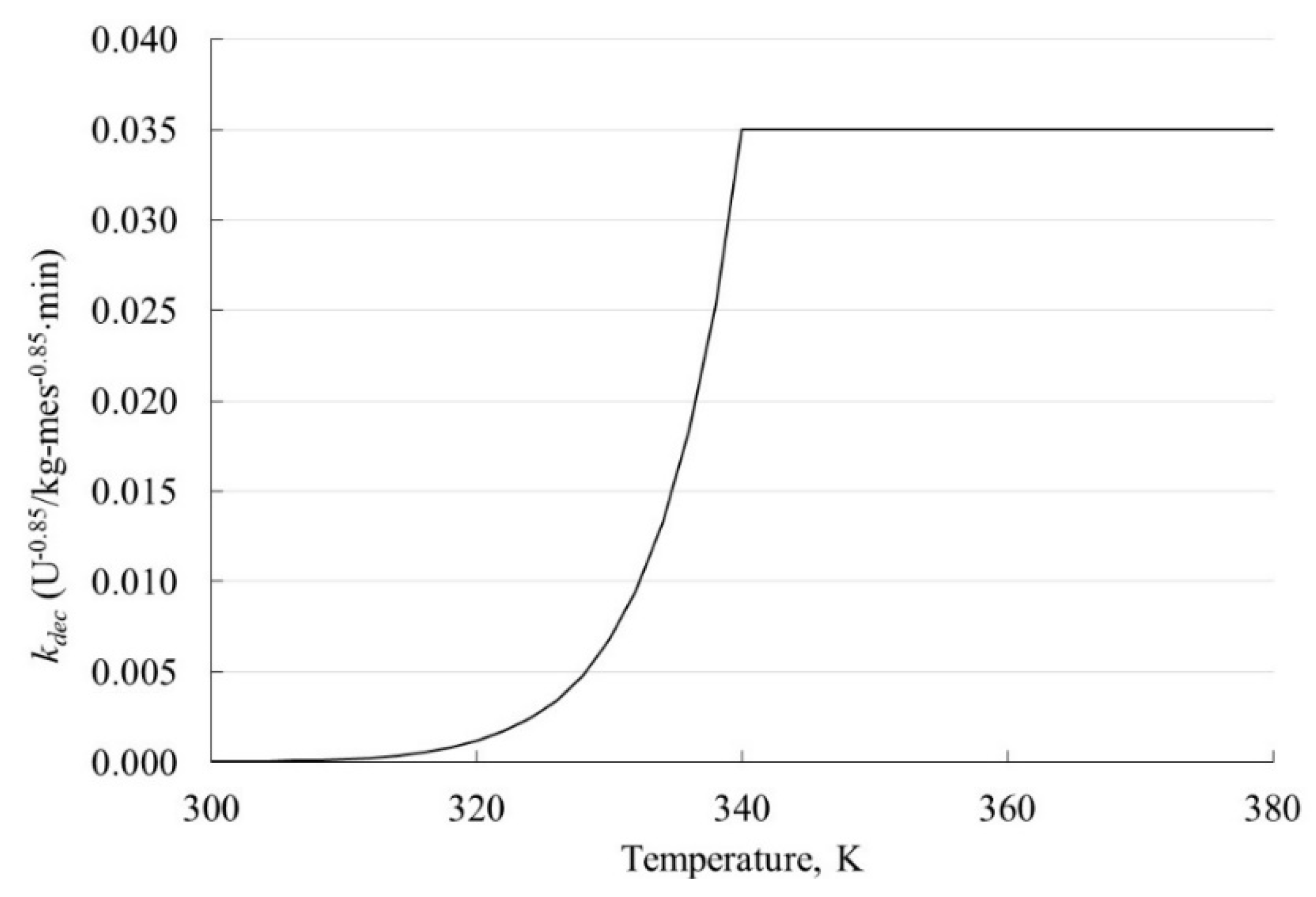
4.2.2. Reaction Rate Constant
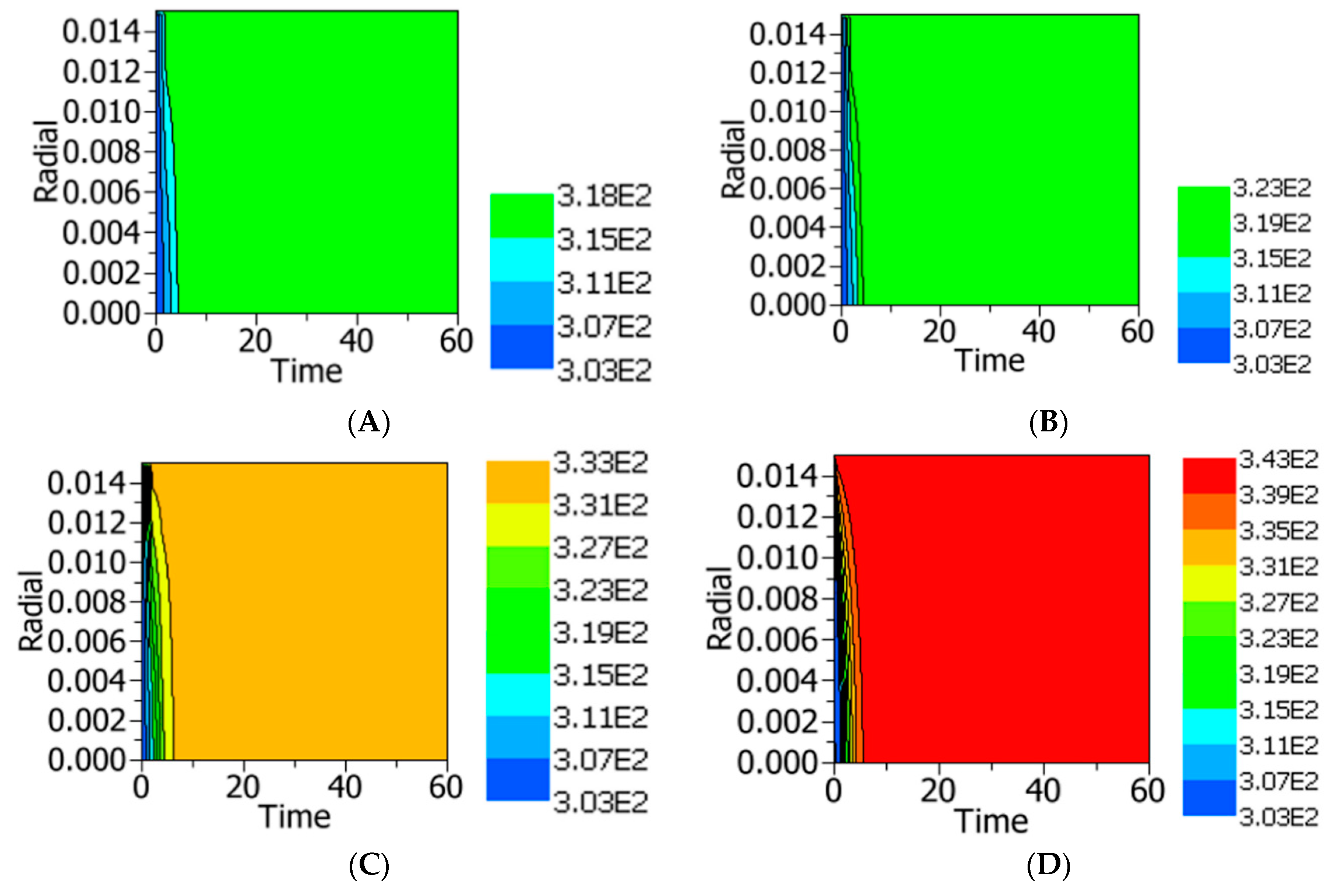
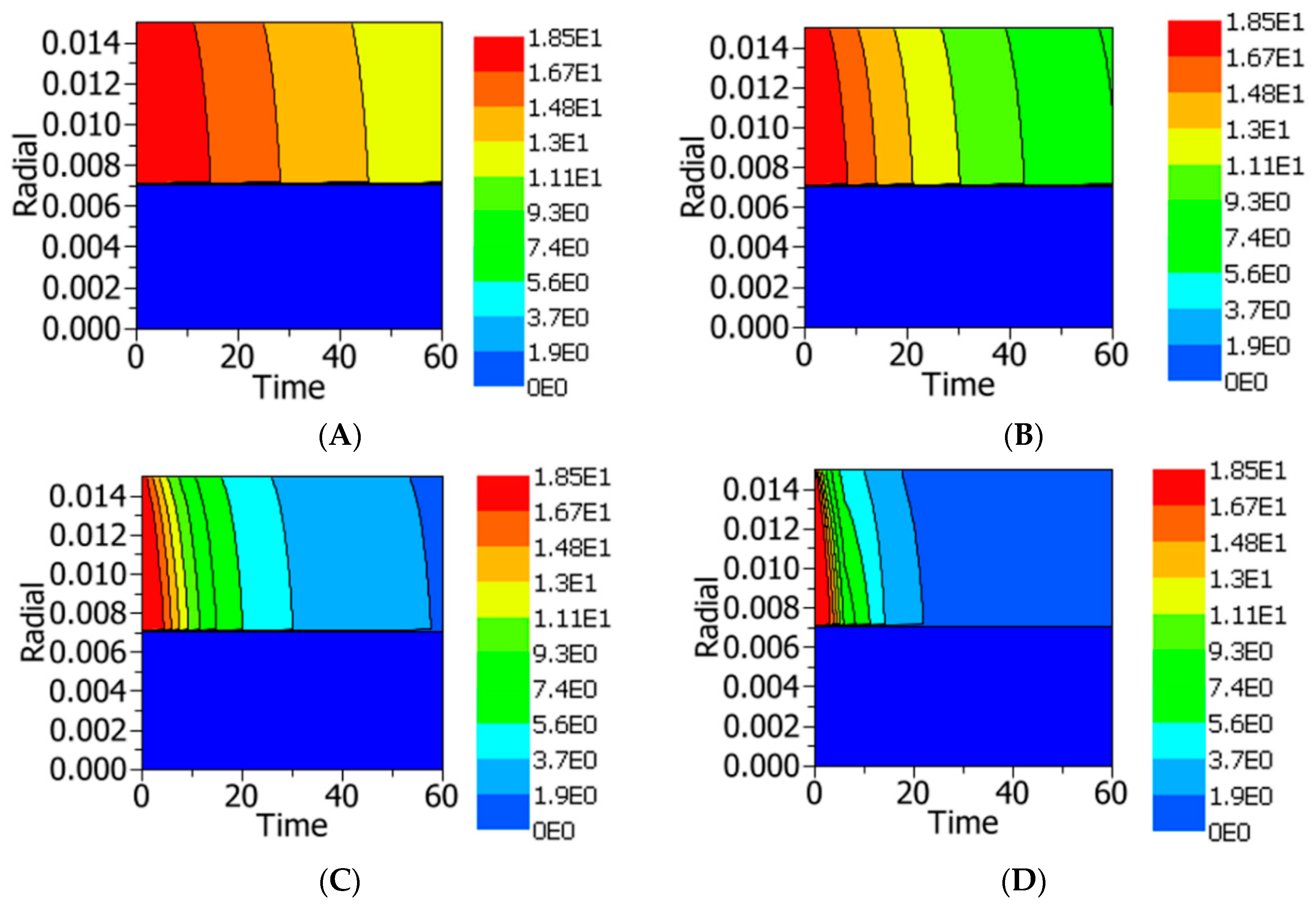
4.2.3. Temperature and Residual Lipase Activity Distribution
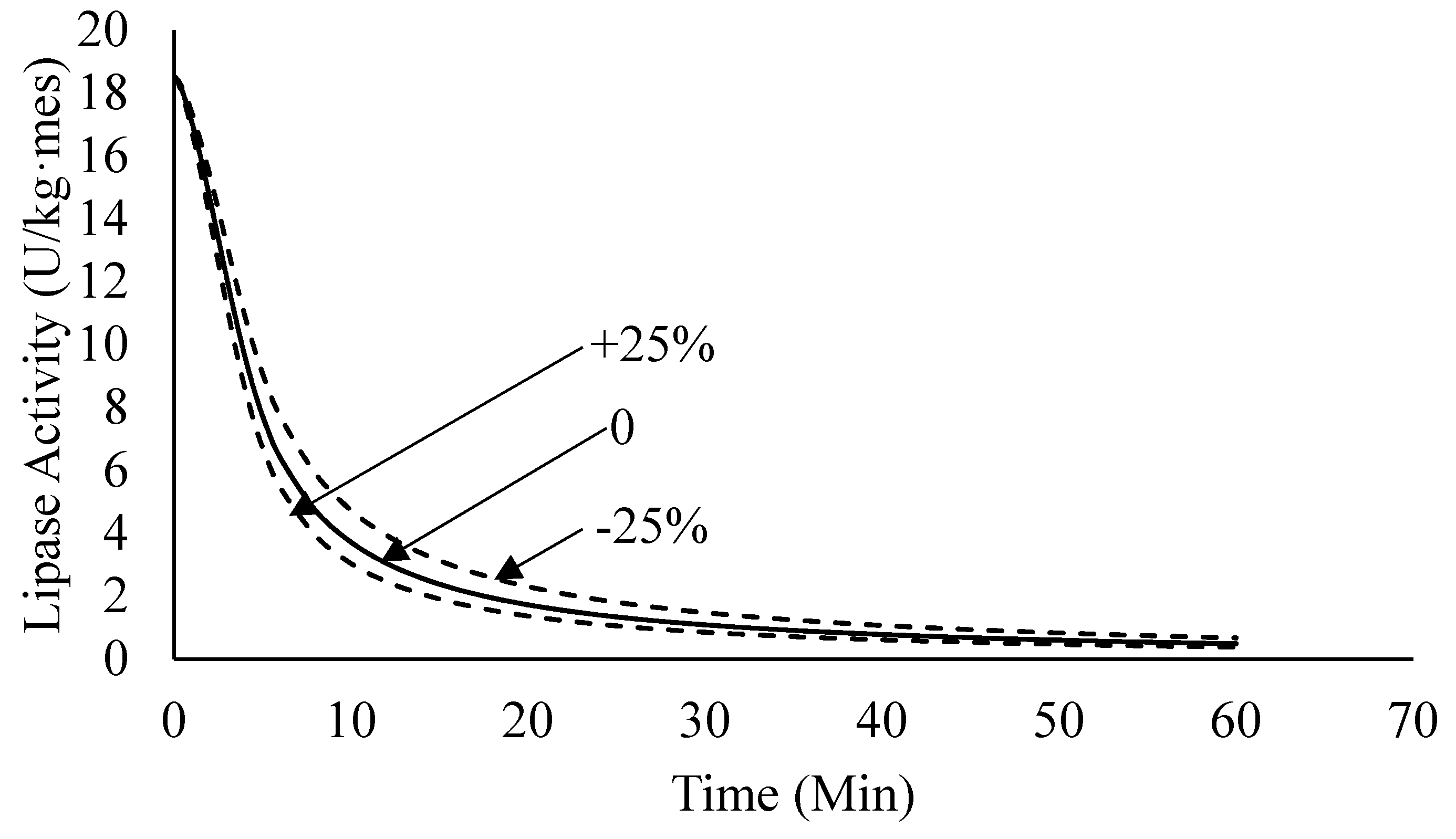
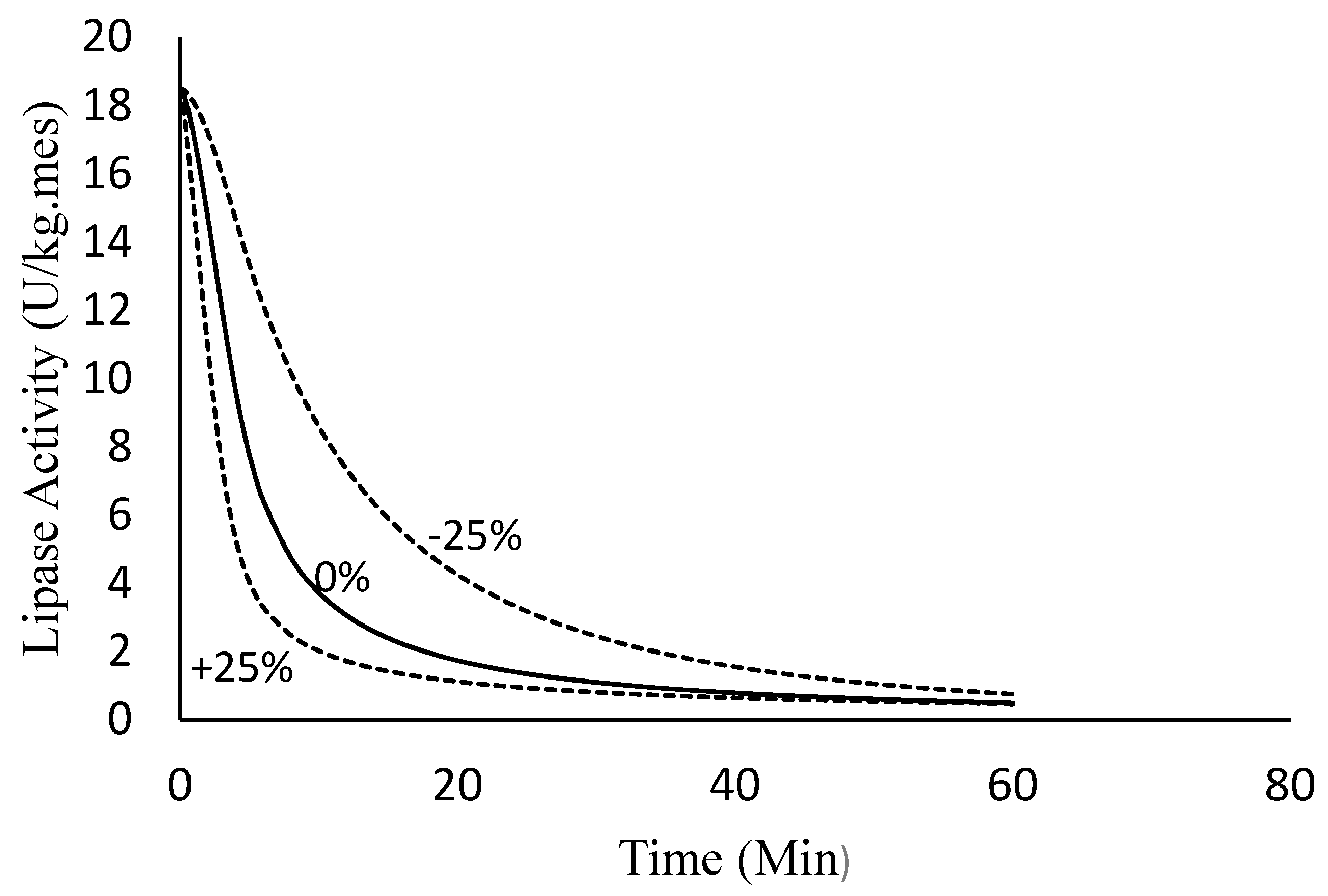
4.2.4. Sensitivity Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Nomenclatures
| cE | Lipase activity (U/kg-mes) |
| cEt0 | Lipase activity (U/kg-mes) |
| cEt | Lipase activity (U/kg-mes) |
| Cpm | Specific heat capacity of mesocarp (kJ/kg⋅K) |
| Cps | Specific heat capacity of shell (kJ/kg⋅K) |
| Cpk | Specific heat capacity of kernel (kJ/kg⋅K) |
| Edec | Activation energy (kJ/kmol) |
| FFA | Free fatty acid (%) |
| h | Convective heat transfer coefficients (kJ/m2⋅K⋅min) |
| kdec | Inactivation rate constant (U−0.85/kg-mes−0.85⋅min) |
| k0dec | Initial inactivation rate constant (U−0.85/kg-mes−0.85⋅min) |
| Mm | Mass of mesocarp (kg-mes) |
| NE | Number of measured data |
| nd | Reaction order |
| Pj | Value of measured data j |
| Average value of measured data j | |
| RA | Relative activity (%) |
| Rg | Gas constant (kJ/kmol·K) |
| Rm | Radius of fruit (m) |
| Rk | Radius of kernel (m) |
| Rs | Radius of shell (m) |
| T | Temperature of fruit (K) |
| Tw | Temperature of water (K) |
| Subscripts | |
| j | number of data |
References
- Ngando-Ebongue, F.G.; Mpondo, M.; Albert, E.; Ekwe, D.; Laverdure, E.; Paul, K. Assessment of the quality of crude palm oil from smallholders in Cameroon. J. Stored Prod. Postharvest Res. 2011, 2, 52–58. [Google Scholar]
- Mohankumar, C.; Arumughan, C.; Kaleysa raj, R. Histological localization of oil palm fruit lipase. J. Am. Oil Chem. Soc. 1990, 67, 665–669. [Google Scholar] [CrossRef]
- Ngando-Ebongue, G.F.; Dhouib, R.; Carrière, F.; Amvam Zollo, P.H.; Arondel, V. Assaying lipase activity from oil palm fruit (Elaeis guineensis Jacq.) mesocarp. Plant Physiol. Biochem. 2006, 44, 611–617. [Google Scholar] [CrossRef] [PubMed]
- Sambanthamurthi, R.; Sundram, K.; Tan, Y.A. Chemistry and biochemistry of palm oil. Prog. Lipid Res. 2000, 39, 507–558. [Google Scholar] [CrossRef]
- FAO Agro-Industry Profiles: OIL PALM 1985. Available online: http://documents.worldbank.org/curated/en/479851468182957759/Agro-industry-profiles-oil-palm (accessed on 5 May 2019).
- Pahoja, V.M.; Sethar, M.A. A Review of Enzymatic Properties of Lipase in Plants, Animals and Microorganisms. J. Appl. Sci. 2002, 2, 474–484. [Google Scholar]
- Makky, M.; Soni, P. In-situ Quality Assessment of Intact Oil Palm Fresh Fruit Bunches using Rapid Portable Non- contact and Non-destructive Approach. J. Food Eng. 2014, 120, 248–259. [Google Scholar] [CrossRef]
- Mba, O.I.; Dumont, M.-J.; Ngadi, M. Palm oil: Processing, characterization and utilization in the food industry—A review. Food Biosci. 2015, 10, 26–41. [Google Scholar] [CrossRef]
- Gupta, M.K. Trans Fat alternatives and challenges. In Practical Guide to Vegetable Oil Processing; AOCS Press: Champaign, IL, USA, 2017; pp. 341–374. [Google Scholar]
- Kannan, P.K.P.; Gundappa, G.K.A. Impact of different deacidification methods on quality characteristics and composition of olein and stearin in crude red palm oil. J. Oleo Sci. 2014, 63, 1209–1221. [Google Scholar]
- Ngando-Ebongue, G.F.; Koona, P.; Nouy, B.; Zok, S.; Carrière, F.; Zollo, P.H.A.; Arondel, V. Identification of oil palm breeding lines producing oils with low acid values. Eur. J. Lipid Sci. Technol. 2008, 110, 505–509. [Google Scholar] [CrossRef]
- Silvamany, H.; Jahim, J.M. Enhancement of Palm Oil Extraction Using Cell Wall Degrading Enzyme Formulation. Malaysian J. Anal. Sci. 2015, 19, 77–87. [Google Scholar]
- Ali, F.S.; Shamsudin, R.; Yunus, R. The Effect of Storage Time of Chopped Oil Palm Fruit Bunches on the Palm Oil Quality. Agric. Agric. Sci. Procedia 2014, 2, 165–172. [Google Scholar] [CrossRef]
- Han, N.M.; May, C.Y.; Nga, M.A. Dry Heating of Palm Fruits: Effect on Selected Parameters. Am. J. Eng. Appl. Sci. 2012, 5, 128–131. [Google Scholar]
- Chow, M.C.; Ma, A.N. Processing of Fresh Palm Fruits Using Microwaves. J. Microw. Power Electromagn. Energy 2007, 40, 165–173. [Google Scholar] [CrossRef] [PubMed]
- Vincent, C.J.; Shamsudin, R.; Baharuddin, A.S. Pre-treatment of oil palm fruits: A review. J. Food Eng. 2014, 143, 123–131. [Google Scholar] [CrossRef]
- Sarah, M.; Rozainee Taib, M. Microwave Sterilization of Oil Palm Fruits: Effect of Power, Temperature and D-value on Oil Quality. J. Med. Bioeng. 2013, 2, 153–156. [Google Scholar] [CrossRef]
- Umudee, I.; Chongcheawchamnan, M.; Kiatweerasakul, M.; Tongurai, C. Sterilization of Oil Palm Fresh Fruit Using Microwave Technique. Int. J. Chem. Eng. Appl. 2013, 4, 111–113. [Google Scholar] [CrossRef]
- Nokkaew, R.; Punsuvon, V. Sterilization of Oil Palm Fruits by Microwave Heating for Replacing Steam Treatment in Palm Oil Mill Process. Adv. Mater. Res. 2014, 1025–1026, 470–475. [Google Scholar] [CrossRef]
- Martins, C.P.C.; Cavalcanti, R.N.; Couto, S.M.; Moraes, J.; Esmerino, E.A.; Silva, M.C.; Raices, R.S.L.; Gut, J.A.W.; Ramaswamy, H.S.; Tadini, C.C.; et al. Microwave Processing: Current Background and Effects on the Physicochemical and Microbiological Aspects of Dairy Products. Compr. Rev. Food Sci. Food Saf. 2019, 18, 67–83. [Google Scholar] [CrossRef]
- Chandrasekaran, S.; Ramanathan, S.; Basak, T. Microwave food processing—A review. Food Res. Int. 2013, 52, 243–261. [Google Scholar] [CrossRef]
- Khan, M.K.; Ahmad, K.; Hassan, S.; Imran, M.; Ahmad, N.; Xu, C. Effect of novel technologies on polyphenols during food processing. Innov. Food Sci. Emerg. Technol. 2018, 45, 361–381. [Google Scholar] [CrossRef]
- Wang, S.; Tang, J.; Cavalieri, R.P. Modeling fruit internal heating rates for hot air and hot water treatments. Postharvest Biol. Technol. 2001, 22, 257–270. [Google Scholar] [CrossRef]
- Hong, S.I.; Lee, H.H.; Kim, D. Effects of hot water treatment on the storage stability of satsuma mandarin as a postharvest decay control. Postharvest Biol. Technol. 2007, 43, 271–279. [Google Scholar] [CrossRef]
- Galedar, M.N.; Jafari, A.; Tabatabaeefar, A. Some physical properties of wild pistachio (Pistacia vera L.) nut and kernel as a function of moisture content. Int. Agrophysics 2008, 22, 117–124. [Google Scholar]
- Tang, M.; Xia, Q.; Holland, B.J.; Wang, H.; Zhang, Y.; Li, R.; Cao, H. Effects of Different Pretreatments to Fresh Fruit on Chemical and Thermal Characteristics of Crude Palm Oil. J. Food Sci. 2017, 82, 2857–2863. [Google Scholar] [CrossRef] [PubMed]
- AOCS. Official Methods and Recommended Practices of the American Oil Chemists’ Society, 4th ed.; AOCS Press: Champaign, IL, USA, 1989. [Google Scholar]
- Ho, L.S.; Nair, A.; Mohd Yusof, H.; Kulaveerasingam, H.; Jangi, M.S. Morphometry of Lipid Bodies in Embryo, Kernel and Mesocarp of Oil Palm: Its Relationship to Yield. Am. J. Plant Sci. 2014, 05, 1163–1173. [Google Scholar] [CrossRef]
- Silva, C.L.M.; Oliveira, F.A.R.; Hendrickx, M. Modelling optimum processing conditions for the sterilization of prepackaged foods. Food Control 1993, 4, 67–78. [Google Scholar] [CrossRef]
- Yu, B.; Jin, Z.; Deng, L.; Xu, X.; He, L.; Wang, J.; Tian, Y.; Chen, H. Kinetic study of thermal inactivation of potato peroxidase during high-temperature short-time processing. J. Food Sci. Technol. 2010, 47, 67–72. [Google Scholar] [CrossRef][Green Version]
- Ab Hadi, A.; Mohammad, A.W.; Takrif, M.S. The Study of Temperature Distribution for Fresh Fruit Bunch during Sterilization. J. Ind. Eng. Res. 2015, 1, 16–24. [Google Scholar]
- Noerhidajat; Yunus, R.; Zurina, Z.A.; Syafiie, S.; Ramanaidu, V.; Rashid, U. Effect of high pressurized sterilization on oil palm fruit digestion operation. Int. Food Res. J. 2016, 23, 129–134. [Google Scholar]
- Owolarafe, O.K.; Olabige, M.T.; Faborode, M.O. Physical and mechanical properties of two varieties of fresh oil palm fruit. J. Food Eng. 2007, 78, 1228–1232. [Google Scholar] [CrossRef]
- Davies, R. Physical and mechanical properties of palm fruit, kernel and nut. J. Agric. Technol. 2016, 8, 2147–2156. [Google Scholar]
- Owolarafe, O.K.; Faborode, M.O. Micro-structural characterisation of palm fruit at sterilisation and digestion stages in relation to oil expression. J. Food Eng. 2008, 85, 598–605. [Google Scholar] [CrossRef]
- Wi, S.G.; Singh, A.P.; Lee, K.H.; Kim, Y.S. The pattern of distribution of pectin, peroxidase and lignin in the middle lamella of secondary xylem fibres in alfalfa (Medicago sativa). Ann. Bot. 2005, 95, 863–868. [Google Scholar] [CrossRef] [PubMed]
- Roongsattham, P.; Morcillo, F.; Fooyontphanich, K.; Jantasuriyarat, C.; Tragoonrung, S.; Amblard, P.; Collin, M.; Mouille, G.; Verdeil, J.-L.; Tranbarger, T.J. Cellular and Pectin Dynamics during Abscission Zone Development and Ripe Fruit Abscission of the Monocot Oil Palm. Front. Plant Sci. 2016, 7, 1–15. [Google Scholar] [CrossRef]
- Flutto, L. Pectin: Properties and Determination. Encycl. Food Sci. Nutr. (Second Ed.) 2003, 4440–4449. [Google Scholar] [CrossRef]
- Khalid, K.; Zakaria, Z.; Wan-Yusof, W.D. Variation of dielectric properties of oil palm mesocarp with moisture content and fruit maturity at microwave frequencies. J. Oil Palm Res. 1996, 8, 83–91. [Google Scholar]
- Owusu, R.K.; Makhzoum, A.; Knapp, J.S. Heat inactivation of lipase from psychrotrophic Pseudomonas fluorescens P38: Activation parameters and enzyme stability at low or ultra-high temperatures. Food Chem. 1992, 44, 261–268. [Google Scholar] [CrossRef]
- Seyhan, F.; Tijskens, L.M.M.; Evranuz, O. Modelling temperature and pH dependence of lipase and peroxidase activity in Turkish hazelnuts. J. Food Eng. 2002, 52, 387–397. [Google Scholar] [CrossRef]
| Property | Symbol | Value |
|---|---|---|
| Density (kg/m3) | 611 a | |
| 560 a | ||
| 1203 b | ||
| Specific heat capacity (kJ/kg⋅K) | 2.816 b | |
| 2.291 b | ||
| 1.980 a | ||
| Thermal conductivity (kJ/m·K·min) | 0.02082 b | |
| 0.04080 b | ||
| 0.03174 a | ||
| Heat transfer coefficient (kJ/m2·K·min) | h | 150 c |
| Part | Dimension (m) | Standard Deviation |
|---|---|---|
| Fruit radius (Rm) | 1.495 × 10−2 | ±4 × 10−3 |
| Endocarp radius (Rs) | 7.1 × 10−3 | ±2 × 10−4 |
| Kernel radius (Rk) | 6.1 × 10−3 | ±1 × 10−4 |
| Water Temp (K) | 308 | 313 | 318 | 323 | 333 | 343 |
|---|---|---|---|---|---|---|
| rRMSE (%) | 1.57 | 4.56 | 3.17 | 5.83 | 5.62 | 7.70 |
| Parameter | Value | Unit |
|---|---|---|
| Initial reaction rate constant (k0dec) | 0.035 | U−0.85/kg-mes−0.85⋅min |
| Reaction order (nd) | 1.85 | - |
| Inactivation energy (Edec) | 153,052 | kJ/kmol |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shehu, U.E.; Mokhtar, M.N.; Mohd Nor, M.Z.; Baharuddin, A.S.; Nawi, N.M. A Study on the Use of Water as a Medium for the Thermal Inactivation of Endogenous Lipase in Oil of Palm Fruit. Energies 2019, 12, 3981. https://doi.org/10.3390/en12203981
Shehu UE, Mokhtar MN, Mohd Nor MZ, Baharuddin AS, Nawi NM. A Study on the Use of Water as a Medium for the Thermal Inactivation of Endogenous Lipase in Oil of Palm Fruit. Energies. 2019; 12(20):3981. https://doi.org/10.3390/en12203981
Chicago/Turabian StyleShehu, Umar Etsu, Mohd Noriznan Mokhtar, Mohd Zuhair Mohd Nor, Azhari Samsu Baharuddin, and Nazmi Mat Nawi. 2019. "A Study on the Use of Water as a Medium for the Thermal Inactivation of Endogenous Lipase in Oil of Palm Fruit" Energies 12, no. 20: 3981. https://doi.org/10.3390/en12203981
APA StyleShehu, U. E., Mokhtar, M. N., Mohd Nor, M. Z., Baharuddin, A. S., & Nawi, N. M. (2019). A Study on the Use of Water as a Medium for the Thermal Inactivation of Endogenous Lipase in Oil of Palm Fruit. Energies, 12(20), 3981. https://doi.org/10.3390/en12203981