Experimental Study on Water Shutoff Technology Using In-Situ Ion Precipitation for Gas Reservoirs
Abstract
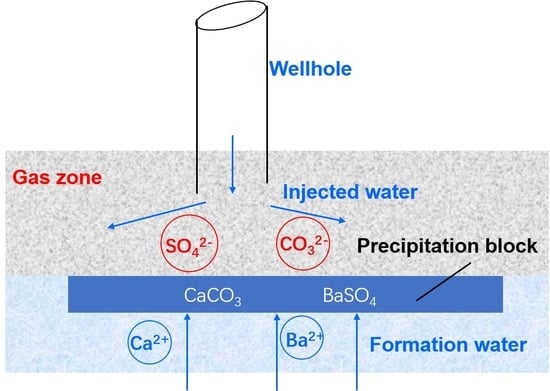
:1. Introduction
2. Experimental Section
2.1. Materials
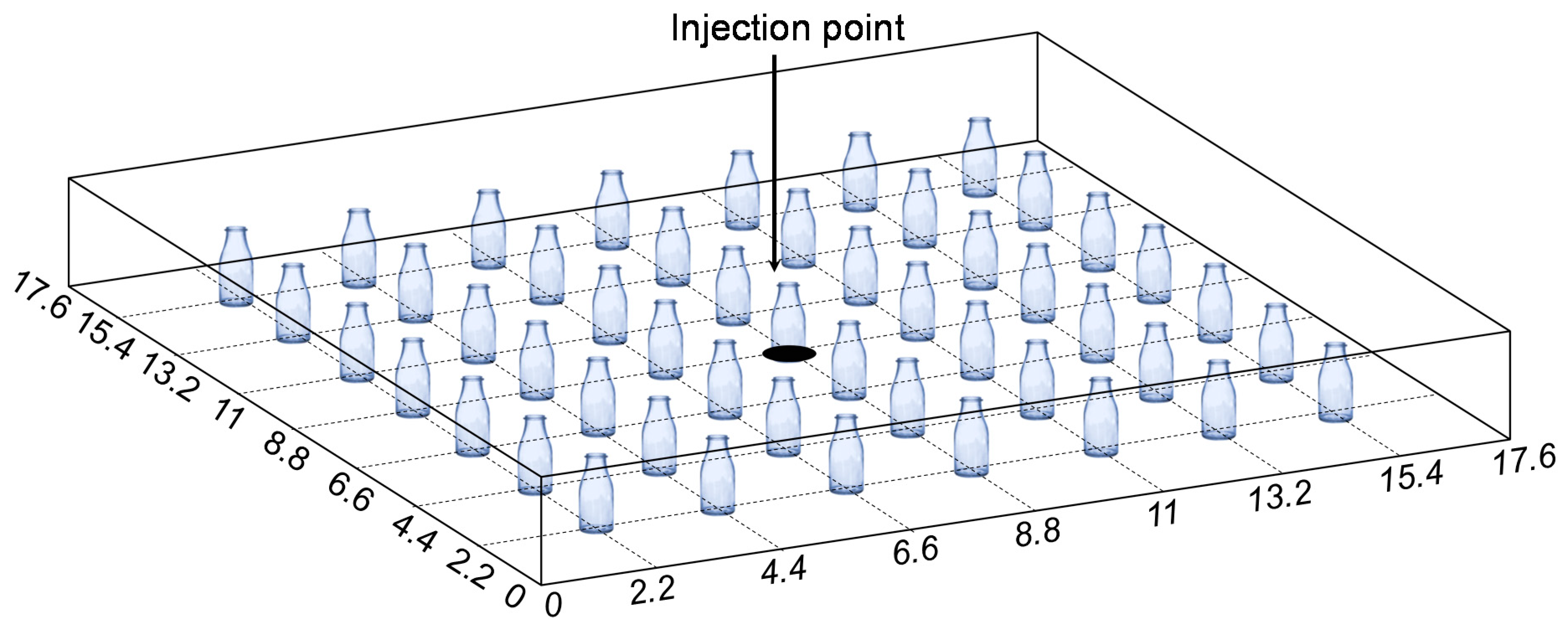
2.2. Experiments on the Mechanism of Ion Precipitation Distribution
2.2.1. Experimental Setup
2.2.2. Experimental Procedures
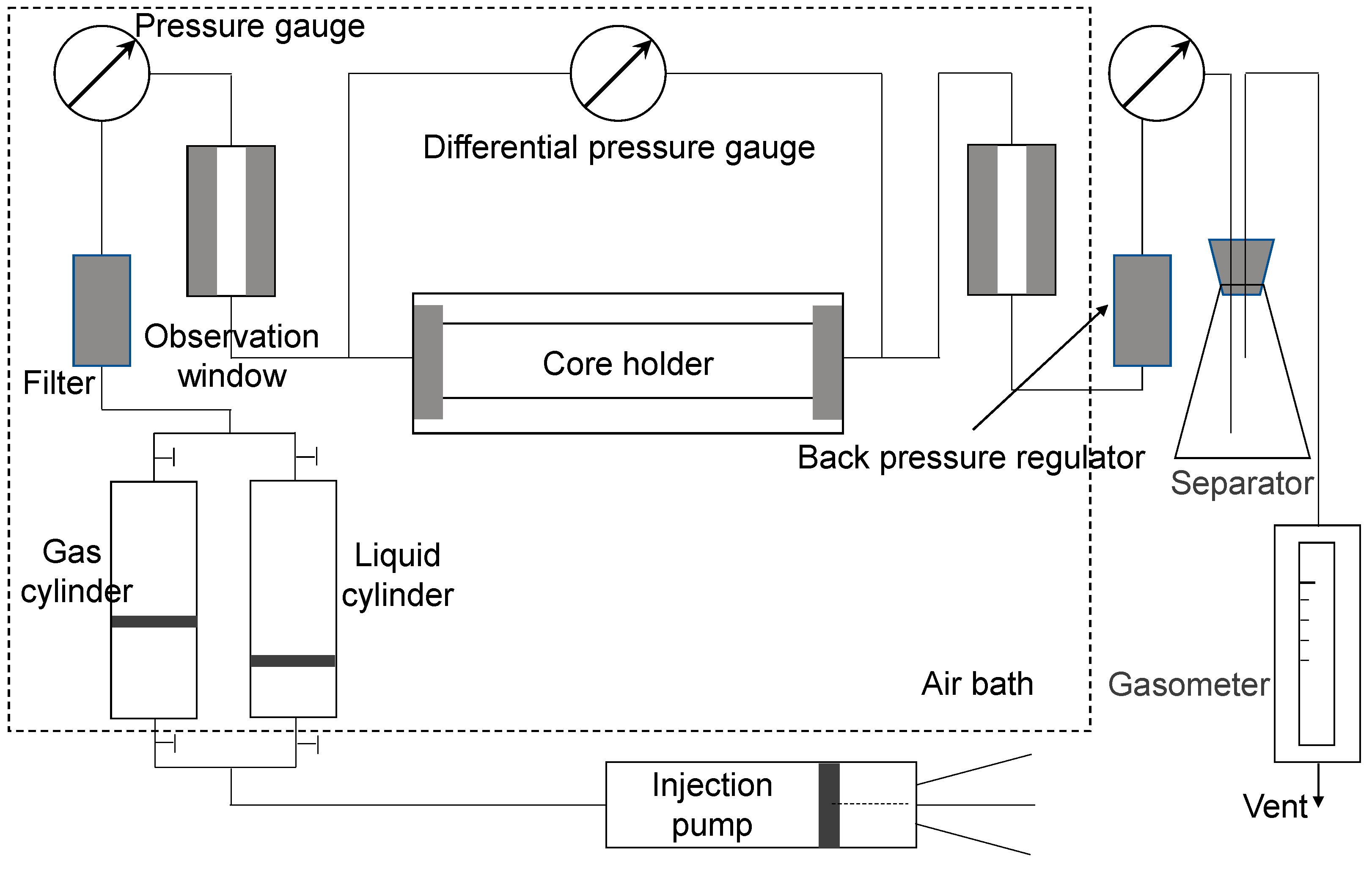
2.3. Core Displacement Experiments
2.3.1. Experimental Setup
2.3.2. Experimental Procedures
3. Results and Discussion
3.1. Experiments on the Mechanism of Ion Precipitation Distribution
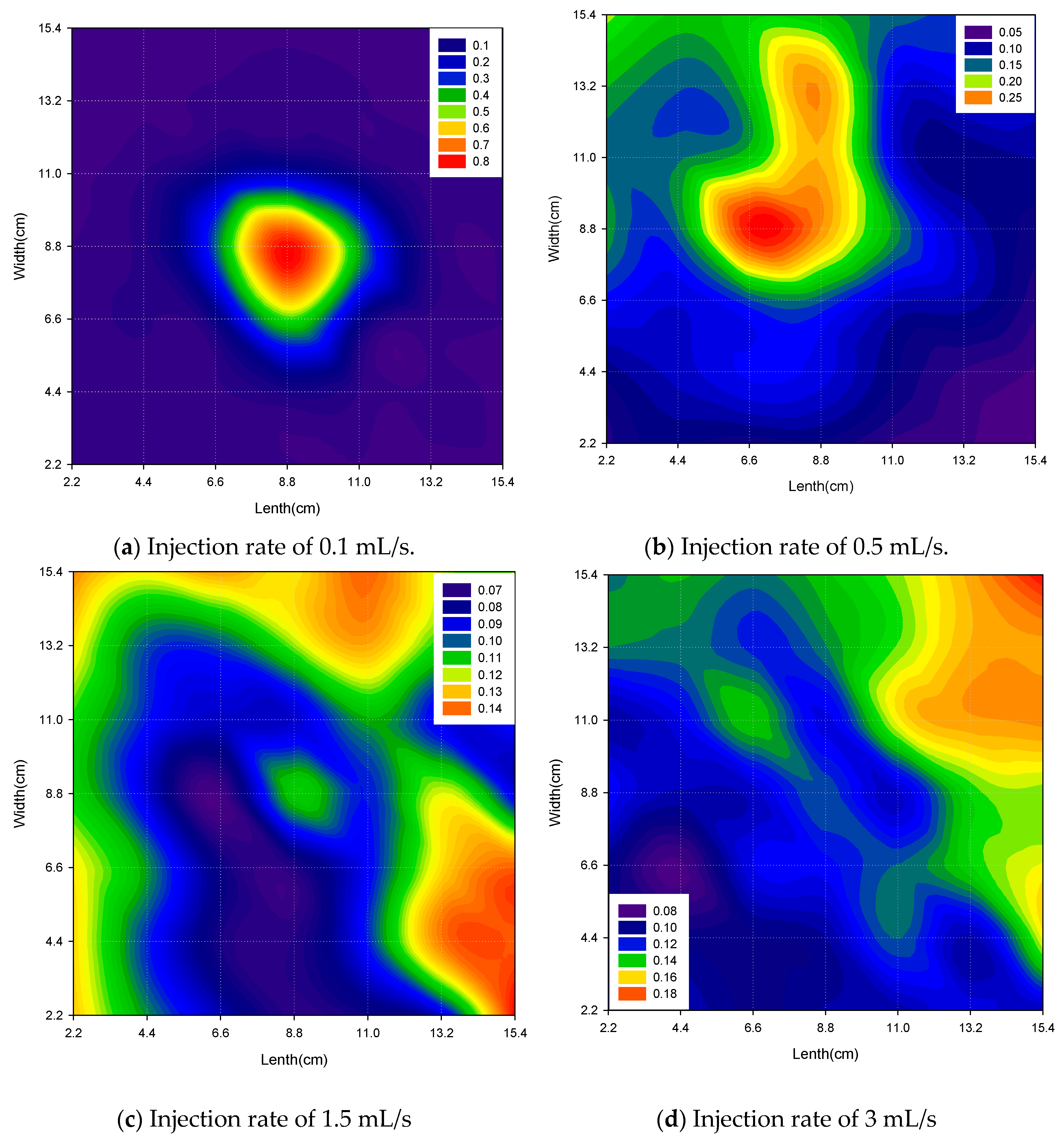
3.1.1. Effect of Injection Flow Rates
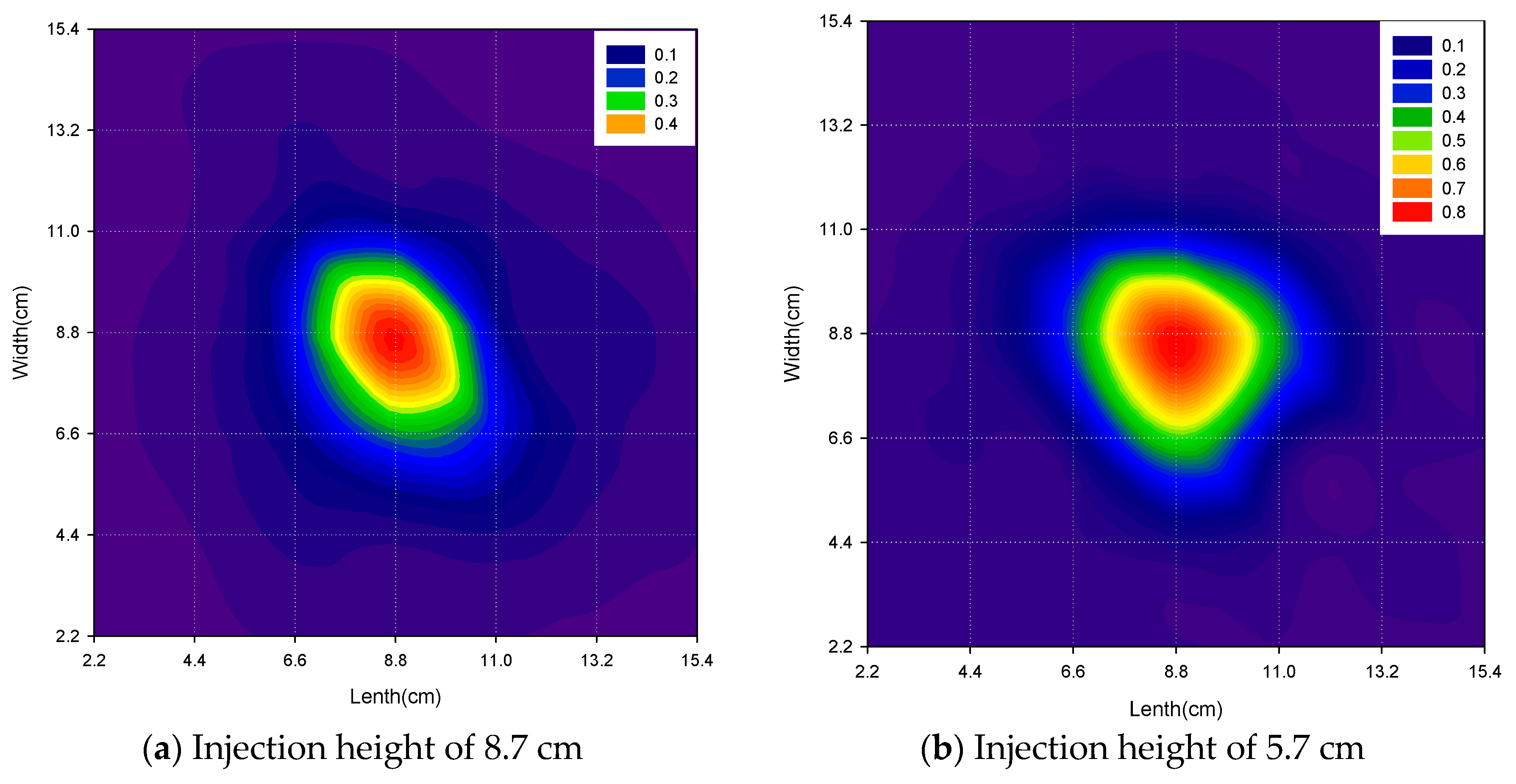
3.1.2. Effect of Injection Heights
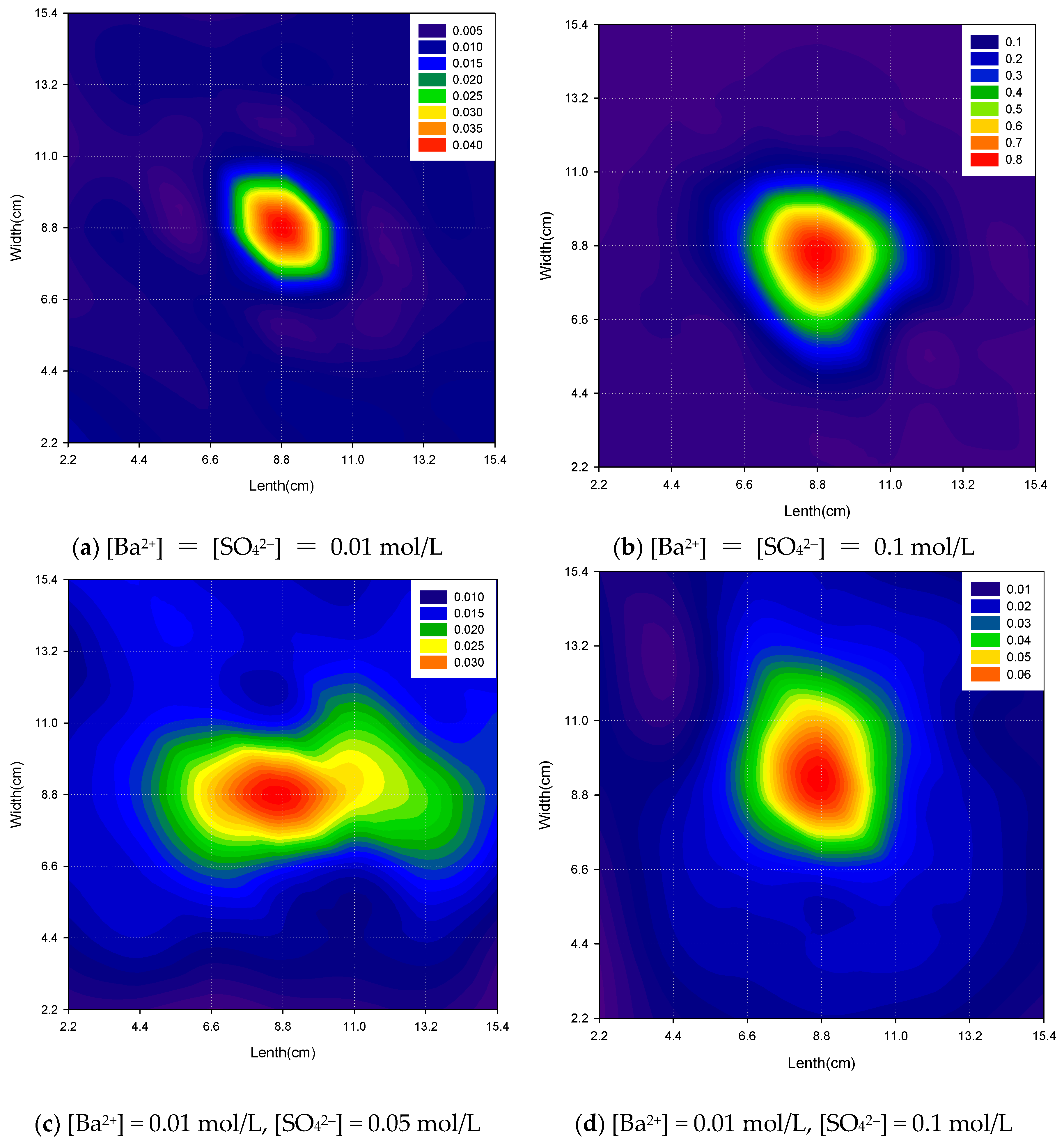
3.1.3. Effect of Solution Concentrations
3.1.4. Gas-Field Water Experiment
3.2. Core Displacement Experiments
3.2.1. Flooding Experiments at Constant Pressure
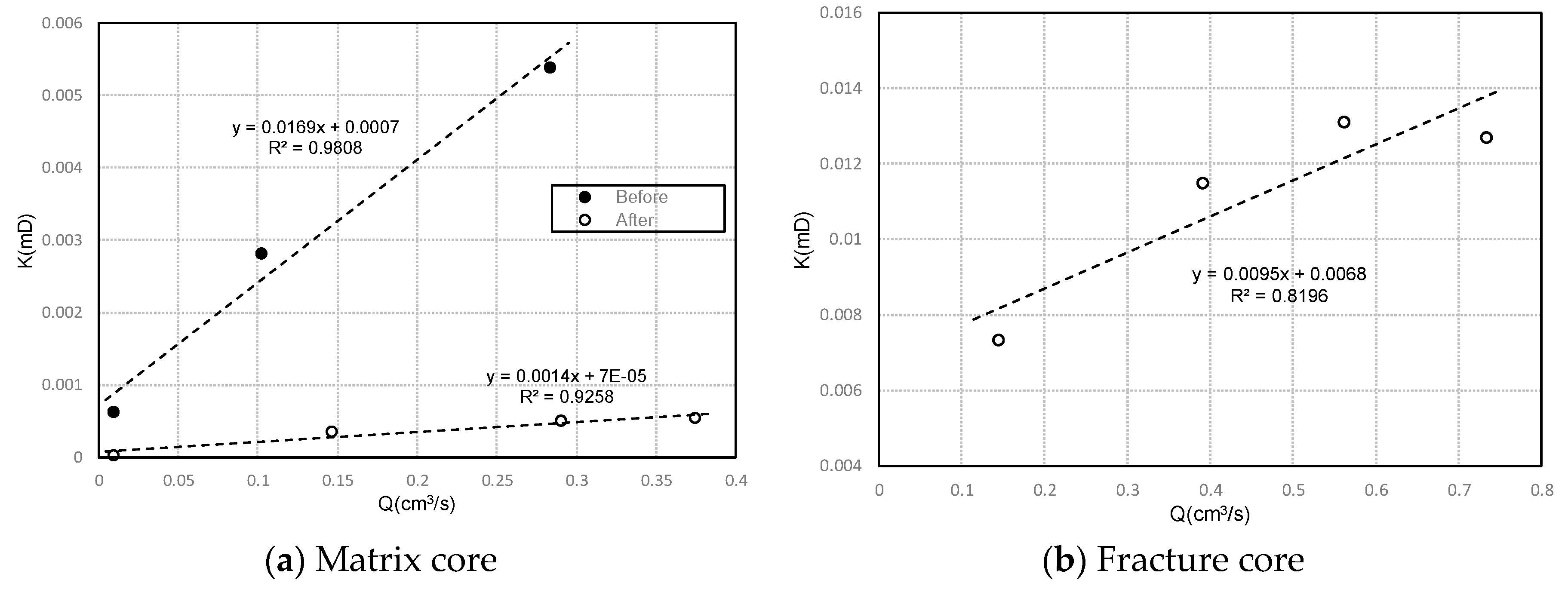
3.2.2. Effect of Ion Precipitation on Permeability
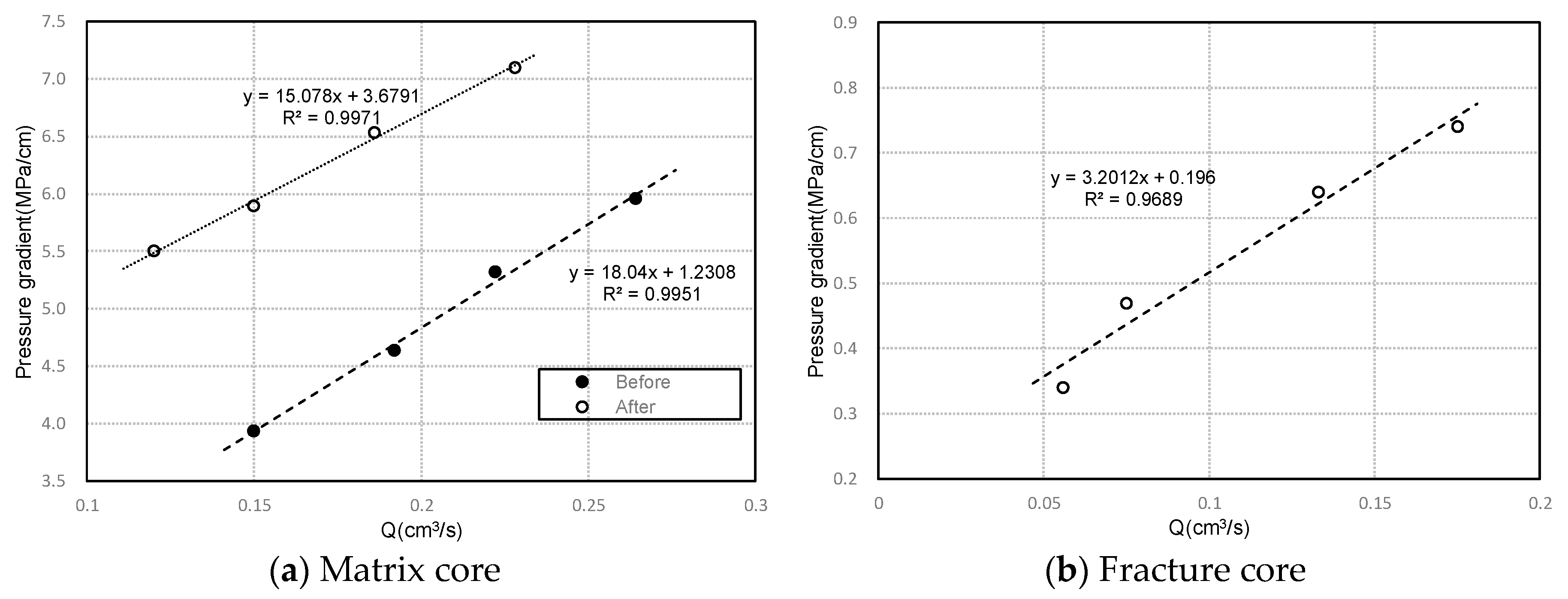
3.2.3. Comparing Experiments of Starting Pressure
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lea, J.F.; Nickens, H.V. Solving Gas-Well Liquid-Loading Problems. J. Pet. Technol. 2004, 56, 30–36. [Google Scholar] [CrossRef]
- Coleman, S.B.; Clay, H.B.; McCurdy, D.G.; Norris, L.H. A New Look at Predicting Gas-Well Load-Up. J. Pet. Technol. 1991, 43, 329–333. [Google Scholar] [CrossRef]
- Guo, B.; Ghalambor, A.; Xu, C. A Systematic Approach to Predicting Liquid Loading in Gas Wells. SPE Prod. Oper. 2005, 21, 81–88. [Google Scholar]
- Yusuf, R.; Group, S.P.T.; Veeken, K.; Hu, B. Investigation of Gas Well Liquid Loading with a Transient Multiphase Flow Model. In Proceedings of the SPE Oil and Gas India Conference and Exhibition, Mumbai, India, 20–22 January 2010. [Google Scholar]
- Lea, J.F., Jr.; Tighe, R.E. Gas Well Operation with Liquid Production. In Proceedings of the SPE Production Operations Symposium, Oklahoma City, OK, USA, 27 February–1 March 1983. [Google Scholar] [CrossRef]
- Oyewole, P.O.; Lea, J.F.; Llc, P.L.T.; Swabing, W.; Ejector, D.E. Artificial Lift Selection Strategy for the Life of a Gas Well with some Liquid Production. In Proceedings of the SPE Annual Technical Conference and Exhibition, Denver, CO, USA, 21–24 September 2008. [Google Scholar] [CrossRef]
- Boswell, J.T.; Hacksma, J.D. Controlling Liquid Load-Up With “Continuous Gas Circulation”. In Proceedings of the SPE Production Operations Symposium, Oklahoma City, OK, USA, 9–11 March 1997; pp. 325–332. [Google Scholar] [CrossRef]
- Willis, M.J.; Horsup, D.I.; Nguyen, D.T. Chemical Foamers for Gas Well Deliquification. In Proceedings of the SPE Asia Pacific Oil and Gas Conference and Exhibition, Perth, Australia, 20–22 October 2008. [Google Scholar] [CrossRef]
- Tang, Y.; Liang, Z. A New Method of Plunger Lift Dynamic Analysis and Optimal Design for Gas Well Deliquification. In Proceedings of the SPE Annual Technical Conference and Exhibition, Denver, CO, USA, 21–24 September 2008. [Google Scholar] [CrossRef]
- Tang, Y. Plunger Lift Dynamic Characteristics in Single Well and Network System for Tight Gas Well Deliquification. In Proceedings of the SPE Annual Technical Conference and Exhibition, New Orleans, LA, USA, 4–7 October 2009. [Google Scholar] [CrossRef]
- Gilbert, W. Flowing and gas-lift well performance. In Proceedings of the Drilling and Production Practice, New York, NY, USA, 1 January 1954; pp. 126–157. [Google Scholar]
- Hearn, W. Gas Well Deliquification Application Overview. In Proceedings of the Abu Dhabi International Petroleum Exhibition and Conference, Abu Dhabi, UAE, 1–4 November 2010. [Google Scholar] [CrossRef]
- Hutchins, R.D.; Dovan, H.T.; Sandiford, B.B. Field Applications of High Temperature Organic Gels for Water Control. In Proceedings of the SPE/DOE Improved Oil Recovery Symposium, Tulsa, OK, USA, 21–24 April 1996. [Google Scholar] [CrossRef]
- Al-Muntasheri, G.A.; Sierra, L.; Garzon, F.O.; Lynn, J.D.; Izquierdo, G.A. Water Shut-off With Polymer Gels in a High Temperature Horizontal Gas Well: A Success Story. In Proceedings of the SPE Improved Oil Recovery Symposium, Tulsa, OK, USA, 24–28 April 2010. [Google Scholar] [CrossRef]
- Zaitoun, A.; Kohler, N.; Marrast, J.; Guerrini, Y. On the use of polymers to reduce water production from gas wells. In Situ 1990, 14, 133–146. [Google Scholar]
- Avery, M.R.; Wells, T.A.; Chang, P.W.; Millican, J.D. Field Evaluation of a New Gelant for Water Control in Production Wells. In Proceedings of the SPE Annual Technical Conference and Exhibition, Houston, TX, USA, 2–5 October 1988. [Google Scholar] [CrossRef]
- Zaitoun, A.; Kohler, N. Two-Phase Flow Through Porous Media: Effect of an Adsorbed Polymer Layer. In Proceedings of the SPE Annual Technical Conference and Exhibition, Houston, TX, USA, 2–5 October 1988. [Google Scholar] [CrossRef]
- Lakatos, I.; Tóth, J.; Kosztin, B. Application of Silicone Microemulsion for Restriction of Water Production in Gas Wells. In Proceedings of the European Petroleum Conference, Aberdeen, UK, 29–31 October 2002. [Google Scholar] [CrossRef]
- Chaabouni, H.; Enkababian, P.G.; Chan, K.S.; Cheneviere, P.; Falxa, P.; Urbanczyk, C. Successful Innovative Water Shutoff Operations in Low-Permeability Gas Wells. In Proceedings of the SPE Middle East Oil and Gas Show and Conference, Manama, Bahrain, 11–14 March 2007. [Google Scholar] [CrossRef]


| Well | Na+ + K+ | Ca2+ | Mg2+ | Ba2+ | Cl− | SO42− | HCO3− | CO32− | OH− | PH |
|---|---|---|---|---|---|---|---|---|---|---|
| Well-I | 27389 | 1619 | 455 | 879 | 47556 | 0 | 385 | 0 | 0 | 6.56 |
| Well-II | 33318 | 2326 | 1586 | 0 | 59693 | 347 | 260 | 0 | 0 | 6.38 |
| Core No. | Length (cm) | Diameter (cm) | Porosity (%) | Permeability (mD) | Crack Volume (cm3) |
|---|---|---|---|---|---|
| 1 | 5.346 | 2.45 | 2.36 | 0.0007 | - |
| 2 | 5 | 2.45 | - | - | 1.12 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, X.; Liu, W.; Yang, L.; Zhou, X.; Yang, P. Experimental Study on Water Shutoff Technology Using In-Situ Ion Precipitation for Gas Reservoirs. Energies 2019, 12, 3881. https://doi.org/10.3390/en12203881
Zhang X, Liu W, Yang L, Zhou X, Yang P. Experimental Study on Water Shutoff Technology Using In-Situ Ion Precipitation for Gas Reservoirs. Energies. 2019; 12(20):3881. https://doi.org/10.3390/en12203881
Chicago/Turabian StyleZhang, Xu, Weihua Liu, Lilong Yang, Xiang Zhou, and Ping Yang. 2019. "Experimental Study on Water Shutoff Technology Using In-Situ Ion Precipitation for Gas Reservoirs" Energies 12, no. 20: 3881. https://doi.org/10.3390/en12203881
APA StyleZhang, X., Liu, W., Yang, L., Zhou, X., & Yang, P. (2019). Experimental Study on Water Shutoff Technology Using In-Situ Ion Precipitation for Gas Reservoirs. Energies, 12(20), 3881. https://doi.org/10.3390/en12203881