3.1. Physicochemical Properties and Fatty Acid Composition of the CCMO
The physicochemical properties of the
CCMO are summarized in
Table 5. The viscosity at 40 °C and the density at 15 °C were 32.83 mm
2/s and 920.6 kg/m
3, respectively. The acid number, the iodine number, and the flash point were 24.52 mg KOH/g, 122.6 mg I2/100 g, and 150.5 °C, respectively. The fatty acid composition of the
CCMO was determined according to the EN 14214 standard test method, and the results are presented in
Table 1.
CCMO contained a higher proportion of unsaturated fatty acids (68.3 wt. %) compared with saturated fatty acids (31.7 wt. %). Oleic acid (C18:1) appeared to be the dominant fatty acid for this oil, followed by palmitic acid (C16:0) and linoleic acid (C18:2), with values of 45.0, 22.8, and 22.4 wt. %, respectively. The acid value of crude
C. manghas oil was 13.41 mg KOH/g. The result obtained from this research was much higher than that presented by Ong et al. [
26], and the obtained viscosity of
CCMO in this study was similar with the previous study. It could be seen that
CCMO had very high viscosity. High viscosity indicated good lubrication properties, which are favorable for diesel engines due to the large molecular mass and the chemical structure However, high viscosity also causes problems, and especially regarding fuel flow and injection spray characteristics in the engine. It is recommended that crude oils should be converted to biodiesel through transesterification in order to minimize the viscosity.
The
CMME from
CCMO was successfully produced through the esterification-transesterification process. Indeed, it was found that the investigated parameters affected the acid value of the
C. manghas oil. Likewise, KOH catalyst concentration, methanol to oil molar ratio, reaction temperature, reaction time, and stirring speed affected the
CMME yield. Optimizing these process parameters maximized the conversion of
CCMO into
CMME. It was also found that the
CMME yield was dependent on the physicochemical properties of the
CCMO. Optimization of the process parameters for acid-catalyzed esterification and base-catalyzed transesterification was achieved using ANN models integrated with ACO. When performing ANNs, choosing a suitable number of neurons in the hidden layer in a cross-validation scheme in order to prevent overfitting to training data for the complexity of the experimental dataset was imperative [
25,
32]. The highest
C. Manghas methyl ester yield was obtained through optimization parameter of ANN coupled with ACO models for esterification and transesterification processes. To prove the credibility of the optimization results, the experimental data for the four input variables used for the esterification process and the five input variables used for the transesterification process were included.
In total, our data comprised 29 and 46 models for the esterification and the transesterification processes, respectively, using ANN. For both of these processes, 70% of the data points were used for training, whereas the remaining 30% were used for validation and testing (i.e., validation and testing phases each consisted of 15% of the data points). A multi-layer perception (MLP) network with one hidden layer and the Levenberg–Marquardt training algorithm were used for testing, which varied the number of neurons from five to 10. Thus, we determined the correlation coefficients (R) and the mean squared errors (MSE) used for all cases, as shown in
Table 6.
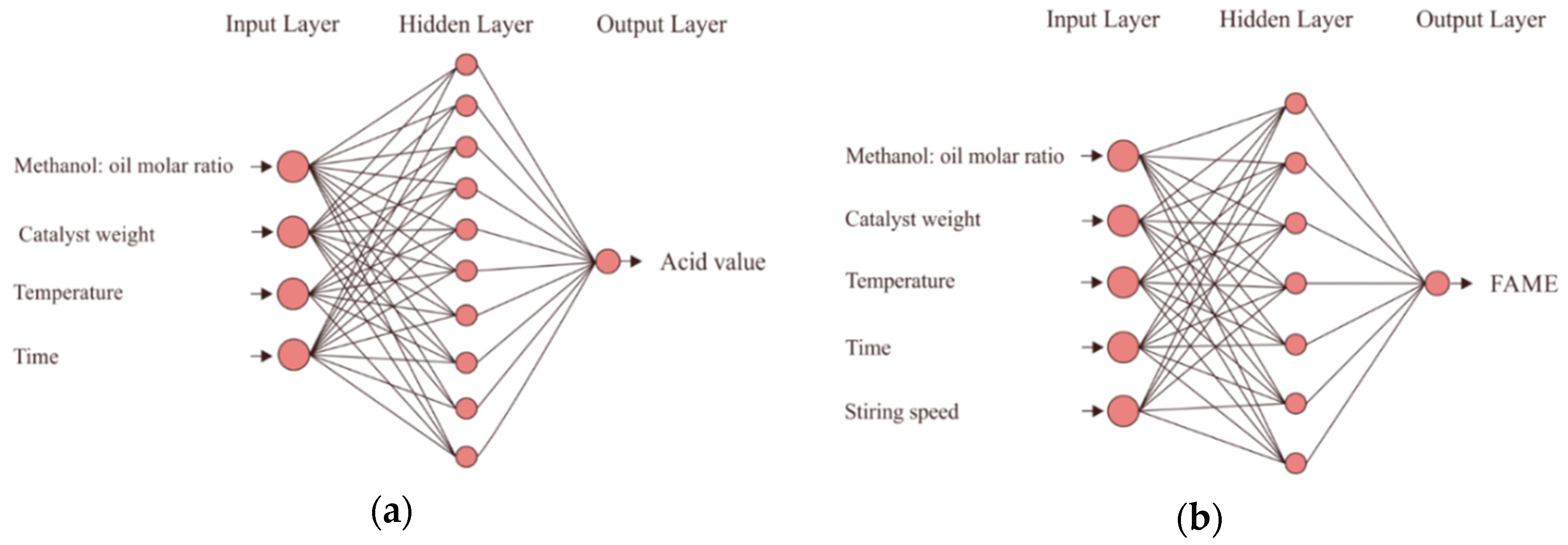
The neural network architecture for the esterification and the transesterification processes is shown in
Figure 1a,b, respectively. For the esterification process, the number of neurons in the input layer was four, whereas the number of neurons in the output layer was one (
Figure 1a). Here, the inputs were catalyst concentration, methanol to oil molar ratio, reaction temperature, and reaction time, whereas the output was the acid number of the
C. manghas oil.
There were five neurons in the input layers, whereas there was one output layer for the transesterification process, as shown in
Figure 1b. Here, the inputs were the same as those for the esterification process but with one additional input (stirring speed), and the output was the
CMME yield. The optimum numbers of hidden neurons were 4-10-1 and 5-7-1 for the esterification and the transesterification processes, respectively. The number of hidden neurons that resulted in the highest R and the lowest MSE represented the optimum number of hidden neurons, as shown in
Table 6.
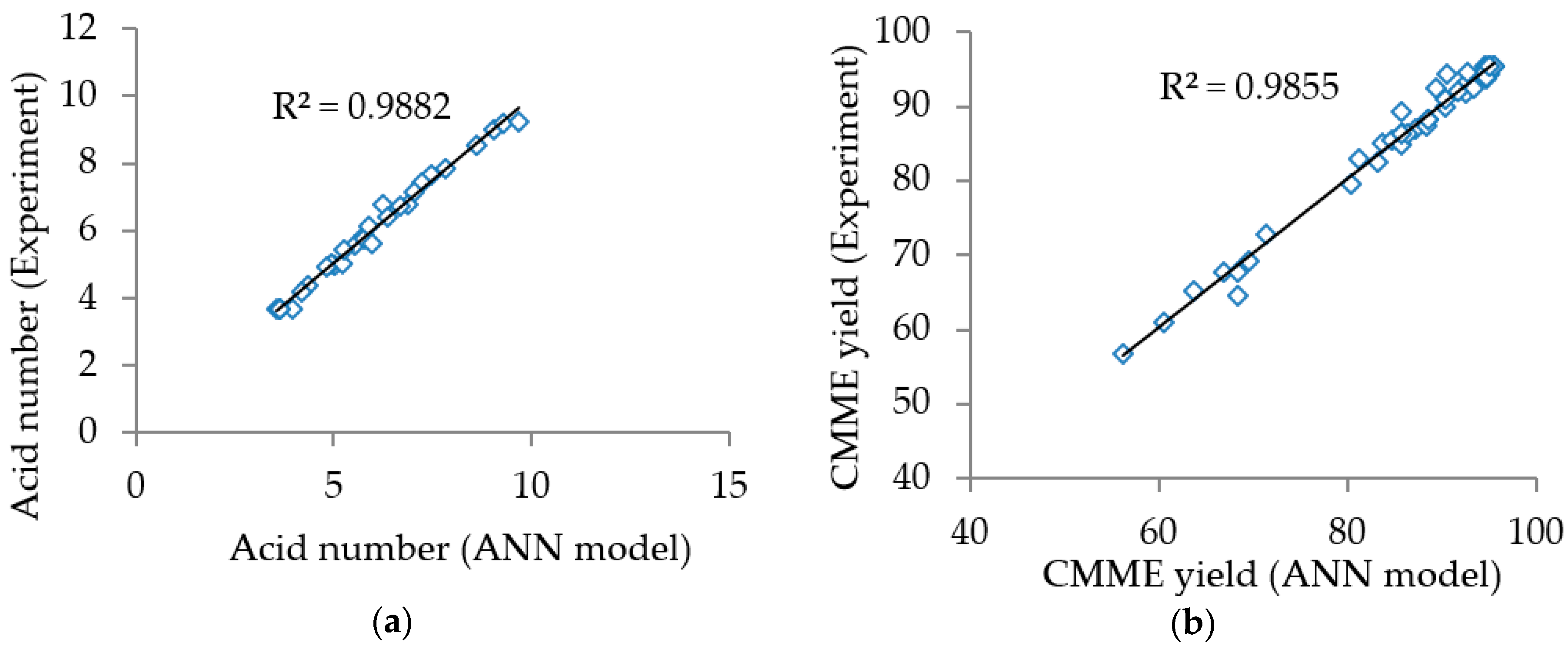
Figure 2a,b shows the acid number and the
CMME yield obtained from experiments plotted against those predicted by the ANN models for the esterification and the transesterification processes, respectively. The coefficients of determination (R
2) were 0.9882 and 0.9855 for esterification and transesterification, respectively. The high R
2 values (more than 95%) indicated that the ANN models described more than 95% of the variability in the acid number and the
CMME yield. In addition, there was excellent agreement between the experimental and the predicted data for both models judging from the plots, since the experimental values fell close to the regression lines. Hence, it could be concluded that the ANN models were reliable to predict the acid number and the
CMME yield in parameters affecting the process parameters for the esterification and the transesterification processes.
Next, the ANN model was integrated with ACO to predict the optimum process parameters for CMME production. For the esterification process, it was found that optimum H2SO4 catalyst concentration, methanol to oil molar ratio, reaction temperature, and reaction time were 0.94 vol %, 10.5:1, 54.5 °C, and 71 min, respectively. The acid value predicted by the ANN model under these optimum process parameters was 3.27 mg KOH/g. The ANN model was validated by conducting four independent experiments for the esterification process using these process parameters, and it was found that the average acid value of the C. manghas oil was 3.42 mg KOH/g.
For the transesterification process, it was found that optimum KOH catalyst concentration, methanol to oil molar ratio, reaction temperature, reaction time, and stirring speed were 1.1 wt. %, 10.9:1, 55 °C, 72 min, and 1100 rpm, respectively. The corresponding
CMME yield predicted by the ANN model was 98.72%. Likewise, the ANN model was validated by carrying out five independent experiments with these optimum process parameters, and it was found that the average
CMME yield was 97.89%. The results shown in
Figure 2 indicate that there was excellent agreement between experimental and predicted data for both acid value and
CMME yield. ANN coupled with ACO models were reliable in predicting the parameters involved for esterification and transesterification processes.
3.3. Kinetic Study
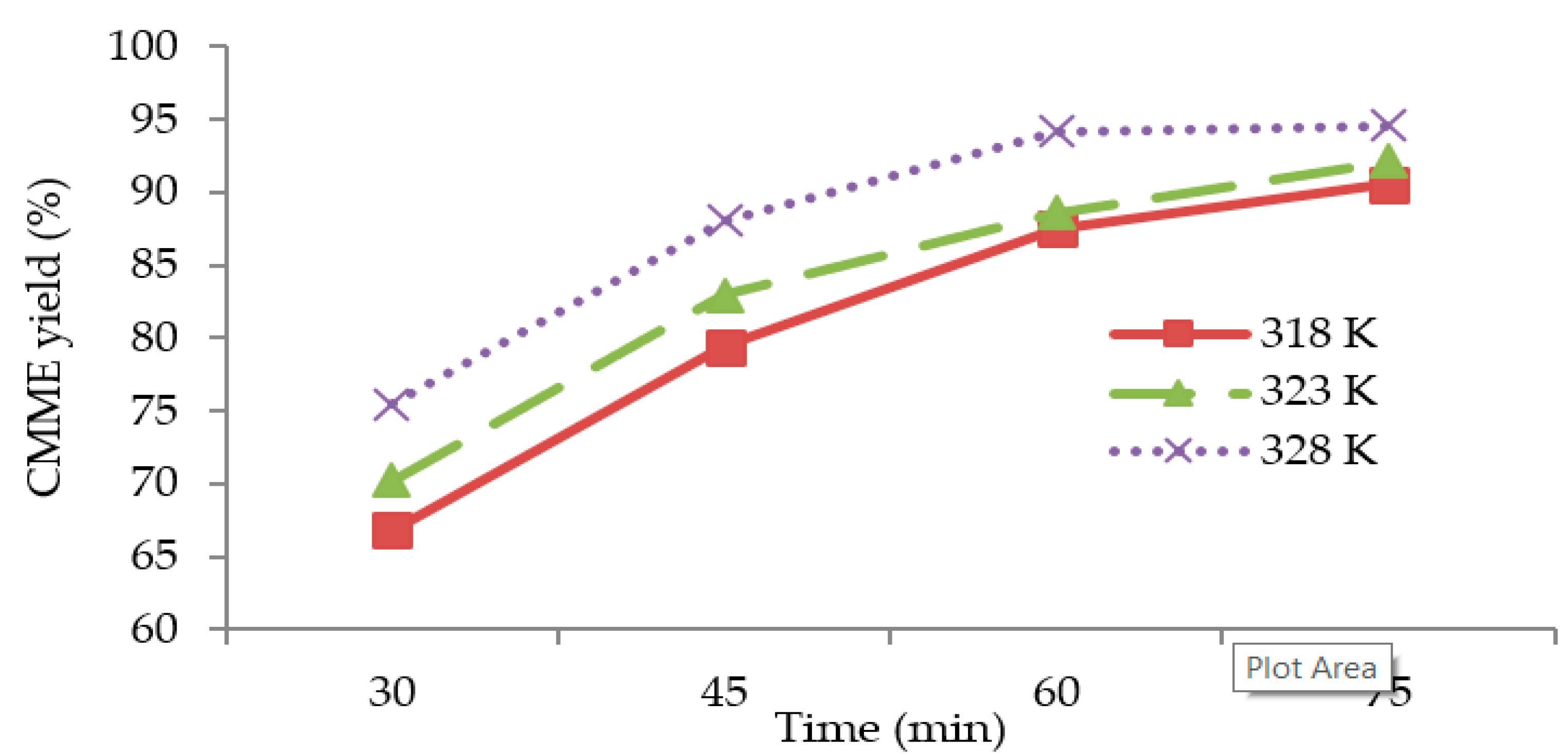
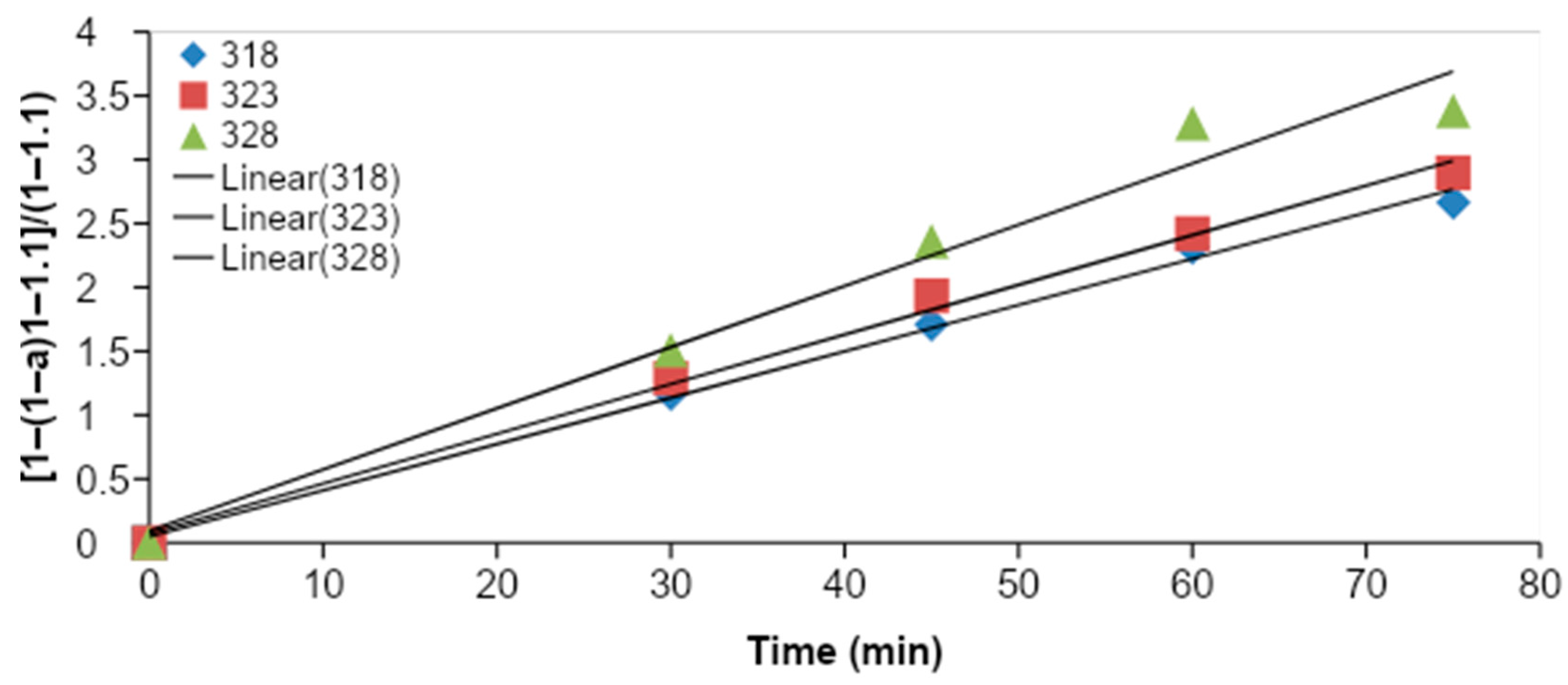
Figure 4 shows the
CMME yield as a function of the time for three different reaction temperatures (318, 323, and 328 K). The graphs were plotted based on optimum KOH catalyst concentration (1.2 wt %), methanol to oil molar ratio (11:1), and stirring speed (1000 rpm) for the transesterification process. It was observed that the transesterification process was characterized by a decreasing rate of reaction, since the slope of each plot was steeper during the first 45 min of transesterification, and the slope became flatter with an increase in time. It was observed that the
CMME yield was higher for higher reaction temperatures. The highest
CMME yield was obtained at 328 K. The higher
CMME yields at higher reaction temperatures may have been due to the higher global reaction rate constant, which was derived from the Arrhenius law.
Most transesterification processes involved a three-step reaction, where one mol of triglycerides required 3 mol of methanol and then produced three mol of methyl ester [
61,
62]. In this study, a one-step kinetic model was used to represent the complete transesterification reaction. The global reaction rate constant for the overall reaction was obtained. Hence, the three reaction steps (with formation of diglyceride, monoglyceride, and glycerol) combined into a single step [
63]. The highest
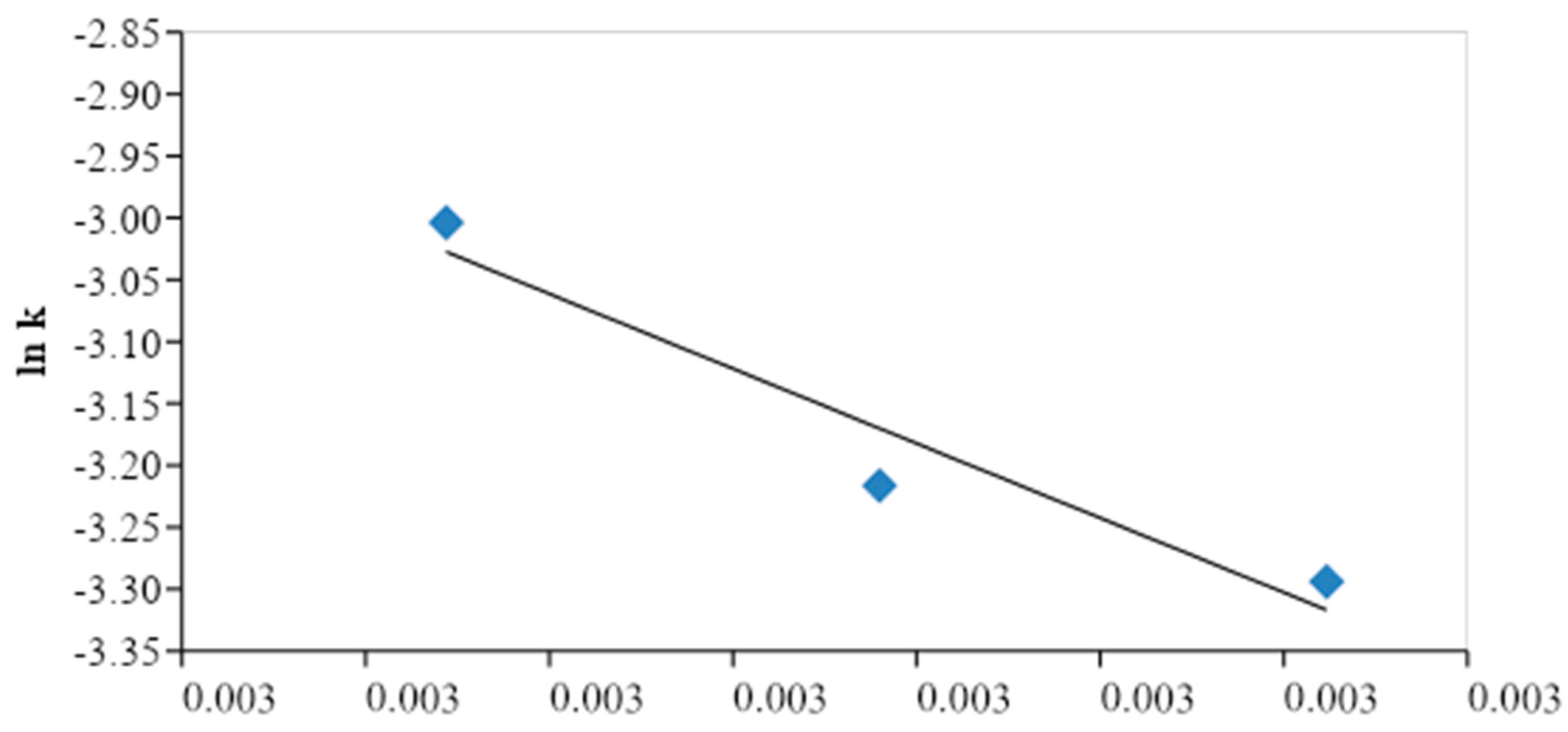
CMME yield found was attained when the KOH concentration and the methanol to oil molar ratio were 2.0 wt. % and 6.03:1, respectively. We plotted ln
k versus 1/
T plot and determined the activation energy and the frequency factor, which are represented by the slope and the intercept of plot, respectively. It was found that the activation energy was 79 kJ/mol, whereas the frequency factor was 2.98 (1010) min
-1. The activation energy for transesterification of the
C. manghas oil was within the acceptable range (33.6–84.0 kJ/mol). However, the decrease in the transesterification reaction may have been due to the slow reaction rate and the deactivation of the KOH catalyst with an increase in time as well as contamination of the KOH catalyst, which reduced the reactivity of the reactants.
3.6. Physicochemical Properties of the CMME
The physicochemical properties of the
CMME were produced and measured under optimum conditions in accordance with the ASTM D6751 and the EN 14214 standard test methods, and the results are summarized in
Table 9.
It was observed that the physicochemical properties of the CMME were improved, and the results obtained were optimized process parameters predicted by the developed ANN models for esterification and transesterification processes. The results indicate that it is imperative to optimize the process parameters for esterification and transesterification processes in order to enhance the properties of the CMME.
The kinematic viscosity at 40 °C of the
CMME was 3.92 mm
2/s. The density at 15 °C of the
CMME was 838.5 kg/m
3, which was within the range (860–880 kg/m
3) given in the ASTM D6751 standard. In addition, the acid value of the
CMME was 0.15 mg KOH/g, which was significantly lower than the maximum limit (0.50 mg KOH/g) stated in the ASTM D6751 standard [
24,
25,
54]. From
Table 9, it can be seen that
CCMO obtained in the present study possessed lower kinematic viscosity, density, and acid value compared to previous results. Moreover, it was found that the optimization process resulted in a slight improvement of the properties of
CMME.
The flash point of the CMME was 150.5 °C, which was lower compared to the previous study of 159.5 °C. This is an important property, as this indicates that biodiesel is not flammable and is safe to be used, especially during transportation, handling, and storage. Therefore, C. manghas biodiesel is desirable because it has a higher flash point than diesel, which reduces the risk of fire hazards when the biodiesel is stored and transported.
The Fatty acid Methyl Ester content of the CMME was 99.5 wt. %, which was slightly higher than the minimum value (96.5 wt. %) specified in the ASTM D6751 standard. CMME consisted of 73.0 wt. % of unsaturated Fatty Acid Methyl Ester compared with 26.5 wt. % of saturated Fatty Acid Methyl Ester content. The major Fatty Acid Methyl Ester contents of CMME were oleic acid (59.94%), followed by palmitic acid (18.75 wt. %), linoleic acid (13.06%), and stearic acid (7.78 wt. %). In general, the physicochemical properties of the CMME fulfilled the fuel specifications stipulated in the ASTM D6751 standard. This indicates that C. manghas oil is a potential feedstock for biodiesel production, provided that the optimum process parameters are used for esterification and transesterification.