Screening of Spontaneous Ignition Feasibility During Air Injection EOR Process Based on Thermal Experiments
Abstract
1. Introduction
2. Methodology
2.1. Spontaneous Ignition Theory
2.2. Application of the Frank-Kamenetskii Theory
2.3. Experimental Method and Kinetic Theory
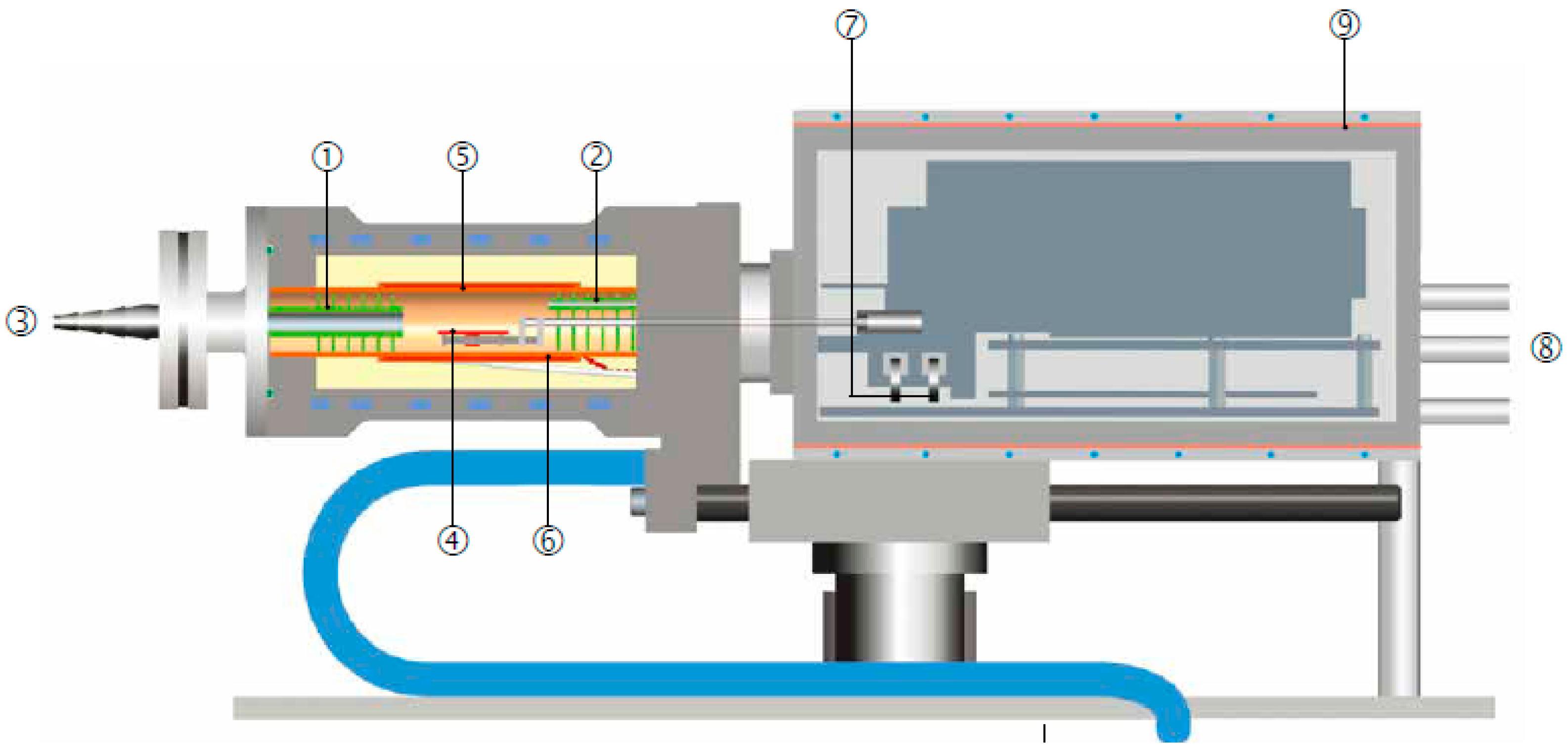
2.3.1. Experimental Method
2.3.2. Kinetic Theory
3. Application of the Proposed Method
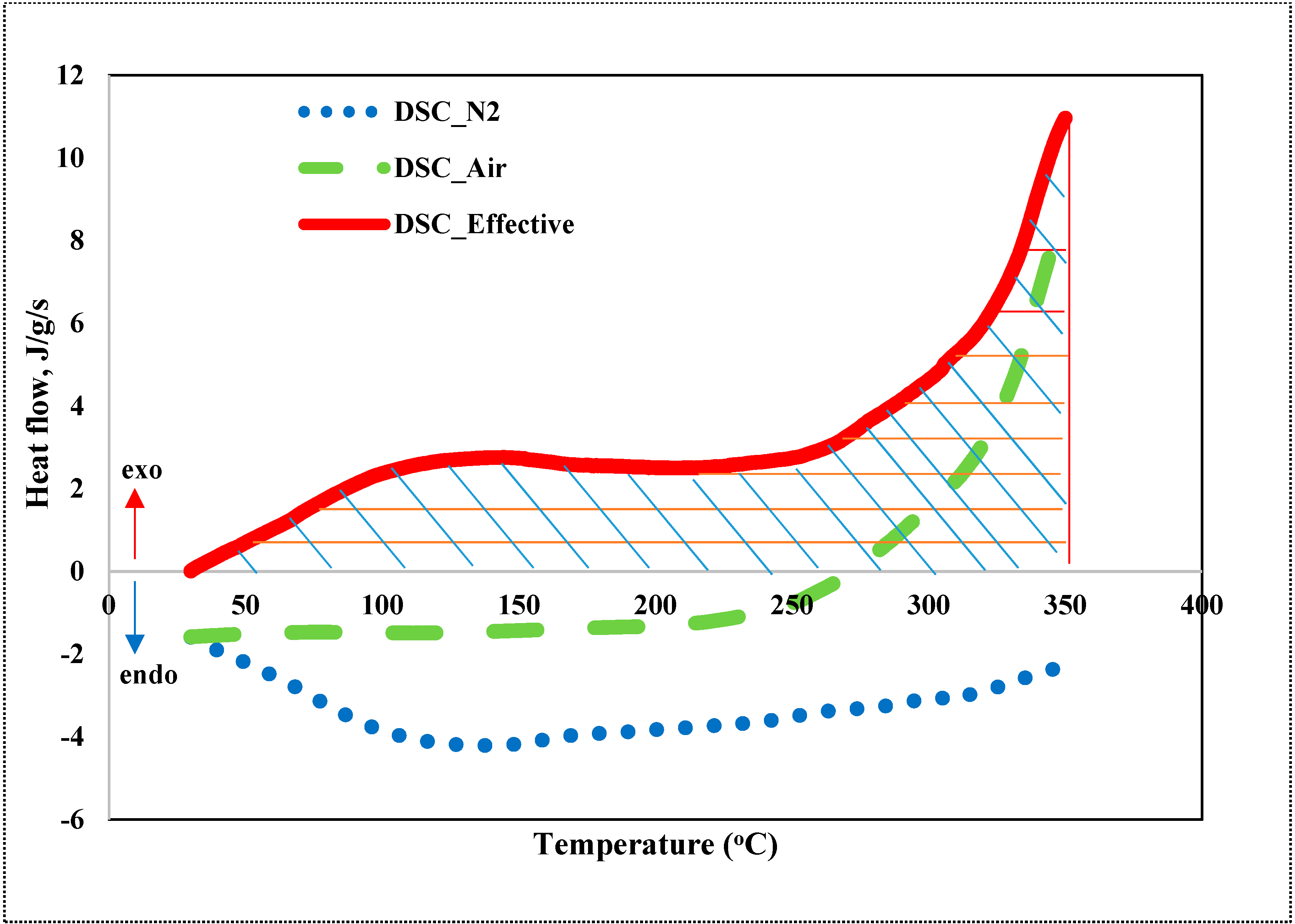
3.1. Experiment and Kinetic Analysis of a Mixture of Sand with Crude Oil
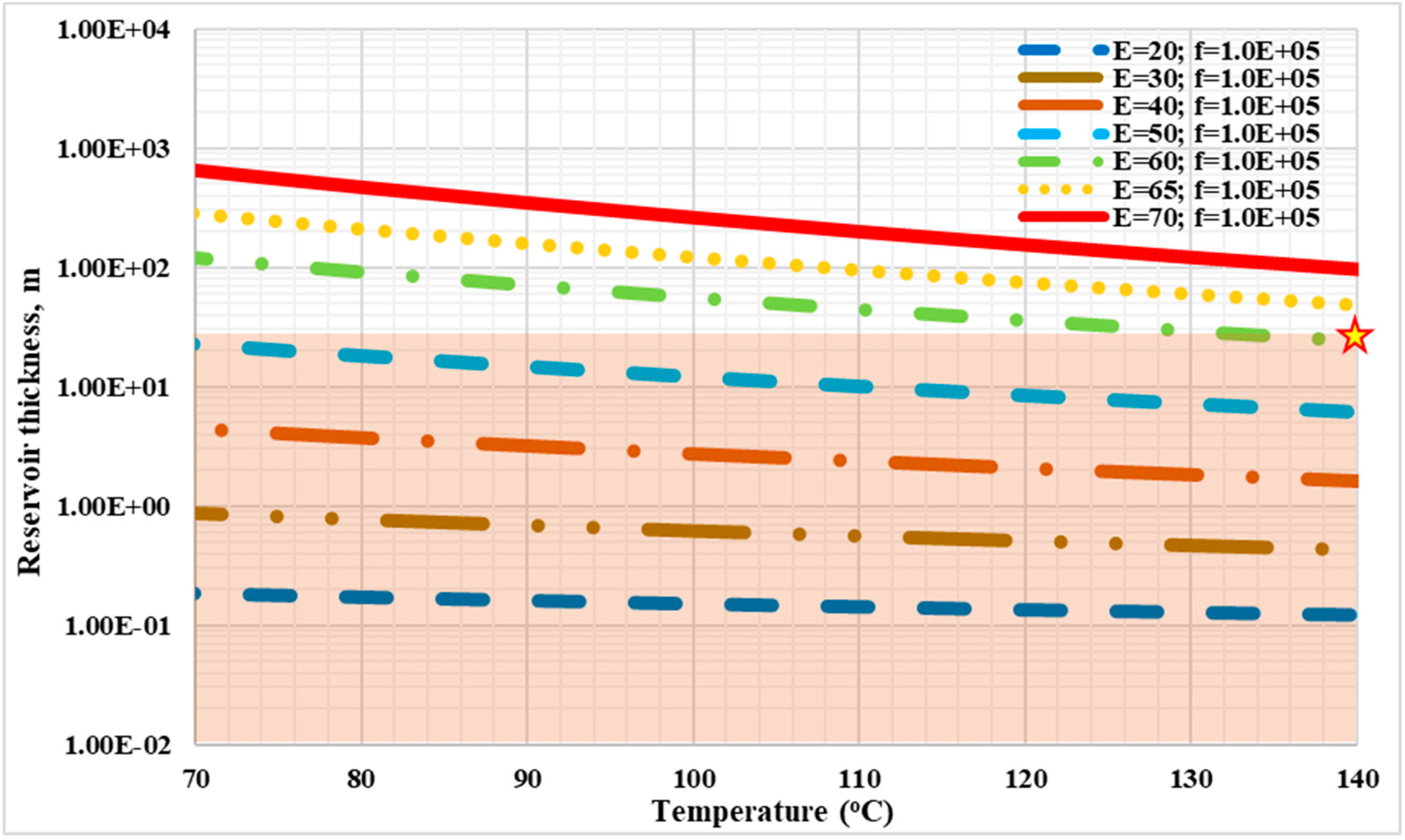
3.2. Spontaneous Ignition Feasibility by the Frank-Kamenetskii Method
4. Discussion of Screening Criteria for Spontaneous Ignition during AIP
5. Conclusions
- ➢
- The Frank-Kamenetskii method can be applied as an effective method to screen crude oil for AIP in terms of spontaneous ignition feasibility, based on thermal experiments such as TGA and DSC.
- ➢
- The proposed method could screen out the mixture of oil and sand that cannot achieve spontaneous ignition due to an insufficient reactivity. However, if the tested sample shows the potential to achieve spontaneous ignition, further experiments need to be applied in order to prove the existence of spontaneous ignition.
- ➢
- At a possible reservoir condition with a temperature from 70 °C to 140 °C and a reservoir thickness from 1 m to 30 m, in order to achieve spontaneous ignition for an AIP the activation energy of the crude oil should be less than 60 kJ/mole, and the frequency factor should be greater than 2 s−1.
Author Contributions
Funding
Conflicts of Interest
Nomenclature
| α | Fractional conversion |
| β | Heating rate, k/min |
| A | Arrhenius constant, min−1 |
| E | Activation energy, J/mol |
| f(co) | value of the mass action law, mole/(m3·s) |
| H | The fraction of the enthalpy yet to be released, kJ |
| k | Temperature dependent rate constant. |
| L | one half of the smallest dimension of the body, m |
| mi | Initial mass of the sample, mg. |
| mf | The final mass of the sample, mg. |
| mt | The sample mass at temperature T, mg. |
| n | The order of reaction. |
| Q | heat of reaction per fuel mass, W/m3 |
| R | Gas constant, 8.314 J/mol K |
| Tp | Peak temperature, K |
| Ta | Ambient temperature, K. |
| Z | Pre-exponential factor, 1/s. |
References
- Kumar, V.K.; Gutierrez, D.; Moore, R.G.; Mehta, S.A. Case history and appraisal of the West Buffalo Red River Unit high-pressure air injection project. In Proceedings of the Hydrocarbon Economics and Evaluation Symposium, Paper SPE 107715, Dallas, TX, USA, 1–3 April 2007; pp. 1–3. [Google Scholar]
- Gutiérrez, D.; Miller, R.J.; Taylor, A.R.; Thies, B.P.; Kumar, V.K. Buffalo Field High-Pressure Air Injection Projects: Technical Performance and Operational Challenges (SPE-113254). In Proceedings of the SPE/DOE Symposium on Improved Oil Recovery, Tulsa, OK, USA, 19–23 April 2008; pp. 20–23. [Google Scholar]
- Gates, C.F.; Ramey , H.J., Jr. Field results of south belridge thermal recovery experiment. J. Petroleum Technol. 1958, 213, 236–244. [Google Scholar]
- Gutierrez, D.; Skoreyko, F.; Moore, R.G.; Mehta, S.A.; Ursenbach, M.G. The challenge of predicting field performance of air injection projects based on laboratory and numerical modelling. J. Can. Pet. Technol. 2009, 48, 23–33. [Google Scholar] [CrossRef]
- Gray, B.F. Spontaneous combustion and self-heating. In SFPE Handbook of Fire Protection Engineering; Springer: New York, NY, USA, 2016; pp. 604–632. [Google Scholar]
- Abu-Khamsin, S.A.; Iddris, A.; Aggour, M.A. The spontaneous ignition potential of a super-light crude oil. Fuel 2001, 80, 1415–1420. [Google Scholar] [CrossRef]
- Jia, H.; Zhao, J.Z.; Pu, W.F.; Li, Y.M.; Yuan, Z.T.; Yuan, C.D. Laboratory investigation on the feasibility of light-oil autoignition for application of the high-pressure air injection (HPAI) process. Energy Fuels 2012, 26, 5638–5645. [Google Scholar] [CrossRef]
- Huang, S.; Sheng, J.J. Discussion of thermal experiments’ capability to screen the feasibility of air injection. Fuel 2017, 195, 151–164. [Google Scholar] [CrossRef]
- Stracher, G.B.; Taylor, T.P. Coal fires burning out of control around the world: Thermodynamic recipe for environmental catastrophe. Int. J. Coal Geol. 2004, 59, 7–17. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, X.; Sugai, Y.; Sasaki, K. Determination of critical self-ignition temperature of low-rank coal using a 1 m wire-mesh basket and extrapolation to industrial coal piles. Energy Fuels 2017, 31, 6700–6710. [Google Scholar] [CrossRef]
- Restuccia, F.; Huang, X.; Rein, G. Self-ignition of natural fuels: Can wildfires of carbon-rich soil start by self-heating? Fire Saf. J. 2017, 91, 828–834. [Google Scholar] [CrossRef]
- Restuccia, F.; Ptak, N.; Rein, G. Self-heating behavior and ignition of shale rock. Combust. Flame 2017, 176, 213–219. [Google Scholar] [CrossRef]
- Drysdale, D. An Introduction to Fire Dynamics; John Wiley & Sons: Hoboken, NJ, USA, 2011. [Google Scholar]
- Huang, S.; Jia, H.; Sheng, J.J. Research on oxidation kinetics of tight oil from Wolfcamp field. Pet. Sci. Technol. 2016, 34, 903–910. [Google Scholar] [CrossRef]
- Huang, S.; Jia, H.; Sheng, J.J. Effect of shale core on combustion reactions of tight oil from Wolfcamp reservoir. Pet. Sci. Technol. 2016, 34, 1172–1179. [Google Scholar] [CrossRef]
- Huang, S.; Jia, H.; Sheng, J.J. Exothermicity and oxidation behavior of tight oil with cuttings from the Wolfcamp shale reservoir. Pet. Sci. Technol. 2016, 34, 1735–1741. [Google Scholar] [CrossRef]
- Huang, S.; Sheng, J.J. An innovative method to build a comprehensive kinetic model for air injection using TGA/DSC experiments. Fuel 2017, 210, 98–106. [Google Scholar] [CrossRef]
- Jia, H.; Ni, J.; Pu, W.; Yue, P.; Jiang, H.; Yang, J. New view on the oxidation mechanisms of crude oil through combined thermal analysis methods. J. Therm. Anal. Calorim. 2014, 118, 1707–1714. [Google Scholar] [CrossRef]
- Jia, H.; Zhao, J.Z.; Pu, W.F.; Zhao, J.; Kuang, X.Y. Thermal study on light crude oil for application of high-pressure air injection (HPAI) process by TG/DTG and DTA tests. Energy Fuels 2012, 26, 1575–1584. [Google Scholar] [CrossRef]
- Ren, S.R.; Greaves, M.; Rathbone, R.R. Oxidation kinetics of North Sea light crude oils at reservoir temperature. Chem. Eng. Res. Des. 1999, 77, 385–394. [Google Scholar] [CrossRef]
- Hou, S.M.; Liu, Y.H.; Yu, H.M.; Niu, B.L.; Ren, S.R. Kinetics of low temperature oxidation of light oil in air injection process. J. China Univ. Pet. 2011, 35, 169–173. [Google Scholar]
- Huang, S.; Sheng, J.J. A practical method to obtain kinetic data from TGA (thermogravimetric analysis) experiments to build an air injection model for enhanced oil recovery. Fuel 2017, 206, 199–209. [Google Scholar] [CrossRef]
- Gundogar, A.S.; Kok, M.V. Thermal characterization, combustion and kinetics of different origin crude oils. Fuel 2014, 123, 59–65. [Google Scholar] [CrossRef]
- Stadler, M.P.; Deo, M.D.; Orr, F.M., Jr. Crude oil characterization using gas chromatography and supercritical fluid chromatography. In Proceedings of the SPE International Symposium on Oilfield Chemistry, New Orleans, LA, USA, 2–5 March 1993. [Google Scholar]
- Jia, H.; Sheng, J.J. Discussion of the feasibility of air injection for enhanced oil recovery in shale oil reservoirs. Petroleum 2017, 3, 249–257. [Google Scholar] [CrossRef]



| f | k | Q | E | |||
|---|---|---|---|---|---|---|
| / | 1/s | mole/m3 | mole/(m3 × s) | W/(m × K) | kJ/kg | kJ/mole |
| 0.878 | 86,300 | 4.736 | 408,685 | 0.5 | 6040 | 69 |
| k | Q | E | ||
|---|---|---|---|---|
| / | mole/m3 | W/(m × K) | kJ/kg | kJ/mole |
| 0.878 | 4.736 | 0.5 | 6040 | 69 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, S.; Sheng, J.J.; Jiang, Q.; Liu, J. Screening of Spontaneous Ignition Feasibility During Air Injection EOR Process Based on Thermal Experiments. Energies 2019, 12, 3687. https://doi.org/10.3390/en12193687
Huang S, Sheng JJ, Jiang Q, Liu J. Screening of Spontaneous Ignition Feasibility During Air Injection EOR Process Based on Thermal Experiments. Energies. 2019; 12(19):3687. https://doi.org/10.3390/en12193687
Chicago/Turabian StyleHuang, Siyuan, James J. Sheng, Qi Jiang, and Jiali Liu. 2019. "Screening of Spontaneous Ignition Feasibility During Air Injection EOR Process Based on Thermal Experiments" Energies 12, no. 19: 3687. https://doi.org/10.3390/en12193687
APA StyleHuang, S., Sheng, J. J., Jiang, Q., & Liu, J. (2019). Screening of Spontaneous Ignition Feasibility During Air Injection EOR Process Based on Thermal Experiments. Energies, 12(19), 3687. https://doi.org/10.3390/en12193687






