Production of Hydrogen-Rich Gas by Formic Acid Decomposition over CuO-CeO2/γ-Al2O3 Catalyst
Abstract
:1. Introduction
2. Materials and Methods
3. Results
3.1. Catalyst Characterization
3.2. Catalytic Performance of CuO-CeO2/γ-Al2O3 in FA Decomposition
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Chandan, A.; Hattenberger, M.; El-kharouf, A.; Du, S.; Dhir, A.; Self, V.; Pollet, B.G.; Ingram, A.; Bujalski, W. High temperature (HT) polymer electrolyte membrane fuel cells (PEMFC)—A review. J. Power Sources 2013, 231, 264–278. [Google Scholar] [CrossRef]
- Quartarone, E.; Mustarelli, P. Polymer fuel cells based on polybenzimidazole/H3PO4. Energy Environ. Sci. 2012, 5, 6436–6444. [Google Scholar] [CrossRef]
- Rosli, R.E.; Sulong, A.B.; Daud, W.R.W.; Zulkifley, M.A.; Husaini, T.; Rosli, M.I.; Majlan, E.H.; Haque, M.A. A review of high-temperature proton exchange membrane fuel cell (HT-PEMFC) system. Int. J. Hydrogen Energy 2017, 42, 9293–9314. [Google Scholar] [CrossRef]
- Li, D.; Li, X.; Gong, J. Catalytic Reforming of Oxygenates: State of the Art and Future Prospects. Chem. Rev. 2016, 116, 11529–11653. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Palo, D.R.; Dagle, R.A.; Holladay, J.D. Methanol Steam Reforming for Hydrogen Production. Chem. Rev. 2007, 107, 3992–4021. [Google Scholar] [CrossRef]
- Sá, S.; Silva, H.; Brandão, L.; Sousa, J.M.; Mendes, A. Catalysts for methanol steam reforming—A review. Appl. Catal. B. 2010, 99, 43–57. [Google Scholar] [CrossRef]
- Galvita, V.; Semin, G.; Belyaev, V.; Yurieva, T.; Sobyanin, V. Production of hydrogen from dimethyl ether. Appl. Catal. A. 2001, 216, 85–90. [Google Scholar] [CrossRef]
- Semelsberger, T.A.; Ott, K.C.; Borup, R.L.; Greene, H.L. Generating hydrogen-rich fuel-cell feeds from dimethyl ether (DME) using Cu/Zn supported on various solid-acid substrates. Appl. Catal. A. 2006, 309, 210–223. [Google Scholar] [CrossRef]
- Volkova, G.; Badmaev, S.; Belyaev, V.; Plyasova, L.; Budneva, A.; Paukshtis, E.; Zaikovsky, V.; Sobyanin, V. Bifunctional catalysts for hydrogen production from dimethyl ether. Stud. Surf. Sci. Catal. 2007, 167, 445–450. [Google Scholar]
- Sun, Q.; Auroux, A.; Shen, J. Surface acidity of niobium phosphate and steam reforming of dimethoxymethane over CuZnO/Al2O3–NbP complex catalysts. J. Catal. 2006, 244, 1–9. [Google Scholar] [CrossRef]
- Fu, Y.; Shen, J. Production of hydrogen by catalytic reforming of dimethoxymethane over bifunctional catalysts. J. Catal. 2007, 248, 101–110. [Google Scholar] [CrossRef]
- Badmaev, S.D.; Pechenkin, A.A.; Belyaev, V.D.; Ven’yaminov, S.A.; Snytnikov, P.V.; Sobyanin, V.A.; Parmon, V.N. Steam reforming of dimethoxymethane to hydrogen-rich gas for fuel cell feeding application. Dokl. Phys. Chem. 2013, 452, 251–253. [Google Scholar] [CrossRef]
- Pechenkin, A.A.; Badmaev, S.D.; Belyaev, V.D.; Sobyanin, V.A. Performance of bifunctional CuO–CeO2/γ-Al2O3 catalyst in dimethoxymethane steam reforming to hydrogen-rich gas for fuel cell feeding. Appl. Catal. B. 2015, 166, 535–543. [Google Scholar] [CrossRef]
- Badmaev, S.D.; Pechenkin, A.A.; Belyaev, V.D.; Sobyanin, V.A. Hydrogen production by steam reforming of dimethoxymethane over bifunctional CuO-ZnO/γ-Al2O3 catalyst. Int. J. Hydrogen Energy 2015, 40, 14052–14057. [Google Scholar] [CrossRef]
- Joó, F. Breakthroughs in Hydrogen Storage-Formic Acid as a Sustainable Storage Material for Hydrogen. ChemSusChem 2008, 1, 805–808. [Google Scholar] [CrossRef] [PubMed]
- Bulushev, D.A.; Beloshapkin, S.; Ross, J.R.H. Hydrogen from formic acid decomposition over Pd and Au catalysts. Catal. Today 2010, 154, 7–12. [Google Scholar] [CrossRef]
- Zhou, X.; Huang, Y.; Xing, W.; Liu, C.; Liao, J.; Lu, T. High-quality hydrogen from the catalyzed decomposition of formic acid by Pd–Au/C and Pd–Ag/C. Chem. Commun. 2008, 30, 3540–3542. [Google Scholar] [CrossRef]
- Tedsree, K.; Li, T.; Jones, S.; Chan, C.W.A.; Yu, K.M.K.; Bagot, P.A.J.; Marquis, E.A.; Smith, G.D.W.; Tsang, S.C.E. Hydrogen production from formic acid decomposition at room temperature using a Ag–Pd core–shell nanocatalyst. Nat. Nanotechnol. 2011, 6, 302–307. [Google Scholar] [CrossRef]
- Fellay, C.; Dyson, P.; Laurenczy, G. A Viable Hydrogen-Storage System Based On Selective Formic Acid Decomposition with a Ruthenium Catalyst. Angew. Chem. Int. Ed. 2008, 47, 3966–3968. [Google Scholar] [CrossRef]
- Bulushev, D.A.; Chuvilin, A.L.; Sobolev, V.I.; Stolyarova, S.G.; Shubin, Y.V.; Asanov, I.P.; Ishchenko, A.V.; Magnani, G.; Riccò, M.; Okotrub, A.V.; et al. Copper on carbon materials: Stabilization by nitrogen doping. J. Mater. Chem. A. 2017, 5, 10574–10583. [Google Scholar] [CrossRef]
- Gazsi, A.; Bánsági, T.; Solymosi, F. Decomposition and Reforming of Formic Acid on Supported Au Catalysts: Production of CO-Free H2. J. Phys. Chem. C. 2011, 115, 15459–15466. [Google Scholar] [CrossRef]
- Solymosi, F.; Koós, Á.; Liliom, N.; Ugrai, I. Production of CO-free H2 from formic acid. A comparative study of the catalytic behavior of Pt metals on a carbon support. J. Catal. 2011, 279, 213–219. [Google Scholar] [CrossRef]
- Iglesia, E.; Boudart, M. Decomposition of formic acid on copper, nickel, and copper-nickel alloys: II. Catalytic and temperature-programmed decomposition of formic acid on Cu/SiO2, Cu/Al2O3, and Cu powder. J. Catal. 1983, 81, 214–223. [Google Scholar] [CrossRef]
- Caputo, T.; Lisi, L.; Pirone, R.; Russo, G. On the role of redox properties of CuO/CeO2 catalysts in the preferential oxidation of CO in H2-rich gases. Appl. Catal. A. Gen. 2008, 348, 42–53. [Google Scholar] [CrossRef]
- Jiang, X.Y.; Lou, L.P.; Chen, Y.X.; Zheng, X.M. Effects of CuO/CeO2 and CuO/γ-Al2O3 catalysts on NO + CO reaction. J. Mol. Catal. A. Chem. 2003, 197, 193–205. [Google Scholar]
- Menon, U.; Poelman, H.; Bliznuk, V.; Galvita, V.V.; Poelman, D.; Marin, G.B. Nature of the active sites for the total oxidation of toluene by CuO-CeO2/Al2O3. J. Catal. 2012, 295, 91–103. [Google Scholar] [CrossRef]

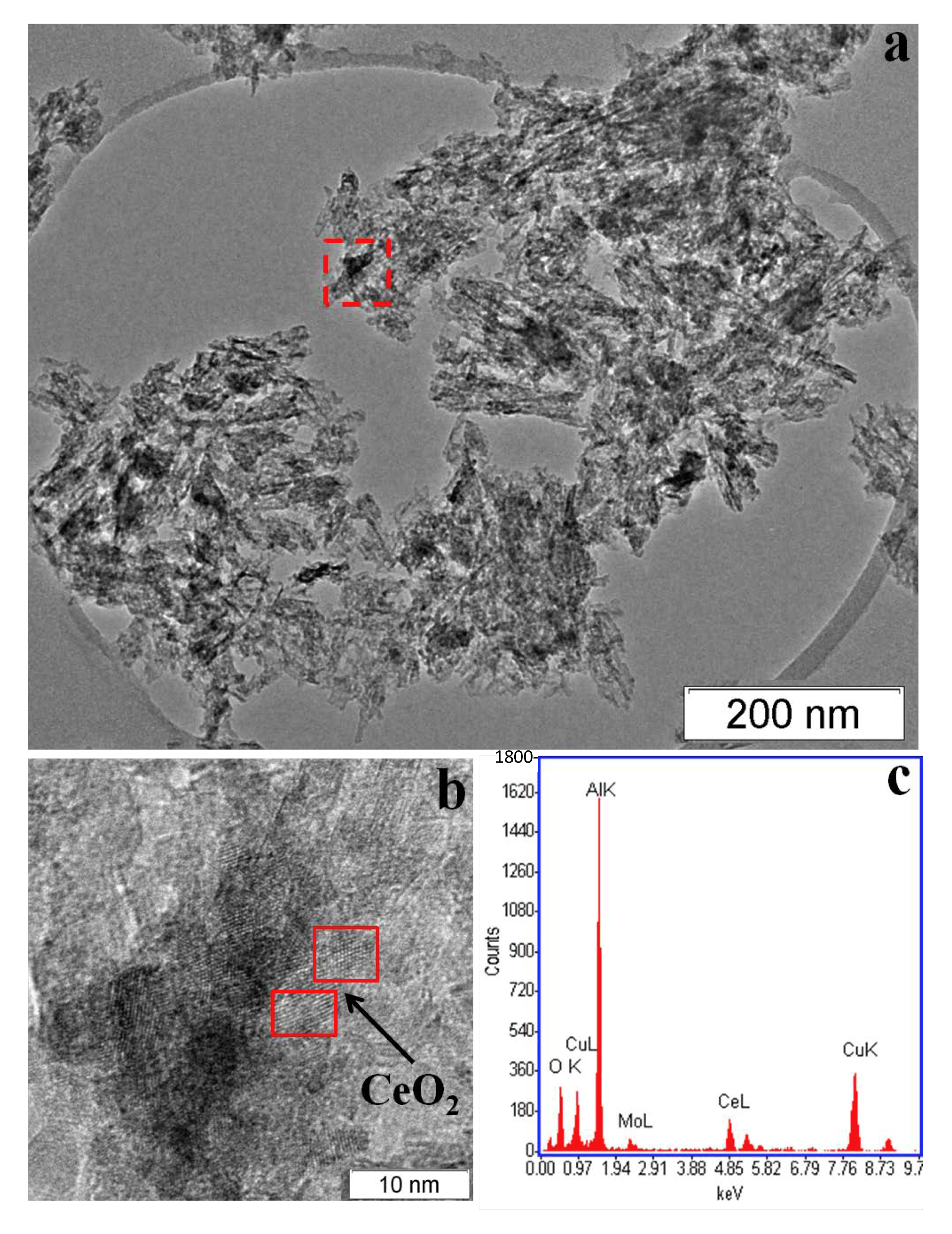

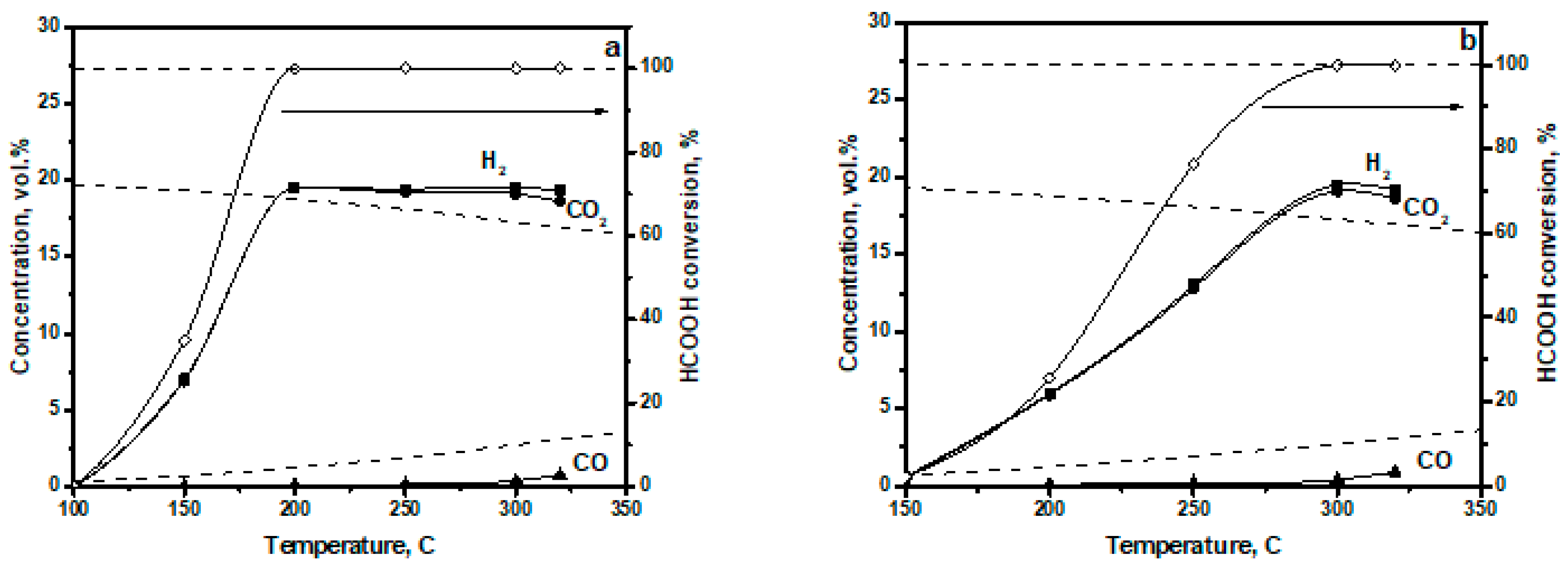
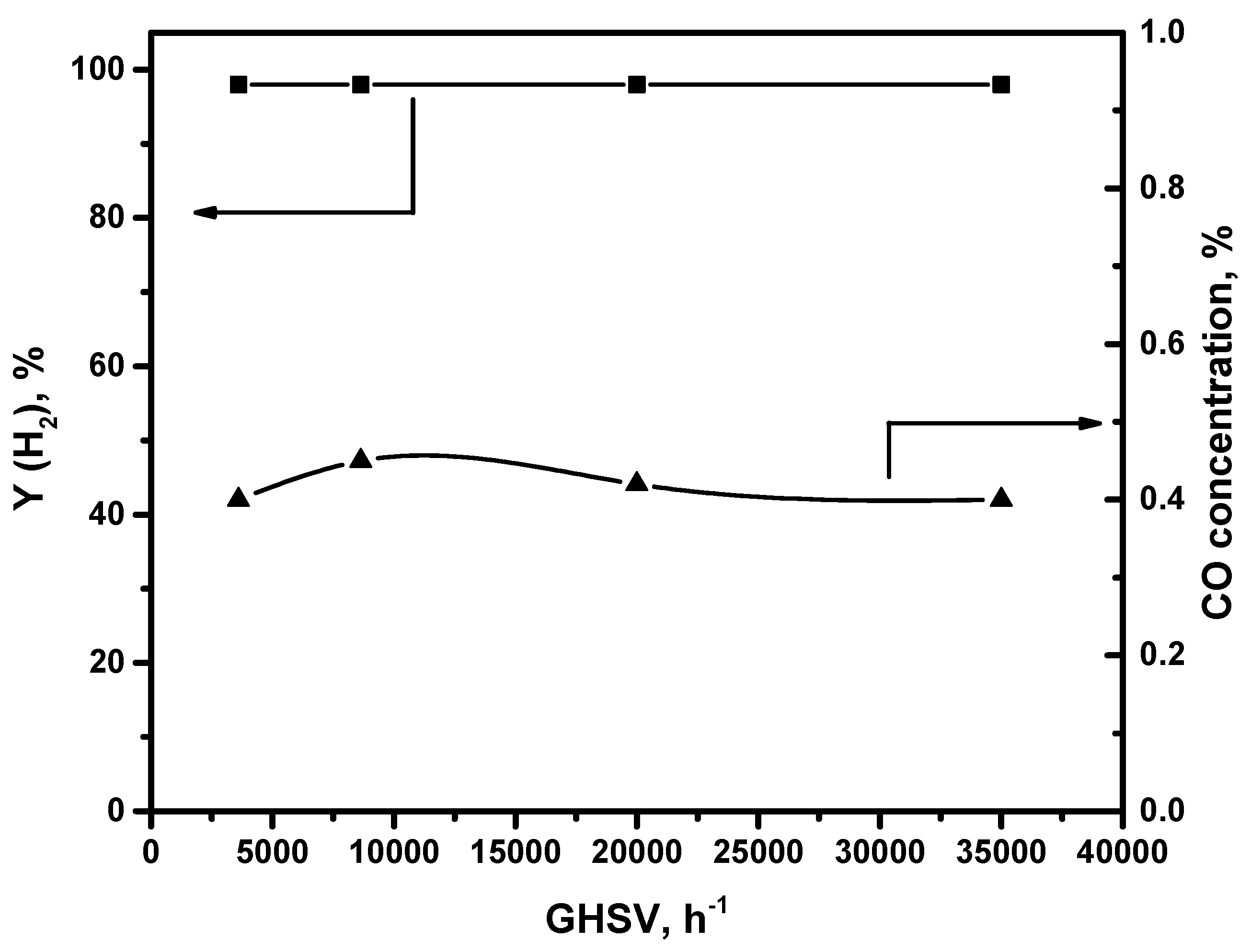
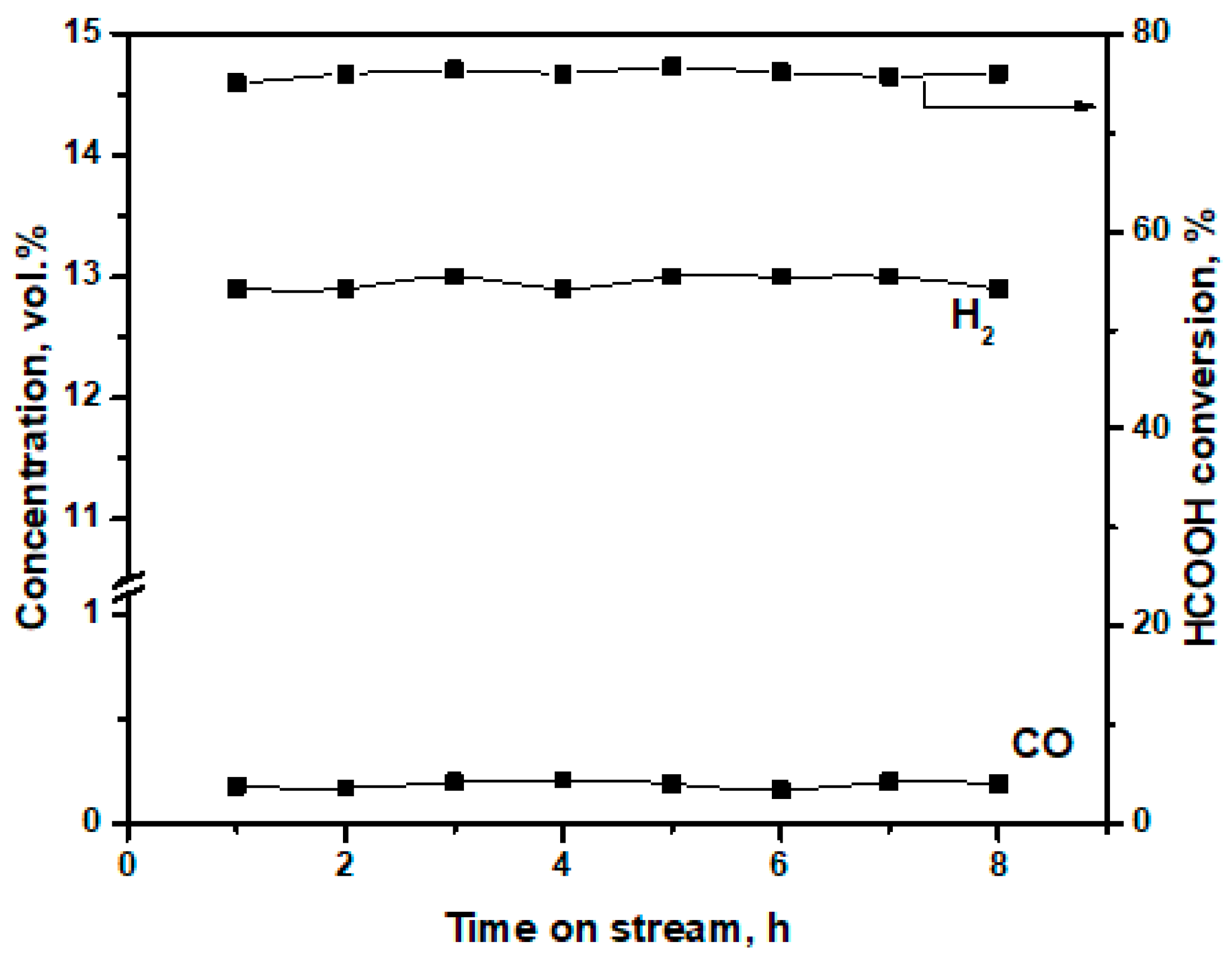
| Catalyst | Phase Composition | Unit Cell Parameters, Å | CSR (Å) |
|---|---|---|---|
| Fresh | γ-Al2O3 | 7.918 | 50 |
| CeO2 | 5.390 | 40 | |
| Used | γ-Al2O3 | 7.918 | 50 |
| CeO2 | 5.395 | 30 | |
| Cu | n.d. | highly dispersed |
| Catalysts | T, °C | Reaction Condition | Y (H2), % | Refs | |
|---|---|---|---|---|---|
| HCOOH: Inert vol.%:vol.% | FA Flow Rate, h−1 | ||||
| 10% CuO-5% CeO2/γ-Al2O3 | 200 | 25:75 | 875 | 98 | This work |
| 300 | 25:75 | 8750 | 98 | ||
| Cu/C | 270 | 5:95 | 3150 1 | 97.4 | [20] |
| 1% Au/SiO2 | 250 | 7:93 | 560 | 99.5 | [21] |
| 2% Ir/C 2% Pt/C 2% Rh/C 2% Pd/C | 200 | 5:95 | 400 | 99.5 98.6 97 91.7 | [22] |
| Reactions | Inlet Composition | T | GHSV | Y (H2) | CO | W (H2) | Refs |
|---|---|---|---|---|---|---|---|
| vol.% | °C | h−1 | % | vol.% | l/(h gcat) | ||
| DMM SR | C3H8O2:H2O:N2 = 14:70:16 | 300 | 10,000 | 95 | 0.5 | 15.5 | [12] |
| DME SR | C2H6O:H2O:N2 = 20:60:20 | 370 | 90 | 1 | 15 | ||
| Methanol SR | CH3OH:H2O:N2 = 40:40:20 | 300 | 95 | 1 | 15 | ||
| FA decomposition | HCOOH:N2 = 25:75 | 300 | 35,000 | 98 | 0.45 | 15 | This work |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pechenkin, A.; Badmaev, S.; Belyaev, V.; Sobyanin, V. Production of Hydrogen-Rich Gas by Formic Acid Decomposition over CuO-CeO2/γ-Al2O3 Catalyst. Energies 2019, 12, 3577. https://doi.org/10.3390/en12183577
Pechenkin A, Badmaev S, Belyaev V, Sobyanin V. Production of Hydrogen-Rich Gas by Formic Acid Decomposition over CuO-CeO2/γ-Al2O3 Catalyst. Energies. 2019; 12(18):3577. https://doi.org/10.3390/en12183577
Chicago/Turabian StylePechenkin, Alexey, Sukhe Badmaev, Vladimir Belyaev, and Vladimir Sobyanin. 2019. "Production of Hydrogen-Rich Gas by Formic Acid Decomposition over CuO-CeO2/γ-Al2O3 Catalyst" Energies 12, no. 18: 3577. https://doi.org/10.3390/en12183577
APA StylePechenkin, A., Badmaev, S., Belyaev, V., & Sobyanin, V. (2019). Production of Hydrogen-Rich Gas by Formic Acid Decomposition over CuO-CeO2/γ-Al2O3 Catalyst. Energies, 12(18), 3577. https://doi.org/10.3390/en12183577




