An Experimental Study on the Heat and Mass Transfer of Liquid Nitrogen in a Loose Medium
Abstract
:1. Introduction
2. Experimental Setup and Methods
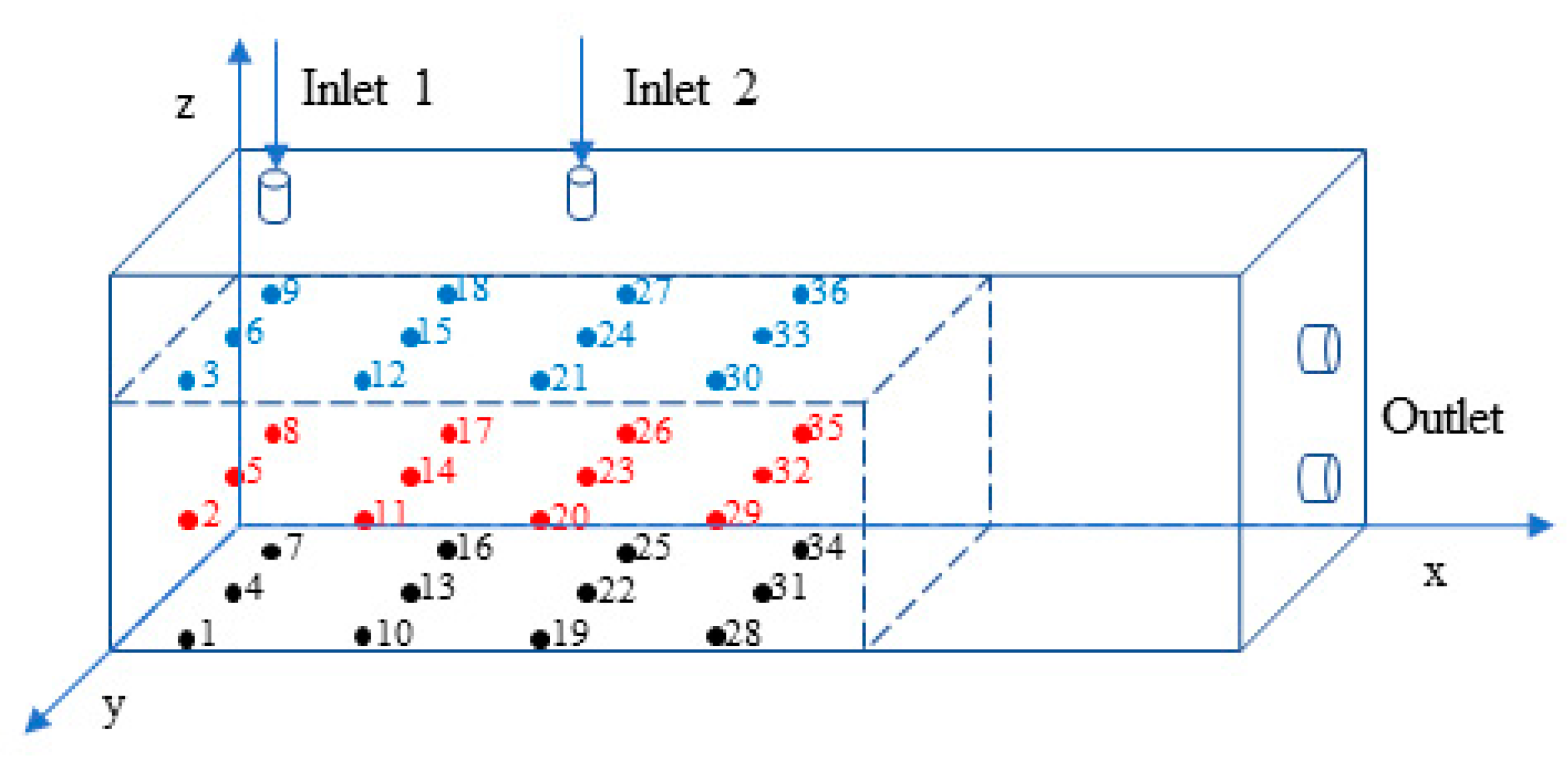
2.1. Experimental Setup
2.2. Experimental Procedure and Scheme
2.3. Leidenfrost Effect
3. Results and Discussion
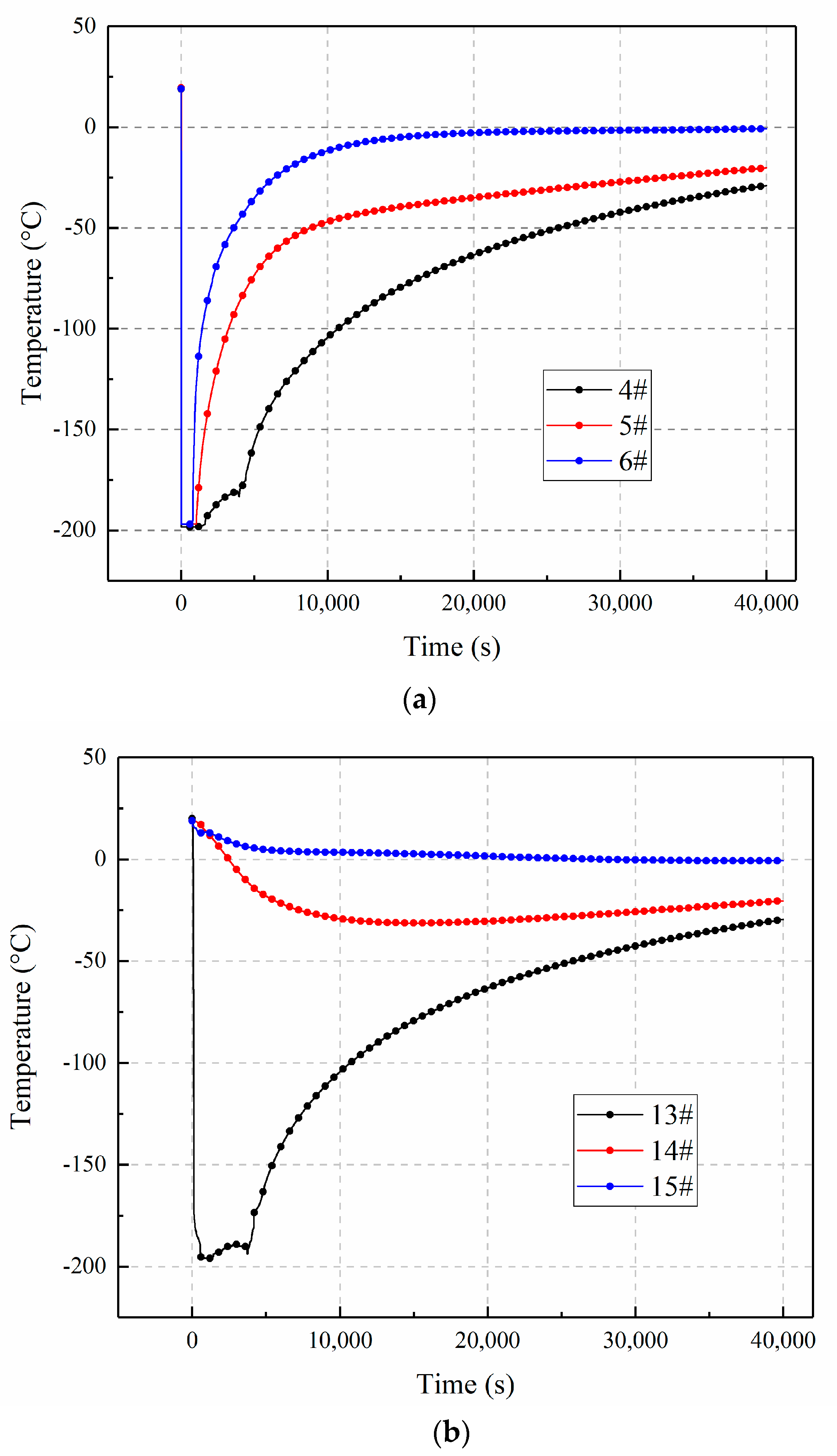
3.1. The Temperature Distributions in a Loose Medium along the X-Axis
3.2. The Temperature Distributions in a Loose Medium along the Z-Axis
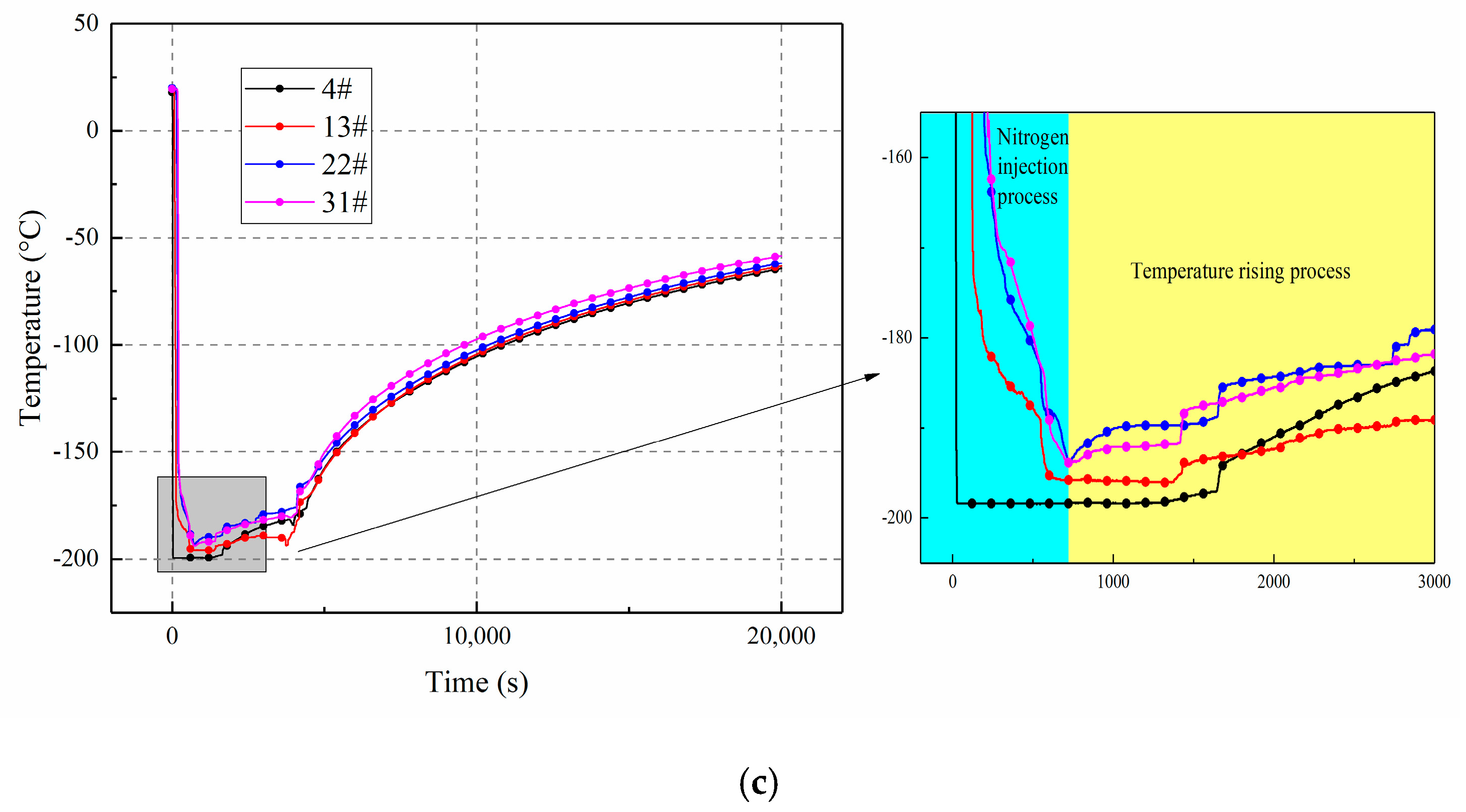
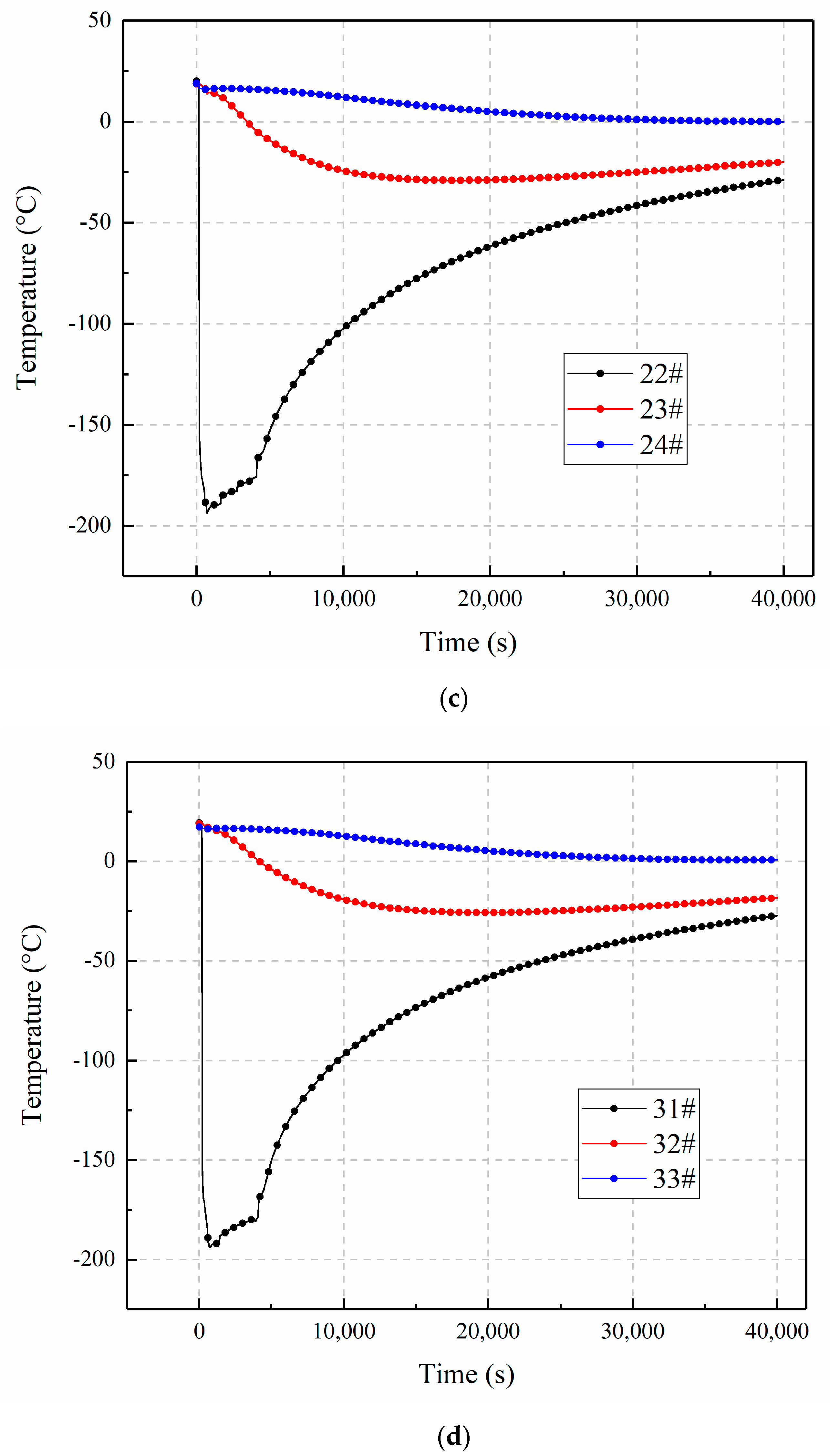
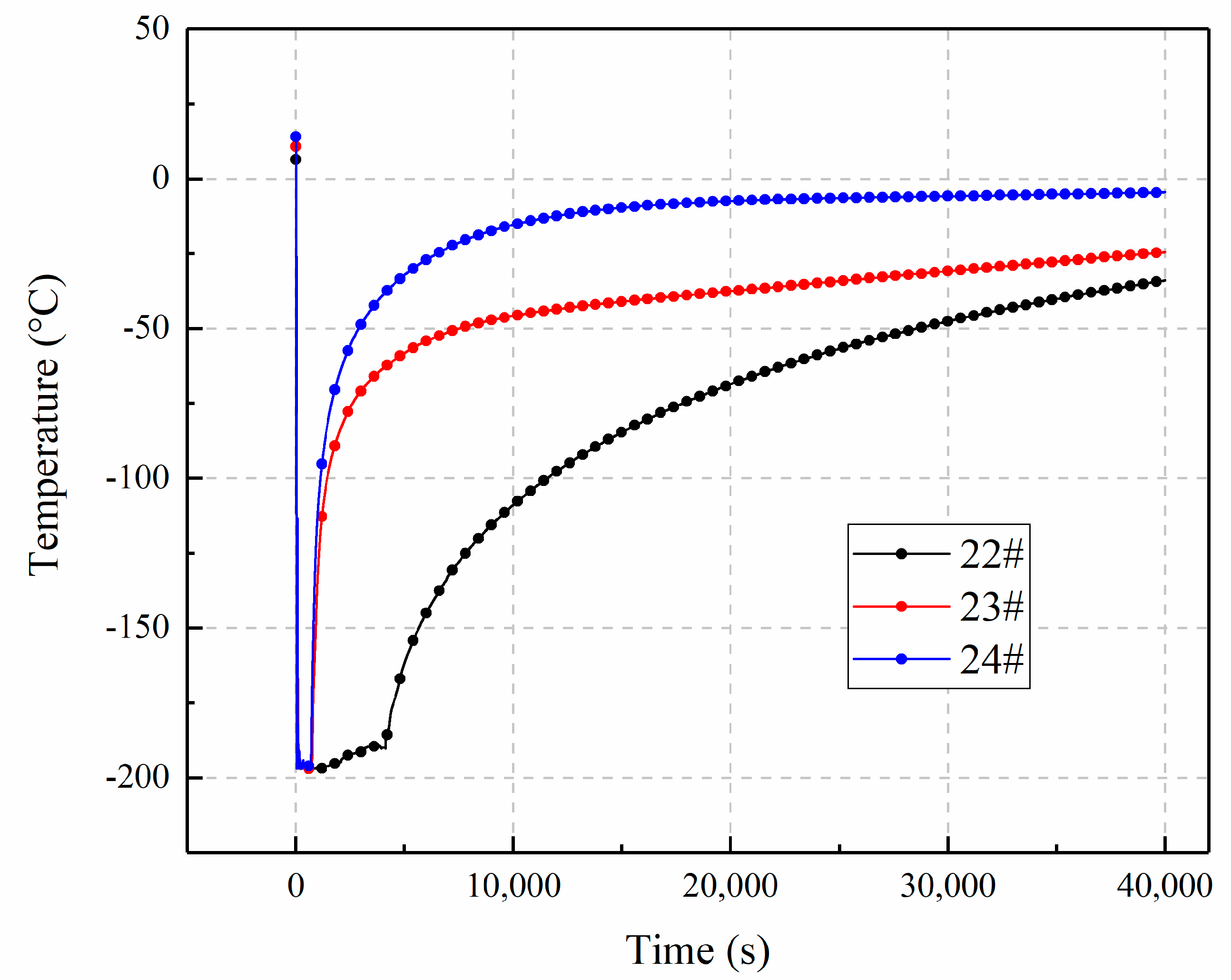
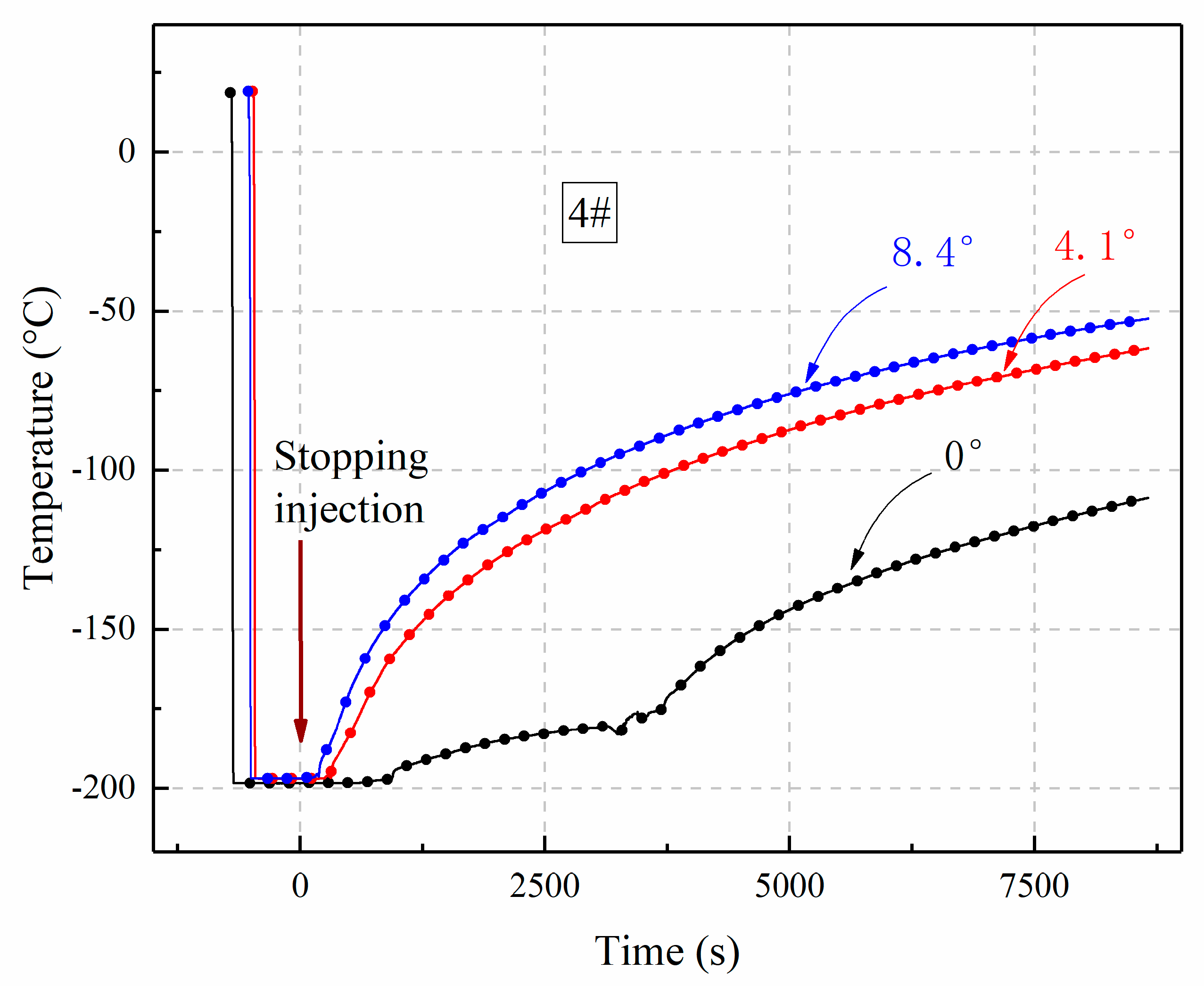
3.3. The Influence of Inclination Angle Variation on Temperature
3.4. Effective Chilldown Zone
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Li, X.; Wang, H.; Liu, B. Chemical characteristics of coal gangue as filter material in simulated coal mine goaf. Asian J. Chem. 2013, 25, 9409–9410. [Google Scholar] [CrossRef]
- Liang, Y.; Wang, S. Prediction of coal mine goaf self-heating with fluid dynamics in porous media. Fire Saf. J. 2017, 87, 49–56. [Google Scholar] [CrossRef]
- Yang, Y.; Li, Z.; Si, L.; Hou, S.; Li, Z.; Li, J. Study on test method of heat release intensity and thermophysical parameters of loose coal. Fuel 2018, 229, 34–43. [Google Scholar] [CrossRef]
- Yuan, L.; Smith, A.C. The effect of ventilation on spontaneous heating of coal. J. Loss Prev. Process Ind. 2012, 25, 131–137. [Google Scholar] [CrossRef]
- Song, Z.; Kuenzer, C. Fires in China over the last decade: A comprehensive review. Int. J. Coal Geol. 2014, 133, 72–99. [Google Scholar] [CrossRef]
- Kong, B.; Li, Z.; Yang, Y.; Liu, Z.; Yan, D. A review on the mechanism, risk evaluation, and prevention of coal spontaneous combustion in China. Environ. Sci. Pollut. Res. 2017, 23453–23470. [Google Scholar] [CrossRef]
- Wang, G.; Wang, Y.; Sun, L.; Song, X.; Liu, Q.; Xu, H.; Du, W. Study on the low-temperature oxidation law in the co-mining face of coal and oil shale in a goaf–A case study in the Liangjia coal mine, China. Energies 2018, 11, 174. [Google Scholar] [CrossRef]
- Du, W.; Wang, G.; Wang, Y.; Liu, X. Thermal degradation of bituminous coal with both model-free and model-fitting methods. Appl. Therm. Eng. 2019, 152, 169–174. [Google Scholar] [CrossRef]
- Feng, G.; Wu, Y.; Zhang, C.; Hu, S.; Shao, H.; Xu, G.; Ren, X.; Wang, Z. Changes on the low-temperature oxidation characteristics of coal after CO2 adsorption: A case study. J. Loss Prev. Process Ind. 2017, 49, 536–544. [Google Scholar] [CrossRef]
- Hao, C.; Chen, Y.; Wang, J.; Deng, C.; Xu, G.; Dai, F.; Si, R.; Wang, H.; Wang, H. Study on the effect of iron-based deoxidizing inhibitors for coal spontaneous combustion prevention. Energies 2018, 11, 789. [Google Scholar] [CrossRef]
- Li, S.; Zhou, G.; Wang, Y.; Jing, B.; Qu, Y. Synthesis and characteristics of fire extinguishing gel with high water absorption for coal mines. Process Saf. Environ. Prot. 2019, 125, 207–218. [Google Scholar] [CrossRef]
- Qin, B.; Lu, Y.; Li, Y.; Wang, D. Aqueous three-phase foam supported by fly ash for coal spontaneous combustion prevention and control. Adv. Powder Technol. 2014, 25, 1527–1533. [Google Scholar] [CrossRef]
- Wang, G.; Yan, G.; Zhang, X.; Du, W.; Huang, Q.; Sun, L.; Zhang, X. Research and development of foamed gel for controlling the spontaneous combustion of coal in coal mine. J. Loss Prev. Process Ind. 2016, 44, 474–486. [Google Scholar] [CrossRef]
- Zhou, F.; Ren, W.; Wang, D.; Song, T.; Li, X.; Zhang, Y. Application of three-phase foam to fight an extraordinarily serious coal mine fire. Int. J. Coal Geol. 2006, 67, 95–100. [Google Scholar] [CrossRef]
- Dong, S.; Lu, X.; Wang, D.; Wang, H.; Zheng, K.; Shi, Q.; Chen, M. Experimental investigation of the fire-fighting characteristics of aqueous foam in underground goaf. Process Saf. Environ. Prot. 2017, 106, 239–245. [Google Scholar] [CrossRef]
- Wu, J.; Yan, H.; Wang, J.; Wu, Y.; Zhou, C. Flame retardant polyurethane elastomer nanocomposite applied to coal mines as air-leak sealant. J. Appl. Polym. Sci. 2013, 129, 3390–3395. [Google Scholar] [CrossRef]
- Cheng, W.; Hu, X.; Xie, J.; Zhao, Y. An intelligent gel designed to control the spontaneous combustion of coal: Fire prevention and extinguishing properties. Fuel 2017, 210, 826–835. [Google Scholar] [CrossRef]
- Shi, B.; Zhou, G.; Ma, L. Normalizing fire prevention technology and a ground fixed station for underground mine fires using liquid nitrogen: A case study. Fire Technol. 2018, 54, 1887–1893. [Google Scholar] [CrossRef]
- Zhou, F.; Shi, B.; Cheng, J.; Ma, L. A new approach to control a serious mine fire with using liquid nitrogen as extinguishing media. Fire Technol. 2015, 51, 325–334. [Google Scholar]
- Shi, B.; Zhou, F. Impact of heat and mass transfer during the transport of nitrogen in coal porous media on coal mine fires. Sci. World J. 2014, 2014. [Google Scholar] [CrossRef]
- Lu, L.; Xin, C.; Liu, X. Heat and mass transfer of liquid nitrogen in coal porous media. Heat Mass Transf. 2017, 54, 1101–1111. [Google Scholar]
- Adamus, A. Review of the use of nitrogen in mine fires. Min. Technol. 2014, 111, 89–98. [Google Scholar] [CrossRef]
- Yu, Z. Study on The Process of Heat and Mass Transfer in Loose Coal with Phase Transition for Liquid Carbon Dioxide. Ph.D. Thesis, Xi’an University of Science and Technology, Xi’an, China, June 2017. [Google Scholar]
- Ramesh, P.; Torrance, K. Boiling in a porous layer heated from below: Effects of natural convection and a moving liquid/two-phase interface. J. Fluid Mech. 1993, 257, 289–309. [Google Scholar] [CrossRef]
- Ramesh, P.; Torrance, K. Numerical algorithm for problems involving boiling and natural convection in porous materials. Numer. Heat Transf. Part B Fundam. 1990, 17, 1–24. [Google Scholar] [CrossRef]
- Yuki, K.; Abei, J.; Hashizume, H.; Toda, S. Numerical investigation of thermofluid flow characteristics with phase change against high heat flux in porous media. J. Heat Transfer 2008, 130, 1–12. [Google Scholar] [CrossRef]
- Shaeffer, R.; Hu, H.; Chung, J.N. An experimental study on liquid nitrogen pipe chilldown and heat transfer with pulse flows. Int. J. Heat Mass Transf. 2013, 67, 955–966. [Google Scholar] [CrossRef]
- Surtaev, A.S.; Pavlenko, A.N.; Kuznetsov, D.V.; Kalita, V.I.; Komlev, D.I.; Ivannikov, A.Y.; Radyuk, A.A. Heat transfer and crisis phenomena at pool boiling of liquid nitrogen on the surfaces with capillary-porous coatings. Int. J. Heat Mass Transf. 2017, 108, 146–155. [Google Scholar] [CrossRef]
- Kandlikar, S.G. A general correlation for saturated two-phase flow boiling heat transfer inside horizontal and vertical tubes. J. Heat Transf. 1990, 112, 219–228. [Google Scholar] [CrossRef]
- Steiner, D. Heat transfer during flow boiling of cryogenic fluids in vertical and horizontal tubes. Cryogenics 1986, 26, 309–318. [Google Scholar] [CrossRef]
- Jiang, R.; Wang, Y.; Li, H.; Yang, M. A numerical simulation on the impact of coal seam dip on productivity of CBM horizontal well. J. China Coal Soc. 2015, 40, 151–157. [Google Scholar]
- Sun, J. Failure characteristics of floor “three-zone” along the inclined direction of coal seam. J. Min. Saf. Eng. 2014, 31, 115–121. [Google Scholar]
- Leidenfrost, J.G. On the fixation of water in diverse fire. Int. J. Heat Mass Transf. 1966, 9, 1153–1166. [Google Scholar] [CrossRef]
- Hartwig, J.; Hu, H.; Styborski, J.; Chung, J.N. Comparison of cryogenic flow boiling in liquid nitrogen and liquid hydrogen chilldown experiments. Int. J. Heat Mass Transf. 2015, 88, 662–673. [Google Scholar] [CrossRef]
- Berenson, P.J. Film-boiling heat transfer from a horizontal surface. J. Heat Transfer 2012, 83, 351. [Google Scholar] [CrossRef]





| Formula | Melting Point | Boiling Point | Latent Heat of Vaporization | Gas/Liquid Ratio (Gas at 21.1 °C and 101.3 kPa, Liquid at Boiling Point) |
|---|---|---|---|---|
| N2 | −210.01 °C | −195.79 °C | 199.1 kJ/kg | 696.5 (volume ratio) |
| Test No. | The Injection Location | The Inclination Angle of Model/° | The Mass of LN2 Injection/kg | The Time of LN2 Injection/s | The Average Mass Flow Rate/(kg/s) |
|---|---|---|---|---|---|
| 1 | 1 | 0 | 13.2 | 710 | 0.0186 |
| 2 | 2 | 0 | 13.1 | 690 | 0.0190 |
| 3 | 1 | 4.1 | 9.1 | 482 | 0.0189 |
| 4 | 2 | 4.1 | 14.9 | 765 | 0.0190 |
| 5 | 1 | 8.4 | 9.5 | 530 | 0.0180 |
| 6 | 2 | 8.4 | 10.1 | 543 | 0.0186 |
| Test No. | Ratio | Test No. | Ratio |
|---|---|---|---|
| Test 1 | 1 | Test 2 | 1.056 |
| Test 3 | 0.151 | Test 4 | 0.704 |
| Test 5 | 0.116 | Test 6 | 0.120 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Lei, B.; Wu, B.; Meng, Y.; He, B. An Experimental Study on the Heat and Mass Transfer of Liquid Nitrogen in a Loose Medium. Energies 2019, 12, 3464. https://doi.org/10.3390/en12183464
Zhang Y, Lei B, Wu B, Meng Y, He B. An Experimental Study on the Heat and Mass Transfer of Liquid Nitrogen in a Loose Medium. Energies. 2019; 12(18):3464. https://doi.org/10.3390/en12183464
Chicago/Turabian StyleZhang, Yang, Baiwei Lei, Bing Wu, Yu Meng, and Binbin He. 2019. "An Experimental Study on the Heat and Mass Transfer of Liquid Nitrogen in a Loose Medium" Energies 12, no. 18: 3464. https://doi.org/10.3390/en12183464
APA StyleZhang, Y., Lei, B., Wu, B., Meng, Y., & He, B. (2019). An Experimental Study on the Heat and Mass Transfer of Liquid Nitrogen in a Loose Medium. Energies, 12(18), 3464. https://doi.org/10.3390/en12183464






