Enhanced CO2 Conversion to Acetate through Microbial Electrosynthesis (MES) by Continuous Headspace Gas Recirculation
Abstract
1. Introduction
2. Materials and Methods
2.1. MES Reactor Setup
2.2. Influents and Inoculum
2.3. Experimental Procedure
2.4. Measurements and Analytical Techniques
2.5. Microbial Community Analysis
3. Results
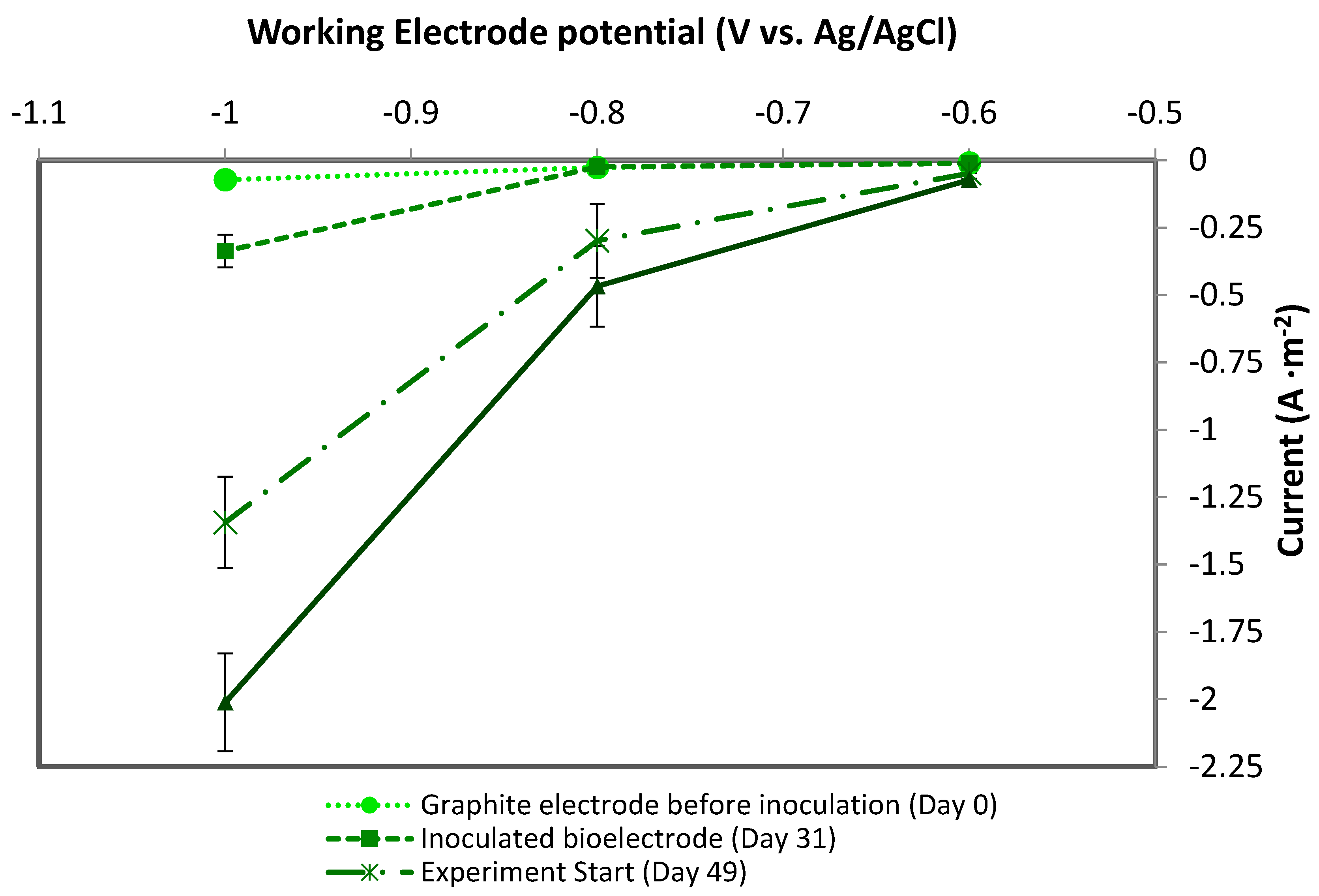
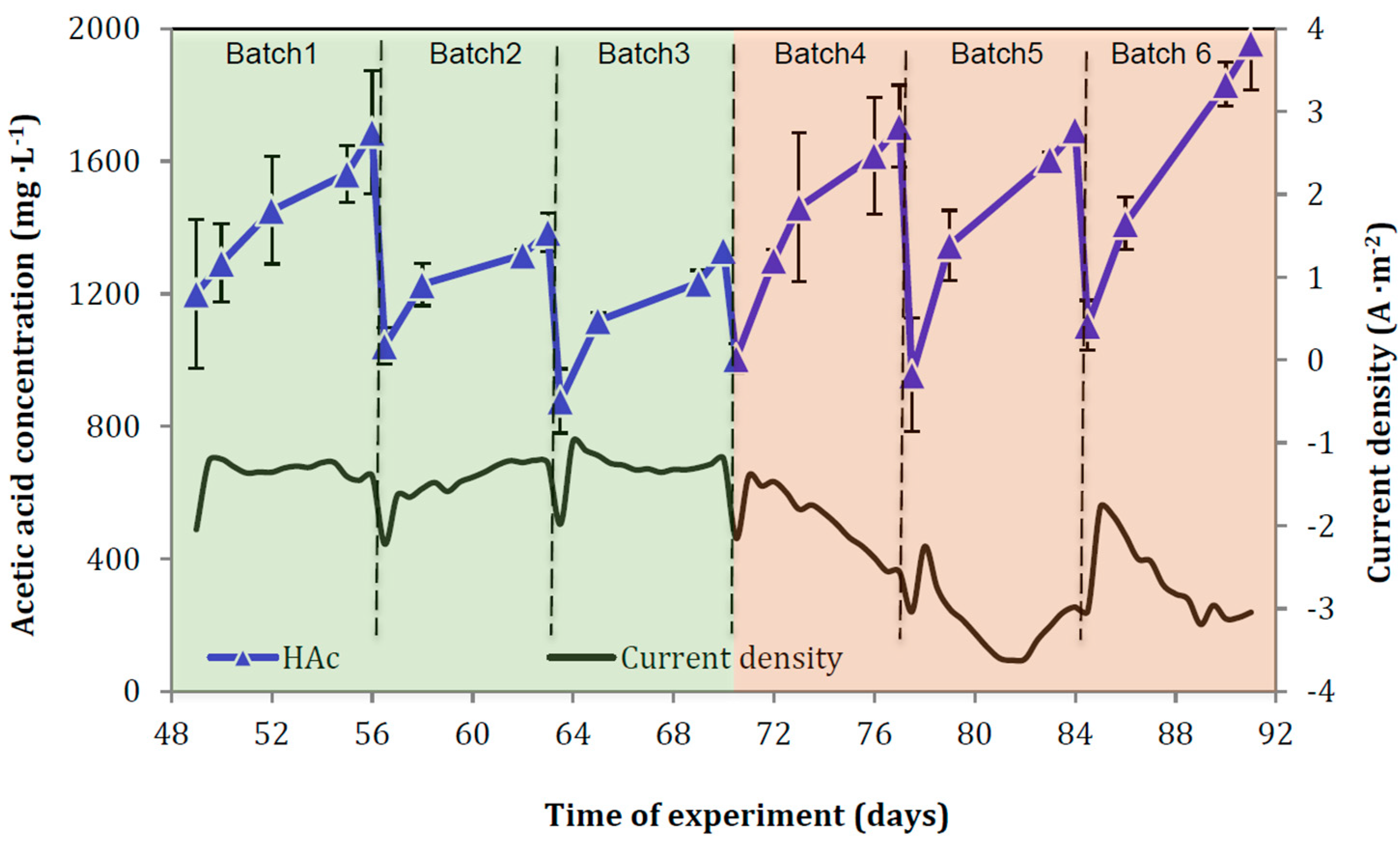
3.1. CO2 Recirculation Effect on MES
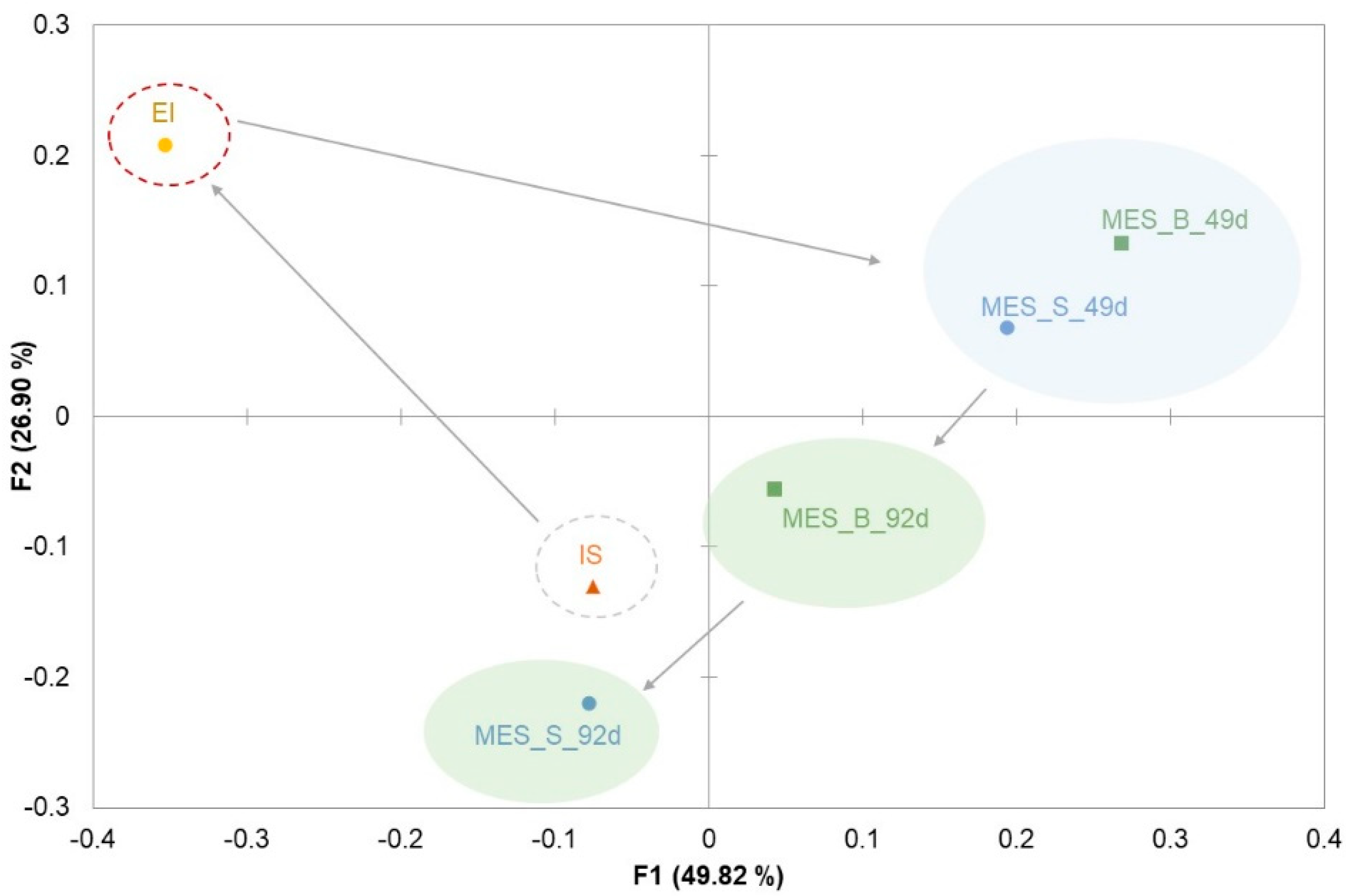
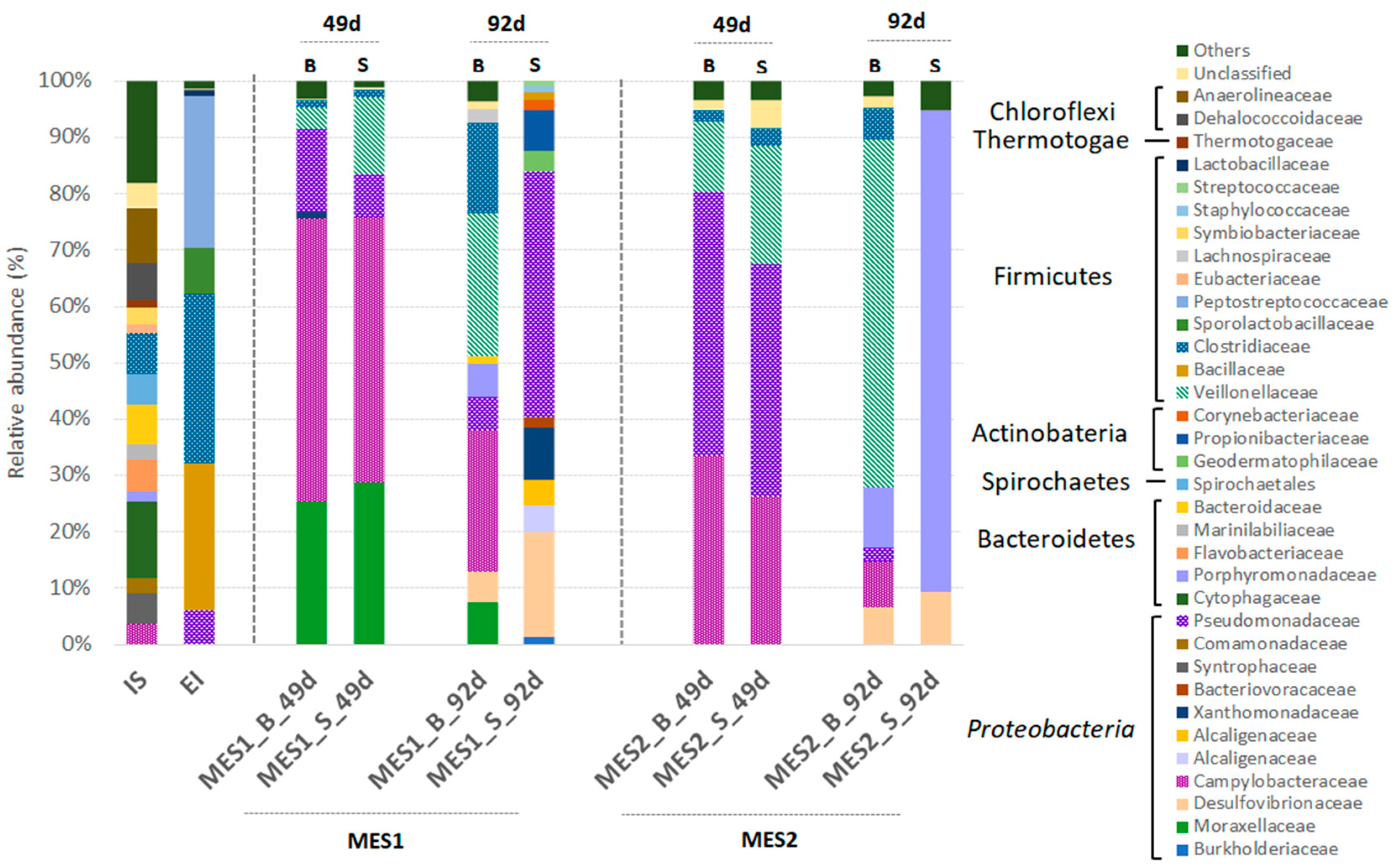
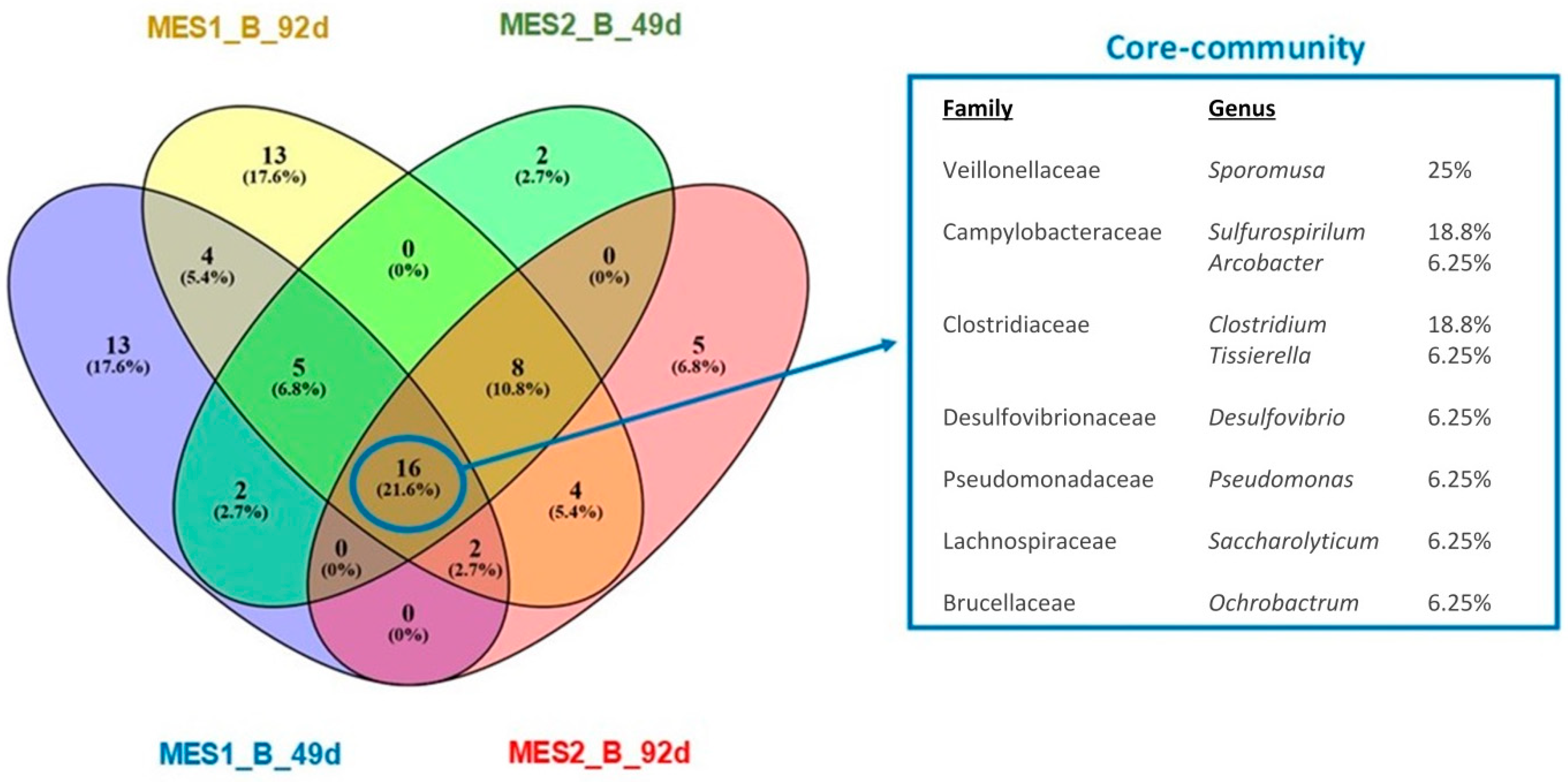
3.2. Microbial Communities Involved in the Process
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Pearson, R.J.; Eisaman, M.D.; Turner, J.W.G.; Edwards, P.P.; Jiang, Z.; Kuznetsov, V.L.; Littau, K.A.; Di Marco, L.; Taylor, S.R.G. Energy Storage via Carbon-Neutral Fuels Made from CO₂, Water, and Renewable Energy. Proc. IEEE 2012, 100, 440–460. [Google Scholar] [CrossRef]
- Manan, Z.A.; Mohd Nawi, W.N.R.; Wan Alwi, S.R.; Klemeš, J.J. Advances in Process Integration research for CO₂ emission reduction—A review. J. Clean. Prod. 2017, 167, 1–13. [Google Scholar] [CrossRef]
- Otto, A.; Grube, T.; Schiebahn, S.; Stolten, D. Closing the loop: Captured CO₂ as a feedstock in the chemical industry. Energy Environ. Sci. 2015, 8, 3283–3297. [Google Scholar] [CrossRef]
- Zdeb, J.; Howaniec, N.; Smoliński, A.; Zdeb, J.; Howaniec, N.; Smoliński, A. Utilization of Carbon Dioxide in Coal Gasification—An Experimental Study. Energies 2019, 12, 140. [Google Scholar] [CrossRef]
- Thakur, I.S.; Kumar, M.; Varjani, S.J.; Wu, Y.; Gnansounou, E.; Ravindran, S. Sequestration and utilization of carbon dioxide by chemical and biological methods for biofuels and biomaterials by chemoautotrophs: Opportunities and challenges. Bioresour. Technol. 2018, 256, 478–490. [Google Scholar] [CrossRef] [PubMed]
- Tremblay, P.L.; Zhang, T. Electrifying microbes for the production of chemicals. Front. Microbiol. 2015, 6, 201. [Google Scholar] [CrossRef] [PubMed]
- Rabaey, K.; Rozendal, R.A. Microbial electrosynthesis—Revisiting the electrical route for microbial production. Nat. Rev. Microbiol. 2010, 8, 706–716. [Google Scholar] [CrossRef] [PubMed]
- Bajracharya, S.; Srikanth, S.; Mohanakrishna, G.; Zacharia, R.; Strik, D.P.; Pant, D. Biotransformation of carbon dioxide in bioelectrochemical systems: State of the art and future prospects. J. Power Sources 2017, 356, 256–273. [Google Scholar] [CrossRef]
- Nevin, K.P.; Hensley, S.A.; Franks, A.E.; Summers, Z.M.; Ou, J.; Woodard, T.L.; Snoeyenbos-West, O.L.; Lovley, D.R. Electrosynthesis of organic compounds from carbon dioxide is catalyzed by a diversity of acetogenic microorganisms. Appl. Environ. Microbiol. 2011, 77, 2882–2886. [Google Scholar] [CrossRef]
- Jafary, T.; Daud, W.R.W.; Ghasemi, M.; Kim, B.H.; Md Jahim, J.; Ismail, M.; Lim, S.S. Biocathode in microbial electrolysis cell; present status and future prospects. Renew. Sustain. Energy Rev. 2015, 47, 23–33. [Google Scholar] [CrossRef]
- May, H.D.; Evans, P.J.; LaBelle, E.V. The bioelectrosynthesis of acetate. Curr. Opin. Biotechnol. 2016, 42, 225–233. [Google Scholar] [CrossRef]
- Aryal, N.; Tremblay, P.L.; Lizak, D.M.; Zhang, T. Performance of different Sporomusa species for the microbial electrosynthesis of acetate from carbon dioxide. Bioresour. Technol. 2017, 233, 184–190. [Google Scholar] [CrossRef]
- Bajracharya, S.; ter Heijne, A.; Dominguez Benetton, X.; Vanbroekhoven, K.; Buisman, C.J.N.; Strik, D.P.B.T.; Pant, D. Carbon dioxide reduction by mixed and pure cultures in microbial electrosynthesis using an assembly of graphite felt and stainless steel as a cathode. Bioresour. Technol. 2015, 195, 14–24. [Google Scholar] [CrossRef]
- Bajracharya, S.; Yuliasni, R.; Vanbroekhoven, K.; Buisman, C.J.N.; Strik, D.P.; Pant, D. Long-term operation of microbial electrosynthesis cell reducing CO₂ to multi-carbon chemicals with a mixed culture avoiding methanogenesis. Bioelectrochemistry 2017, 113, 26–34. [Google Scholar] [CrossRef]
- Ali, J.; Sohail, A.; Wang, L.; Rizwan Haider, M.; Mulk, S.; Pan, G.; Ali, J.; Sohail, A.; Wang, L.; Rizwan Haider, M.; et al. Electro-Microbiology as a Promising Approach Towards Renewable Energy and Environmental Sustainability. Energies 2018, 11, 1822. [Google Scholar] [CrossRef]
- Desloover, J.; Arends, J.B.A.; Hennebel, T.; Rabaey, K. Operational and technical considerations for microbial electrosynthesis. Biochem. Soc. Trans. 2012, 40, 1233–1238. [Google Scholar] [CrossRef]
- Mateos, R.; Escapa, A.; San-Martín, M.I.; De Wever, H.; Sotres, A.; Pant, D. Long-term open circuit microbial electrosynthesis system promotes methanogenesis. J. Energy Chem. 2020, 41, 3–6. [Google Scholar] [CrossRef]
- Muñoz-Aguilar, R.; Molognoni, D.; Bosch-Jimenez, P.; Borràs, E.; Della Pirriera, M.; Luna, Á.; Muñoz-Aguilar, R.S.; Molognoni, D.; Bosch-Jimenez, P.; Borràs, E.; et al. Design, Operation, Modeling and Grid Integration of Power-to-Gas Bioelectrochemical Systems. Energies 2018, 11, 1947. [Google Scholar] [CrossRef]
- Zaybak, Z.; Pisciotta, J.M.; Tokash, J.C.; Logan, B.E. Enhanced start-up of anaerobic facultatively autotrophic biocathodes in bioelectrochemical systems. J. Biotechnol. 2013, 168, 478–485. [Google Scholar] [CrossRef]
- Mateos, R.; Sotres, A.; Alonso, R.M.; Escapa, A.; Morán, A. Impact of the start-up process on the microbial communities in biocathodes for electrosynthesis. Bioelectrochemistry 2018, 121, 27–37. [Google Scholar] [CrossRef]
- Martínez, E.J.; Gil, M.V.; Rosas, J.G.; Moreno, R.; Mateos, R.; Morán, A.; Gómez, X. Application of thermal analysis for evaluating the digestion of microwave pre-treated sewage sludge. J. Therm. Anal. Calorim. 2016, 127, 1209–1219. [Google Scholar] [CrossRef]
- Rice, E.W.; Bridgewater, L.; American Public Health Association; American Water Works Association; Water Environment Federation. Standard Methods for the Examination of Water and Wastewater; American Public Health Association: Washington, DC, USA, 2012; ISBN 9780875530130. [Google Scholar]
- del Pilar Anzola Rojas, M.; Mateos, R.; Sotres, A.; Zaiat, M.; Gonzalez, E.R.; Escapa, A.; De Wever, H.; Pant, D. Microbial electrosynthesis (MES) from CO₂ is resilient to fluctuations in renewable energy supply. Energy Convers. Manag. 2018, 177, 272–279. [Google Scholar] [CrossRef]
- Callaway, T.R.; Dowd, S.E.; Wolcott, R.D.; Sun, Y.; McReynolds, J.L.; Edrington, T.S.; Byrd, J.A.; Anderson, R.C.; Krueger, N.; Nisbet, D.J. Evaluation of the bacterial diversity in cecal contents of laying hens fed various molting diets by using bacterial tag-encoded FLX amplicon pyrosequencing. Poult. Sci. 2009, 88, 298–302. [Google Scholar] [CrossRef]
- Saratale, R.G.; Saratale, G.D.; Pugazhendhi, A.; Zhen, G.; Kumar, G.; Kadier, A.; Sivagurunathan, P. Microbiome involved in microbial electrochemical systems (MESs): A review. Chemosphere 2017, 177, 176–188. [Google Scholar] [CrossRef]
- LaBelle, E.V.; Marshall, C.W.; Gilbert, J.A.; May, H.D. Influence of Acidic pH on Hydrogen and Acetate Production by an Electrosynthetic Microbiome. PLoS ONE 2014, 9, e109935. [Google Scholar] [CrossRef]
- Gildemyn, S.; Verbeeck, K.; Slabbinck, R.; Andersen, S.J.; Prévoteau, A.; Rabaey, K. Integrated Production, Extraction, and Concentration of Acetic Acid from CO₂ through Microbial Electrosynthesis. Environ. Sci. Technol. Lett. 2015, 2, 325–328. [Google Scholar] [CrossRef]
- Jourdin, L.; Grieger, T.; Monetti, J.; Flexer, V.; Freguia, S.; Lu, Y.; Chen, J.; Romano, M.; Wallace, G.G.; Keller, J. High Acetic Acid Production Rate Obtained by Microbial Electrosynthesis from Carbon Dioxide. Environ. Sci. Technol. 2015, 49, 13566–13574. [Google Scholar] [CrossRef]
- Zou, S.; He, Z. Efficiently “pumping out” value-added resources from wastewater by bioelectrochemical systems: A review from energy perspectives. Water Res. 2018, 131, 62–73. [Google Scholar] [CrossRef]
- Cecconet, D.; Bolognesi, S.; Callegari, A.; Capodaglio, A.G. Simulation tests of in situ groundwater denitrification with aquifer-buried biocathodes. Heliyon 2019, 5, e02117. [Google Scholar] [CrossRef]
- Cooney, M.J.; Roschi, E.; Marison, I.W.; Comminellis, C.; von Stockar, U. Physiologic studies with the sulfate-reducing bacterium Desulfovibrio desulfuricans: Evaluation for use in a biofuel cell. Enzyme Microb. Technol. 1996, 18, 358–365. [Google Scholar] [CrossRef]
- Aulenta, F.; Catapano, L.; Snip, L.; Villano, M.; Majone, M. Linking Bacterial Metabolism to Graphite Cathodes: Electrochemical Insights into the H2-Producing Capability of Desulfovibrio sp. ChemSusChem 2012, 5, 1080–1085. [Google Scholar] [CrossRef]
- Arends, J.B.A.; Patil, S.A.; Roume, H.; Rabaey, K. Continuous long-term electricity-driven bioproduction of carboxylates and isopropanol from CO₂ with a mixed microbial community. J. CO2 Util. 2017, 20, 141–149. [Google Scholar] [CrossRef]
- Patil, S.A.; Arends, J.B.A.; Vanwonterghem, I.; van Meerbergen, J.; Guo, K.; Tyson, G.W.; Rabaey, K. Selective Enrichment Establishes a Stable Performing Community for Microbial Electrosynthesis of Acetate from CO2. Environ. Sci. Technol. 2015, 49, 8833–8843. [Google Scholar] [CrossRef]
- Hari, A.R.; Venkidusamy, K.; Katuri, K.P.; Bagchi, S.; Saikaly, P.E. Temporal microbial community dynamics in microbial electrolysis cells—Influence of acetate and propionate concentration. Front. Microbiol. 2017, 8, 1371. [Google Scholar] [CrossRef]
- Schwartz, E.; Friedrich, B. The H2-metabolizing prokaryotes. In The Prokaryotes; Dworkin, M., Falkow, S., Rosenberg, E., Schleifer, K.H., Stackebrandt, E., Eds.; Springer: New York, NY, USA, 2006. [Google Scholar]
- Koch, C.; Harnisch, F. Is there a specific ecological niche for electroactive microorganisms? ChemElectroChem 2016, 3, 1282–1295. [Google Scholar] [CrossRef]
- Deutzmann, J.S.; Sahin, M.; Spormann, A.M. Extracellular Enzymes Facilitate Electron Uptake in Biocorrosion and Bioelectrosynthesis. MBio 2015, 6, e00496-15. [Google Scholar] [CrossRef]

| Acetic Acid Production | ||||||
|---|---|---|---|---|---|---|
| Operation Mode | Batch | MES1 Rate | MES2 Rate | MES Average | Std. Dev. | Period Average |
| (mg·L−1·d−1) | (mg·L−1·d−1) | (mg·L−1·d−1) | (mg·L−1·d−1) | (mg·L−1·d−1) | ||
| Without recirculation | 1 | 73.5 | 65.5 | 69.5 | 5,6 | 61.1 |
| 2 | 49.5 | 48.4 | 48.9 | 0.7 | ||
| 3 | 55.3 | 74.7 | 65.0 | 13.8 | ||
| With recirculation | 4 | 83.1 | 117.1 | 100.2 | 24.1 | 109.1 |
| 5 | 122.0 | 89.1 | 105.6 | 23.3 | ||
| 6 | 115.0 | 128.25 | 121.6 | 9.4 | ||
| Operation Mode | Batch | CO2 Initial | CO2 Final | Acetate Production | CO2-to-Acetate Conversion | Transferred Charge | Cathodic Efficiency |
|---|---|---|---|---|---|---|---|
| (mol C) | (mol C) | (mol C) | (%) | (C) | (%) | ||
| Without recirculation | 1 | 0.022 | 0.006 | 0.016 | 98% | 14,230 | 89.8% |
| 2 | 0.022 | 0.008 | 0.011 | 81% | 15,670 | 57.4% | |
| 3 | 0.022 | 0.006 | 0.015 | 93% | 13,790 | 86.7% | |
| With recirculation | 4 | 0.027 | 0.001 | 0.023 | 90% | 20,320 | 90.6% |
| 5 | 0.027 | 0.000 | 0.025 | 93% | 33,500 | 57.8% | |
| 6 | 0.027 | 0.000 | 0.028 | 106% | 28,480 | 78.5% |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mateos, R.; Sotres, A.; Alonso, R.M.; Morán, A.; Escapa, A. Enhanced CO2 Conversion to Acetate through Microbial Electrosynthesis (MES) by Continuous Headspace Gas Recirculation. Energies 2019, 12, 3297. https://doi.org/10.3390/en12173297
Mateos R, Sotres A, Alonso RM, Morán A, Escapa A. Enhanced CO2 Conversion to Acetate through Microbial Electrosynthesis (MES) by Continuous Headspace Gas Recirculation. Energies. 2019; 12(17):3297. https://doi.org/10.3390/en12173297
Chicago/Turabian StyleMateos, Raúl, Ana Sotres, Raúl M. Alonso, Antonio Morán, and Adrián Escapa. 2019. "Enhanced CO2 Conversion to Acetate through Microbial Electrosynthesis (MES) by Continuous Headspace Gas Recirculation" Energies 12, no. 17: 3297. https://doi.org/10.3390/en12173297
APA StyleMateos, R., Sotres, A., Alonso, R. M., Morán, A., & Escapa, A. (2019). Enhanced CO2 Conversion to Acetate through Microbial Electrosynthesis (MES) by Continuous Headspace Gas Recirculation. Energies, 12(17), 3297. https://doi.org/10.3390/en12173297








