ATR-FTIR Model Development and Verification for Qualitative and Quantitative Analysis in MDEA–H2O–MEG/TEG–CO2 Blends
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methodology
2.2.1. Calibration Solution Preparation
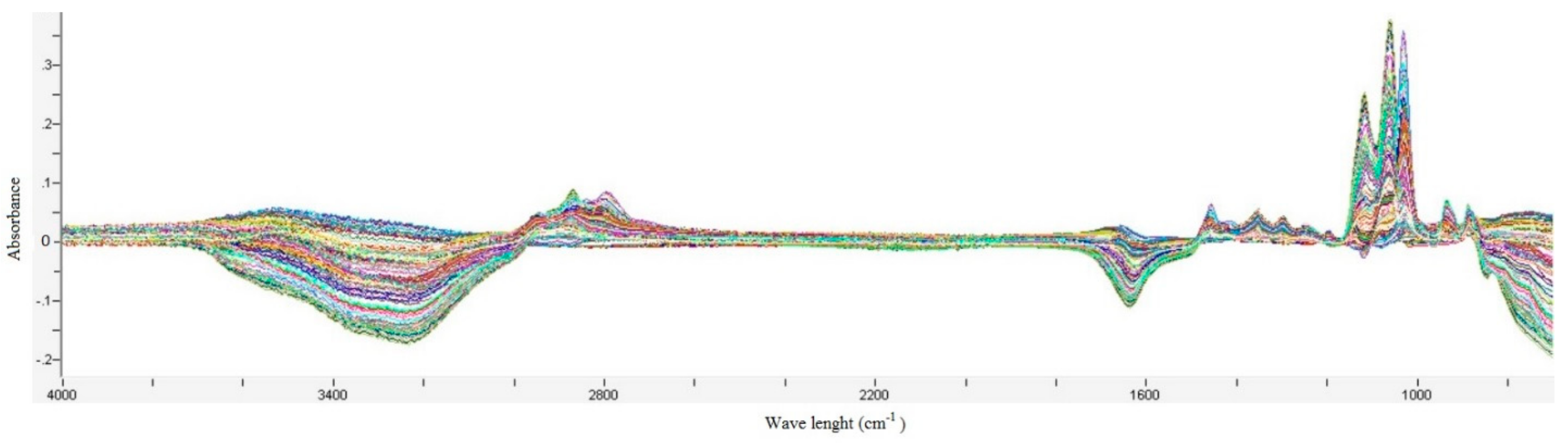
2.2.2. FTIR Analysis
2.2.3. Multivariate Analysis
2.2.4. Thermal Degradation
3. Results and Discussion
3.1. Model Development
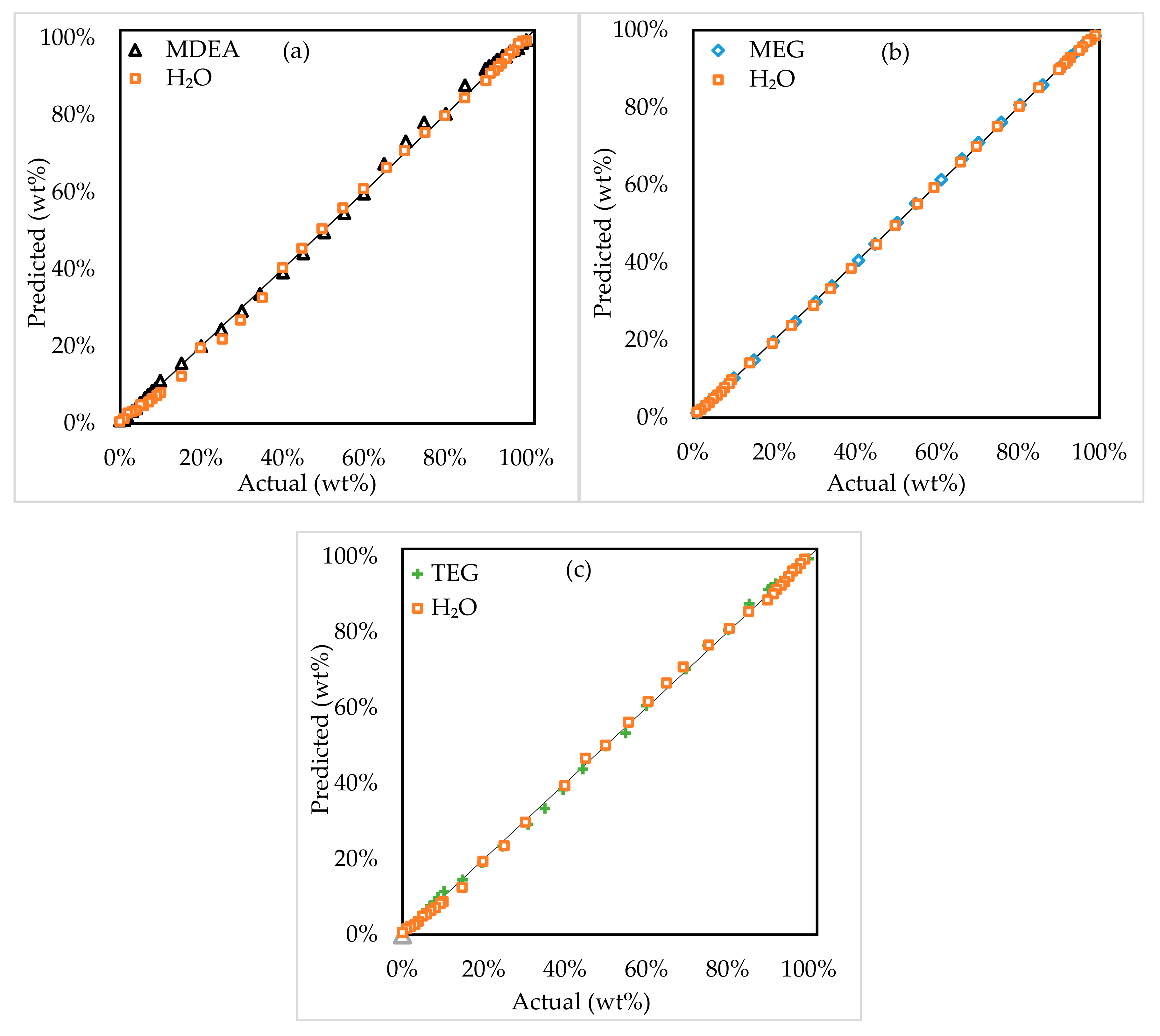
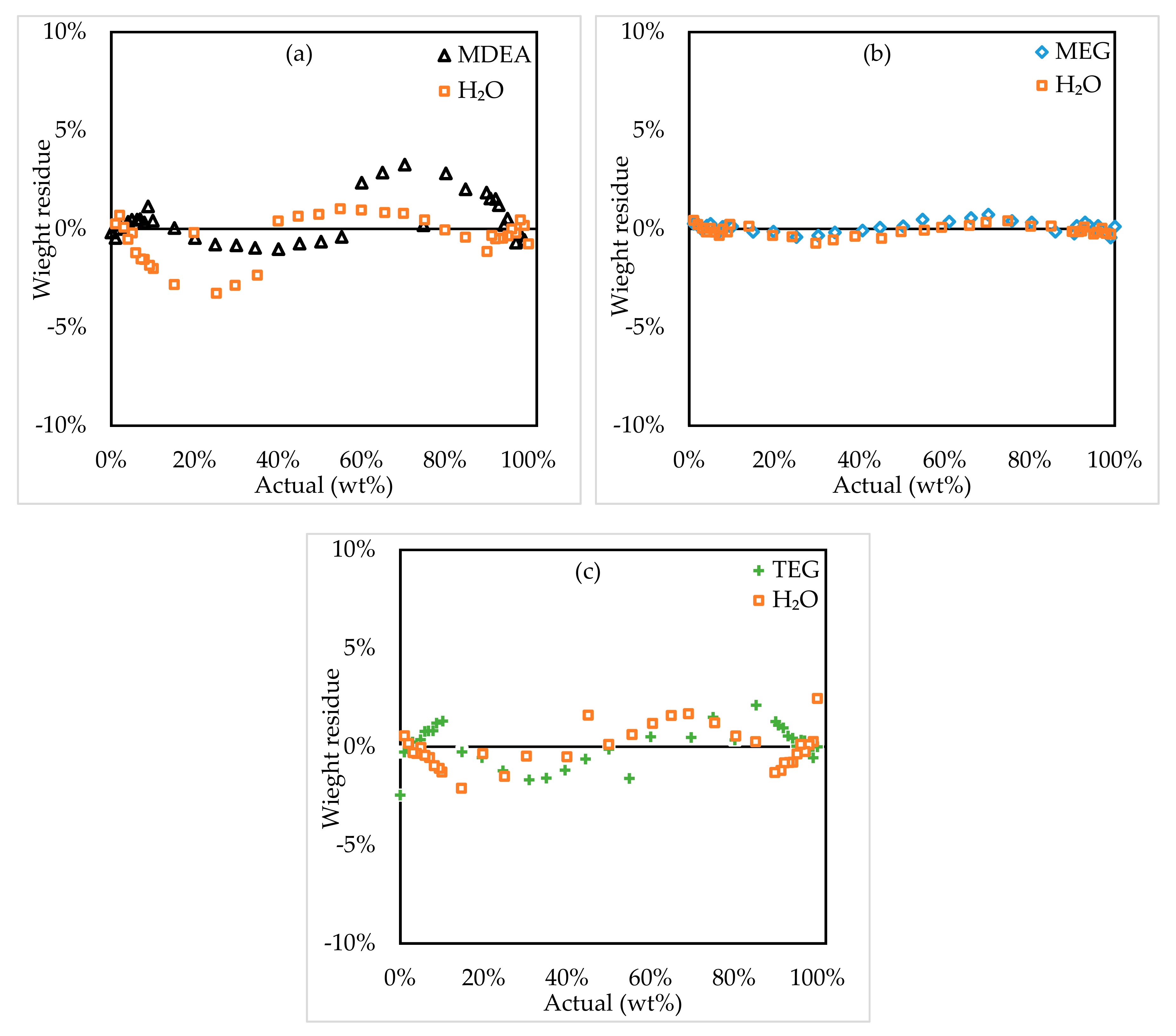
3.1.1. Two-Component Systems
3.1.2. Three-Component Systems
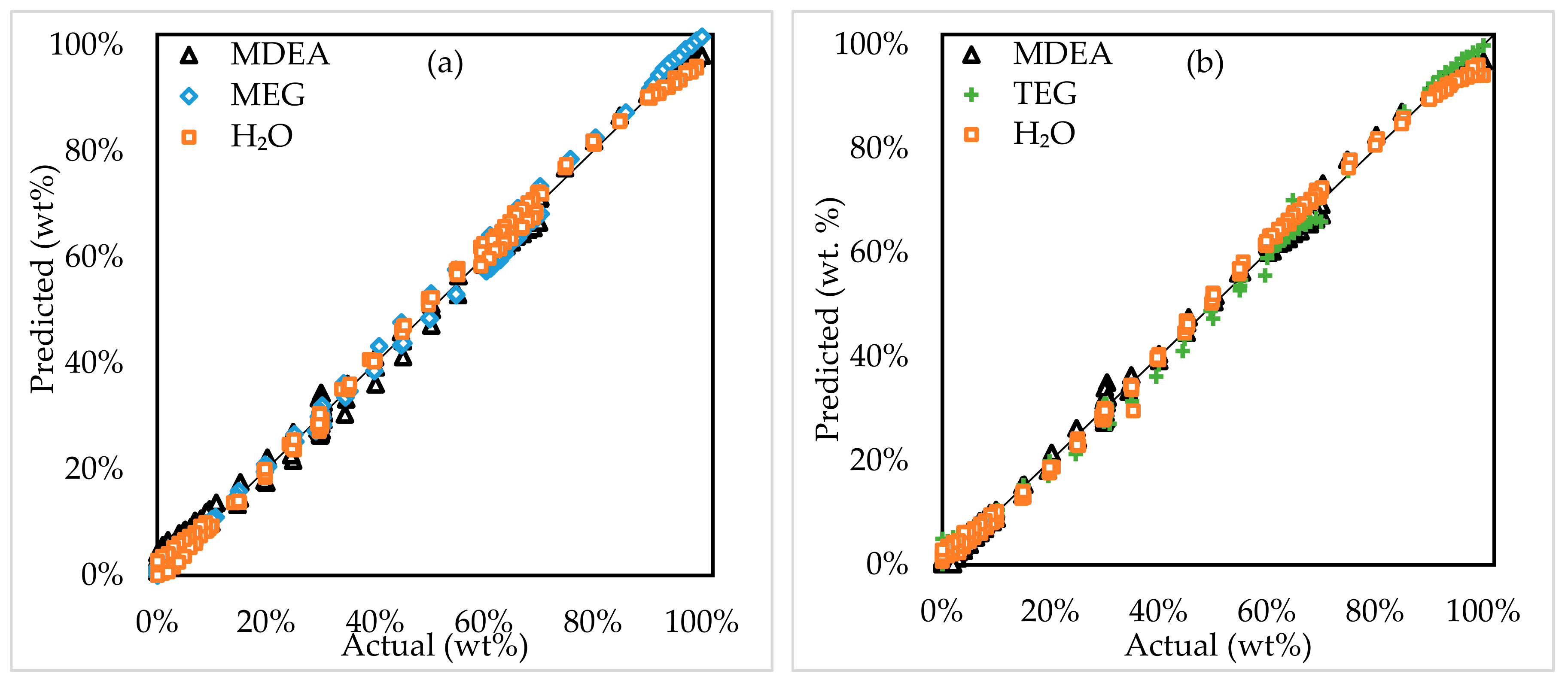
3.1.3. Four-Component Systems
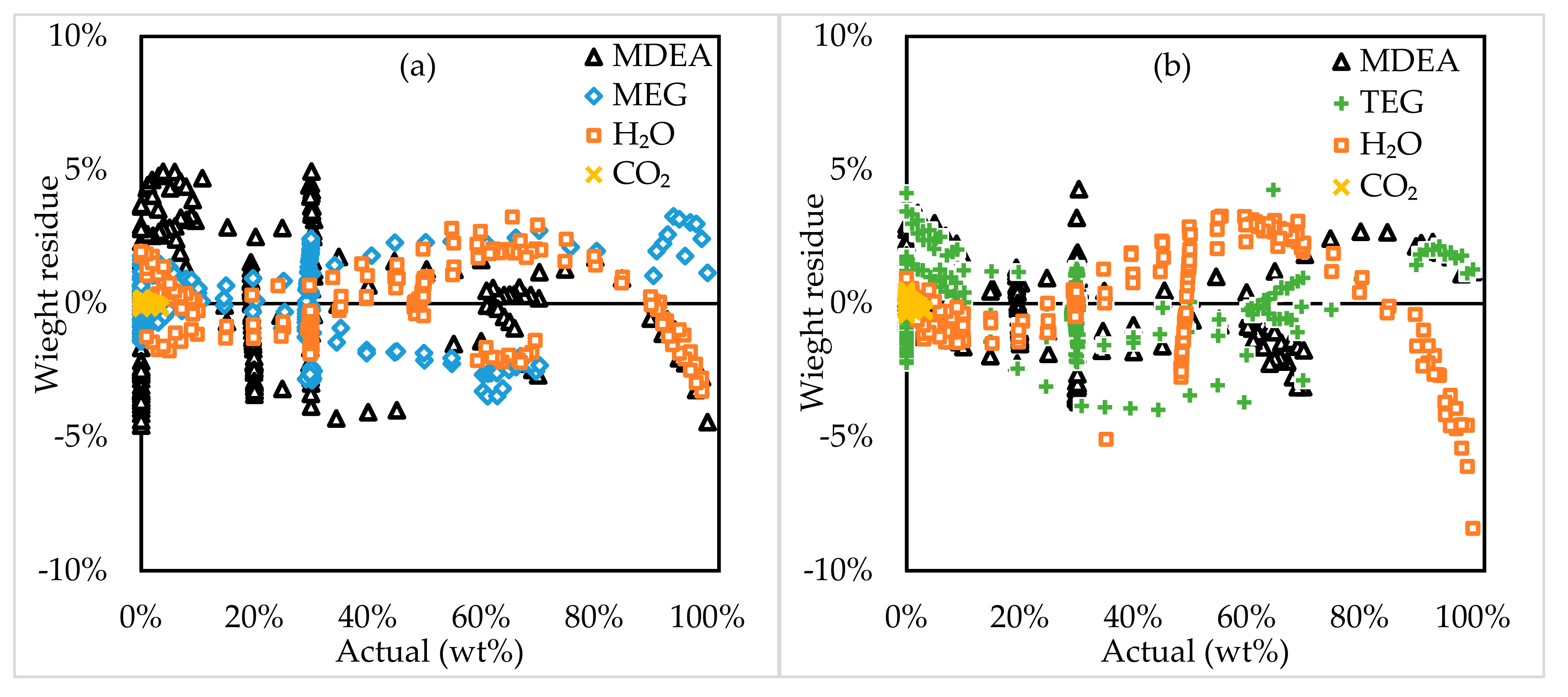
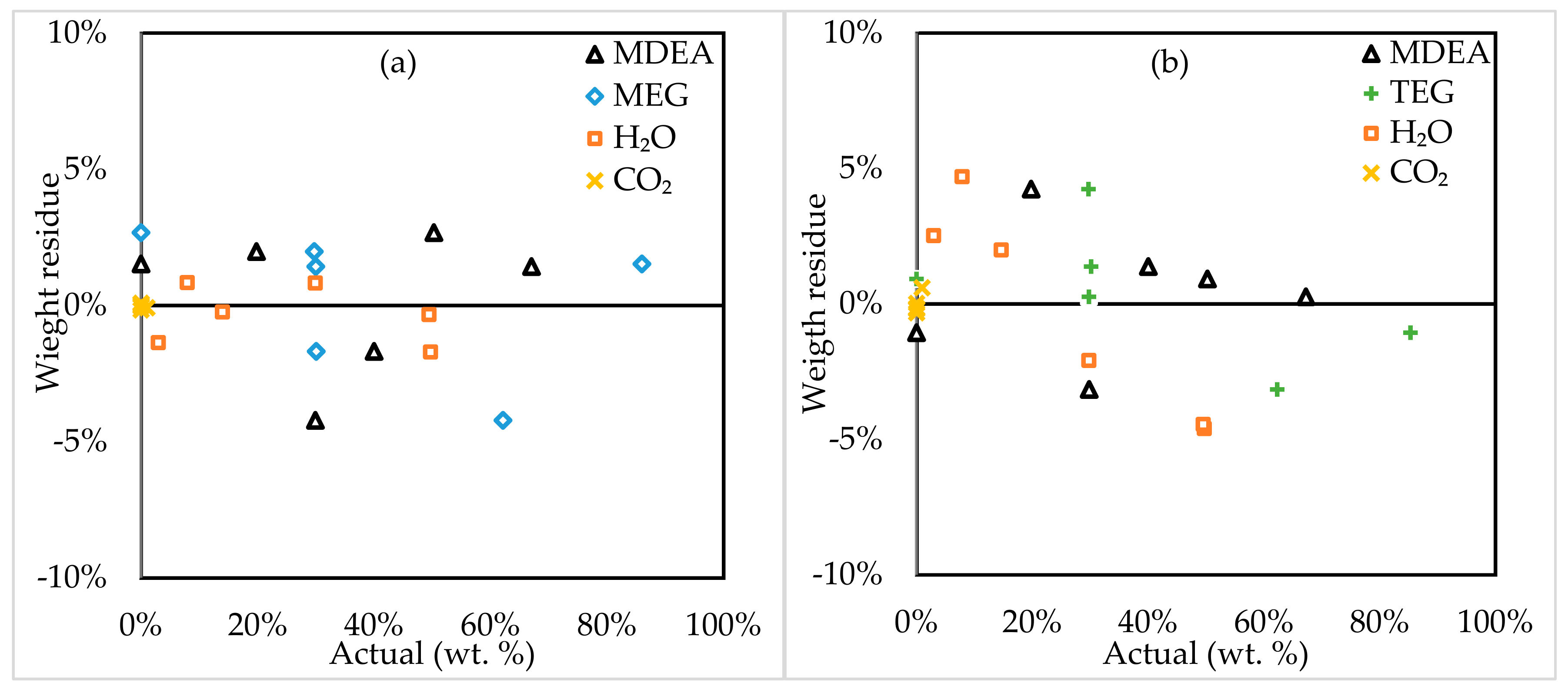
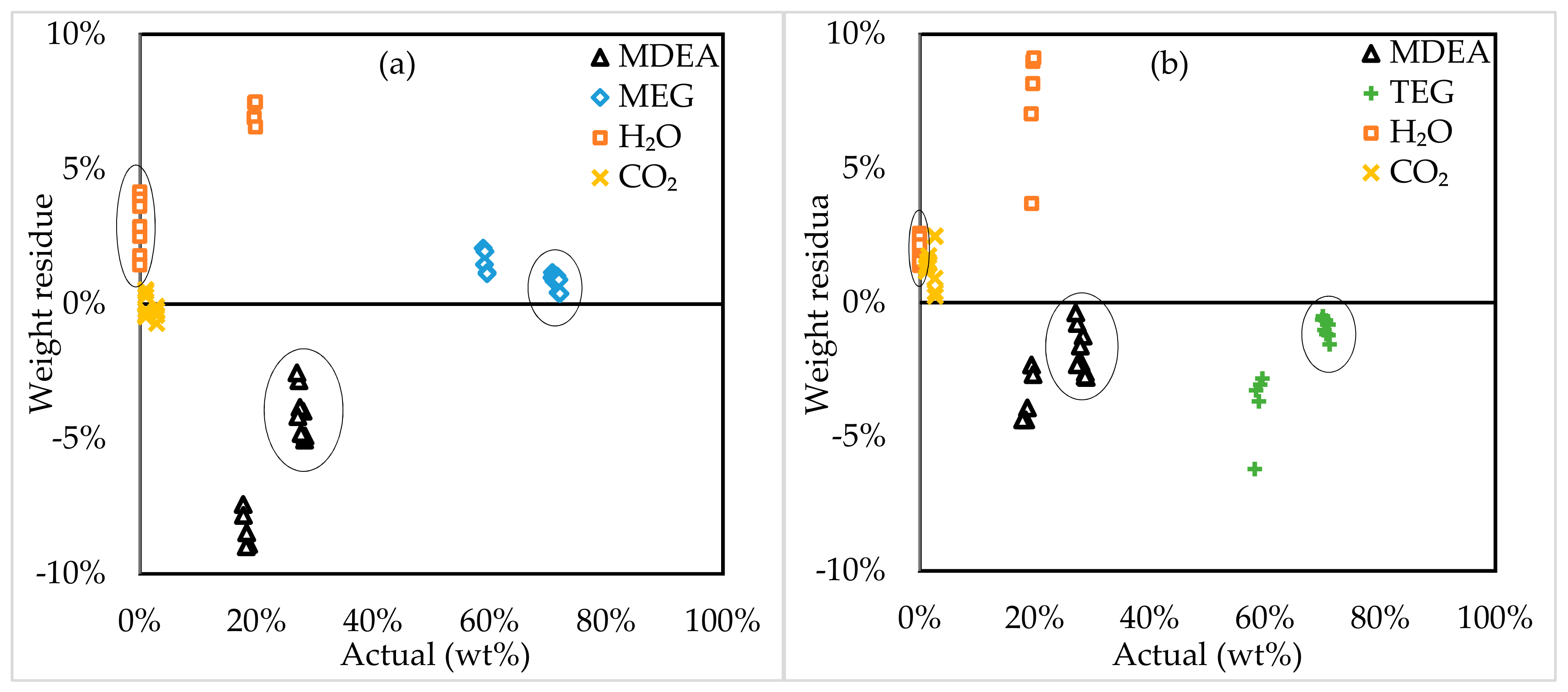
3.2. Model Assessment
3.3. Model Application
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| ATR | Attenuated total reflectance |
| CO2 | Carbon dioxide |
| FTIR | Fourier transform infrared |
| H2O | Water |
| MDEA | N-Methyldiethanolamine |
| MEG | Ethylene glycol |
| PLS | Partial least-squares |
| R2 | Correlation factor |
| RSME | Root square mean error |
| SE | Standard error |
| SECV | Standard error of cross-validation |
| TEG | Triethylene glycol |
| VN | Validation number |
| Wt% | Weight percent |
| xi | Actual value of chemical component |
| yi | Predicted value of chemical component |
References
- Schmeal, W.R.; MacNab, A.J.; Rhodes, P.R. Corrosion in amine/sour gas treating contactors. Chem. Eng. Prog. 1978, 74, 37–42. [Google Scholar]
- Rennie, S. Corrosion and materials selection for amine service. Mater. Forum 2006, 30, 126–130. [Google Scholar]
- Wasiu, A.B.; Aziz, A.R.A.; Heikal, M.R. The Effect of Carbon Dioxide Content-natural Gas on the Performance Characteristics of Engines: A Review. J. Appl. Sci. 2012, 12, 2346–2350. [Google Scholar] [CrossRef]
- Doujaiji, B.; Al-Tawfiq, J.A. Hydrogen sulfide exposure in an adult male. Ann. Saudi Med. 2010, 30, 76–80. [Google Scholar] [CrossRef] [PubMed]
- Amosa, M.; Mohammed, I.; Yaro, S. Sulphide scavengers in oil and gas industry—A review. Nafta 2010, 61, 85–92. [Google Scholar]
- Kohl, A.L.; Nielsen, R.B. Absorption of Water Vapor by Dehydrating Solutions. In Gas Purification, 5th ed.; Kohl, A.L., Nielsen, R.B., Eds.; Gulf Professional Publishing: Houston, TX, USA, 1997; Chapter 11; pp. 946–1021. [Google Scholar]
- Stewart, C.; Hessami, M.A. A study of methods of carbon dioxide capture and sequestration—The sustainability of a photosynthetic bioreactor approach. Energy Convers. Manag. 2005, 46, 403–420. [Google Scholar] [CrossRef]
- Neill, B.C.; Oppenheimer, M. Dangerous Climate Impacts and the Kyoto Protocol. Science 2002, 296, 1971. [Google Scholar] [CrossRef]
- Kohl, A.L.; Nielsen, R.B. Alkanolamines for Hydrogen Sulfide and Carbon Dioxide Removal. In Gas Purification, 5th ed.; Gulf Professional Publishing: Houston, TX, USA, 1997; Chapter 2; pp. 40–186. [Google Scholar]
- Netusil, M.; Ditl, P. Comparison of three methods for natural gas dehydration. J. Nat. Gas Chem. 2011, 20, 471–476. [Google Scholar] [CrossRef]
- Hutchinson, A.J. Process for Treating Gases. U.S. Patent 2,177,068, 24 October 1939. [Google Scholar]
- McCartney, E.R. Gas Purification and Dehydration Process. U.S. Patent 2,435,089, 27 January 1948. [Google Scholar]
- Chapin, W.F. Purification and Dehydration of Gases. U.S. Patent 2,518,752, 15 August 1950. [Google Scholar]
- McCartney, E.R. Extraction of Acidic Impurities and Moisture from Gases. U.S. Patent 2,547,278, 3 April 1951. [Google Scholar]
- Shoukat, U.; Fytianos, G.; Knuutila, H.K. Thermal stability and corrosion studies of amines for combined acid gas removal and hydrate control for subsea gas treatment systems. In Proceedings of the 2016 Techno-Ocean (Techno-Ocean), Kobe, Japan, 6–8 October 2016; pp. 176–180. [Google Scholar]
- Shoukat, U.; Pinto, D.D.D.; Knuutila, H.K. Study of Various Aqueous and Non-Aqueous Amine Blends for Hydrogen Sulfide Removal from Natural Gas. Processes 2019, 7, 160. [Google Scholar] [CrossRef]
- Eimer, D. Simultaneous removal of water and hydrogen sulphide from natural gas. In Department of Chemical Engineering; Norwegian University of Science and Technology: Trondheim, Norway, 1994. [Google Scholar]
- Carter, C.F.; Lange, H.; Ley, S.V.; Baxendale, I.R.; Wittkamp, B.; Goode, J.G.; Gaunt, N.L. ReactIR Flow Cell: A New Analytical Tool for Continuous Flow Chemical Processing. Org. Process. Res. Dev. 2010, 14, 393–404. [Google Scholar] [CrossRef]
- Richner, G.; Puxty, G. Assessing the Chemical Speciation during CO2 Absorption by Aqueous Amines Using in Situ FTIR. Ind. Eng. Chem. Res. 2012, 51, 14317–14324. [Google Scholar] [CrossRef]
- Einbu, A.; Ciftja, A.F.; Grimstvedt, A.; Zakeri, A.; Svendsen, H.F. Online Analysis of Amine Concentration and CO2 Loading in MEA Solutions by ATR-FTIR Spectroscopy. Energy Procedia 2012, 23, 55–63. [Google Scholar] [CrossRef]
- Geers, L.F.G.; Van De Runstraat, A.; Joh, R.; Schneider, R.; Goetheer, E.L.V. Development of an Online Monitoring Method of a CO2 Capture Process. Ind. Eng. Chem. Res. 2011, 50, 9175–9180. [Google Scholar] [CrossRef]
- Morken, A.K.; Nenseter, B.; Pedersen, S.; Chhaganlal, M.; Feste, J.K.; Tyborgnes, R.B.; Ullestad, Ø.; Ulvatn, H.; Zhu, L.; Mikoviny, T.; et al. Emission Results of Amine Plant Operations from MEA Testing at the CO2 Technology Centre Mongstad. Energy Procedia 2014, 63, 6023–6038. [Google Scholar] [CrossRef]
- Mertens, J.; Knudsen, J.; Thielens, M.L.; Andersen, J. On-line monitoring and controlling emissions in amine post combustion carbon capture: A field test. Int. J. Greenh. Gas Control 2012, 6, 2–11. [Google Scholar] [CrossRef]
- Chahen, L.; Huard, T.; Cuccia, L.; Cuzuel, V.; Dugay, J.; Pichon, V.; Vial, J.; Gouedard, C.; Bonnard, L.; Cellier, N.; et al. Comprehensive monitoring of MEA degradation in a post-combustion CO2 capture pilot plant with identification of novel degradation products in gaseous effluents. Int. J. Greenh. Gas Control 2016, 51, 305–316. [Google Scholar] [CrossRef]
- Milella, F.; Mazzotti, M. Estimating speciation of aqueous ammonia solutions of ammonium bicarbonate: Application of least squares methods to infrared spectra. React. Chem. Eng. 2019, 4, 1284–1302. [Google Scholar] [CrossRef]
- Rogers, W.J.; Bullin, J.A.; Davison, R.R. FTIR measurements of acid-gas–methyldiethanolamine systems. AIChE J. 1998, 44, 2423–2430. [Google Scholar] [CrossRef]
- Cuccia, L.; Dugay, J.; Bontemps, D.; Louis-Louisy, M.; Vial, J. Analytical methods for the monitoring of post-combustion CO2 capture process using amine solvents: A review. Int. J. Greenh. Gas Control 2018, 72, 138–151. [Google Scholar] [CrossRef]
- Handojo, L.; Prihartoni, M.D.; Susanti, R.F.; Yaswari, Y.; Raksajati, A.; Indarto, A. Non-oxidative thermal degradation of amines: GCMS/FTIR spectra analysis and molecular modeling. Sep. Sci. Technol. 2018, 53, 2259–2266. [Google Scholar] [CrossRef]
- Haghi, R.K.; Yang, J.; Tohidi, B.; Haghi, R.K. Integrated Near Infrared and Ultraviolet Spectroscopy Techniques for Determination of Hydrate Inhibitors in the Presence of NaCl. Ind. Eng. Chem. Res. 2018, 57, 11728–11737. [Google Scholar] [CrossRef]
- Ma’Mun, S.; Svendsen, H.F.; Hoff, K.A.; Juliussen, O. Selection of new absorbents for carbon dioxide capture. Energy Convers. Manag. 2007, 48, 251–258. [Google Scholar] [CrossRef]
- Monteiro, J.G.S.; Pinto, D.D.; Zaidy, S.A.; Hartono, A.; Svendsen, H.F. VLE data and modelling of aqueous N,N-diethylethanolamine (DEEA) solutions. Int. J. Greenh. Gas Control 2013, 19, 432–440. [Google Scholar] [CrossRef]
- Maitra, S.; Yan, J. Principle Component Analysis and Partial Least Squares: Two Dimension Reduction Techniques for Regression. Appl. Multivar. Stat. Model 2008, 79, 79–90. [Google Scholar]
- Du, Y.; Liang, Y.; Jiang, J.; Berry, R.; Ozaki, Y. Spectral regions selection to improve prediction ability of PLS models by changeable size moving window partial least squares and searching combination moving window partial least squares. Anal. Chim. Acta 2004, 501, 183–191. [Google Scholar] [CrossRef]
- Dupuy, N.; Galtier, O.; Ollivier, D.; Vanloot, P.; Artaud, J. Comparison between NIR, MIR, concatenated NIR and MIR analysis and hierarchical PLS model. Application to virgin olive oil analysis. Anal. Chim. Acta 2010, 666, 23–31. [Google Scholar] [CrossRef] [PubMed]
- Zanone, A.; Tavares, D.T.; De Paiva, J.L. An FTIR spectroscopic study and quantification of 2-amino-2-methyl-1-propanol, piperazine and absorbed carbon dioxide in concentrated aqueous solutions. Vib. Spectrosc. 2018, 99, 156–161. [Google Scholar] [CrossRef]
- Bro, R.; Smilde, A.K. Principal component analysis. Anal. Methods 2014, 6, 2812–2831. [Google Scholar] [CrossRef]
- Ballabio, D.; Consonni, V. Classification tools in chemistry. Part 1: Linear models. PLS-DA. Anal. Methods 2013, 5, 3790. [Google Scholar] [CrossRef]
- Shoukat, U.; Baumeister, E.; Pinto, D.D.; Knuutila, H.K. Thermal stability and corrosion of tertiary amines in aqueous amine and amine-glycol-water solutions for combined acid gas and water removal. J. Nat. Gas Sci. Eng. 2019, 62, 26–37. [Google Scholar] [CrossRef]
- Ciftja, A.F. Quantitative characterization of absorbents for CO2 capture. In Depart of Chemical Engineering; Norwegian Unversity of Science and Technology: Trondheim, Norway, 2013. [Google Scholar]
- Jackson, P.; Robinson, K.; Puxty, G.; Attalla, M. In situ Fourier Transform-Infrared (FT-IR) analysis of carbon dioxide absorption and desorption in amine solutions. Energy Procedia 2009, 1, 985–994. [Google Scholar] [CrossRef]
- Falk, M.; Miller, A.G. Infrared spectrum of carbon dioxide in aqueous solution. Vib. Spectrosc. 1992, 4, 105–108. [Google Scholar] [CrossRef]
- Diab, F.; Provost, E.; Laloué, N.; Alix, P.; Souchon, V.; Delpoux, O.; Fürst, W. Quantitative analysis of the liquid phase by FT-IR spectroscopy in the system CO2/diethanolamine (DEA)/H2O. Fluid Phase Equilibria 2012, 325, 90–99. [Google Scholar] [CrossRef]
- Robinson, K.; McCluskey, A.; Attalla, M.I. An ATR-FTIR Study on the Effect of Molecular Structural Variations on the CO2 Absorption Characteristics of Heterocyclic Amines, Part II. ChemPhysChem 2012, 13, 2331–2341. [Google Scholar] [CrossRef] [PubMed]
- Davis, J. Thermal Degradation of Aqueous Amines Used for Carbon Dioxide Capture; University of Texas Libraries: Austin, TX, USA, 2009. [Google Scholar]
- Islam, M.S.; Yusoff, R.; Ali, B.S.; Islam, M.N.; Chakrabarti, M.H. Degradation studies of amines and alkanolamines during sour gas treatment process. Int. J. Phys. Sci. 2011, 6, 5883–5896. [Google Scholar]
- Gouedard, C.; Picq, D.; Launay, F.; Carrette, P.L. Amine degradation in CO2 capture. I. A review. Int. J. Greenh. Gas Control 2012, 10, 244–270. [Google Scholar] [CrossRef]
- Da Silva, E.F.; Grimstvedt, A.; Vevelstad, S.J.; Vernstad, K.; Lepaumier, H.; Einbu, A.; Svendsen, H.F.; Zahlsen, K. Understanding 2-Ethanolamine Degradation in Postcombustion CO2 Capture. Ind. Eng. Chem. Res. 2012, 51, 13329–13338. [Google Scholar] [CrossRef]
- Zhou, S.; Wang, S.; Chen, C. Thermal Degradation of Monoethanolamine in CO2 Capture with Acidic Impurities in Flue Gas. Ind. Eng. Chem. Res. 2012, 51, 2539–2547. [Google Scholar] [CrossRef]
- Zoannou, K.S.; Sapsford, D.J.; Griffiths, A.J. Thermal degradation of monoethanolamine and its effect on CO2 capture capacity. Int. J. Greenh. Gas Control 2013, 17, 423–430. [Google Scholar] [CrossRef]
- Rao, A.B.; Rubin, E.S. A Technical, Economic, and Environmental Assessment of Amine-Based CO2 Capture Technology for Power Plant Greenhouse Gas Control. Environ. Sci. Technol. 2002, 36, 4467–4475. [Google Scholar] [CrossRef]
- Freeman, S.A.; Davis, J.; Rochelle, G.T. Degradation of aqueous piperazine in carbon dioxide capture. Int. J. Greenh. Gas Control 2010, 4, 756–761. [Google Scholar] [CrossRef]





| Chemical | CAS | Purity | Chemical Structure |
|---|---|---|---|
| Carbon dioxide (CO2) | 124-38-9 | ≥99.9% | 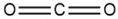 |
| Ethylene glycol (MEG) | 107-21-1 | ≥99.5% |  |
| N-Methyldiethanolamine (MDEA) | 105-59-9 | ≥99.0% | 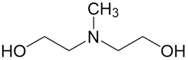 |
| Triethylene glycol (TEG) | 112-27-6 | ≥99.8% | 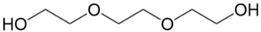 |
| System | Name | Range of the Components (wt%) | ||||
|---|---|---|---|---|---|---|
| MDEA (wt%) | H2O (wt%) | MEG (wt%) | TEG (wt%) | CO2 Loading (mol CO2/mol MDEA) | ||
| Two component system | MDEA-H2O | 0–100 | 0–100 | - | - | - |
| MEG-H2O | - | 0–100 | 0–100 | - | - | |
| TEG-H2O | - | 0–100 | - | 0–100 | - | |
| Three component system | MDEA-MEG-H2O | 0–70 | 0–70 | 30 | - | - |
| 30 | 0–70 | 0–70 | - | - | ||
| 0–70 | 30 | 0–70 | - | - | ||
| MDEA-TEG-H2O | 0–70 | 0–70 | - | 30 | - | |
| 30 | 0–70 | - | 0–70 | - | ||
| 0–70 | 30 | - | 0–70 | - | ||
| Four component system | MDEA-MEG-H2O-CO2 | 20 | 50 | 30 | - | 0–0.5 |
| MDEA-TEG-H2O-CO2 | 20 | 50 | - | 30 | 0–0.4 | |
| Component | Factors | Standard Error of Cross-Validation (SECV) (wt%) | Root Mean Square (RMSE) (wt%) | Maximum Possible Correlation Coefficient (R2) | Bias (wt%) | Standard Error (SE) (wt%) | Validation Number (VN) |
|---|---|---|---|---|---|---|---|
| MDEA | 3 | 0.0122 | 0.0122 | 0.999 | 0.0002 | 0.0109 | 22.96 |
| H2O | 3 | 0.0122 | 0.0122 | 0.999 | 0.0089 | 0.0109 | 22.96 |
| MEG | 3 | 0.0120 | 0.0027 | 1 | 0.0007 | 0.0026 | 93.85 |
| H2O | 3 | 0.0120 | 0.0027 | 1 | −0.0009 | 0.0026 | 93.85 |
| TEG | 3 | 0.0073 | 0.0098 | 0.999 | 0.0022 | 0.0136 | 18.36 |
| H2O | 3 | 0.0073 | 0.0098 | 0.999 | 0.0068 | 0.0136 | 18.36 |
| Component | Factors | SECV (wt%) | RMSE (wt%) | R2 | Bias (wt%) | SE (wt%) | VN |
|---|---|---|---|---|---|---|---|
| MDEA | 4 | 0.0264 | 0.0261 | 0.992 | 0.0033 | 0.0298 | 8.38 |
| MEG | 4 | 0.0157 | 0.0157 | 0.997 | 0.0005 | 0.0171 | 14.56 |
| H2O | 4 | 0.0149 | 0.0149 | 0.998 | 0.0009 | 0.0151 | 16.36 |
| MDEA | 4 | 0.0136 | 0.0141 | 0.998 | 0.001 | 0.0141 | 17.65 |
| TEG | 4 | 0.0155 | 0.0151 | 0.997 | 0.001 | 0.0152 | 16.47 |
| H2O | 4 | 0.0166 | 0.0165 | 0.997 | 0.0011 | 0.0166 | 15.09 |
| Component | Factors | SECV (wt%) | RMSE (wt%) | R2 | Bias (wt%) | SE (wt%) | VN |
|---|---|---|---|---|---|---|---|
| MDEA | 5 | 0.0259 | 0.0259 | 0.991 | 0.0031 | 0.0259 | 9.64 |
| MEG | 5 | 0.0162 | 0.0162 | 0.996 | 0.001 | 0.0154 | 16.26 |
| H2O | 5 | 0.0128 | 0.0129 | 0.998 | 0.0012 | 0.0129 | 19.16 |
| CO2 | 5 | 0.001 | 0.0012 | 0.982 | 0.000004 | 0.0012 | 7.38 |
| MDEA | 4 | 0.0167 | 0.0167 | 0.996 | 0.0012 | 0.0167 | 14.95 |
| TEG | 4 | 0.0154 | 0.0154 | 0.997 | 0.001 | 0.0154 | 16.23 |
| H2O | 4 | 0.0201 | 0.0201 | 0.994 | 0.0022 | 0.0201 | 12.41 |
| CO2 | 4 | 0.002 | 0.0019 | 0.936 | 0.0002 | 0.0020 | 4.04 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shoukat, U.; Baumeister, E.; Knuutila, H.K. ATR-FTIR Model Development and Verification for Qualitative and Quantitative Analysis in MDEA–H2O–MEG/TEG–CO2 Blends. Energies 2019, 12, 3285. https://doi.org/10.3390/en12173285
Shoukat U, Baumeister E, Knuutila HK. ATR-FTIR Model Development and Verification for Qualitative and Quantitative Analysis in MDEA–H2O–MEG/TEG–CO2 Blends. Energies. 2019; 12(17):3285. https://doi.org/10.3390/en12173285
Chicago/Turabian StyleShoukat, Usman, Eva Baumeister, and Hanna K. Knuutila. 2019. "ATR-FTIR Model Development and Verification for Qualitative and Quantitative Analysis in MDEA–H2O–MEG/TEG–CO2 Blends" Energies 12, no. 17: 3285. https://doi.org/10.3390/en12173285
APA StyleShoukat, U., Baumeister, E., & Knuutila, H. K. (2019). ATR-FTIR Model Development and Verification for Qualitative and Quantitative Analysis in MDEA–H2O–MEG/TEG–CO2 Blends. Energies, 12(17), 3285. https://doi.org/10.3390/en12173285






