1. Introduction
The increasing threat of global warming has scientists rushing for immediate and sustainable solutions to carbon emissions. According to the United Nations (UN) Environment Emissions Gap Report, the total carbon emissions in 2017 had reached 53.5 GtCO
2e, a record high and 0.6 GtCO
2e more than the 2016 emissions [
1]. Atmospheric CO
2 levels have also reached the highest levels since the Pliocene, recorded at 413.5 ppm as of April 2019 [
2]. To achieve the target of limiting global warming to 1.5 °C in accordance to the Paris Agreement, emissions therefore must be cut by 55% by 2030 to achieve the least cost pathway [
1,
3]. However, with the policies and measures in place, it is estimated that the Paris Agreement target will not be met, and global warming is projected to be at 3.1–3.5 °C above pre-industrial levels by 2100 [
4]. Given these stark indicators, measures in order to reduce carbon emissions must be performed.
Carbon capture and sequestration (CCS) techniques have been perceived as one of the main solutions to the increasing threat of anthropogenic emissions. CCS techniques, methods, and technologies mainly involve the use of a carbon sink, wherein carbon dioxide can be diverted from the atmosphere whilst being stored in a secure and reliable manner [
5]. These CCS methods can be classified into three main categories according to their sinks—namely oceanic, terrestrial, and geologic [
6]. Oceanic carbon sequestration involves either injecting CO
2 deep into the ocean or promoting photosynthetic CO
2 fixation by aquatic organisms to sequester CO
2 [
7], whereas soil and vegetation (such as forests) are considered the sinks for terrestrial carbon sequestration [
6]. On the other hand, geologic carbon sequestration utilizes rock formations and deep land traps, seams, and aquifers to securely store carbon [
6].
Most of these methods are highly efficient as carbon capture and sequestration can reach up to 100% efficiency for most processes; however, some of these technologies are energy-intensive and cost-ineffective, and thus, cannot be used for industrial application [
5].
One particular method stands out among others in terms of its capability to balance the efficiency and energy considerations. CCS via mineralization (CCS-M) is a process in which carbon is stored in geological formations, particularly minerals, which act as the carbon sink. These materials are more stable and reasonably inert, and are thus viable options [
8]. CCS-M can be divided primarily into two types—direct or in situ mineralization, and indirect or ex situ mineralization. In situ mineralization involves directly injecting carbon dioxide (CO
2) deep beneath earth’s crust in order to form carbonates with the ions present, storing CO
2 underground [
8]. This process requires a relatively low amount of energy; however, as utilization of the products is perceived in the long run, this method is not popular as the process yields products of low purity [
9]. CO
2 conversion is very slow as well, as it relies solely on natural weathering, and is thus the main drawback of this method [
8]. On the other hand, ex situ mineralization has become a more popular method recently due to its promising potential for industrial use. In this method, carbonation is performed above the ground, meaning minerals are excavated first and are subjected to a series of steps [
8]. Among these, the pH swing method has showed great potential in recent years, due to its high reaction rates in comparison to other ex situ methods, as well as its ability to form products with very high purity even for low-grade raw materials [
8].
In this study, the applicability of locally available and unutilized mine waste, i.e., mixed dump from a nickel laterite ore mine site, for carbon sequestration is to be determined by varying the process parameters mentioned in leaching tests using the pH swing method. Nickel mine waste contains a substantial amount of iron, particularly in the form of nickel oxides, such as goethite [
10]. Nickel ions are hosted in goethite mainly through adsorption or substitution in the structure; thus, its abundance [
10]. The report by the Philippine Statistical Authority and the Department of Environment and Natural Resources Mines and Geosciences Bureau also mentioned that the ratio amount of ore processed to actual nickel extracted is estimated to be at 77:1, which implies the huge amount of wastes generated in nickel mine sites [
11]. With the growing need for waste utilization and conversion to valuable resources, tailings and residues from nickel mine sites must therefore be processed in order to reduce the risk that it poses to the environment, as well as to convert these materials into something usable and valuable.
Aside from determining the significance of process parameters, the study also aimed to establish the mineralogical, elemental, and morphological properties of the mixed dump. The study also aimed to optimize the parameters for possible industrial application, and lastly, to establish the carbon sequestration potential of the mixed dump through calculation of the theoretical amount of CO2 sequestered per amount of sample.
2. Theoretical Framework
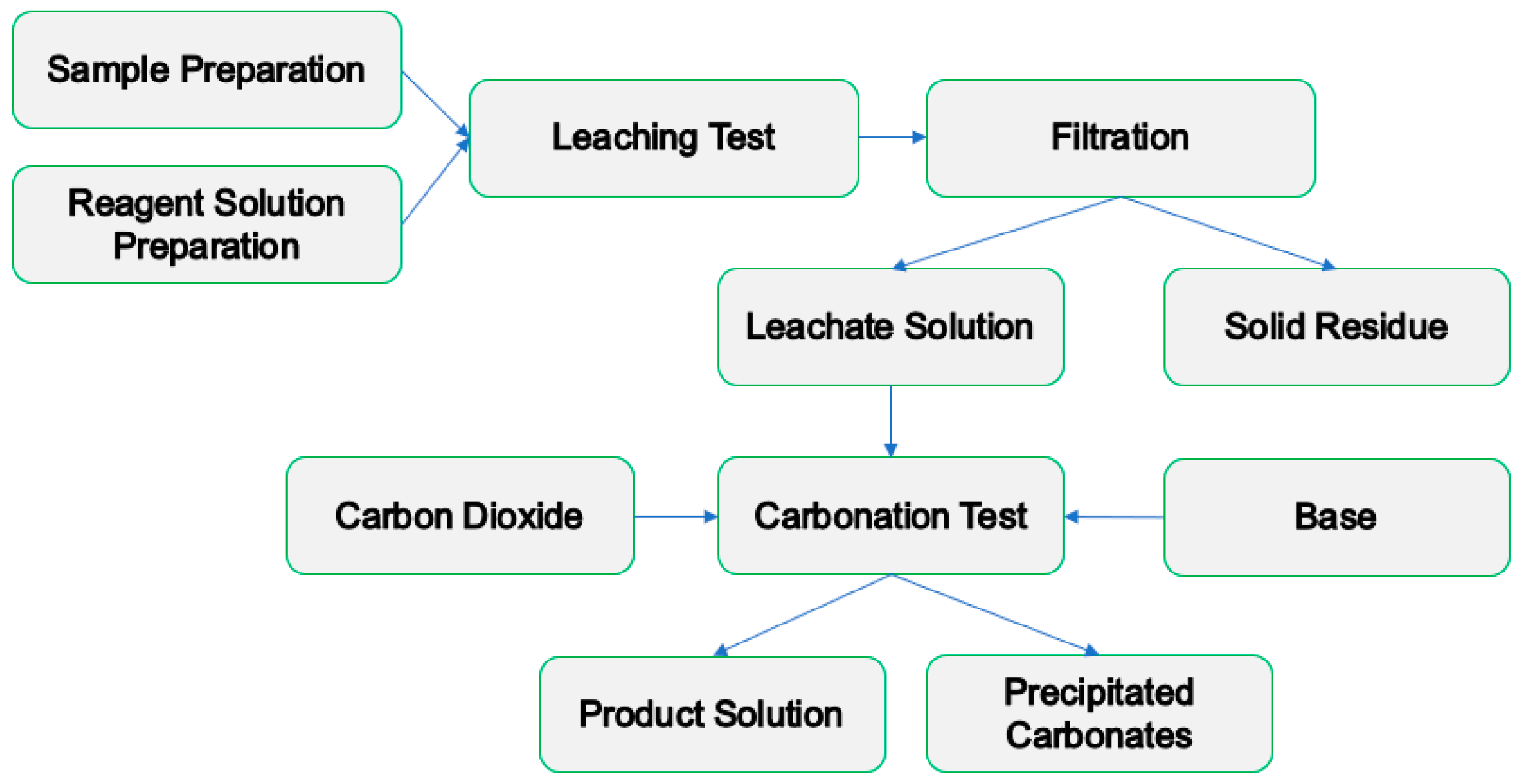
The pH swing method is a type of indirect carbon sequestration that is comprised of two steps—extraction and carbonation, both of which performed in an aqueous setup. A simplified diagram of the pH swing method is shown in
Figure 1. The material primarily is subjected to an extracting medium, to obtain as much ions as possible for use in the carbonation step. Whilst containing undesired ions, pH is usually adjusted prior to carbonation, to precipitate the undesired ions out of the solution. Once only the desired ions remain, CO
2 is injected at high pH (pH 8 to 12) to achieve maximum CO
2 absorption [
12,
13,
14,
15]. Through this manner, high quality of products is formed, making it feasible for actual industrial application [
9].
Extensive studies have been performed for various raw materials using the pH swing method. Industrial wastes, particularly, have been studied in-depth to address the growing concern on the volume of wastes generated globally and for their possible utilization in other industries and processes. Other materials of low market value, such as low-grade ores, have also been explored for possible conversion to valuable and profitable products. Utilization of high-grade material, on the other hand, was primarily abandoned due to the fact that these materials entail additional cost which would make carbon sequestration even more costly, as well as possible increased competition with industries that utilize these materials, making CCS more undesirable.
For industrial wastes, some of the raw materials used were waste cement powder and blast furnace slag, which are abundant sources of calcium (Ca) [
12]. For these materials, Ca is present in the form of calcium silicate (CaSiO
3), and reacts with an acid, e.g., acetic acid (CH
3COOH), in a two-step scheme (Equations (1) and (2)) to form stable calcite [
16]:
Other potential raw materials derived from waste that have been identified for calcium carbonation are electric arc furnace (EAF) slag [
17,
18], basic oxygen furnace (BOF) slag [
17,
18], cement kiln dust [
19], pulverized-fuel ash [
19,
20], oil shale ash [
21,
22], air pollution control residue [
23], and paper sludge incineration ash [
19] which have shown experimental CO
2 uptakes ranging from 1.7–25 wt.% [
24].
For magnesium carbonation, various studies have utilized serpentine-rich materials, particularly chrysotile- and antigorite-bearing ores. These materials have shown a lot of potential by posting high extraction rates at high temperatures of 70–100 °C [
25,
26]. Serpentines, with a general chemical formula of Mg
3Si
2O
5(OH)
4, react with the leaching agent to form magnesite in the sequence of reactions below (Equations (3) to (5)) [
14]:
Olivines, another group of magnesium silicate minerals, have also manifested the same results, particularly for materials bearing forsterite [
27]. Forsterite forms stable magnesite in the following series of steps (Equations (6) to (8)) [
28]:
Nonetheless, in the case of iron-rich minerals, limited studies have been performed for their viability in carbon sequestration via indirect mineral carbonation, particularly through the pH swing method. One study has utilized fayalite, a type of olivine with chemical formula Fe
2SiO
4, in carbon sequestration by subjecting the sample in a water-supercritical CO
2 setup (Equations (9) to (11)) [
29]:
Although siderite (FeCO
3) formation was reported, low concentrations were observed even for an extended period of time (80 days), mainly due to the limited dissolution of the iron silicates in the aqueous medium [
29]. Basalts, which are formed from the solidification of lava from volcanic eruptions, have also been sought as possible site for CO
2 injection as these deposits contain huge quantities of Ca, Mg, and Fe; however, the challenge of injecting supercritical CO
2 underground, and its accompanying reactions remain to be clearly defined [
30]. Subsequently, studies have also been devoted to the utilization of iron for carbon sequestration in saline aquifers and sea beds due to its inherent abundance in the form of hematite and goethite [
31,
32,
33,
34,
35]; nonetheless, these studies cited the slow rate of iron release to the solution as a possible hindrance to the carbonation process.
It is evident from the studies mentioned that the efficiency of carbon dioxide sequestration is highly dependent on the availability of ions capable of forming carbonates. A study on mine tailings obtained from a platinum group mineral (PGM) ore processing plant highlighted that, although carbonation rates of near completion (96–98%) for calcium and iron have been achieved, the transfer of ions in solution remained to be the determining step [
13]. Process improvements have been performed in order to address this drawback; several leaching agents have been analyzed, both organic and inorganic acids, in order to increase the transfer of carbonation ions into the liquid matrix. Among these, hydrochloric acid (HCl) and sulfuric acid (H
2SO
4) have been sought as possible leaching agents for Mg and Fe rich materials [
25,
36], whereas maximum extraction of Ca has been observed using acetic acid (CH
3COOH) in the addition of 0.1 wt.% ethylenediamine tetra-acetic acid (EDTA) [
12,
18].
Aside from the choice of leaching agent, impurities in the minerals were also reported to play a main role in the carbon dioxide sequestration process. Silica formation, as observed in serpentines and olivines, hinders further release of ions in the aqueous solution due to the formation of a passivating layer that coats the surface of the material [
28]. The presence of aluminum in the form of Al
3+ also decreases the leachability of magnesium in the sample as the ion promotes layer-to-layer linkage by H-bonding, which hinders the release of the Mg
+ ions [
37]. Similarly, the presence of Fe
3+ in magnesium rich samples has been observed to hinder the release of Mg
+ due to preferential leaching of iron in the solution [
37].
Process parameters, such as reaction temperature, reaction time, and reagent concentration, have been reported to have significant effects in the effective transfer of carbonation ions, although the magnitude of significance varies across a range of values. A three-fold increase in Mg extraction, from 28% to 75%, has been observed from a serpentinized lherzolite when the temperature was increased from 50 °C to 100 °C at a reaction time of 180 min, whereas the same study reported no increase in Mg extraction when the reaction temperature was further increased to 120 °C and 140 °C at the same timeframe [
38]. A different study reported the effect of reaction time on the extraction of Mg and Fe in antigorites; for Mg, 55% of the initial content of the sample has been extracted at the first 30 min using 1.4 M NH
4SO
4, whereas a total of 70% extraction or only an additional 15% has been extracted after 3 h [
39]. For Fe, the similar study has reported 40–45% Fe extraction after 30 min and further increase after an additional 2.5 h [
39]. The study of Teir et al. (2007), on the other hand, has observed the extraction profile of natural serpentinite for HCl at different concentrations; an increase in the extraction efficiency of Mg from 16% to 24% has been observed when HCl concentration was increased from 1 M to 4 M at 20 °C and 1 h; for Fe, an increase from 9% to 16% has been observed [
25]. The study of Sanna et al. focused on another reagent, NH
4SO
4, and observed a three-fold increase for Fe extraction, from 37.9% to 100.0% when the concentration was increased from 1 M to 3 M after 3 h [
38]. The same study reported an increase in Mg extraction of about 15% from 66% to 82% at the same period [
38]. Although the behavior of different combinations of process parameters has been reported in previous studies, the optimum set of parameters for different raw materials varies individually and therefore needs to be established.
3. Methodology
3.1. Sample Description and Preparation
The mixed dump sample used in this experiment was obtained from a nickel laterite ore mine in Agusan Del Norte, one of the provinces in Southern Philippines. Mixed dump is basically a type of mine waste referring to combined, accumulated, or collected mine wastes in solid form. As received, the sample had a brownish black color and had a composition of agglomerated fine particles (due to moisture) and small rock-like components ranging from 5–30 mm in diameter.
Prior to analysis and testing, the samples were dried in a Shellab 1600 Hafo Series convection oven at 105 °C for 24 h. The dried mixed dump samples were then grounded in an MRC Scientific RJM-102 ball mill for 4 h at 350 rpm and sieved in a W.S. Tyler RX-182 Ro-Tap Sieve Shaker for 30 min to obtain a particle size range of 75 to 150 µm. Once the desired particle size range was obtained, the grounded and sieved samples were stored in an airtight container at room temperature to avoid contamination by moisture.
3.2. Sample Characterization
The mineralogical characteristics of the sample were first established through X-Ray powder diffraction (XRD), using Multiflex Rigaku 150 (range [°2Th] = 5°–60°, scan step time = 0.6000 s, step size [°2Th] = 0.02) available at Tokyo Institute of Technology. The oxide composition of the sample was determined via X-ray fluorescence microscopy through the Horiba Scientific XGT-7200 X-ray Analytical Microscope also available at Tokyo Institute of Technology, whereas the elemental composition was determined by fusing the sample with sodium peroxide, leaching it with HCl, and subjecting it to Agilent 5100 for Inductively-Coupled Plasma Optical Emission Spectroscopy (ICP-OES). This was performed by a third-party laboratory (Intertek Testing Services Philippines, Inc).
The morphological properties of the mixed dump were also analyzed in the Phenom XL Scanning Electron Microscope-Energy Dispersive X-Ray (SEM-EDX) at De La Salle University, with a magnification range of 2750×–10000×, a voltage of 15 kV, and gold sputtering. Brunauer–Emmett–Teller (BET) surface analysis was otherwise performed at the University of the Philippines–Los Baños using the Quantachrome Nova Station A to determine the pore volume, pore distribution and surface area of the sample.
3.3. Reagent Solution Preparation
Hydrochloric acid (HCl) solutions with concentration of 1.0 M, 2.5 M, and 4.0 M were prepared using Duksan AR Grade Hydrochloric Acid (12.38 M). Fresh solutions were prepared every run to prevent degradation of the reagent.
3.4. Reactor Setup
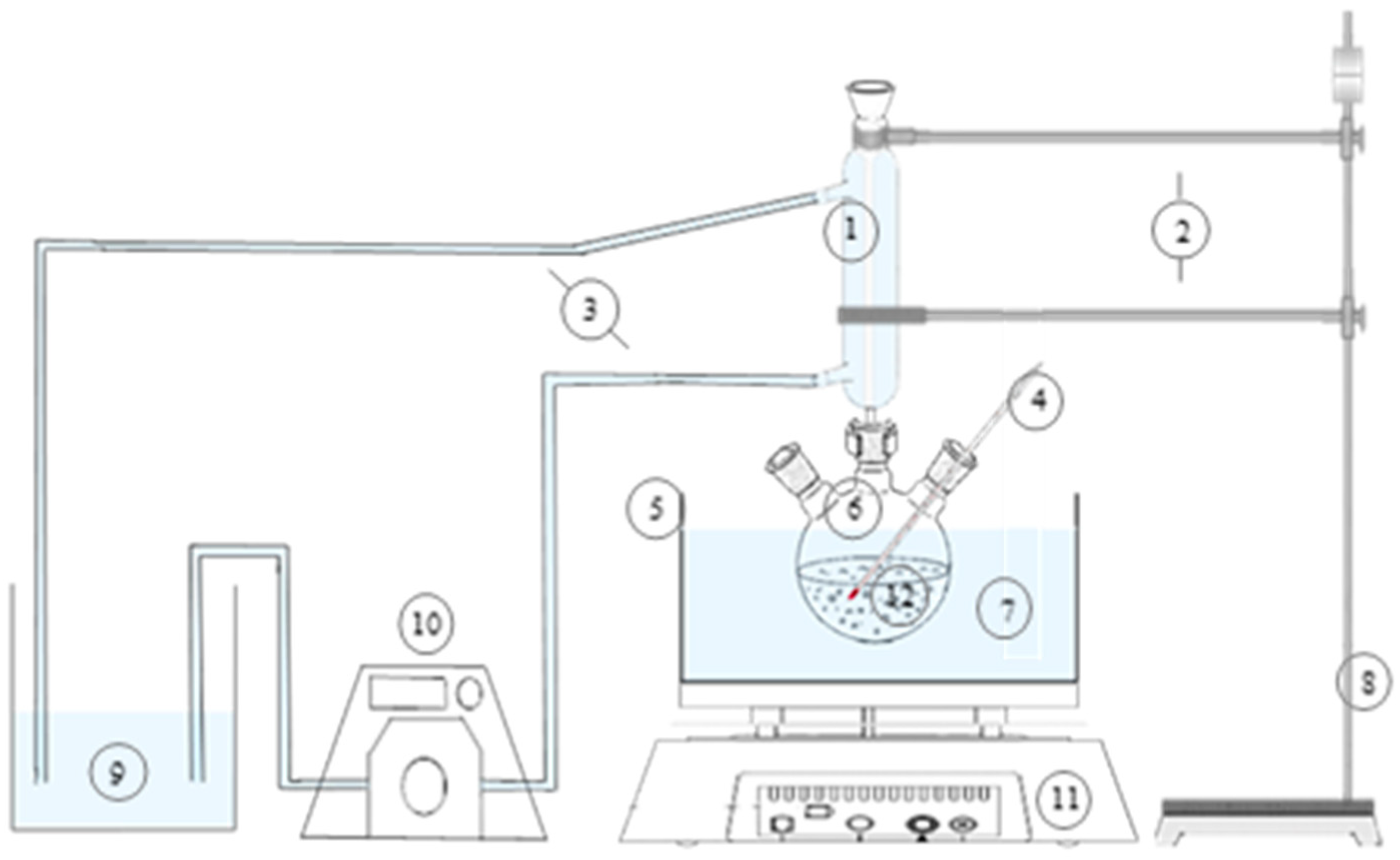
The setup of the reactor was based on the study of Teir et al. (2007) [
25] but with some modifications as shown in
Figure 2. The leaching experiment was conducted in a Pyrex 500 mL angular three-neck spherical round bottom flask submerged in a 1000 cP silicone oil bath. The temperature of the bath was controlled by the Corning Laboratory Hot Plate, and the temperature inside the reactor setup was monitored regularly by an alcohol thermometer within ±1.0 °C. The contents of the reactor were mixed using a magnetic bar stirrer, regulated also by the hot plate. To prevent excess evaporation, the reactor was coupled with a 300 mm Graham condenser, with cooling water coming from the nearby water supply.
3.5. Experimental Design
The study considered three parameters as possible significant factors in the leaching test—namely, solvent concentration, reaction temperature, and reaction time. The values for these factors were varied to three (3) levels, as shown in
Table 1, and were established through precedent studies. The range of reagent concentrations used in the study was based on the studies that utilized HCl as the leaching agent [
14,
18], whereas for reaction time, the range of values was determined through a compilation of studies which examined the extraction efficiency as a function of time [
14,
18,
38,
39]. Lastly, the reaction temperature was established through a variety of studies that employed the pH swing method for carbon sequestration [
12,
13,
38,
39].
For this experiment, the Face-Centered Cube (FCC) Design of Response Surface Methodology (RSM) was employed as the experimental design, as optimization of the process parameters was desired. In this design, seventeen treatment combinations were considered, with the central point in triplicates to establish the deviation. The treatment combinations for the experiment are shown in
Table 2.
3.6. Leaching Test
Two hundred milliliters (200 mL) of HCl solution at the desired concentration was first poured into the reaction vessel and heated to the required temperature. Subsequently, 10 g of the mixed dump sample in powder form was added and mixed by a magnetic stirrer at a speed of 650 rpm. Temperature was monitored constantly throughout the reaction, and it was ensured that the deviation is within tolerance. The leaching test was allowed to proceed until the desired reaction time, and once completed, the angular three-neck spherical round bottom flask was detached from the setup and the reaction mixture was cooled down by dipping the flask in tap water for 20 min. The solution was then transferred to a beaker and filtered using an Advantec (Tokyo, Japan) 0.45 µm syringe filter. The filtrates were stored in polypropylene falcon tubes and kept refrigerated to reduce the corrosive properties of the filtrate prior testing.
3.7. Leachate Characterization and Ion Extraction Efficiency Calculation
Similar to the raw sample, the ion concentrations in the filtrate were determined through Agilent 5100 ICP-OES. Four ions were analyzed—namely iron (Fe), magnesium (Mg), aluminum (Al), and silicon (Si).
To determine the efficiency of the extraction, individual ion extraction efficiencies were calculated using Equation (12), which was derived from the study of Daval et al. (2013) [
40].
where:
| Xi | is the extraction efficiency for element i; |
| Mt | is the initial mass of the sample used in the experiment (mg); |
| %iMt | is the percent elemental composition of element i in the raw sample; |
| Vsol | is the leachate solution volume after the desired reaction time (L); |
| Ci | is the concentration of element i in the leachate determined through ICP-OES (mg/L). |
5. Conclusions and Recommendations
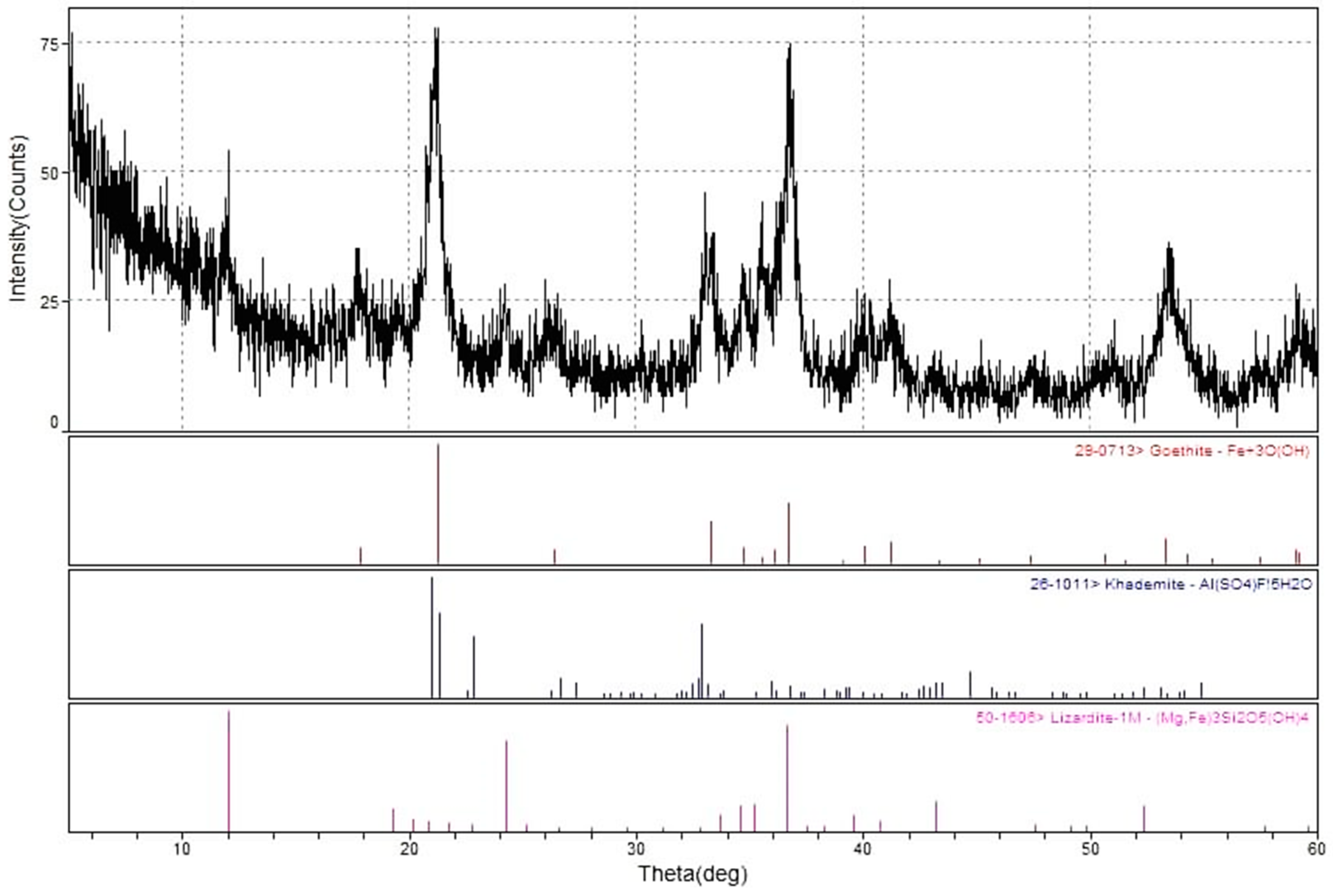
X-ray diffraction analysis suggested three major minerals present in the mixed dump, namely: goethite, α-FeO(OH), khademite, AlSO4F·5H2O, and lizardite-1M, Mg3Si2O5(OH)4. XRF and ICP-OES results further established goethite as the main mineral present for the sample, due to the dominance of iron recorded for both analyses.
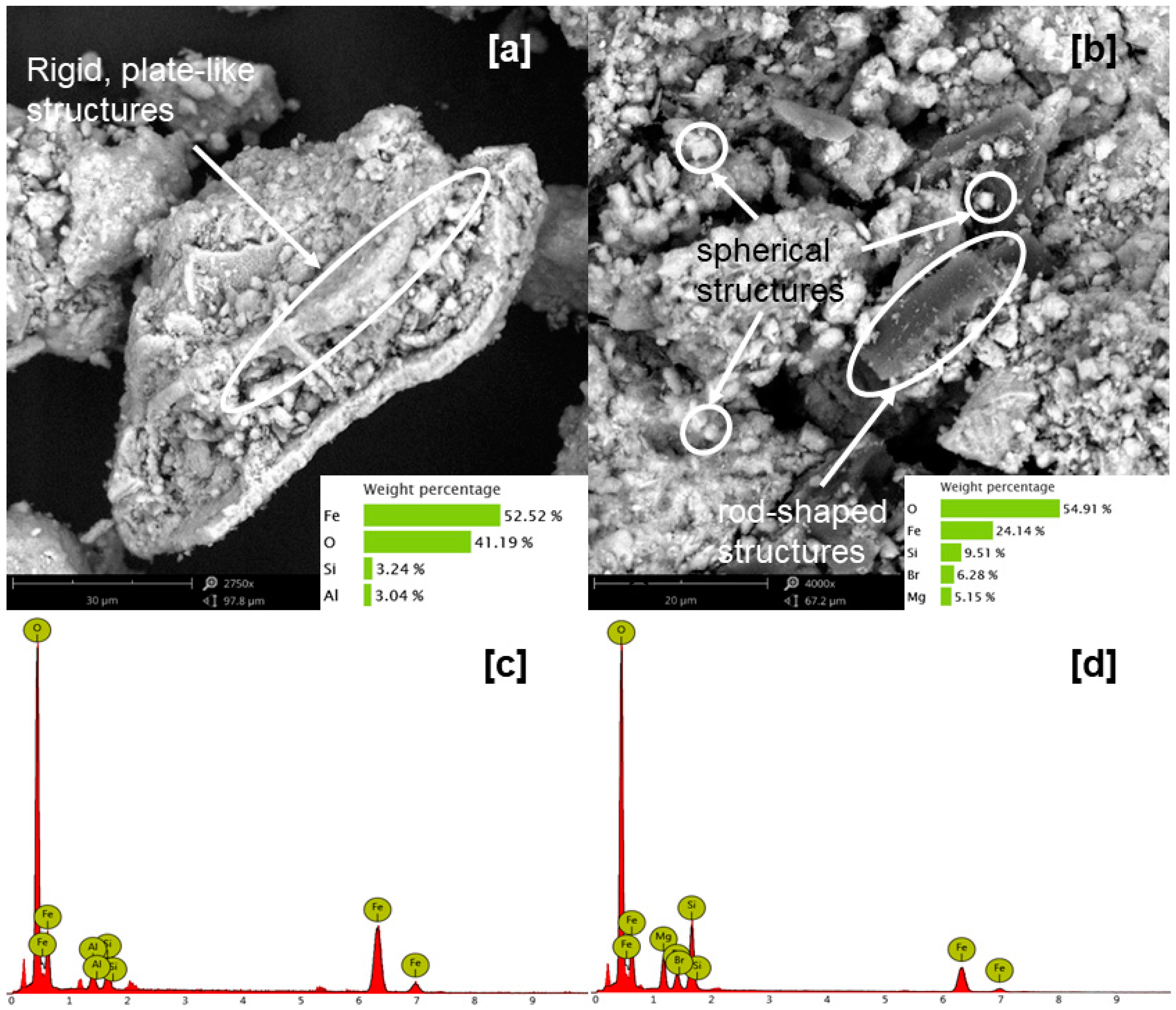
The morphological characteristics of the sample were also analyzed, and SEM-EDX results reported high heterogeneity in the sample. Through region analysis, it was found that the composition differs from particle to particle; although Fe remained to be consistently dominant, Al and Mg appeared to be concentrated in specific regions in the sample. On the other hand, BET results suggested high accessible surface for the sample as the surface area-to-volume ratio is much higher to those previously reported.
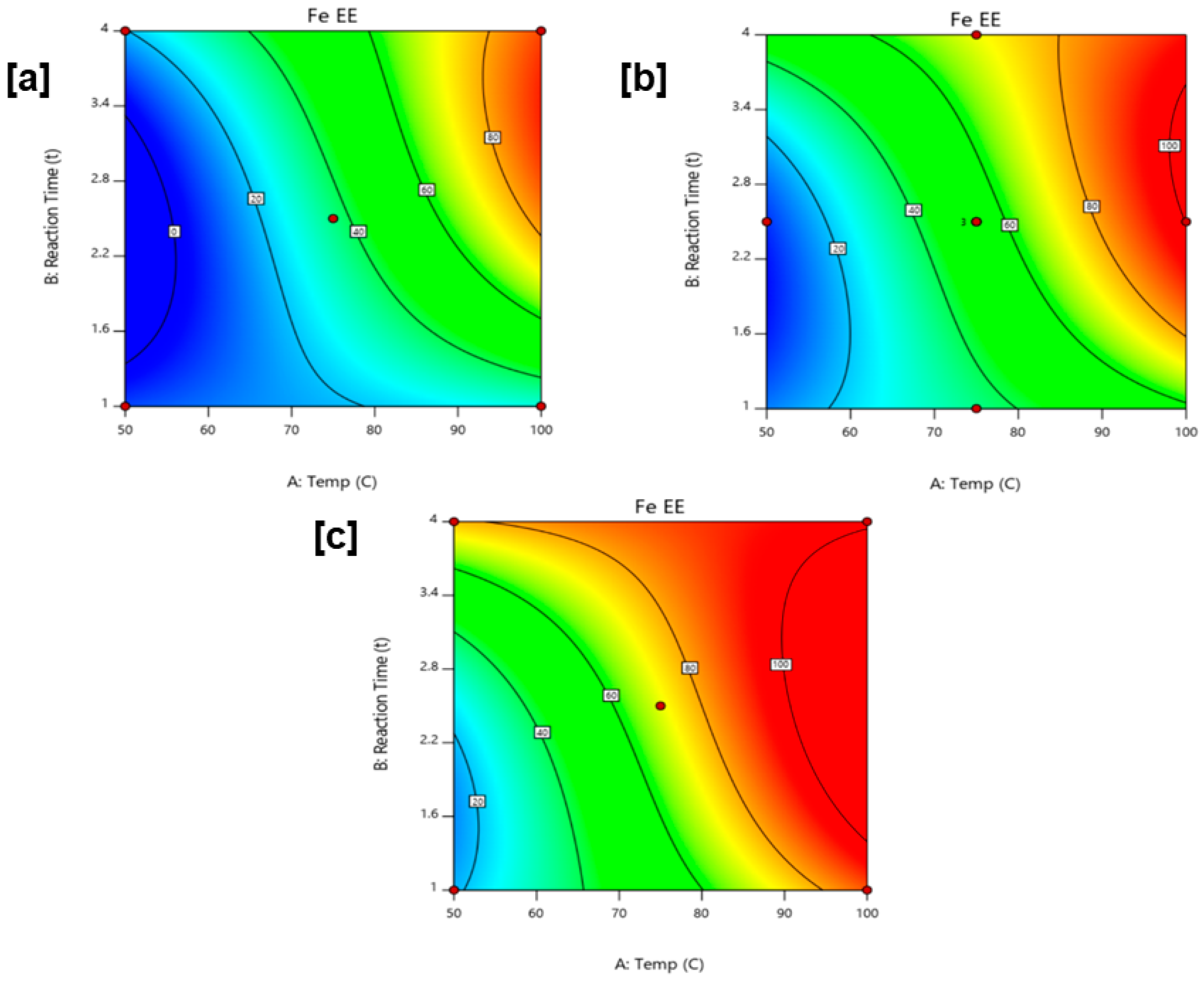
Ion extraction efficiencies, particularly for iron, posted very high results, with the highest calculated extraction efficiency of 95.37% obtained at 100 °C, 2.5 h, and 2.5 M HCl. Promising results for ion extraction can highly be attributed to the accessible surface available for the sample, as well as the increased solubility of goethite in HCl at high temperature and high reagent concentration.
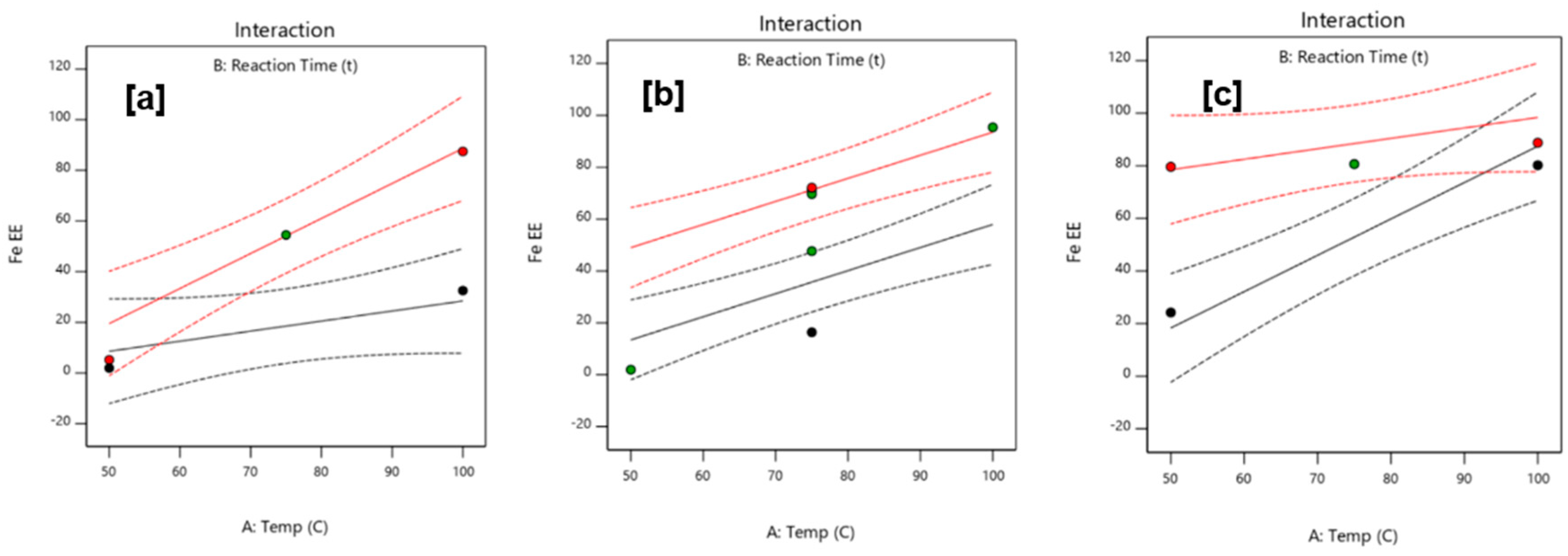
Significance of the parameters was determined using a face-centered cube design for response surface methodology. Outcomes of ANOVA revealed that all parameters were significant for iron extraction efficiency, with interactions highly pronounced at low and high reagent concentrations due to the dependence of the leaching process in reaction time and temperature.
Lastly, the carbon sequestration potential of the mixed dump was quantified through the theoretical amount of CO2 that can be sequestered per amount of sample. It is reported that the mixed dump can sequester a maximum of 327.2 mg CO2/g sample at 100 °C, 2.5 h and 2.5 M HCl, which is higher than that of waste cement, blast furnace slag, fly ash, and grounded serpentinite. The challenge posted in this process however is, for iron carbonation to proceed, particularly in the case of goethite, an additional reduction step is required as Fe3+ cannot be readily utilized to form carbonates. Sulfur dioxide, which forms sulfide ions in aqueous solutions, is one of the reducing agents considered and is existent in combination with CO2 in industrial flue gas. Therefore, although iron carbonation requires an additional step to proceed into completion, the inherent presence of the reducing agent together with CO2 means that there is great potential for iron-rich materials in carbon dioxide sequestration via mineralization.
The authors suggest that material selection be evaluated concisely to make the pH swing process viable and cost-appealing. A thorough investigation of the energy penalties associated to the sample preparation and its subsequent effect on the efficiency of the carbon sequestration process, is also recommended.