Butyronitrile-Based Electrolytes for Fast Charging of Lithium-Ion Batteries
Abstract
:1. Introduction
2. Experimental Section
2.1. Electrolyte Formulation
2.2. Preparation of T44 Graphite and Lithium Manganese Oxide Electrodes
2.3. Electrochemical Measurements
2.3.1. Cell Set-Up
2.3.2. Linear Sweep Voltammetry Measurements
2.3.3. Cyclic Voltammetry Measurements
2.3.4. Galvanostatic Measurements
2.4. Conductivity Measurements
2.5. X-ray Photoelectron Spectroscopy (XPS) Analysis
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Winter, M.; Brodd, R.J. What are batteries, fuel cells, and supercapacitors? Chem. Rev. 2004, 104, 4245–4269. [Google Scholar] [CrossRef] [PubMed]
- Andre, D.; Kim, S.-J.; Lamp, P.; Lux, S.F.; Maglia, F.; Paschos, O.; Stiaszny, B. Future generations of cathode materials: an automotive industry perspective. J. Mater. Chem. A 2015, 3, 6709–6732. [Google Scholar] [CrossRef]
- Schmuch, R.; Wagner, R.; Hörpel, G.; Placke, T.; Winter, M. Performance and cost of materials for lithium-based rechargeable automotive batteries. Nat. Energy 2018, 3, 267. [Google Scholar] [CrossRef]
- Winter, M.; Barnett, B.; Xu, K. Before Li ion batteries. Chem. Rev. 2018, 118, 11433–11456. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.; Han, X.; Li, J.; Hua, J.; Ouyang, M. A review on the key issues for lithium-ion battery management in electric vehicles. J. Power Sources 2013, 226, 272–288. [Google Scholar] [CrossRef]
- Placke, T.; Kloepsch, R.; Dühnen, S.; Winter, M. Lithium ion, lithium metal, and alternative rechargeable battery technologies: the odyssey for high energy density. J. Solid State Electrochem. 2017, 21, 1939–1964. [Google Scholar] [CrossRef]
- Betz, J.; Bieker, G.; Meister, P.; Placke, T.; Winter, M.; Schmuch, R. Theoretical versus Practical Energy: A Plea for More Transparency in the Energy Calculation of Different Rechargeable Battery Systems. Adv. Energy Mater. 2019, 9, 1803170. [Google Scholar] [CrossRef]
- Wagner, R.; Preschitschek, N.; Passerini, S.; Leker, J.; Winter, M. Current research trends and prospects among the various materials and designs used in lithium-based batteries. J. Appl. Electrochem. 2013, 43, 481–496. [Google Scholar] [CrossRef]
- Xu, K. Nonaqueous liquid electrolytes for lithium-based rechargeable batteries. Chem. Rev. 2004, 104, 4303–4417. [Google Scholar] [CrossRef]
- Wen, J.; Yu, Y.; Chen, C. A Review on Lithium-Ion Batteries Safety Issues: Existing Problems and Possible Solutions. Mater. Express 2012, 2, 197–212. [Google Scholar] [CrossRef]
- Wrodnigg, G.H.; Besenhard, J.O.; Winter, M. Ethylene sulfite as electrolyte additive for lithium-ion cells with graphitic anodes. J. Electrochem. Soc. 1999, 146, 470–472. [Google Scholar] [CrossRef]
- Chawla, N.; Bharti, N.; Singh, S. Recent advances in non-flammable electrolytes for safer lithium-ion batteries. Batteries 2019, 5, 19. [Google Scholar] [CrossRef]
- Xu, K. Electrolytes and Interphases in Li-Ion Batteries and Beyond. Chem. Rev. 2014, 114, 11503–11618. [Google Scholar] [CrossRef] [PubMed]
- Cekic-Laskovic, I.; von Aspern, N.; Imholt, L.; Kaymaksiz, S.; Oldiges, K.; Rad, B.R.; Winter, M. Synergistic effect of blended components in nonaqueous electrolytes for lithium ion batteries. Top. Curr. Chem. 2017, 375, 37. [Google Scholar] [CrossRef] [PubMed]
- Xue, L.; Lee, S.-Y.; Zhao, Z.; Angell, C.A. Sulfone-carbonate ternary electrolyte with further increased capacity retention and burn resistance for high voltage lithium ion batteries. J. Power Sources 2015, 295, 190–196. [Google Scholar] [CrossRef] [Green Version]
- Lewandowski, A.; Kurc, B.; Stepniak, I.; Swiderska-Mocek, A. Properties of Li-graphite and LiFePO4 electrodes in LiPF6–sulfolane electrolyte. Electrochim. Acta 2011, 56, 5972–5978. [Google Scholar] [CrossRef]
- Abu-Lebdeh, Y.; Davidson, I. High-Voltage Electrolytes Based on Adiponitrile for Li-Ion Batteries. J. Electrochem. Soc. 2009, 156, A60–A65. [Google Scholar] [CrossRef]
- Winter, M.; Novák, P. Chloroethylene Carbonate, a Solvent for Lithium-Ion Cells, Evolving CO2 during Reduction. J. Electrochem. Soc. 1998, 145, L27–L30. [Google Scholar] [CrossRef]
- Wrodnigg, G.H.; Besenhard, J.O.; Winter, M. Cyclic and acyclic sulfites: new solvents and electrolyte additives for lithium ion batteries with graphitic anodes? J. Power Sources 2001, 97, 592–594. [Google Scholar] [CrossRef]
- Krämer, E.; Passerini, S.; Winter, M. Dependency of aluminum collector corrosion in lithium ion batteries on the electrolyte solvent. ECS Electrochem. Lett. 2012, 1, C9–C11. [Google Scholar] [CrossRef]
- Winter, M.; Moeller, K.-C.; Besenhard, J.O. Lithium Batteries: Science and Technology; Nazri, G.A., Pistoia, G., Eds.; Kluwer Academic Publishers: New York, NY, USA, 2004; p. 144. [Google Scholar]
- Safa, M.; Chamaani, A.; Chawla, N.; El-Zahab, B. Polymeric ionic liquid gel electrolyte for room temperature lithium battery applications. Electrochim. Acta 2016, 213, 587–593. [Google Scholar] [CrossRef]
- Safa, M.; Adelowo, E.; Chamaani, A.; Chawla, N.; Herndon, M.; Baboukani, A.; Wang, C.; El-Zahab, B. Poly (Ionic Liquid) based Composite Gel Electrolyte for Lithium Batteries. ChemElectroChem 2019, 6, 3319–3332. [Google Scholar] [CrossRef]
- Hilbig, P.; Ibing, L.; Wagner, R.; Winter, M.; Cekic-Laskovic, I. Ethyl methyl sulfone-based electrolytes for lithium ion battery applications. Energies 2017, 10, 1312. [Google Scholar] [CrossRef]
- Yamada, Y.; Furukawa, K.; Sodeyama, K.; Kikuchi, K.; Yaegashi, M.; Tateyama, Y.; Yamada, A. Unusual Stability of Acetonitrile-Based Superconcentrated Electrolytes for Fast-Charging Lithium-Ion Batteries. J. Am.Chem. Soc. 2014, 136, 5039–5046. [Google Scholar] [CrossRef]
- Isken, P.; Dippel, C.; Schmitz, R.; Schmitz, R.W.; Kunze, M.; Passerini, S.; Winter, M.; Lex-Balducci, A. High flash point electrolyte for use in lithium-ion batteries. Electrochim. Acta 2011, 56, 7530–7535. [Google Scholar] [CrossRef]
- Schmitz, R.W.; Murmann, P.; Schmitz, R.; Müller, R.; Krämer, L.; Kasnatscheew, J.; Isken, P.; Niehoff, P.; Nowak, S.; Röschenthaler, G.-V.; et al. Investigations on novel electrolytes, solvents and SEI additives for use in lithium-ion batteries: Systematic electrochemical characterization and detailed analysis by spectroscopic methods. Prog. Solid State Chem. 2014, 42, 65–84. [Google Scholar] [CrossRef]
- Korepp, C.; Santner, H.; Fujii, T.; Ue, M.; Besenhard, J.; Möller, K.-C.; Winter, M. 2-Cyanofuran—A novel vinylene electrolyte additive for PC-based electrolytes in lithium-ion batteries. J. Power Sources 2006, 158, 578–582. [Google Scholar] [CrossRef]
- Brox, S.; Röser, S.; Streipert, B.; Hildebrand, S.; Rodehorst, U.; Qi, X.; Wagner, R.; Winter, M.; Cekic-Laskovic, I. Innovative, Non-Corrosive LiTFSI Cyanoester-Based Electrolyte for Safer 4 V Lithium-Ion Batteries. ChemElectroChem 2017, 4, 304–309. [Google Scholar] [CrossRef]
- Brox, S.; Röser, S.; Husch, T.; Hildebrand, S.; Fromm, O.; Korth, M.; Winter, M.; Cekic-Laskovic, I. Alternative Single-Solvent Electrolytes Based on Cyanoesters for Safer Lithium-Ion Batteries. ChemSusChem 2016, 9, 1704–1711. [Google Scholar] [CrossRef]
- Pohl, B.; Grünebaum, M.; Drews, M.; Passerini, S.; Winter, M.; Wiemhöfer, H.D. Nitrile functionalized silyl ether with dissolved LiTFSI as new electrolyte solvent for lithium-ion batteries. Electrochim. Acta 2015, 180, 795–800. [Google Scholar] [CrossRef]
- Kirshnamoorthy, A.N.; Oldiges, K.; Winter, M.; Heuer, A.; Cekic-Laskovic, I.; Holm, C.; Smiatek, J. Electrolyte solvents for high voltage lithium ion batteries: ion correlation and specific anion effects in adiponitrile. Chem. Chem. Phys. 2018, 20, 25701–25715. [Google Scholar] [CrossRef] [PubMed]
- Oldiges, K.; von Aspern, N.; Cekic-Laskovic, I.; Winter, M.; Brunklaus, G. Impact of Trifluoromethylation of Adiponitrile on Aluminum Dissolution Behavior in Dinitrile-Based Electrolytes. J. Electrochem. Soc. 2018, 165, A3773–A3781. [Google Scholar] [CrossRef]
- Santner, H.J.; Möller, K.C.; Ivanco, J.; Ramsey, M.G.; Netzer, F.P.; Yamaguchi, S.; Besenhard, J.O.; Winter, M. Acrylic acid nitrile, a film-forming electrolyte component for lithium-ion batteries, which belongs to the family of additives containing vinyl groups. J. Power Sources 2003, 119, 368–372. [Google Scholar] [CrossRef]
- Peled, E. The Electrochemical Behavior of Alkali and Alkaline Earth Metals in Nonaqueous Battery Systems—The Solid Electrolyte Interphase Model. J. Electrochem. Soc. 1979, 126, 2047–2051. [Google Scholar] [CrossRef]
- Peled, E.; Golodnitsky, D.; Ardel, G. Advanced model for solid electrolyte interphase electrodes in liquid and polymer electrolytes. J. Electrochem. Soc. 1997, 144, L208–L210. [Google Scholar] [CrossRef]
- Zhang, S.S.; Ding, M.S.; Xu, K.; Allen, J.; Jow, T.R. Understanding solid electrolyte interface film formation on graphite electrodes. Electrochem. Solid State Lett. 2001, 4, A206–A208. [Google Scholar] [CrossRef]
- Winter, M.; Appel, W.K.; Evers, B.; Hodal, T.; Moller, K.C.; Schneider, I.; Wachtler, M.; Wagner, M.R.; Wrodnigg, G.H.; Besenhard, J.O. Studies on the anode/electrolyte interface in lithium ion batteries. Chem. Mon. 2001, 132, 473–486. [Google Scholar] [CrossRef]
- Li, F.S.; Wu, Y.S.; Chou, J.; Winter, M.; Wu, N.L. A mechanically robust and highly ion-conductive polymer-blend coating for high-power and long-life lithium-ion battery anodes. Adv. Mater 2015, 27, 130–137. [Google Scholar] [CrossRef]
- Winter, M. The solid electrolyte interphase–the most important and the least understood solid electrolyte in rechargeable Li batteries. Z. Phys. Chem. 2009, 223, 1395–1406. [Google Scholar] [CrossRef]
- Santner, H.J.; Korepp, C.; Winter, M.; Besenhard, J.O.; Möller, K.-C. In-situ FTIR investigations on the reduction of vinylene electrolyte additives suitable for use in lithium-ion batteries. Anal. Bioanal. Chem. 2004, 379, 266–271. [Google Scholar] [CrossRef]
- Märkle, W.; Lu, C.-Y.; Novák, P. Morphology of the Solid Electrolyte Interphase on Graphite in Dependency on the Formation Current. J. Electrochem. Soc. 2011, 158, A1478–A1482. [Google Scholar] [CrossRef]
- Besenhard, J.O.; Wagner, M.W.; Winter, M.; Jannakoudakis, A.D.; Jannakoudakis, P.D.; Theodoridou, E. Inorganic Film-Forming Electrolyte Additives Improving the Cycling Behavior of Metallic Lithium Electrodes and the Self-Discharge of Carbon Lithium Electrodes. J. Power Sources 1993, 44, 413–420. [Google Scholar] [CrossRef]
- Wang, K.; Xing, L.; Zhi, H.; Cai, Y.; Yan, Z.; Cai, D.; Zhou, H.; Li, W. High stability graphite/electrolyte interface created by a novel electrolyte additive: A theoretical and experimental study. Electrochim. Acta 2018, 262, 226–232. [Google Scholar] [CrossRef]
- Wang, Y.; Nakamura, S.; Tasaki, K.; Balbuena, P.B. Theoretical Studies to Understand Surface Chemistry on Carbon Anodes for Lithium-Ion Batteries: How Does Vinylene Carbonate Play Its Role as an Electrolyte Additive? J. Am.Chem. Soc. 2002, 124, 4408–4421. [Google Scholar] [CrossRef] [PubMed]
- Aurbach, D.; Gamolsky, K.; Markovsky, B.; Gofer, Y.; Schmidt, M.; Heider, U. On the use of vinylene carbonate (VC) as an additive to electrolyte solutions for Li-ion batteries. Electrochim. Acta 2002, 47, 1423–1439. [Google Scholar] [CrossRef]
- Zhang, L.; Ma, Y.; Cheng, X.; Zuo, P.; Cui, Y.; Guan, T.; Du, C.; Gao, Y.; Yin, G. Enhancement of high voltage cycling performance and thermal stability of LiNi1/3Co1/3Mn1/3O2 cathode by use of boron-based additives. Solid State Ionics 2014, 263, 146–151. [Google Scholar] [CrossRef]
- Meister, P.; Qi, X.; Kloepsch, R.; Krämer, E.; Streipert, B.; Winter, M.; Placke, T. Anodic Behavior of the Aluminum Current Collector in Imide-Based Electrolytes: Influence of Solvent, Operating Temperature, and Native Oxide-Layer Thickness. ChemSusChem 2017, 10, 804–814. [Google Scholar] [CrossRef]
- Markevich, E.; Salitra, G.; Aurbach, D. Fluoroethylene carbonate as an important component for the formation of an effective solid electrolyte interphase on anodes and cathodes for advanced Li-ion batteries. ACS Energy Lett. 2017, 2, 1337–1345. [Google Scholar] [CrossRef]
- Zugmann, S.; Moosbauer, D.; Amereller, M.; Schreiner, C.; Wudy, F.; Schmitz, R.; Schmitz, R.; Isken, P.; Dippel, C.; Muller, R.; et al. Electrochemical characterizaiton of electrolytes for lithium-ion batteries based on lithium difluoromono(oxalato)borate. J. Power Sources 2011, 196, 1417–1424. [Google Scholar] [CrossRef]
- Dagger, T.; Grützke, M.; Reichert, M.; Haetge, J.; Nowak, S.; Winter, M.; Schappacher, F.M. Investigation of lithium ion battery electrolytes containing flame retardants in combination with the film forming electrolyte additives vinylene carbonate, vinyl ethylene carbonate and fluoroethylene carbonate. J. Power Sources 2017, 372, 276–285. [Google Scholar] [CrossRef]
- Heine, J.; Hilbig, P.; Qi, X.; Niehoff, P.; Winter, M.; Bieker, P. Fluoroethylene carbonate as electrolyte additive in tetraethylene glycol dimethyl ether based electrolytes for application in lithium ion and lithium metal batteries. J. Electrochem. Soc. 2015, 162, A1094–A1101. [Google Scholar] [CrossRef]
- Kasnatscheew, J.; Schmitz, R.W.; Wagner, R.; Winter, M.; Schmitz, R. Fluoroethylene Carbonate as an Additive for γ-Butyrolactone Based Electrolytes. J. Electrochem. Soc. 2013, 160, A1369–A1374. [Google Scholar] [CrossRef]
- Winter, M.; Novak, P.; Monnier, A. Graphites for lithium-ion cells: The correlation of the first-cycle charge loss with the Brunauer-Emmett-Teller surface area. J. Electrochem. Soc. 1998, 145, 428–436. [Google Scholar] [CrossRef]
- Hall, D.S.; Eldesoky, A.; Logan, E.R.; Tonita, E.M.; Ma, X.; Dahn, J.R. Exploring Classes of Co-Solvents for Fast-Charging Lithium-Ion Cells. J. Electrochem. Soc. 2018, 165, A2365–A2373. [Google Scholar] [CrossRef]
- Pal, A.; Kumar, A. Excess Molar Volumes, Viscosities, and Refractive Indices of Diethylene Glycol Dimethyl Ether with Dimethyl Carbonate, Diethyl Carbonate, and Propylene Carbonate at (298.15, 308.15, and 318.15) K. J. Chem. Eng. Data 1998, 43, 143–147. [Google Scholar] [CrossRef]
- Smart, M.C.; Ratnakumar, B.V.; Surampudi, S. Electrolytes for Low-Temperature Lithium Batteries Based on Ternary Mixtures of Aliphatic Carbonates. J. Electrochem. Soc. 1999, 146, 486–492. [Google Scholar] [CrossRef]
- Ue, M.; Sasaki, Y.; Tanaka, Y.; Morita, M. Nonaqueous Electrolytes with Advances in Solvents. In Electrolytes for Lithium and Lithium-Ion Batteries; Jow, T.R., Xu, K., Borodin, O., Ue, M., Eds.; Springer: New York, NY, USA, 2014; pp. 93–165. [Google Scholar]
- D’Aprano, A.; Fuoss, R.M. Conductance in isodielectric mixtures. I.n-butyronitrile with dioxane, benzene, and carbon tetrachloride. J. Solut. Chem. 1974, 3, 45–55. [Google Scholar] [CrossRef]
- Wyman, J. Polarization and Dielectric Constant of Liquids. J. Am.Chem. Soc. 1936, 58, 1482–1486. [Google Scholar] [CrossRef]
- Grünebaum, M.; Buchheit, A.; Lürenbaum, C.; Winter, M.; Wiemhöfer, H.-D. Ester-Based Battery Solvents in Contact with Metallic Lithium: Effect of Water and Alcohol Impurities. J. Phys. Chem. 2019, 123, 7033–7044. [Google Scholar] [CrossRef]
- Bieker, G.; Winter, M.; Bieker, P. Electrochemical in situ investigations of SEI and dendrite formation on the lithium metal anode. Phys. Chem. Chem. Phys. 2015, 17, 8670–8679. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, K.; Ding, S.P.; Jow, T.R. Toward reliable values of electrochemical stability limits for electrolytes. J. Electrochem. Soc. 1999, 146, 4172–4178. [Google Scholar] [CrossRef]
- Kasnatscheew, J.; Streipert, B.; Röser, S.; Wagner, R.; Laskovic, I.C.; Winter, M. Determining oxidative stability of battery electrolytes: validity of common electrochemical stability window (ESW) data and alternative strategies. Phys. Chem. Chem. Phys. 2017, 19, 16078–16086. [Google Scholar] [CrossRef] [PubMed]
- Hilbig, P.; Ibing, L.; Streipert, B.; Wagner, R.; Winter, M.; Cekic-Laskovic, I. Acetonitrile-Based Electrolytes for Lithium-Ion Battery Application. Curr. Top. Electrochem. 2018, 20, 1–30. [Google Scholar]
- Meister, P.; Jia, H.; Li, J.; Kloepsch, R.; Winter, M.; Placke, T. Best Practice: Performance and Cost Evaluation of Lithium Ion Battery Active Materials with Special Emphasis on Energy Efficiency. Chem. Mater. 2016, 28, 7203–7217. [Google Scholar] [CrossRef]
- Besenhard, J.O.; Winter, M. Insertion reactions in advanced electrochemical energy storage. Pure Appl. Chem. 1998, 70, 603–608. [Google Scholar] [CrossRef] [Green Version]
- Mller, T. Lithium ion battery automotive applications and requirements. In Proceedings of the Seventeenth Annual Battery Conference on Applications and Advances, Long Beach, CA, USA, 18 January 2002; pp. 113–118. [Google Scholar]
- Wachtler, M.; Wagner, M.R.; Schmied, M.; Winter, M.; Besenhard, J.O. The effect of the binder morphology on the cycling stability of Li–alloy composite electrodes. J. Electroanal. Chem. 2001, 510, 12–19. [Google Scholar] [CrossRef]
- Niehoff, P.; Passerini, S.; Winter, M. Interface Investigations of a Commercial Lithium Ion Battery Graphite Anode Material by Sputter Depth Profile X-ray Photoelectron Spectroscopy. Langmuir 2013, 29, 5806–5816. [Google Scholar] [CrossRef] [PubMed]
- Niehoff, P.; Winter, M. Composition and Growth Behavior of the Surface and Electrolyte Decomposition Layer of/on a Commercial Lithium Ion Battery LixNi1/3Mn1/3Co1/3O2 Cathode Determined by Sputter Depth Profile X-ray Photoelectron Spectroscopy. Langmuir 2013, 29, 15813–15821. [Google Scholar] [CrossRef]
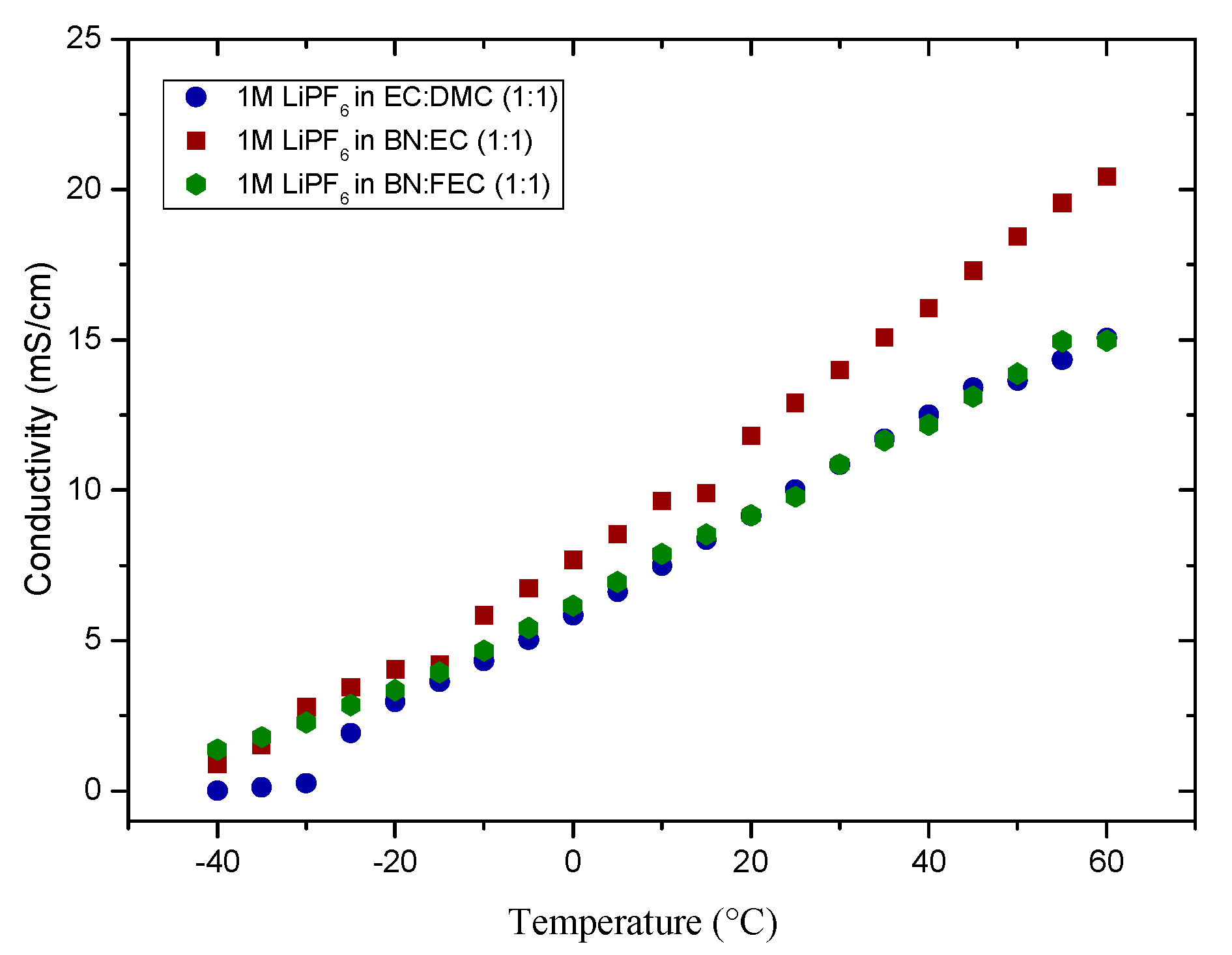
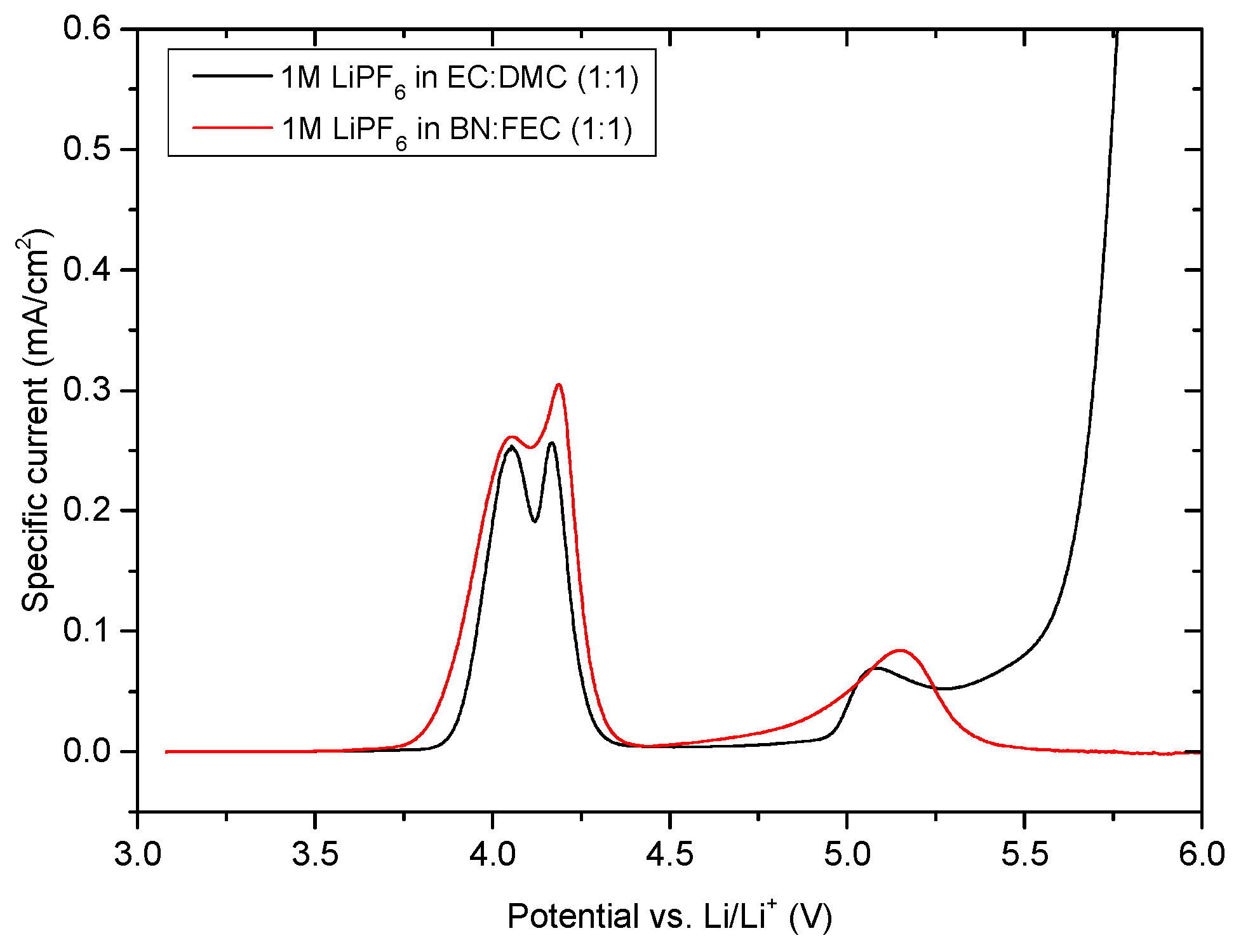

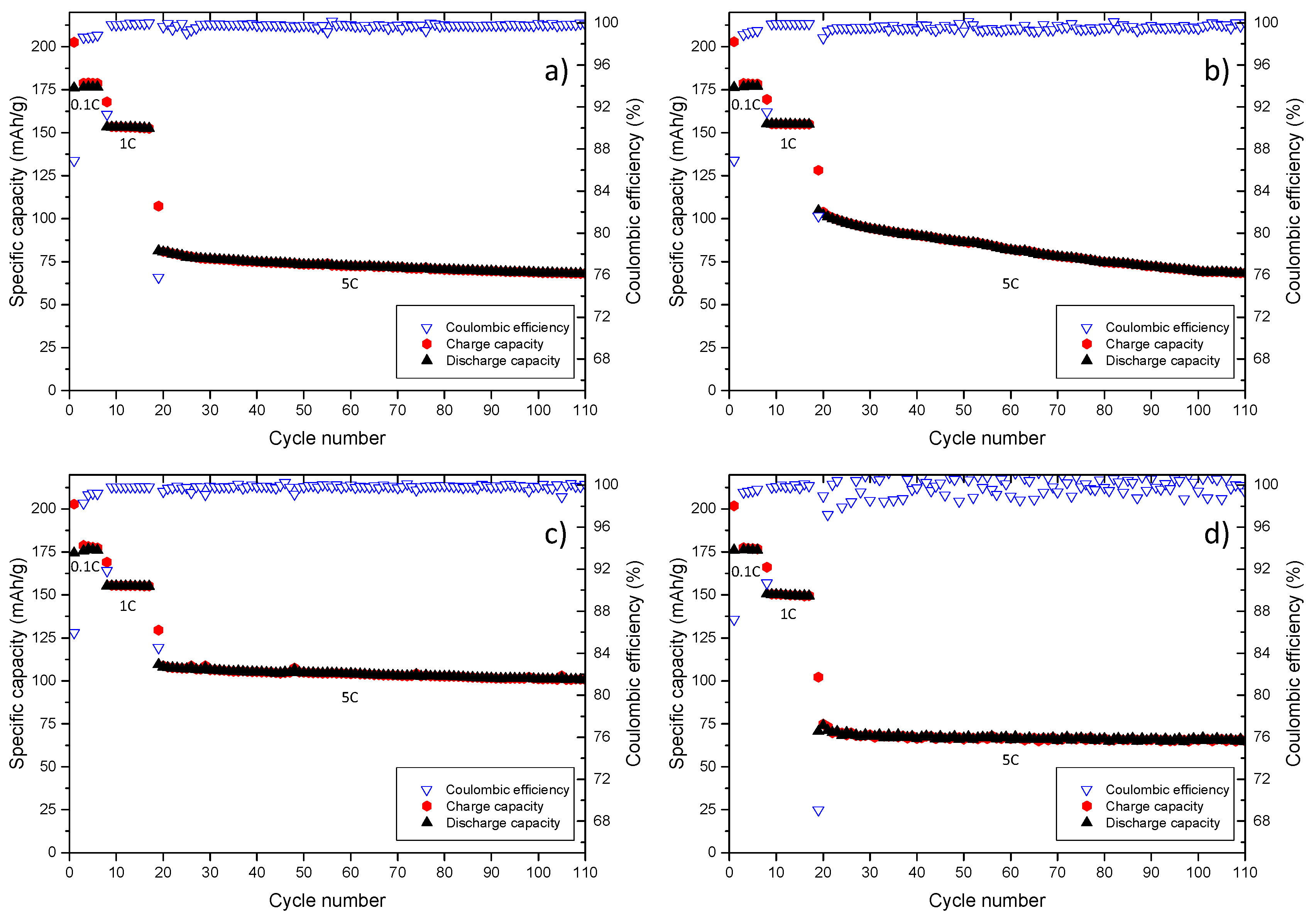
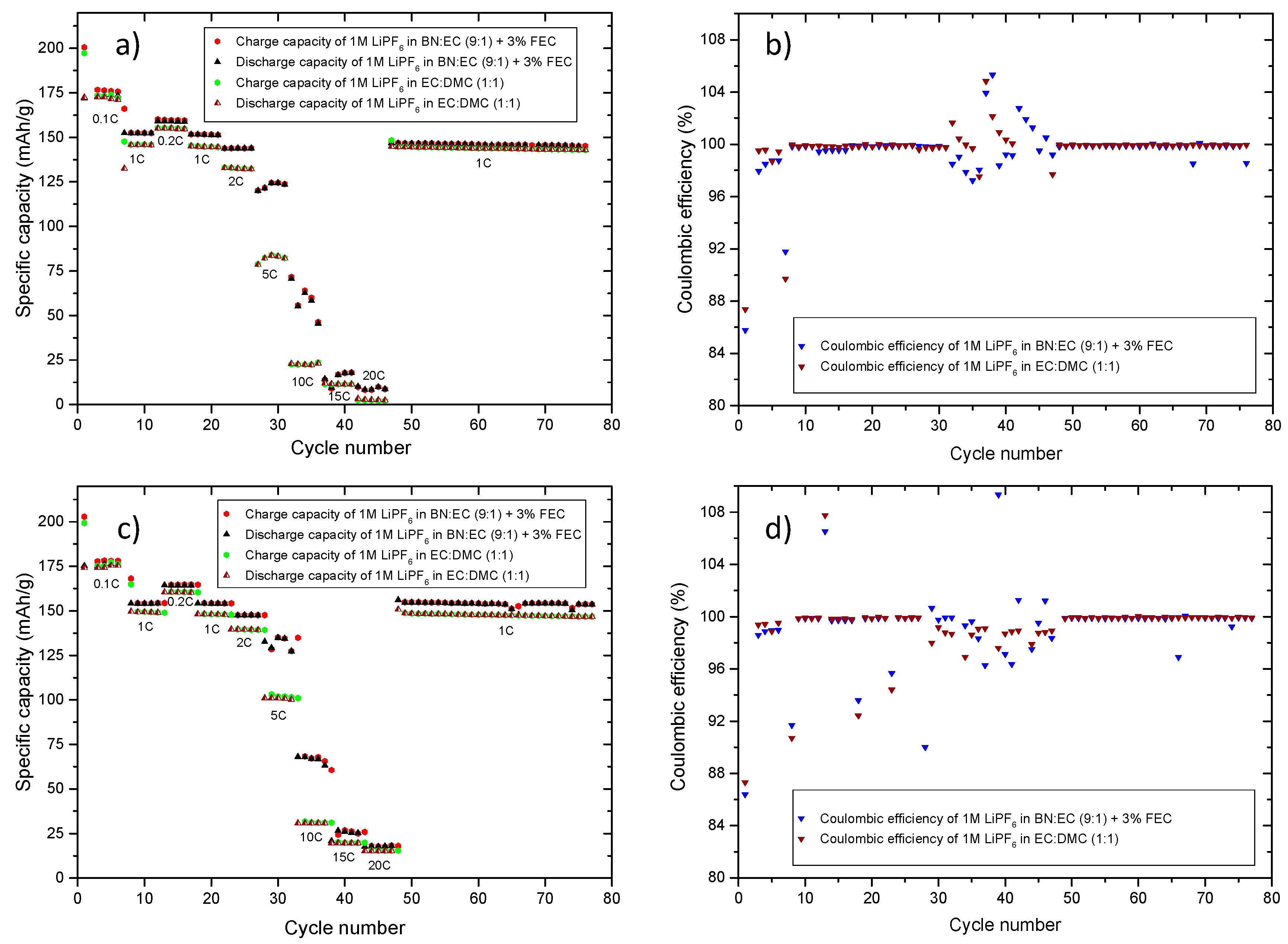
| Structure | Compound | Abbreviation | η(cP) | εr |
|---|---|---|---|---|
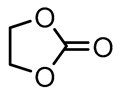 | Ethylene carbonate | EC | 1.9 a [13] | 89.8 a [9] |
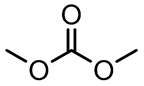 | Dimethyl carbonate | DMC | 0.59 [56] | 3.12 [57] |
 | Fluoroethylene carbonate | FEC | 4.1 [58] | 107 [58] |
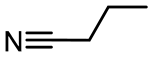 | Butyronitrile | BN | 0.553 [59] | 20.7 [60] |
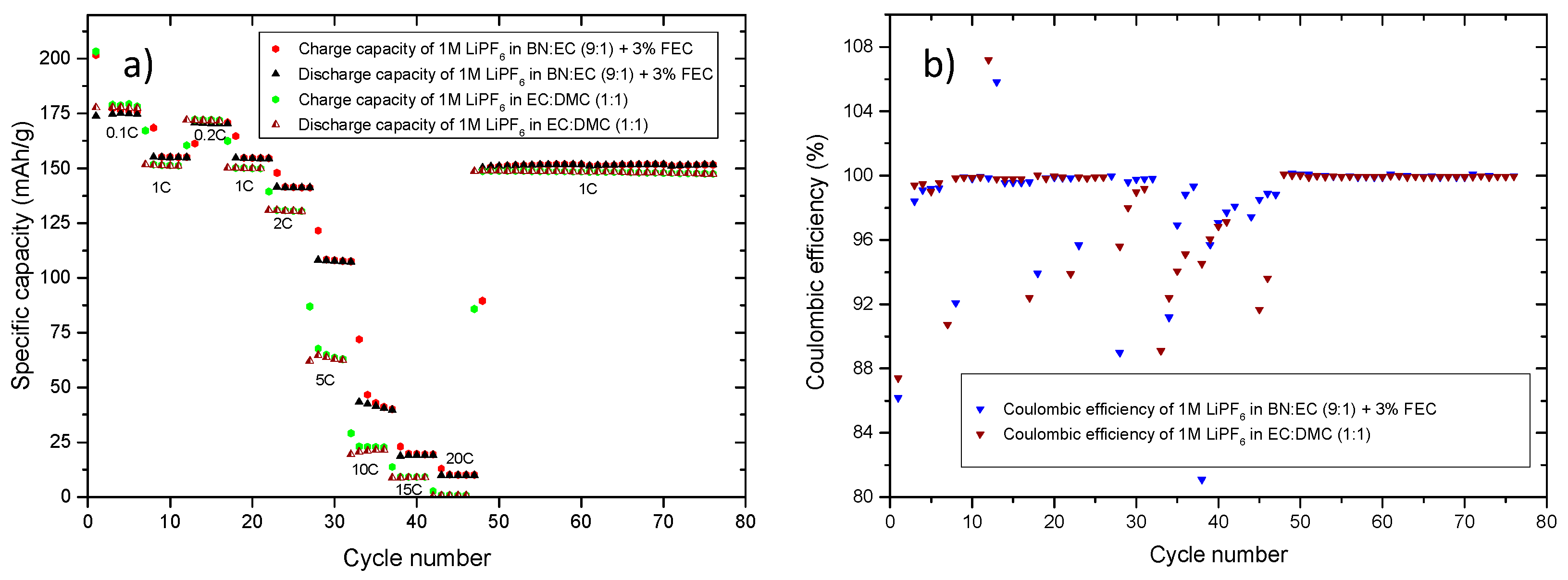
| C-Rate | C-Rate Performance (Charge) Specific Capacity (mAh/g) | C-Rate Performance (Discharge) Specific Capacity (mAh/g) | C-Rate Performance (Charge/Discharge) Specific Capacity (mAh/g) | |||
|---|---|---|---|---|---|---|
| Cell a | Cell b | Cell a | Cell b | Cell a | Cell b | |
| 0.1C | 176 | 172 | 177 | 174 | 175 | 177 |
| 1C | 152 | 145 | 154 | 149 | 154 | 150 |
| 2C | 144 | 133 | 148 | 139 | 140 | 129 |
| 5C | 125 | 84 | 134 | 99 | 108 | 63 |
| 10C | 62 | 23 | 68 | 31 | 42 | 22 |
| 20C | 9 | 2 | 18 | 15 | 9 | 1 |
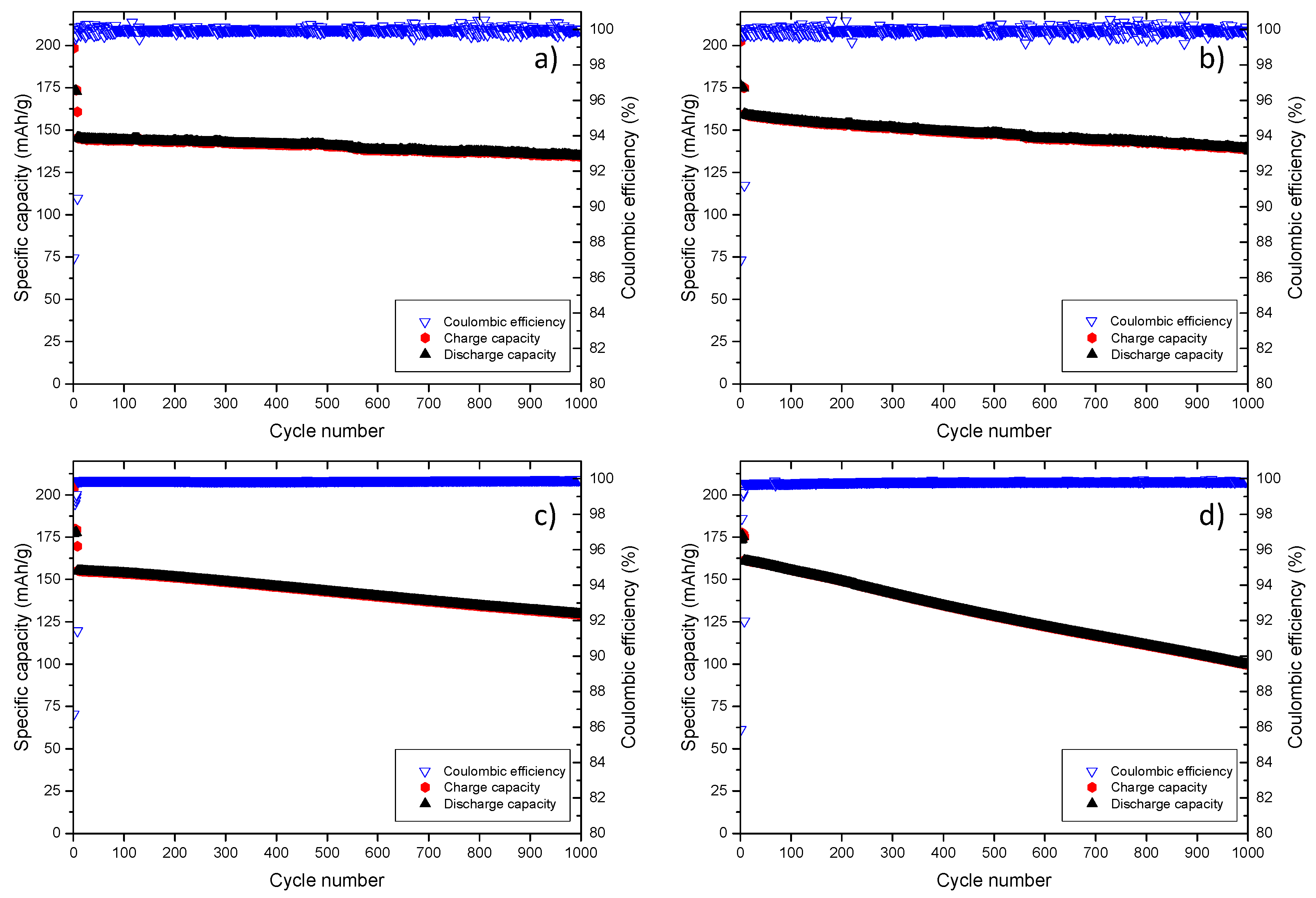
| Selected Parameters | Cell a | Cell b (CV) | Cell c | Cell d (CV) |
|---|---|---|---|---|
| (Reference Electrolyte) | (Reference Electrolyte) | (BN-Based Electrolyte) | (BN-Based Electrolyte) | |
| 1st Coulombic efficiency (%) | 87 | 87 | 87 | 86 |
| Discharge capacity in the10th cycle (mAh/g) | 145 | 159 | 155 | 161 |
| Discharge capacity in the 1000th cycle (mAh/g) | 135 | 139 | 129 | 100 |
| Capacity retention (%) | 93 | 87 | 83 | 62 |
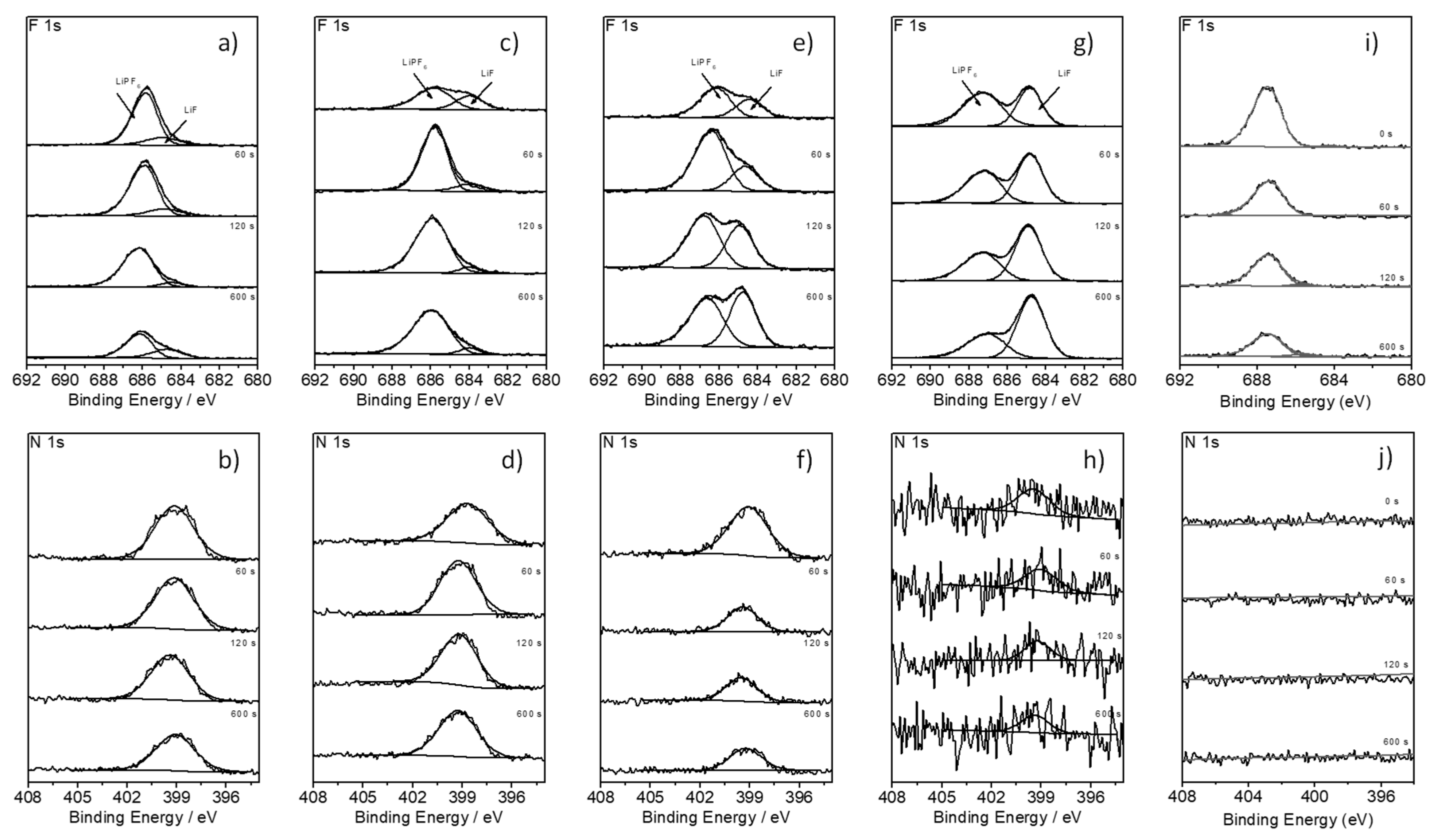
| Pure BN-Based Electrolyte: Surface Concentration (a.u.) | ||||
| Sputter time | 0 s | 60 s | 120 s | 600 s |
| LIF | 2.13 (0.25) | 0.99 (0.71) | 1.28 (0.05) | 1.84 (0.66) |
| N 1 s | 10.74 (0.98) | 10.06 (1.24) | 8.69 (2.07) | 9.14 (0.38) |
| BN + 5%FEC based electrolyte: surface concentration (a.u.) | ||||
| Sputter time | 0 s | 60 s | 120 s | 600 s |
| LIF | 3.80 (1.02) | 2.14 (2.43) | 1.26 (0.45) | 4.00 (0.53) |
| N 1 s | 11.83 (0.58) | 12.26 (0.99) | 9.37 (0.32) | 9.78 (0.73) |
| BN:EC (9:1) + 2%FEC based electrolyte: surface concentration (a.u.) | ||||
| Sputter time | 0 s | 60 s | 120 s | 600 s |
| LIF | 4.37 (0.79) | 7.88 (0.22) | 8.79 (0.28) | 9.90 (0.16) |
| N 1 s | 3.53 (0.35) | 3.71 (0.28) | 3.22 (0.11) | 3.28 (0.21) |
| BN:EC (9:1) + 3%FEC based electrolyte: surface concentration (a.u.) | ||||
| Sputter time | 0 s | 60 s | 120 s | 600 s |
| LIF | 12.15 (0.47) | 16.49 (1.03) | 18.40 (1.16) | 20.61 (0.52) |
| N 1 s | 0.44 (0.11) | 0.38 (0.11) | 0.27 (0.30) | 0.25 (0.25) |
| Reference electrode (pristine): surface concentration (a.u.) | ||||
| Sputter time | 0 s | 60 s | 120 s | 600 s |
| LIF | 0.86 (0.84) | 2.33 (0.73) | 1.52 (1.47) | 1.32 (0.93) |
| N 1 s | 0 (0) | 0 (1.12) | 0 (0) | 0 (0) |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hilbig, P.; Ibing, L.; Winter, M.; Cekic-Laskovic, I. Butyronitrile-Based Electrolytes for Fast Charging of Lithium-Ion Batteries. Energies 2019, 12, 2869. https://doi.org/10.3390/en12152869
Hilbig P, Ibing L, Winter M, Cekic-Laskovic I. Butyronitrile-Based Electrolytes for Fast Charging of Lithium-Ion Batteries. Energies. 2019; 12(15):2869. https://doi.org/10.3390/en12152869
Chicago/Turabian StyleHilbig, Peter, Lukas Ibing, Martin Winter, and Isidora Cekic-Laskovic. 2019. "Butyronitrile-Based Electrolytes for Fast Charging of Lithium-Ion Batteries" Energies 12, no. 15: 2869. https://doi.org/10.3390/en12152869
APA StyleHilbig, P., Ibing, L., Winter, M., & Cekic-Laskovic, I. (2019). Butyronitrile-Based Electrolytes for Fast Charging of Lithium-Ion Batteries. Energies, 12(15), 2869. https://doi.org/10.3390/en12152869






