Large-Scale Waste Bio-Remediation Using Microalgae Cultivation as a Platform
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Design
2.2. Waste-Based Media Preparation and Characterization
2.2.1. Agriculture Waste
2.2.2. Aquaculture Waste
2.2.3. Anaerobic Digested (AD) Municipal Waste
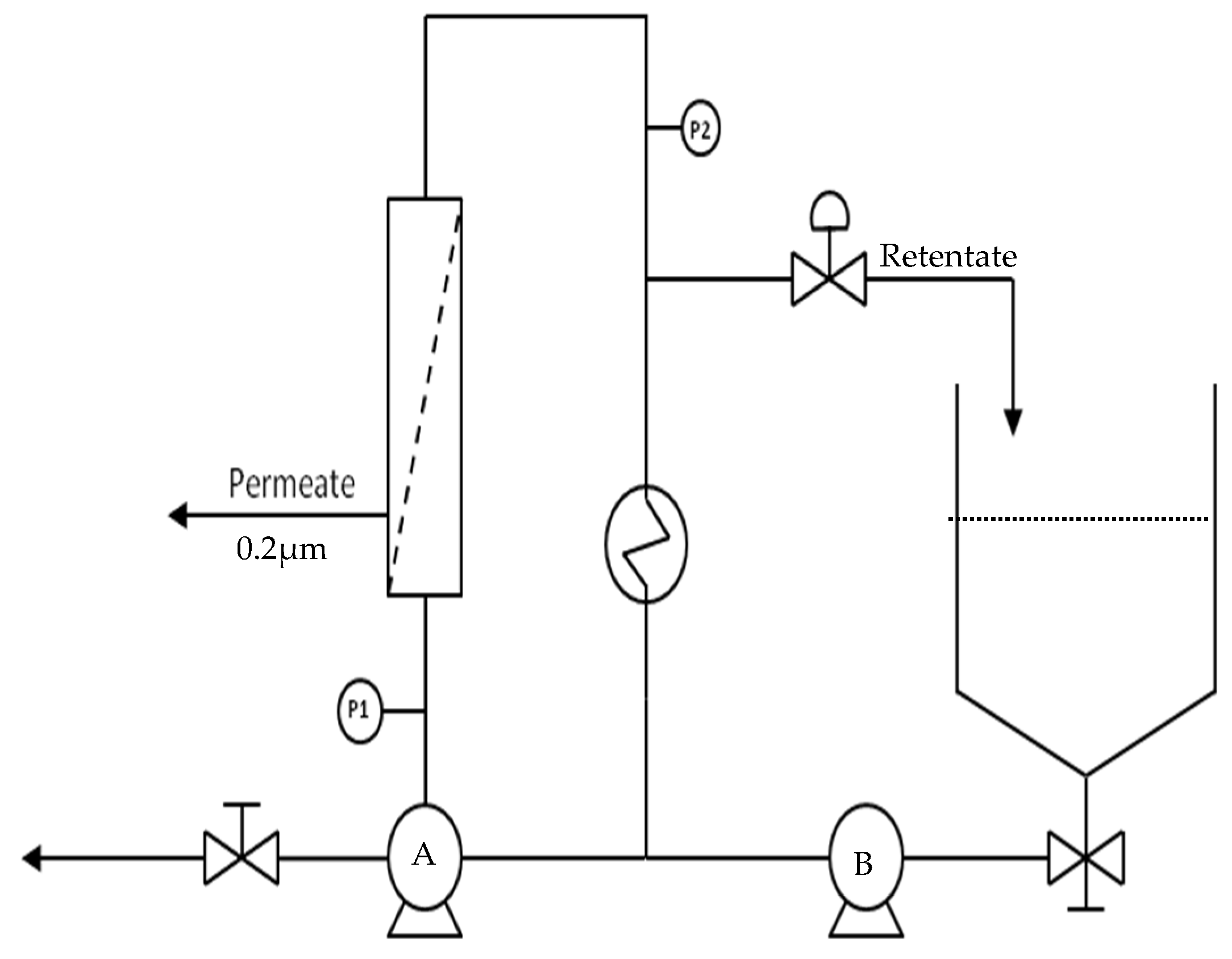
2.2.4. Membrane Separation Unit
2.3. Strain and Culture Medium
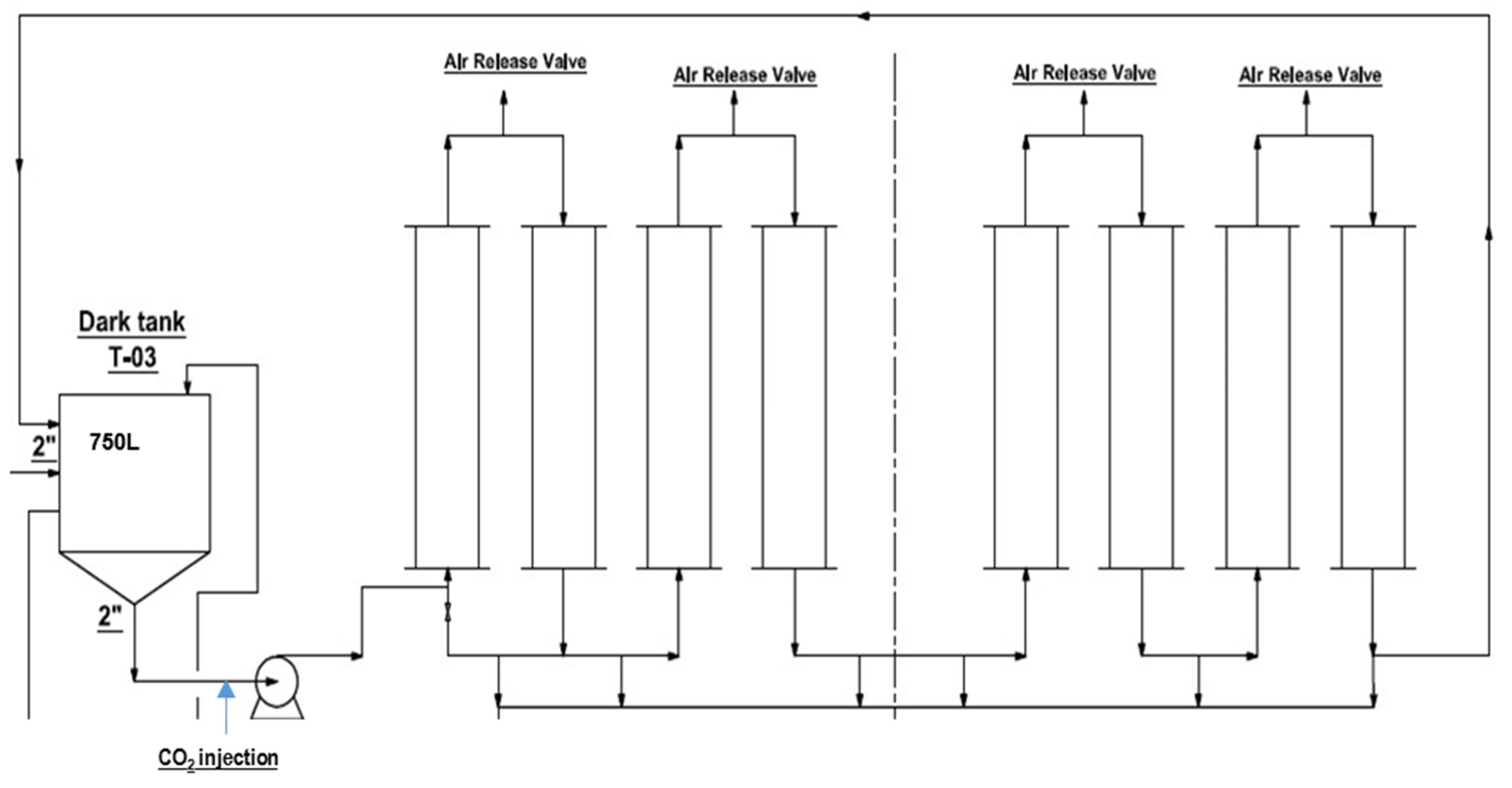
2.4. Cultivation System and Conditions
2.5. Sampling and Analysis
2.6. Nutrient Uptake Rate Calculation.
2.7. Statistical Analyses
3. Results
3.1. Algal Waste-Based Growth Experiments
3.2. Waste Remediation Efficiency
3.3. Biomass Evaluation and Characteristic for Energy and High Value Products Development
4. Discussion
4.1. Cultivation
4.2. Waste Remediation
4.3. Product Development, Fertilisers and Feed Development.
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- European Commision. Directive 2000/60/EC of the European Parliament and of the Council Establishing a Framework for the Community Action in the Field of Water Policy. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=celex%3A32000L0060 (accessed on 10 July 2019).
- UKTI. Water and Treated Water. Available online: https://www.gov.uk/government/publications/water-and-treated-water/water-and-treated-water (accessed on 10 July 2019).
- Ministerio de Obras Públicas. Norma de emision para la regulacion de contaminantes asociados a las descargas de residuos industriales liquidos a sistemas de alcantarillado; Ministerio de Obras Públicas: Santiago, Chile, 1998; Available online: https://www.leychile.cl/Navegar?idNorma=121486 (accessed on 16 July 2019).
- Ministerio de Desarrollo Sostenible y Medio Ambiente. Reglamentación de la Ley n° 1333 del medio ambiente: Reglamento en materia de contaminación hídrica; Ministerio de Desarrollo Sostenible y Medio Ambiente: La Paz, Bolivia, 1992. Available online: http://biblioteca.unmsm.edu.pe/redlieds/Recursos/archivos/Legislacion/Bolivia/reglamento_contaminacion.pdf (accessed on 16 July 2019).
- Ministerio de Vivienda, Construcción y Saneamiento. Aprueban límites máximos permisibles (lmp) a las descargas de aguas residuales en los sistemas de recolección de alcantarillado sanitario; Ministerio de Vivienda, Construcción y Saneamiento: Lima, Perú, 2010. Available online: https://www.oefa.gob.pe/?wpfb_dl=7827 (accessed on 16 July 2019).
- Ministerio de Salud y del Ambiente. Ley de Prevencion y Control de la Contaminación Ambiental; Ministerio de Salud y del Ambiente: Quito, Ecuador, 2004. Available online: http://www.ambiente.gob.ec/wp-content/uploads/downloads/2012/09/LEY-DE-PREVENCION-Y-CONTROL-DE-LA-CONTAMINACION-AMBIENTAL.pdf (accessed on 16 July 2019).
- Zhou, Y.; Duan, N.; Wu, X.; Fang, H. Cod discharge limits for urban wastewater treatment plants in china based on statistical methods. Water 2018, 10, 777. [Google Scholar] [CrossRef]
- Ministry of Environment Protection of China: Beijing, China. Discharge standard of pollutants for municipal wastewater treatment plant (gb 18918-2002). Available online: http://english.mee.gov.cn/Resources/standards/water_environment/Discharge_standard/200710/t20071024_111808.shtml (accessed on 15 May 2018).
- Perin, G.; Yunus, I.S.; Valton, M.; Alobwede, E.; Jones, P.R. Sunlight-driven recycling to increase nutrient use-efficiency in agriculture. Algal Res. 2019, 41, 101554. [Google Scholar] [CrossRef]
- Rodero, M.d.R.; Lebrero, R.; Serrano, E.; Lara, E.; Arbib, Z.; García-Encina, P.A.; Muñoz, R. Technology validation of photosynthetic biogas upgrading in a semi-industrial scale algal-bacterial photobioreactor. Bioresour. Technol. 2019, 279, 43–49. [Google Scholar] [CrossRef] [PubMed]
- Gerardo, M.L.; Van Den Hende, S.; Vervaeren, H.; Coward, T.; Skill, S.C. Harvesting of microalgae within a biorefinery approach: A review of the developments and case studies from pilot-plants. Algal Res. 2015, 11, 248–262. [Google Scholar] [CrossRef]
- Tolboom, S.N.; Carrillo-Nieves, D.; de Jesús Rostro-Alanis, M.; de la Cruz Quiroz, R.; Barceló, D.; Iqbal, H.M.N.; Parra-Saldivar, R. Algal-based removal strategies for hazardous contaminants from the environment – a review. Sci. Total. Environ. 2019, 665, 358–366. [Google Scholar] [CrossRef] [PubMed]
- Bunce, J.T.; Ndam, E.; Ofiteru, I.D.; Moore, A.; Graham, D.W. A review of phosphorus removal technologies and their applicability to small-scale domestic wastewater treatment systems. Front. Environ. Sci. 2018, 6, 8. [Google Scholar] [CrossRef]
- Capodaglio, A.G.; Hlavínek, P.; Raboni, M. Physico-chemical technologies for nitrogen removal from wastewaters: A review. Rev. Ambiente Agua 2015, 10, 481–498. [Google Scholar]
- Rahman, M.M.; Salleh, M.A.M.; Rashid, U.; Ahsan, A.; Hossain, M.M.; Ra, C.S. Production of slow release crystal fertilizer from wastewaters through struvite crystallization—A review. Arabian J. Chem. 2014, 7, 139–155. [Google Scholar] [CrossRef]
- Neethling, J.B.; Benisch, M. Struvite control through process and facility design as well as operation strategy. Water Sci. Technol. 2004, 49, 191–199. [Google Scholar] [CrossRef]
- Forrest, A.L.; Fattah, K.P.; Mavinic, D.S.; Koch, F.A. Optimizing struvite production for phosphate recovery in wwtp. J. Environ. Eng. 2008, 134, 395–402. [Google Scholar] [CrossRef]
- Cusick, R.D.; Ullery, M.L.; Dempsey, B.A.; Logan, B.E. Electrochemical struvite precipitation from digestate with a fluidized bed cathode microbial electrolysis cell. Water Res. 2014, 54, 297–306. [Google Scholar] [CrossRef] [PubMed]
- Doyle, J.D.; Parsons, S.A. Struvite formation, control and recovery. Water Res. 2002, 36, 3925–3940. [Google Scholar] [CrossRef]
- Mehrabadi, A.; Craggs, R.; Farid, M.M. Wastewater treatment high rate algal ponds (wwt hrap) for low-cost biofuel production. Bioresour. Technol. 2015, 184, 202–214. [Google Scholar] [CrossRef] [PubMed]
- Azubuike, C.C.; Chikere, C.B.; Okpokwasili, G.C. Bioremediation techniques-classification based on site of application: Principles, advantages, limitations and prospects. World J. Microbiol. Biotechnol. 2016, 32, 180. [Google Scholar] [CrossRef] [PubMed]
- Seelen, L.M.S.; Flaim, G.; Jennings, E.; De Senerpont Domis, L.N. Saving water for the future: Public awareness of water usage and water quality. J. Environ. Manag. 2019, 242, 246–257. [Google Scholar] [CrossRef]
- Silkina, A.; Zacharof, M.-P.; Hery, G.; Nouvel, T.; Lovitt, R.W. Formulation and utilisation of spent anaerobic digestate fluids for the growth and product formation of single cell algal cultures in heterotrophic and autotrophic conditions. Bioresour. Technol. 2017, 244, 1445–1455. [Google Scholar] [CrossRef] [PubMed]
- Menke, S.; Sennhenn, A.; Sachse, J.H.; Majewski, E.; Huchzermeyer, B.; Rath, T. Screening of microalgae for feasible mass production in industrial hypersaline wastewater using disposable bioreactors. Clean-Soil Air Water 2012, 40, 1401–1407. [Google Scholar] [CrossRef]
- Vane, L.M. Review: Water recovery from brines and salt-saturated solutions: Operability and thermodynamic efficiency considerations for desalination technologies. J. Chem. Technol. Biotechnol. 2017, 92, 2506–2518. [Google Scholar] [CrossRef] [PubMed]
- APHA. Standard Methods for the Examination of Water and Wastewater, 20th ed.; Amer Public Health Assn: Washington, DC, USA, 1998. [Google Scholar]
- Gerardo, M.L.; Zacharof, M.P.; Lovitt, R.W. Strategies for the recovery of nutrients and metals from anaerobically digested dairy farm sludge using cross-flow microfiltration. Water Res. 2013, 47, 4833–4842. [Google Scholar] [CrossRef]
- Xiao, Y.; Zhang, J.; Cui, J.; Feng, Y.; Cui, Q. Metabolic profiles of nannochloropsis oceanica imet1 under nitrogen-deficiency stress. Bioresour. Technol. 2013, 130, 731–738. [Google Scholar] [CrossRef]
- Guillard, R.R.L. Culture of phytoplankton for feeding marine invertebrates. In Culture of Marine Invertebrate Animals; Smith, W.L., Chanley, M.H., Eds.; Plenum Press: New York, NY, USA, 1975; pp. 26–60. [Google Scholar]
- Mayers, J.J.; Flynn, K.J.; Shields, R.J. Influence of the n:P supply ratio on biomass productivity and time-resolved changes in elemental and bulk biochemical composition of Nannochloropsis sp. Bioresour. Technol. 2014, 169, 588–595. [Google Scholar] [CrossRef] [PubMed]
- Fuentes-Gruenewald, C.; Bayliss, C.; Zanain, M.; Pooley, C.; Scolamacchia, M.; Silkina, A. Evaluation of batch and semi-continuous culture of porphyridium purpureum in a photobioreactor in high latitudes using fourier transform infrared spectroscopy for monitoring biomass composition and metabolites production. Bioresour. Technol. 2015, 189, 357–363. [Google Scholar] [CrossRef] [PubMed]
- Mayers, J.J.; Flynn, K.J.; Shields, R.J. Rapid determination of bulk microalgal biochemical composition by fourier-transform infrared spectroscopy. Bioresour. Technol. 2013, 148, 215–220. [Google Scholar] [CrossRef] [PubMed]
- Folch, J.; Lees, M.; Stanley, G.H.S. A simple method for the isolation and purification of total lipides from animal tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar]
- Dubois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric method for determination of sugars and related substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Safi, C.; Charton, M.; Pignolet, O.; Silvestre, F.; Vaca-Garcia, C.; Pontalier, P.-Y. Influence of microalgae cell wall characteristics on protein extractability and determination of nitrogen-to-protein conversion factors. J. Appl. Phycol. 2013, 25, 523–529. [Google Scholar] [CrossRef]
- Lupatsch, I.; Kissil, G.W.; Sklan, D.; Pfeffer, E. Energy and protein requirements for maintenance and growth in gilthead seabream (sparus aurata l.). Aquac. Nutr. 1998, 4, 165–173. [Google Scholar] [CrossRef]
- Bougaran, G.; Bernard, O.; Sciandra, A. Modeling continuous cultures of microalgae colimited by nitrogen and phosphorus. J. Theor. Biol. 2010, 265, 443–454. [Google Scholar] [CrossRef] [PubMed]
- Béchet, Q.; Plouviez, M.; Chambonnière, P.; Guieysse, B. 21 - Environmental impacts of full-scale algae cultivation. In Microalgae-Based Biofuels and Bioproducts; Gonzalez-Fernandez, C., Muñoz, R., Eds.; Woodhead Publishing: Cambridge, UK, 2017; pp. 505–525. [Google Scholar]
- Delrue, F.; Álvarez-Díaz, P.D.; Fon-Sing, S.; Fleury, G.; Sassi, J.F. The environmental biorefinery: Using microalgae to remediate wastewater, a win-win paradigm. Energies 2016, 9, 132. [Google Scholar] [CrossRef]
- Van Den Hende, S. Microalgal bacterial flocs for wastewater treatment: From concept to pilot scale. Ph.D. Thesis, Ghent University, Ghent, Belgium, February 2014. [Google Scholar]
- Yi, D.; Reardon, T.; Stringer, R. Shrimp aquaculture technology change in indonesia: Are small farmers included? Aquaculture 2018, 493, 436–445. [Google Scholar] [CrossRef]
- Yilmazel, Y.D.; Demirer, G.N. Removal and recovery of nutrients as struvite from anaerobic digestion residues of poultry manure. Environ. Technol. 2011, 32, 783–794. [Google Scholar] [CrossRef] [PubMed]
- Chong, N.M.; Thai, Q.M. Optimization and kinetics of nutrient removal from wastewater by chemical precipitation of struvite. Desalin. Water Treat. 2015, 54, 3422–3431. [Google Scholar] [CrossRef]
- Li, Z.; Ren, X.; Zuo, J.; Liu, Y.; Duan, E.; Yang, J.; Chen, P.; Wang, Y. Struvite precipitation for ammonia nitrogen removal in 7-aminocephalosporanic acid wastewater. Molecules 2012, 17, 2126–2139. [Google Scholar] [CrossRef] [PubMed]
- Van Den Hende, S.; Carré, E.; Cocaud, E.; Beelen, V.; Boon, N.; Vervaeren, H. Treatment of industrial wastewaters by microalgal bacterial flocs in sequencing batch reactors. Bioresour. Technol. 2014, 161, 245–254. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez, R.; Ferrer, I.; González-Molina, A.; Salvadó, H.; García, J.; Uggetti, E. Microalgae recycling improves biomass recovery from wastewater treatment high rate algal ponds. Water Res. 2016, 106, 539–549. [Google Scholar] [CrossRef] [PubMed]
- Passos, F.; Gutiérrez, R.; Brockmann, D.; Steyer, J.-P.; García, J.; Ferrer, I. Microalgae production in wastewater treatment systems, anaerobic digestion and modelling using adm1. Algal Res. 2015, 10, 55–63. [Google Scholar] [CrossRef]
- Bohutskyi, P.; Chow, S.; Ketter, B.; Fung Shek, C.; Yacar, D.; Tang, Y.; Zivojnovich, M.; Betenbaugh, M.J.; Bouwer, E.J. Phytoremediation of agriculture runoff by filamentous algae poly-culture for biomethane production, and nutrient recovery for secondary cultivation of lipid generating microalgae. Bioresour. Technol. 2016, 222, 294–308. [Google Scholar] [CrossRef]
- Craggs, R.J.; Adey, W.H.; Jessup, B.K.; Oswald, W.J. A controlled stream mesocosm for tertiary treatment of sewage. Ecol. Eng. 1996, 6, 149–169. [Google Scholar] [CrossRef]
- Khan, M.I.; Shin, J.H.; Kim, J.D. The promising future of microalgae: Current status, challenges, and optimization of a sustainable and renewable industry for biofuels, feed, and other products. Microb. Cell Fact. 2018, 17, 36. [Google Scholar] [CrossRef]
- Lindland, K.M.; Gjerstad, B.; Krøvel, A.V.; Ravagnan, E. Governing for sustainability in the norwegian aquaculture industry. Ocean Coast. Manag. 2019, 179, 104827. [Google Scholar] [CrossRef]
- Stiles, W.A.V.; Styles, D.; Chapman, S.P.; Esteves, S.; Bywater, A.; Melville, L.; Silkina, A.; Lupatsch, I.; Fuentes Grünewald, C.; Lovitt, R.; et al. Using microalgae in the circular economy to valorise anaerobic digestate: Challenges and opportunities. Bioresour. Technol. 2018, 267, 732–742. [Google Scholar] [CrossRef] [PubMed]
- Gbadamosi, O.K.; Lupatsch, I. Effects of dietary nannochloropsis salina on the nutritional performance and fatty acid profile of nile tilapia, oreochromis niloticus. Algal Res. 2018, 33, 48–54. [Google Scholar] [CrossRef]
- Van Den Hende, S.; Claessens, L.; De Muylder, E.; Boon, N.; Vervaeren, H. Microalgal bacterial flocs originating from aquaculture wastewater treatment as diet ingredient for litopenaeus vannamei (boone). Aquac. Res. 2016, 47, 1075–1089. [Google Scholar] [CrossRef]
- Alobwede, E.; Leake, J.R.; Pandhal, J. Circular economy fertilization: Testing micro and macro algal species as soil improvers and nutrient sources for crop production in greenhouse and field conditions. Geoderma 2019, 334, 113–123. [Google Scholar] [CrossRef]



| Media Formulation | mMol L−1 N | mMol L−1 P | N:P Ratio | Dilution Factor mL L−1 | Final N Concentration µMol L−1 | Final P Concentration µMol L−1 | N:P Ratio |
|---|---|---|---|---|---|---|---|
| Agricultural waste | 55.4 | 4.3 | 12.7 | 15.9 | 880.8 | 69.3 | 12.7 |
| Aquaculture waste | 8.8 | 57.4 | 24.5 | 1.92 | 17 (+882) * | 110.3 | 8.15 |
| AD municipal waste | 71.4 | 4.5 | 15.9 | 20 | 1428 | 89.7 | 15.9 |
| F/2 | 1 | 882 | 36.2 | 24.5 |
| Parameters | Agriculture Waste | Aquaculture Waste | AD Municipal Waste |
|---|---|---|---|
| PH Value | 8.5 ± 0.02 | 8.5 ± 0.2 | 8.7 ± 0.015 |
| BOD (mg L−1) | 421 ± 16 | 405 ± 14 | 433 ± 12 |
| COD (mg L−1) | 2500 ± 256 | 2786 ± 385 | 2766 ± 410 |
| Ammonium (mg L−1) | 360 ± 15 | 26 ± 1.5 | 890 ± 30 |
| TSS (total suspended solids) (mg L−1) | 210 ± 6.8 | 472 ± 9 | 1060 ± 12.1 |
| Phosphorus (mg L−1) | 36.5 ± 0.6 | 418 ± 2.8 | 63 ± 0.5 |
| Magnesium (mg L−1) | 5.9 ± 0.8 | 29 ± 2.6 | 8.55 ± 1.65 |
| Dissolved iron (mg L−1) | 2.64 ± 0.06 | 5.93 ± 0.2 | 2.61 ± 0.03 |
| Conductivity (mS cm−1) | 5.16 ± 0.02 | 0.858 ± 0.006 | 4.89 ± 0.2 |
| Waste-Based Media | NH4 Uptake Rate (µMol L−1) | P Uptake Rate (µMol L−1) | Max Biomass Concentration (g L−1) | µ at Exp. Phase (day−1) | Doubling Time | |
|---|---|---|---|---|---|---|
| (day−1) | (H) | |||||
| Agriculture waste | 186 | 8.1 | 1.3 | 0.41 | 1.68 | 40.39 |
| AD municipal waste | 36 | 0.74 | 1.78 | 0.45 | 1.53 | 36.80 |
| Aquaculture waste | N/A | 2.73 | 2.5 | 0.62 | 1.11 | 26.71 |
| Control | 192 | 9.6 | 1.99 | 0.42 | 1.64 | 39.43 |
| Waste-Based Media | Protein % | Lipids % | Ash % | Carbohydrates % | Energy kcal g−1 |
|---|---|---|---|---|---|
| Agriculture waste | 28 ± 1.3 | 21.4 ± 1.5 | 23.0 ± 0.23 | 27.6 ± 1.6 | 5.9 ± 0.1 |
| Aquaculture waste | 21.3 ± 1.2 | 34.8 ± 1.8 | 23.4 ± 0.17 | 19.7 ± 1.5 | 6.4 ± 0.3 |
| Municipal waste | 36.6 ± 1.7 | 17.9 ± 0.4 | 23.9 ± 0.18 | 23.5 ± 1.8 | 4.9 ± 0.2 |
| F2 | 36.5 ± 1.8 | 24.5 ± 1.5 | 21.8 ± 0.16 | 17.3 ± 1.5 | 5.5 ± 0.4 |
| Author | Effluent Type | Parameter | Influent | Effluent | % Recovered | RT (Retention Time) |
|---|---|---|---|---|---|---|
| STRUVITE METHOD | ||||||
| Yilmazel & Demirer, 2011 | Poultry Manure | Ammonia | 3.6 | Unknown | 86% | 60 Min |
| Phosphate | 1.2 | Unknown | 31% | 60 Min | ||
| Chong & Thai, 2014 | Synthetic WW | Phosphate | 0.3 | 54 ± 2 | 84% | 120 Min |
| Li et al., 2012 | Swine WW | Ammonia | 4.9 | Unknown | 88% | 60 Min |
| HRAP | ||||||
| Van Den Hende, et al., 2014 | Manure | TN | 4.3 | 0.2 | 43% | 4 days |
| TP | 3.9 | 1.3 | 65% | 4 days | ||
| Gutierrez et al., 2016 | Urban WW | N-NH4+ | 1.5 | 0.2 | 95% | 1 month |
| Passos et al., 2015 | Municipal WW | N-NH4+ | 1.05 | 0.02 | 95% | 1 month |
| ATS | ||||||
| Bohutskyi et al., 2016 | Agriculture WW | TP | 0.004 | 0.003 | 22% | 1 month |
| TN | 0.07 | 0.05 | 6% | 1 month | ||
| Craggs et al., 1996 | Secondary sewage | TP | 2.9 | 1.4 | 20% | 1 day |
| TN | 21.13 | 5.28 | 40% | 1 day | ||
| ALGAL PBR | ||||||
| Current study | Agriculture WW | TON | 0.8 | 0.0006 | 99.5% | 5 days |
| Nitrate | 0.181 | 0.0005 | 99.9% | 5 days | ||
| N-NH4+ | 0.802 | 0.0003 | 99.9% | 5 days | ||
| T P | 0.007 | 0.0002 | 99.9% | 4 days | ||
| Aquaculture WW | TP | 0.11 | 0.00011 | 99.9% | 4 days | |
| AD municipal WW | N-NH4+ | 0.001 | 0.00012 | 99.9% | 5 days | |
| TP | 0.089 | 0.00016 | 99.9% | 4 days | ||
| Results from [52] | % Crude Protein | % Crude Lipid | % Crude Carbohydrate | % Ash | Gross Energy MJ/kg |
|---|---|---|---|---|---|
| Fish meal | 63.0 | 11.0 | – | 15.8 | 20.1 |
| Poultry meal | 58.0 | 11.3 | – | 18.9 | 19.1 |
| Spirulina | 58.0 | 11.6 | 10.8 | 13.4 | 20.1 |
| Chlorella | 52.0 | 7.5 | 24.3 | 8.2 | 19.3 |
| Tetraselmis | 27.2 | 14.0 | 45.4 | 11.5 | 18.0 |
| Nannochloropsis | 42.8 | 16.6 | 33.9 | 6.7 | 22.6 |
| Results from the current study | |||||
| Nannochloropsis oceanica | 21–36 | 17–34 | 19–27 | 23 | 4–6.5 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Silkina, A.; Ginnever, N.E.; Fernandes, F.; Fuentes-Grünewald, C. Large-Scale Waste Bio-Remediation Using Microalgae Cultivation as a Platform. Energies 2019, 12, 2772. https://doi.org/10.3390/en12142772
Silkina A, Ginnever NE, Fernandes F, Fuentes-Grünewald C. Large-Scale Waste Bio-Remediation Using Microalgae Cultivation as a Platform. Energies. 2019; 12(14):2772. https://doi.org/10.3390/en12142772
Chicago/Turabian StyleSilkina, Alla, Naomi E. Ginnever, Fleuriane Fernandes, and Claudio Fuentes-Grünewald. 2019. "Large-Scale Waste Bio-Remediation Using Microalgae Cultivation as a Platform" Energies 12, no. 14: 2772. https://doi.org/10.3390/en12142772
APA StyleSilkina, A., Ginnever, N. E., Fernandes, F., & Fuentes-Grünewald, C. (2019). Large-Scale Waste Bio-Remediation Using Microalgae Cultivation as a Platform. Energies, 12(14), 2772. https://doi.org/10.3390/en12142772







