Physical and Chemical Properties of Waste from PET Bottles Washing as A Component of Solid Fuels
Abstract
1. Introduction
2. Materials and Methods
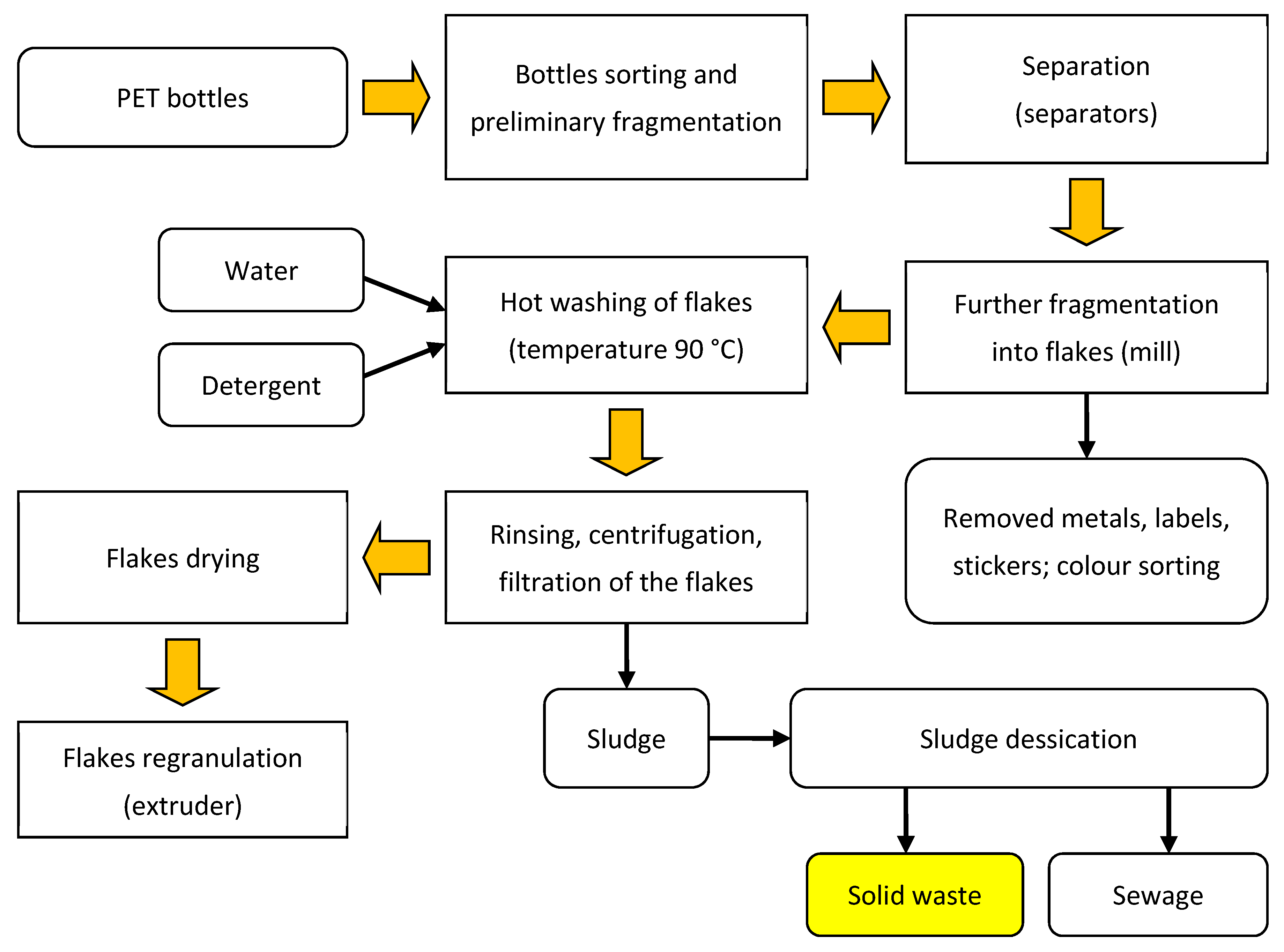
2.1. Material
2.2. Methods
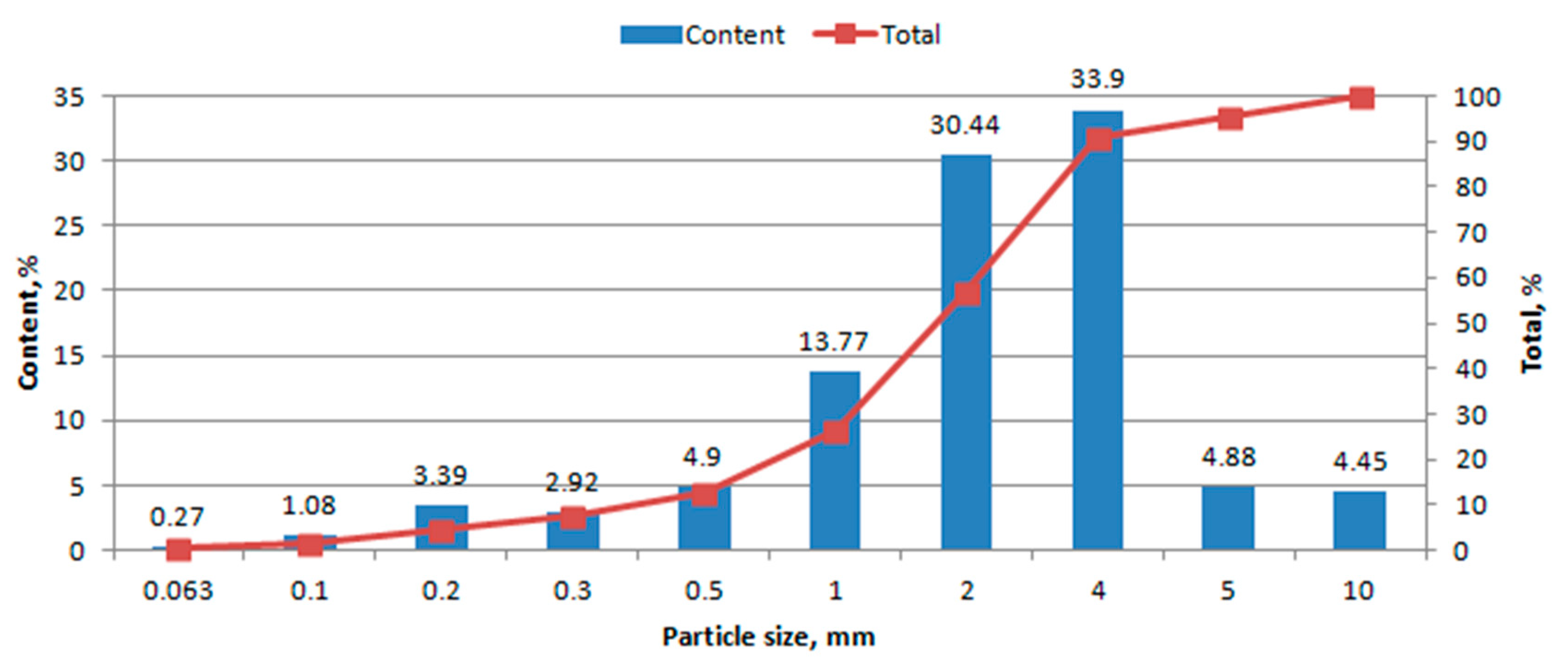
- Granulometric composition was determined by sieve analysis, according to [17]. The analysis was performed on waste dried at 105 °C. Separation of the material into fractions containing grains of various sizes was carried out on an AS 300 vibrating screen (Retsch, Germany). The grain uniformity coefficient was calculated using the Hazen formula U = d60/d10, where d60 is the equivalent diameter corresponding to 60% of the grains on the summation curve, and d10 is the equivalent diameter corresponding to 10% of the grains on the summation curve [18].
- Hygroscopic, transient and analytical moisture was determined according to the standard [19]. Total moisture was determined as transient moisture plus moisture of air-dry fuel, calculated as a percentage of the weight of the working fuel as follows:where: —total moisture (%), —transient moisture (%), —hygroscopic moisture (%). To determine the moisture content, a laboratory dryer (SLW 115 STD, POL-EKO) was used.
- The bulk and specific densities were found in accordance with [20] and [21], respectively. The specific density was determined by the pycnometer method as the ratio of the mass and volume of the solid phase. The bulk density found by determining the mass of the sample and the volume occupied by the sample in the measuring vessel [22]. The mass of the sample is the difference in mass of a measuring vessel of a specified volume, filled with freshly made material and an empty vessel.
- The content of total organic substances was determined from the loss on the scale after calcination at a temperature of 500 °C, according to the method used by Skalmowski et al. [21].
- The chemical composition of the inorganic substance was determined using XRF (Philips PW 1404 sequential spectrometer). The ash basicity index was calculated as follows [23]:
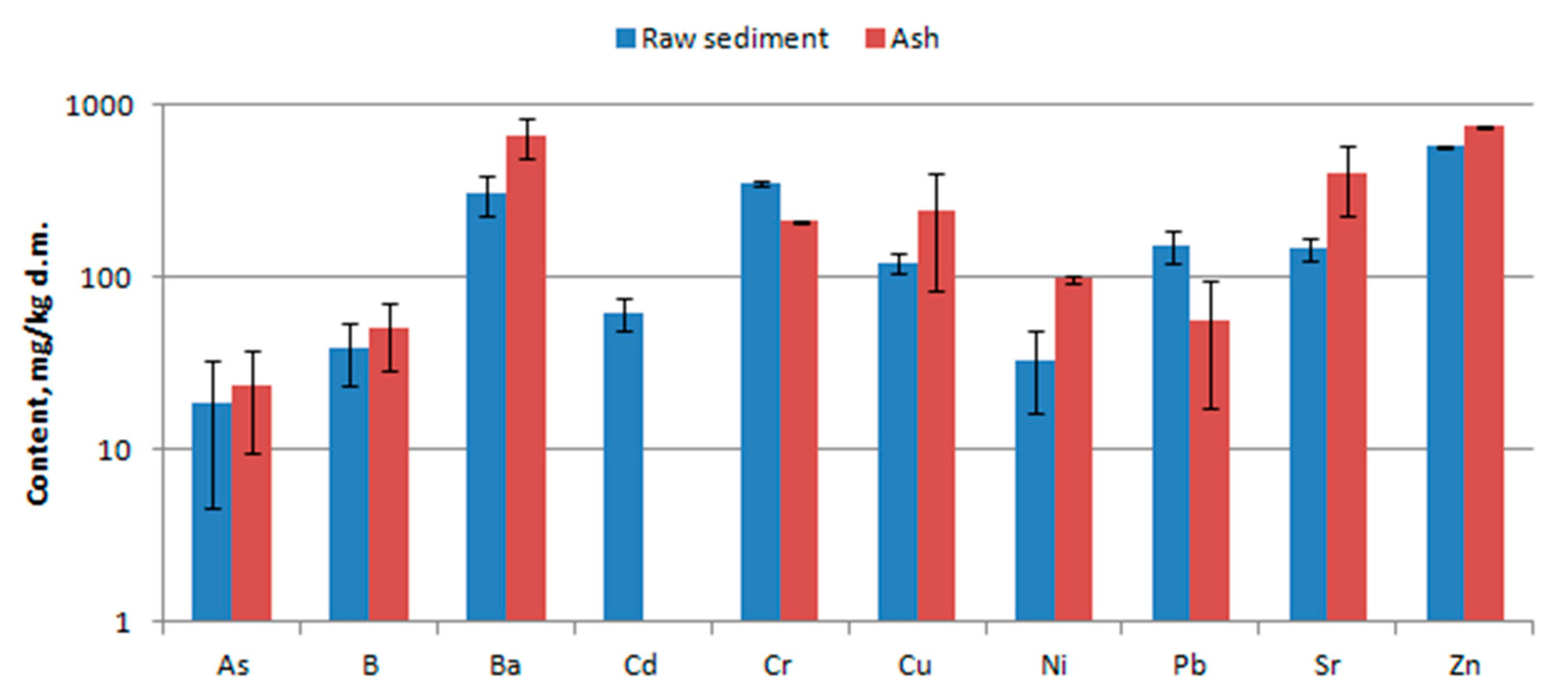
- Leaching tests of trace elements were carried out in accordance with the standard [24]. The test sample was crushed and sieved through a sieve with a mesh size <10 mm. The tests of leaching of impurities from waste were carried out for the ratio liquid to solid phase 10 l/kg. The individual compounds were designated using the reference methods indicated in [25].
- Sulphates, chlorides, fluorides, sodium and potassium were determined in accordance with the existing standard [26], and substances extracted with petroleum ether (PEE) by the specific method used by Hermanowicz et al. [27]. The determination consisted in subjecting the waste sample to extraction with petroleum ether in the Soxhlet apparatus, and then removing the solvent and weighing the remaining extract. The designation was made for a sample of dried and crushed waste.
- The content of organic compounds in aqueous extracts was determined on an N/C 3100 multi analyzer (Analytik Jena). The analyzer directly measures the total carbon (TC) and the total inorganic carbon (TIC), whereas the total organic carbon (TOC) is determined as the difference between TC and TIC.
- The concentration of metal ions was analyzed using an inductively coupled plasma ICP-AES emission spectrometer (Thermo Elemental IRIS Intrepid II XSP DUO). Before the measurements, the waste was subjected to the mineralization process and prepared in the form of solution. Waste samples were pre-dried at 105 °C and crushed. Next, about 2 g of the sample were weighed and placed in a porcelain crucible. Samples prepared in this way were burned for 5 hours at 520 °C in an FCF22S muffle furnace (CZYLOK, Poland). The ash was then dissolved in 20 ml of HCl, poured through a filter into a 100 ml flask and made up to volume with distilled water. Solutions prepared in this way were analyzed for the content of metals.
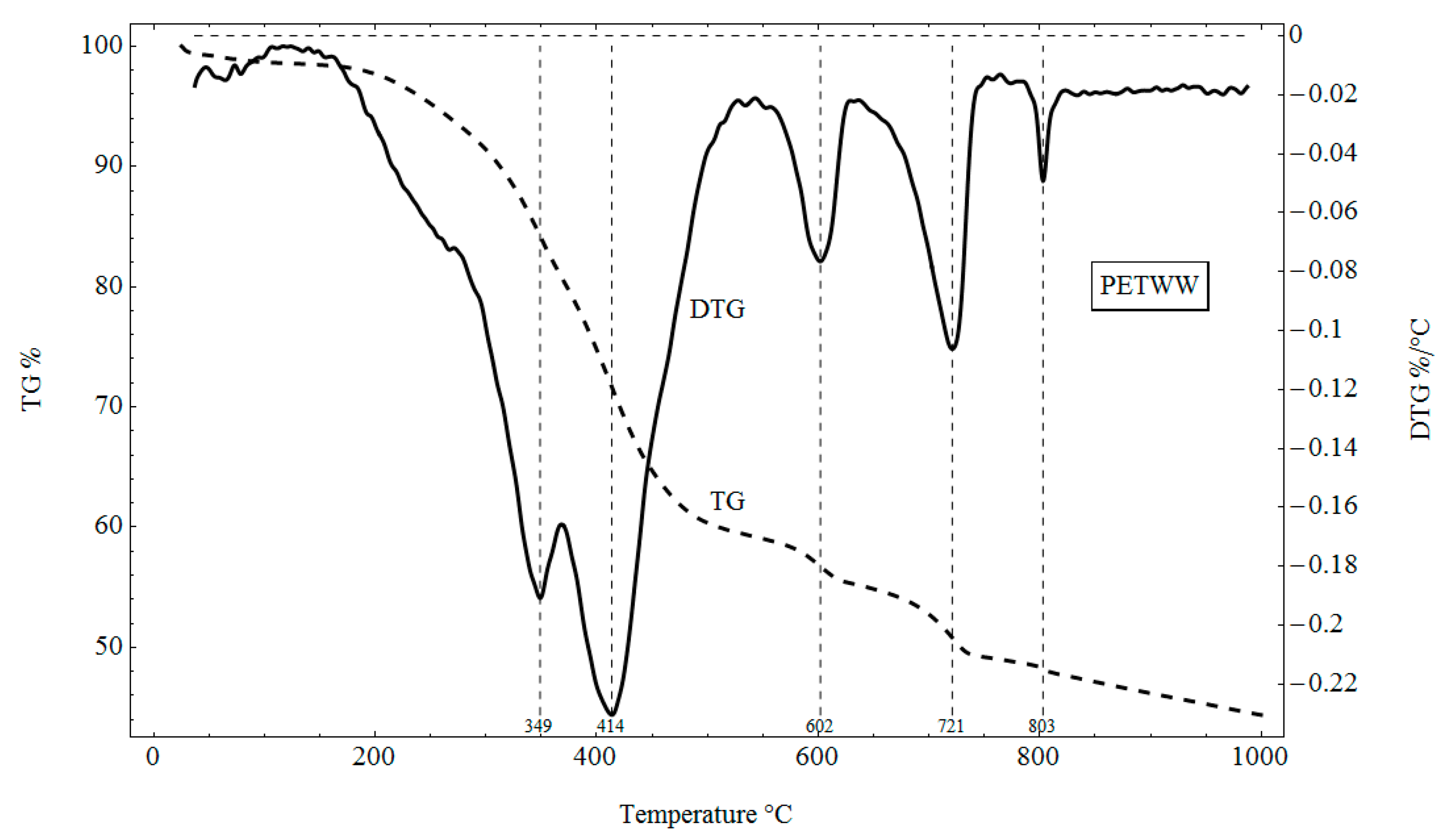
- Thermal analysis was carried out using a LABSYS TG-DTA/DSC type derivative. A sample of 10 mg was heated under nitrogen to 1000 °C at a heating rate of 10 °C/min. The device allowed for simultaneous differential and weight analysis by recording temperature, weight loss curve (TG) and thermogravimetric differential curve (DTG) on one graph, which allowed us to link precisely the duration of analysis, temperature and thermal effects in the sample with quantified weight changes.
- pH measurements were made with an ELMETRON pH/mV CP-401 pH meter (accuracy of +/-0.002 pH).
- Fuel properties, such as the content of combustible, non-flammable and volatile components were determined in accordance with [21]. In the determination of flammable (X1, %) and non-flammable (X2, %) components, the samples were calcined at 800 °C to a constant mass in an FCF22S muffle furnace (CZYLOK, Poland). The content of non-combustible constituents in the samples tested was calculated in relation to the dry mass of waste as follows: X2 = 100 – X1. The volatile components content was determined by heating the dried and crushed waste sample for 7 minutes in a closed crucible at 800 °C, in accordance with the standardized method, and then determining the loss of mass under these conditions.
- The combustion heat was determined on an isoperiobolic IKA C 2000 Basic calorimeter, while the calorific value was determined according to [28].
- The contents of the basic elementary components (C, H, N, S) were determined on a LECO Tru/Spec CHNS elemental analyzer.
- The oxygen content was determined by a calculation method based on the difference according to the equation (dry basis):O(%) = 100% − (Ash(%) + C(%) + N(%) + H(%) + S(%)).
3. Results
3.1. Granulometric Analysis
3.2. Physical Properties
3.3. Chemical Composition
3.4. TG and DTG Analysis
3.5. Fuel Properties
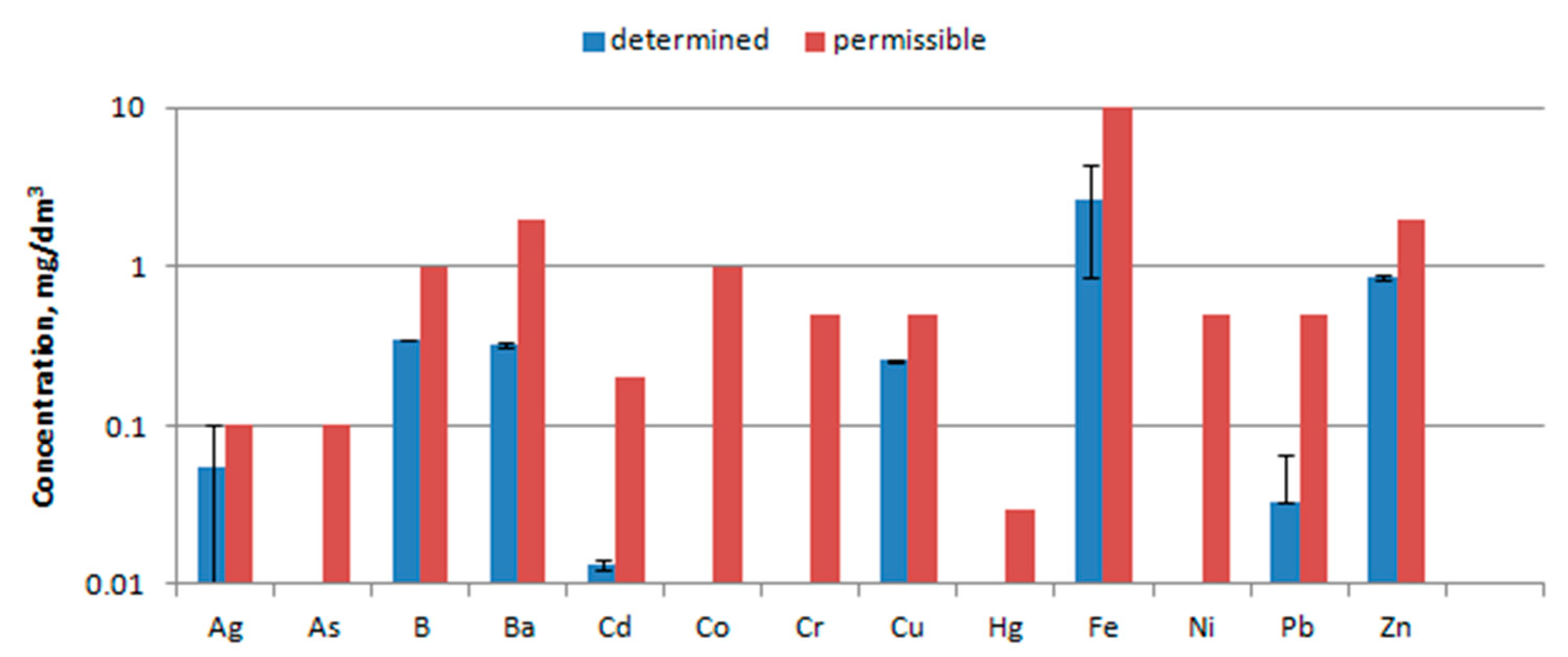
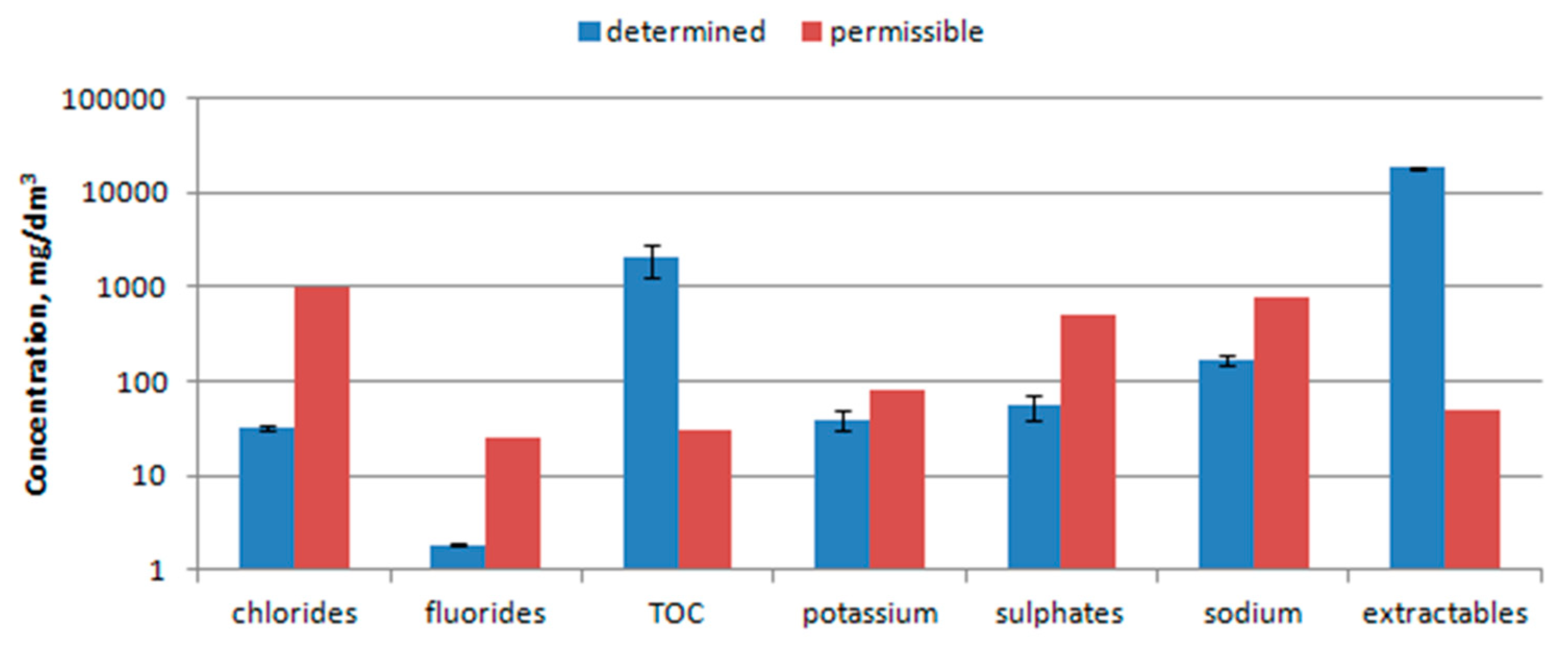
3.6. Water Extract Analysis
4. Discussion
4.1. Granulometric Analysis
4.2. Physical Properties
4.3. Chemical Composition
4.4. TG and DTG Analysis
4.5. Fuel Properties
4.6. Water Extract Analysis
5. Conclusions
- PETWW is a specific waste, which has the potential to be used as a component of alternative fuels, being a source of renewable energy in combustion processes—it is suitably well grained (grain size up to 10 mm), does not contain have heavy metals or other harmful substances in values exceeding the permissible ones, and its calorific value, although noticeably less than that of biomass, is comparable to that of sewage sludge.
- The calorific value is above 10 MJ/kg and can reach up to 13.2 MJ/kg for PETWW of moisture content below 8%. Although low moisture can be cost-ineffective, 10 MJ/kg at moisture of 45% can be assumed to be quite good. However, it is suggested that the wet waste should be mixed with other waste to obtain a new fuel with more satisfactory parameters.
- The waste should not be disposed in landfill due to values of TOC (ca. 2000 mg/dm3), PEE (ca. 18,500 mg/dm3) and its quite good calorific value (above 10 MJ/kg).
- PETWW could be used as a fuel in co-combustion processes in energy-intensive industries, for example, the cement industry, which could, however, require some drying or mixing with other fuel.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Vijaykumar, S.; Patel, M.R.; Patel, J.V. Pet waste management by chemical recycling: A review. J. Polym. Environ. 2010, 18, 8–25. [Google Scholar]
- Cichy, J.; Sobczyk, W. Odpady z tworzyw sztucznych i ich recykling (Plastics waste and its recycling). Edukac. Tech. Inform. 2014, 1, 348–353. [Google Scholar]
- Li, W.C.; Tse, H.F.; Fok, L. Plastic waste in the marine environment: A review of sources, occurrence and effects. Sci. Total Environ. 2016, 566–567, 333–349. [Google Scholar] [CrossRef] [PubMed]
- Plastics Europe. Plastics Europe—The Facts 2018; EPRO Report; Plastics Europe: Frankfurt, Germany, 2018; Available online: https://www.plasticseurope.org/de (accessed on 11 March 2019).
- Seebaluck, V.; Koussa, W.B. Prospects for recycling of waste PET bottles in Mauritius. Univ. Maurit. Res. J. 2009, 15, 334–349. [Google Scholar]
- Czarnecka-Komorowska, D. Aspekty recyklingu tworzyw sztucznych. Recykling 2007, 4, 30–31. [Google Scholar]
- Katami, T.; Yasuhara, A.; Okuda, T.; Shibamoto, T. Formation of PCDDs, PCDFs, and coplanar PCBs from polyvinyl chloride during combustion in an incinerator. Environ. Sci. Technol. 2002, 36, 1320–1324. [Google Scholar] [CrossRef] [PubMed]
- NAPCOR. Report on Postconsumer PET Container Recycling Activity in 2017; NAPCOR Report; NAPCOR: Charlotte, NC, USA, 2017; Available online: https://napcor.com/reports-resources/ (accessed on 11 March 2019).
- European Parliament; The Council of the European Union. Directive (EU) 2018/851 of the European Parliament and of the Council of 30 May 2018 amending Directive 2008/98/EC on waste. Off. J. Eur. Union 2008. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32018L0851&from=EN (accessed on 4 June 2019).
- Krehula, L.K.; Siročić, A.P.; Dukić, M.; Hrnjak-Murgić, Z. Cleaning efficiency of poly (ethylene terephthalate) washing procedure in recycling process. J. Elastomers Plast. 2012, 45, 429–444. [Google Scholar] [CrossRef]
- Wasielewski, R.; Sobolewski, A. Stałe paliwa wtórne - jako element systemu odzysku energii z odpadów. Nowa Energia 2009, 1, 24–29. [Google Scholar]
- Guo, F.; Zhong, Z. Optimization of the co-combustion of coal and composite biomass pellets. J. Clean. Prod. 2018, 185, 399–407. [Google Scholar] [CrossRef]
- Wang, T.; Hou, H.; Ye, Y.; Rong, H.; Li, J.; Xue, Y. Combustion behavior of refuse-derived fuel produced from sewage sludge and rice husk/wood sawdust using thermogravimetric and mass spectrometric analyses. J. Clean. Prod. 2019, 222, 1–11. [Google Scholar] [CrossRef]
- Sobolewski, A.; Wasilewski, R.; Stelmach, S. Wykorzystanie stałych paliw wtórnych w energetyce (Utilization of solid recovered fuels in power industry). Polityka Energetyczna 2007, 10, 379–389. [Google Scholar]
- Polish Std. BN-87/9103-03. Unieszkodliwianie Odpadów Miejskich—Pobieranie, Przechowywanie i Przesyłanie Oraz Wstępne Przygotowywanie Próbek Odpadów do Badań. Available online: http://bc.pollub.pl/dlibra/publication/11242/edition/10220/content?ref=desc (accessed on 6 June 2019).
- Perugini, F.; Mastellone, M.L.; Arena, U. A life cycle assessment of mechanical and feedstock recycling options for management of plastic packaging wastes. Environ. Prog. 2005, 24, 137–154. [Google Scholar] [CrossRef]
- Polish Std. PN-EN 15415-1. Stałe Paliwa Wtórne—Oznaczanie Rozkładu Wielkości Ziaren—Część 1: Metoda Przesiewania dla Cząstek o Małym Rozmiarze; Wydawnictwa Normalizacyjne: Warszawa, Poland, 2011. [Google Scholar]
- Pazdro, Z.; Kozerski, B. Hydrologia Ogólna; Wydawnictwo/Geologiczne: Warszawa, Poland, 1990; pp. 93–95. [Google Scholar]
- Polish Std. PN-80G/04511. Paliwa stałe—Oznaczanie Zawartości Wilgoci; Wydawnictwa Normalizacyjne: Warszawa, Poland, 1980. [Google Scholar]
- CEN/TS 15401 Standard. Solid Recovered Fuels—Determination of Bulk Density; BSI Group: London, UK, 2010. [Google Scholar]
- Skalmowski, K.; Wolska, K.; Pieniak, U.; Roszczyńska, I. Badania Właściwości Technologicznych Odpadów Komunalnych:Ćwiczenia Laboratoryjne; Oficyna Wydawnicza—Politechnika Warszawska: Warszawa, Poland, 2004; pp. 94–97. [Google Scholar]
- Vassilev, S.; Vassileva, C.G.; Karayigit, A.; Bulut, Y.; Alastuey, A.; Querol, X. Phase-mineral and chemical composition of composite samples from feed coals, bottom ashes and fly ashes at the Soma power station, Turkey. Int. J. Coal Geol. 2005, 61, 35–63. [Google Scholar] [CrossRef]
- Dyjakon, A. Analysis of slagging and fouling propensities of biofuels in terms of their combustion and co-combustion in the boilers. Agric. Eng. 2012, 2, 5–18. [Google Scholar]
- Polish Std. PN-EN 12457-4. Charakteryzowanie Odpadów—Wymywanie—Badanie Zgodności w Odniesieniu do Wymywania Ziarnistych Materiałów Odpadowych i Osadów; Wydawnictwa Normalizacyjne: Warszawa, Poland, 2006. [Google Scholar]
- Minister of Environment (Poland). Regulation of the Minister of Environment of 18th November 2014 on the Conditions to be Fulfilled When Introducing Sewage into Waters or into the Ground, and on Substances Particularly Harmful to the Aquatic Environment; (In Polish: Rozporządzenie Ministra Środowiska z dnia 18 listopada 2014 r. w sprawie warunków, jakie należy spełnić przy wprowadzaniu ścieków do wód lub do ziemi, oraz w sprawie substancji szczególnie szkodliwych dla środowiska wodnego, Dz.U. 2014 poz. 1800); Minister of Environment: Warsaw, Poland, 2014.
- Polish Std. PN-EN-ISO 10304-1. Jakość Wody—Oznaczanie Rozpuszczonych Anionów za Pomocą Chromatografii jonowej—Część 1: Oznaczanie Bromków, Chlorków, Fluorków, Azotanów, Azotynów, Fosforanów i Siarczanów; Wydawnictwa Normalizacyjne: Warszawa, Poland, 2009. [Google Scholar]
- Hermanowicz, W.; Dojlido, J.; Dożańska, W.; Koziorowski, B.; Zerbe, J. Fizyczno-Chemiczne Badanie Wody i Ścieków; Wydawnictwo: Warszawa, Poland, 1999. [Google Scholar]
- Polish Std. PN-ISO 1928:2002P. Paliwa Stałe—Oznaczanie Ciepła Spalania Metodą Spalania w Bombie Kalorymetrycznej i Obliczanie Wartości Opałowej; Wydawnictwa Normalizacyjne: Warszawa, Poland, 2002. [Google Scholar]
- Czop, M.; Błaszczyk, E. Determination of the fuel properties of selected packaging waste from the municipal sector. Arch. Waste Environ. Prot. 2015, 17, 131–138. [Google Scholar]
- Wandrasz, J.; Wandrasz, A. Paliwa Formowane Biopaliwa i Paliwa z Odpadów w Procesach Termicznych; Wydawca Seidel-Przywecki Sp. z o.o.: Warszawa, Poland, 2006; pp. 43–78. [Google Scholar]
- Przepiórski, J.; Karolczyk, J.; Takeda, K.; Tsumura, T.; Toyoda, M.; Morawski, A.W. Porous carbon obtained by carbonization of PET mixedwith basic magnesium carbonate: Pore structure and pore creation mechanism. Ind. Eng. Chem. Res. 2009, 48, 7110–7116. [Google Scholar] [CrossRef]
- García, R.; González-Vázquez, M.P.; Pevida, C.; Rubiera, F. Pelletization properties of raw and torrefied pine sawdust: Effect of copelletization, temperature, moisture content and glycerol addition. Fuel 2018, 215, 290–297. [Google Scholar] [CrossRef]
- Fernández, R.G.; García, C.P.; Lavín, A.G.; Bueno de las Heras, J.L. Study of main combustion characteristics for biomass fuels used in boilers. Fuel Process Technol. 2012, 103, 16–26. [Google Scholar] [CrossRef]
- Song, E.; Kim, D.; Jeong, C.J.; Kim, D.Y. A Kinetic study on combustible coastal debris pyrolysis via thermogravimetric analysis. Energies 2019, 12, 836. [Google Scholar] [CrossRef]
- Földvari, M. Handbook of Thermogravimetric System of Minerals and Its use in Geological Practice; Occasional Papers of the Geological Institute of Hungary: Budapest, Hungary, 2011; p. 63. [Google Scholar]
- Przelaskowska, A.; Klaja, J.; Kulinowski, P.; Gaweł, A. Zastosowanie metod analizy termicznej w badaniach skał silikoklastycznych o zróżnicowanym zaileniu (Thermal analysis of siliciclastic rocks of different clay kontent). Nafta-Gaz 2013, 7, 479–487. [Google Scholar]
- Pulka, J.; Manczarski, P.; Koziel, J.A.; Białowiec, A. Torrefaction of sewage sludge, kinetics and fuel properties of biochars. Energies 2019, 12, 565. [Google Scholar] [CrossRef]
- Dmitrienko, M.A.; Nyashina, G.S.; Strizhak, P.A. Major gas emissions from combustion of slurry fuels based on coal, coal waste, and coal derivatives. J. Clean. Prod. 2018, 177, 284–301. [Google Scholar] [CrossRef]
- Vershinina, K.Y.; Shlegel, N.E.; Strizhak, P.A. Relative combustion efficiency of composite fuels based on of woo processing and oil production wastes. Energy 2019, 169, 18–28. [Google Scholar] [CrossRef]
- Chi, H.; Pans, M.A.; Sun, C.; Liu, H. An investigation of lime addition to fuel as a countermeasure to bed agglomeration for the combustion of non-woody biomass fuels in a 20kWth bubbling fluidised bed combustor. Fuel 2019, 240, 349–361. [Google Scholar] [CrossRef]
- Michalak, R. Prawne i techniczne aspekty wytwarzania i stosowania paliw z odpadów przemysłowych. In Paliwa z Odpadów; Wandrasz, J.W., Nadziakiewicz, J., Eds.; Wydawnictwo—Helion: Gliwice, Poland, 2003; Volume 4, pp. 21–28. [Google Scholar]
- Sobolewski, A.; Ilmurzyńska, J.; Iluk, T.; Czaplicki, A. Zgazowanie biomasy. In Nowoczesne Technologie Pozyskania i Energetycznego Wykorzystania Biomasy; Bocian, P., Golec, T., Rakowski, J., Eds.; Wydawnictwo/Instytut Energetyki: Warszawa, Poland, 2010; pp. 280–309. [Google Scholar]
- Pawłowski, P.; Bałazińska, M.; Ignasiak, K.; Robak, J. Przygotowanie odpadów komunalnych do ich energetycznego wykorzystania—Paliwo typu SRF. (Preparation of the selected groups of waste their energy use: Fuel from waste type SRF). Piece Przemysłowe Kotły 2016, 4, 21–27. [Google Scholar]
- Lebecki, K. Właściwości paliwowe stosowanych w energetyce pyłów pochodzących z biomasy (Combustible characteristics of biomass originating dusts applicable in energy sector). Zeszyty Naukowe Wyższej Szkoły Zarządzania Ochroną Pracy Katowicach 2010, 1, 71–79. [Google Scholar]
- Wisz, J.; Matwiejew, A. Biomasa—badania w laboratorium w aspekcie przydatności do energetycznego spalania. Energetyka 2005, 9, 631–636. [Google Scholar]
- Czop, M. Tests of basic fueling properties of polyolefin wastes. Arch. Waste Environ. Prot. 2013, 15, 71–80. [Google Scholar] [CrossRef][Green Version]
- Zakościelna, P.; Sierakowski, M.; Świątkowski, A. Changes in the distribution of heavy metals turing the thermal treatment of municipal sewage sludge (in Polish). Eng. Prot. Environ. 2017, 20, 175–183. [Google Scholar] [CrossRef]
- Sładeczek, F. Aktualny stan współspalania paliw z odpadów w piecach cementowych w Polsce. In Proceedings of the I Forum Paliw Alternatywnych, Lubliniec, Poland, 29–30 September 2011. [Google Scholar]
- Mokrzycki, E.; Uliasz-Bocheńczyk, A.; Sarna, M. Wastes as alternative fuels in cement industry. In Energy 2000: The Beginning of a New Millenium, Proceedings of the 8-th International Energy Forum ENERGEX 2000, Las Vegas, CA, USA, 23–28 July 2000; Catania, P., Ed.; CRC Press: Boca Raton, FL, USA, 2000; 1421p. [Google Scholar]
- Bożym, M. Wymagania jakościowe stawiane osadom ściekowym spalanym w cementowniach. Chemik 2013, 67, 1019–1024. [Google Scholar]
- Latkowska, B.; Fitko, H.; Stelmach, S. Ocena właściwości paliwowych ubocznego produktu z produkcji bioetanolu (Evaluation of fuel properties of by-product from bioethanol production). Inżynieria Ekologiczna 2011, 25, 222–230. [Google Scholar]
- Polish Std. PN-EN 15359. Stałe Paliwa Wtórne—Wymagania Techniczne i Klasy; Wydawnictwa Normalizacyjne: Warszawa, Poland, 2012. [Google Scholar]
- Bilitewski, B.; Härdtle, G.; Marek, K. Podręcznik Gospodarki Odpadami—Teoria i Praktyka; Wydawnictwo—Seidel-Przywecki: Warszawa, Poland, 2006; pp. 454–455. [Google Scholar]
- Manzone, M.; Gioelli, F.; Balsari, P. effects of different storage techniques on round-baled orchard-pruning residues. Energies 2019, 12, 1044. [Google Scholar] [CrossRef]
- Poluszyńska, J. Możliwości zastosowania popiołów ze spalania biomasy w gospodarowaniu osadami ściekowymi. Prace Instytutu Ceramiki Materiałów Budowlanych 2013, 13, 49–59. [Google Scholar]
- Ciesielczuk, T.; Kusza, G.; Nemś, A. Nawożenie popiołami z termicznego przekształcania biomasy źródłem pierwiastków śladowych dla gleb. Ochrona Środowiska Zasobów Naturalnych 2011, 49, 219–227. [Google Scholar]
- Pachowski, J. Popioły Lotne i Ich Zastosowanie w Budownictwie Drogowym; Wydawnictwa Komunikacji i Łączności: Warszawa, Poland, 1976. [Google Scholar]
- Wang, S.; Miller, A.; Llamazos, E.; Fonseca, F.; Baxter, L. Biomass fly ash in concrete: Mixture proportioning and mechanical properties. Fuel 2008, 87, 365–371. [Google Scholar] [CrossRef]
- Hinojosa, M.J.R.; Galvin, A.P.; Agrela, F.; Perianes, M.; Barbudo, A. Potential use of biomass bottom ash as alternative construction material: Conflictive chemical parameters according to technical regulations. Fuel 2014, 128, 248–259. [Google Scholar] [CrossRef]
- Meller, E.; Bilenda, E. Wpływ nawożenia popiołami z biomasy na plon i pobranie składników przez kukurydzę zwyczajną. Polityka Energetyczna 2013, 16, 339–345. [Google Scholar]
- Vassilev, S.V.; Baxter, D.; Andersen, L.K.; Vassileva, C.G. An overview of the composition and application of biomass ash—Part 1: Phase–mineral and chemical composition and classification. Fuel 2013, 105, 40–76. [Google Scholar] [CrossRef]
- Querol, X.; Moreno, N.; Umaña, J.; Alastuey, A.; Hernández, E.; López-Soler, A.; Plana, F. Synthesis of zeolites from coal fly ash: An overview. Int. J. Coal Geol. 2002, 50, 413–423. [Google Scholar] [CrossRef]
- Minister of Economy (Poland). Regulation of the Minister of Economy of 16th July 2015 on the Admission of Waste for Landfills; (in Polish: Rozporządzenie Ministra Gospodarki z dnia 16 lipca 2015 r. w sprawie dopuszczania odpadów do składowania na składowiskach, Dz.U. 2015 poz. 1277); Minister of Economy: Warsaw, Poland, 2015.

| Quantity | Unit | Value |
|---|---|---|
| Hygroscopic moisture | % | 43.7 ± 1.5 |
| Transient moisture | % | 6.0 ± 0.76 |
| Total moisture | % | 45.1 ± 4.03 |
| Analytical moisture | % | 12.1 ± 1.5 |
| Bulk density | kg/m3 | 602.3 ± 63.2 |
| Specific density | kg/dm3 | 1.27 ± 0.14 |
| Component | SiO2 | Al2O3 | Fe2O3 | CaO | Na2O | MgO | K2O | P2O5 | TiO2 | SO3 | MnO | Loss on Ignition |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Value, wt% | 10.32 | 4.49 | 3.13 | 16.0 | 0.67 | 1.32 | 0.3 | 0.69 | 0.07 | 0.27 | 0.11 | 61.2 |
| Parameter | Tested PETWW | Dry Sewage Sludge [37] | Flame Coal [38] | Lignite [38] | Biomass | |
|---|---|---|---|---|---|---|
| Sawdust from Pine [39] | Wheat Straw [40] | |||||
| The calorific value, MJ/kg | 10.1–13.2 | 12.279–13.503 | 24.82 | 22.91 | 19.41 | N/A |
| Non-flammable parts (ash), % | 37.6 ± 1.75 | 40.3 | 8.52 | 4.12 | 1.88 | 7.01 |
| Flammable parts (loss of ignition), % | 62.4 ± 1.05 | 59.7 | 91.48 | 95.88 | 98.12 | 92.99 |
| Volatile matter, % | 57.7 ± 4.6 | N/A | 40.19 | 47.63 | 73.52 | 73.20 |
| Ultimate, Dry Basis | ||||||
| Carbon, C, % | 34.4 ± 1.1 | 29.7 | 70.54 | 70.23 | 46.48 | 42.61 |
| Hydrogen, H, % | 2.9 ± 0.14 | 4.81 | 5.28 | 6.25 | 6.15 | 6.34 |
| Sulphur, S, % | 0.11 ± 0.01 | 0.12 | 0.36 | 0.43 | 0.21 | <0.01 |
| Nitrogen, N, % | 0.39 ± 0.05 | 4.02 | 2.40 | 0.76 | 1.57 | 0.63 |
| Oxygen, O, % | 25.59 ± 1.1 | 21.08 | 12.91 | 18.21 | 43.71 | 43.41 |
| H/C | 1.04 | 1.94 | 0.90 | 1.07 | 1.59 | 1.69 |
| O/C | 0.56 | 0.53 | 0.14 | 0.19 | 0.71 | 0.71 |
| Parameter | Tested PETWW | EACP Guidelines | Domestic Cement Plants Requirements for Refuse Derived Fuel |
|---|---|---|---|
| Hg, mg/kg d.m. | 0 | <10 | <2000 |
| The total for Cd and Tl, mg/kg d.m. | 57–66 | <100 | |
| The total of the remaining metals (As, B, Ba, Cr, Cu, Fe, Ni, Pb, Sr, Zn), mg/kg d.m. | 1677–1817 | <2500 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jabłońska, B.; Kiełbasa, P.; Korenko, M.; Dróżdż, T. Physical and Chemical Properties of Waste from PET Bottles Washing as A Component of Solid Fuels. Energies 2019, 12, 2197. https://doi.org/10.3390/en12112197
Jabłońska B, Kiełbasa P, Korenko M, Dróżdż T. Physical and Chemical Properties of Waste from PET Bottles Washing as A Component of Solid Fuels. Energies. 2019; 12(11):2197. https://doi.org/10.3390/en12112197
Chicago/Turabian StyleJabłońska, Beata, Paweł Kiełbasa, Maroš Korenko, and Tomasz Dróżdż. 2019. "Physical and Chemical Properties of Waste from PET Bottles Washing as A Component of Solid Fuels" Energies 12, no. 11: 2197. https://doi.org/10.3390/en12112197
APA StyleJabłońska, B., Kiełbasa, P., Korenko, M., & Dróżdż, T. (2019). Physical and Chemical Properties of Waste from PET Bottles Washing as A Component of Solid Fuels. Energies, 12(11), 2197. https://doi.org/10.3390/en12112197







