A Self-Powered Glucose Biosensor Operated Underwater to Monitor Physiological Status of Free-Swimming Fish
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
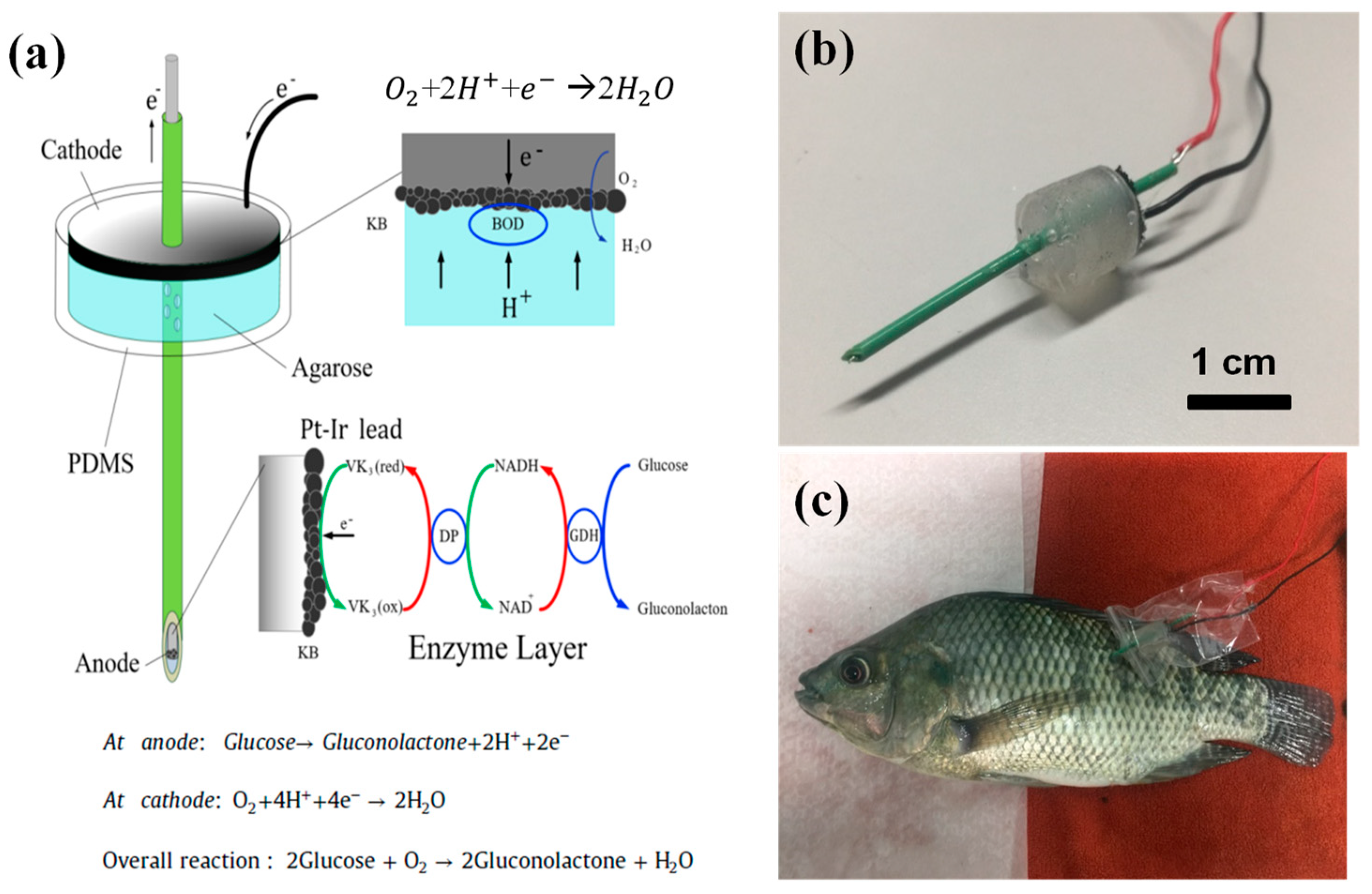
2.2. Design of Needle-Type EFCs
2.3. Preparation of Enzymatic Electrodes
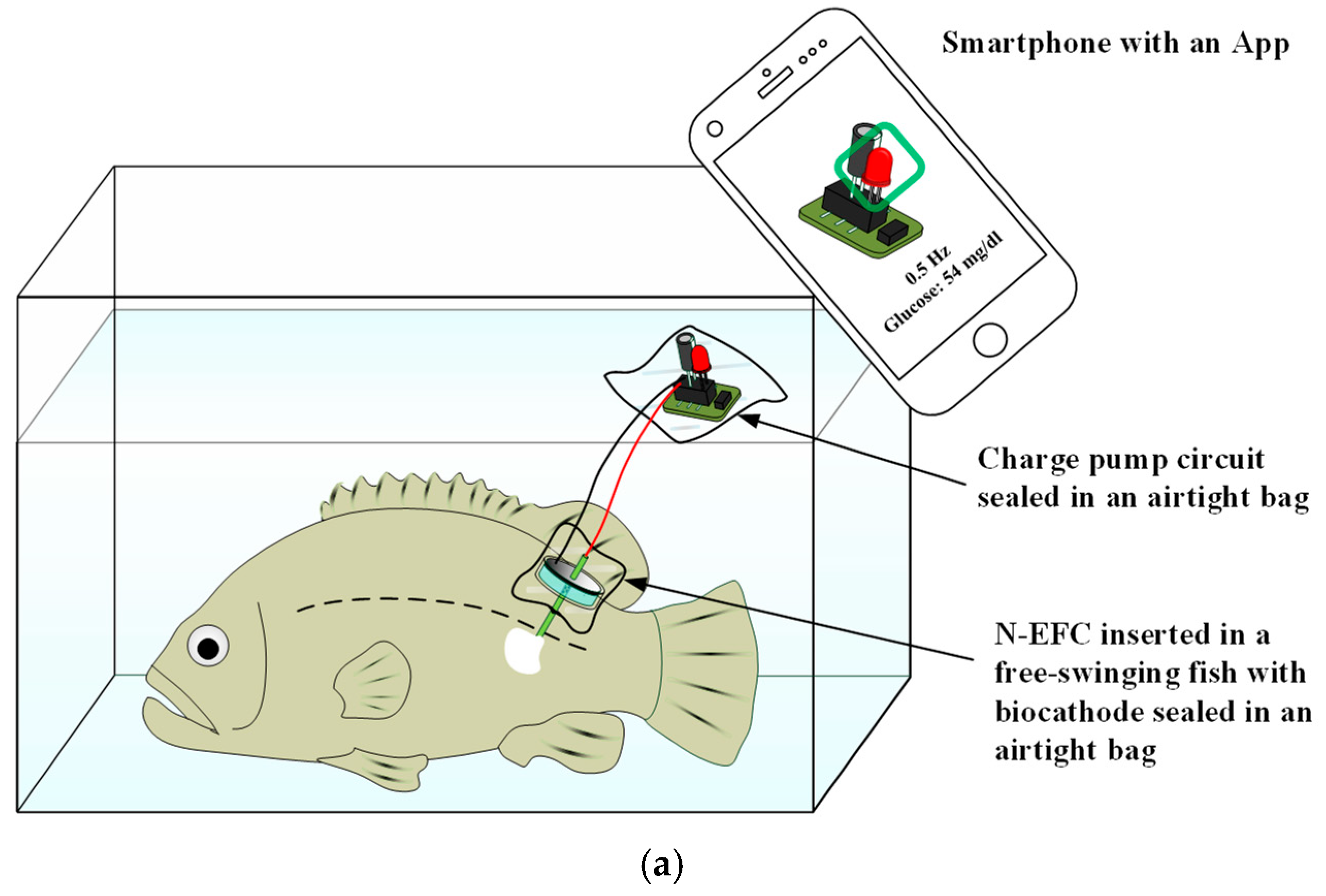
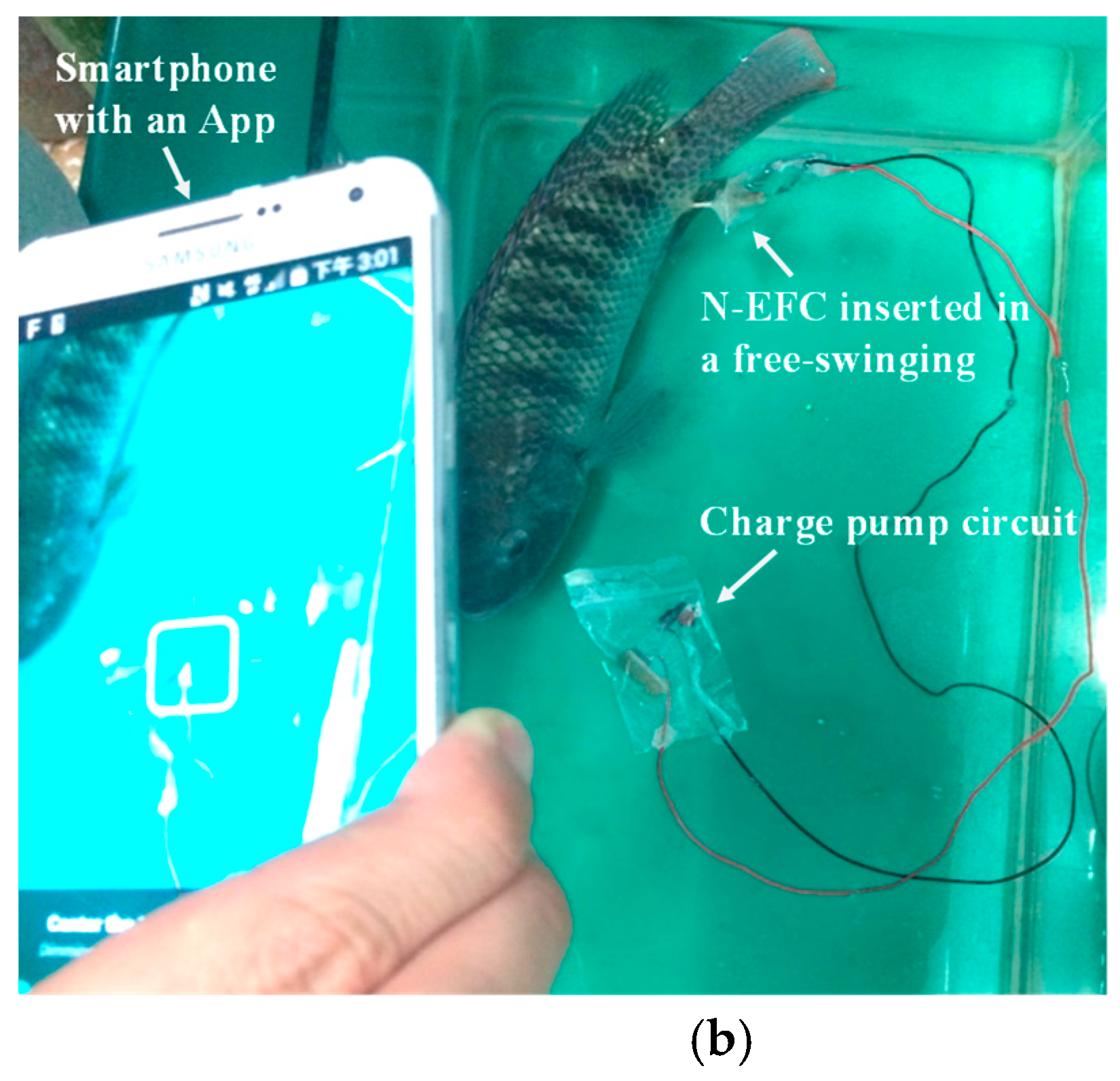
2.4. Insertion of the N-EFC into a Living Fish
3. Results and Discussion
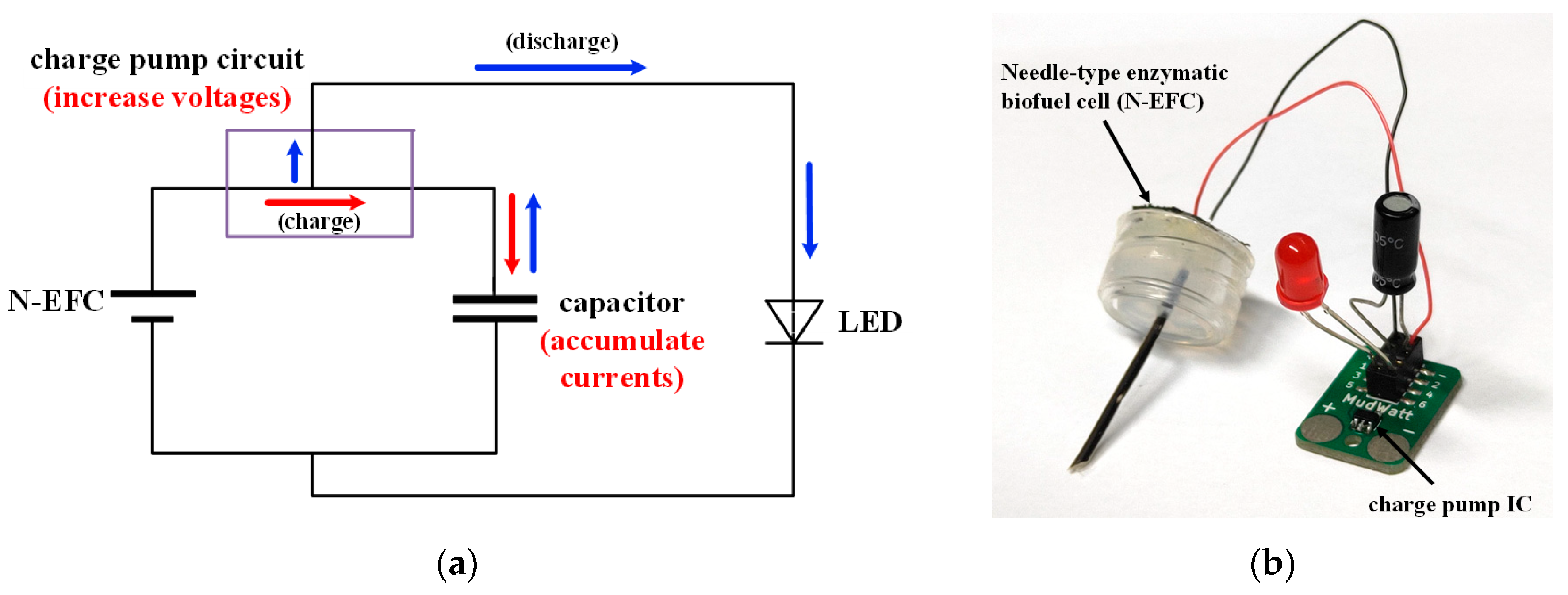
3.1. Power Generation of the N-EFC
3.2. Bioelectricity Generated from a Living Fish with the N-EFC
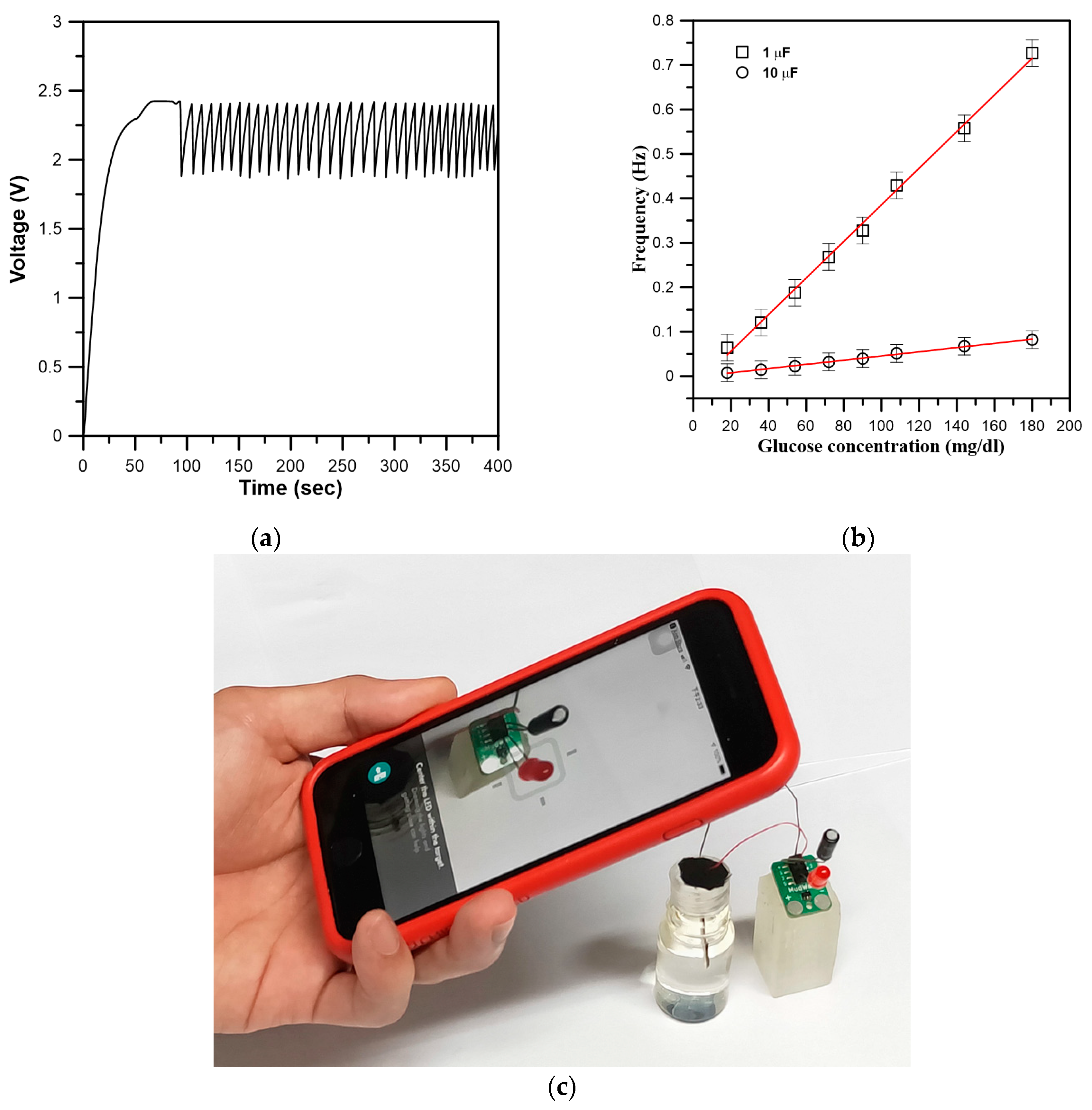
3.3. Characterization of Self-Powered Glucose Biosensor
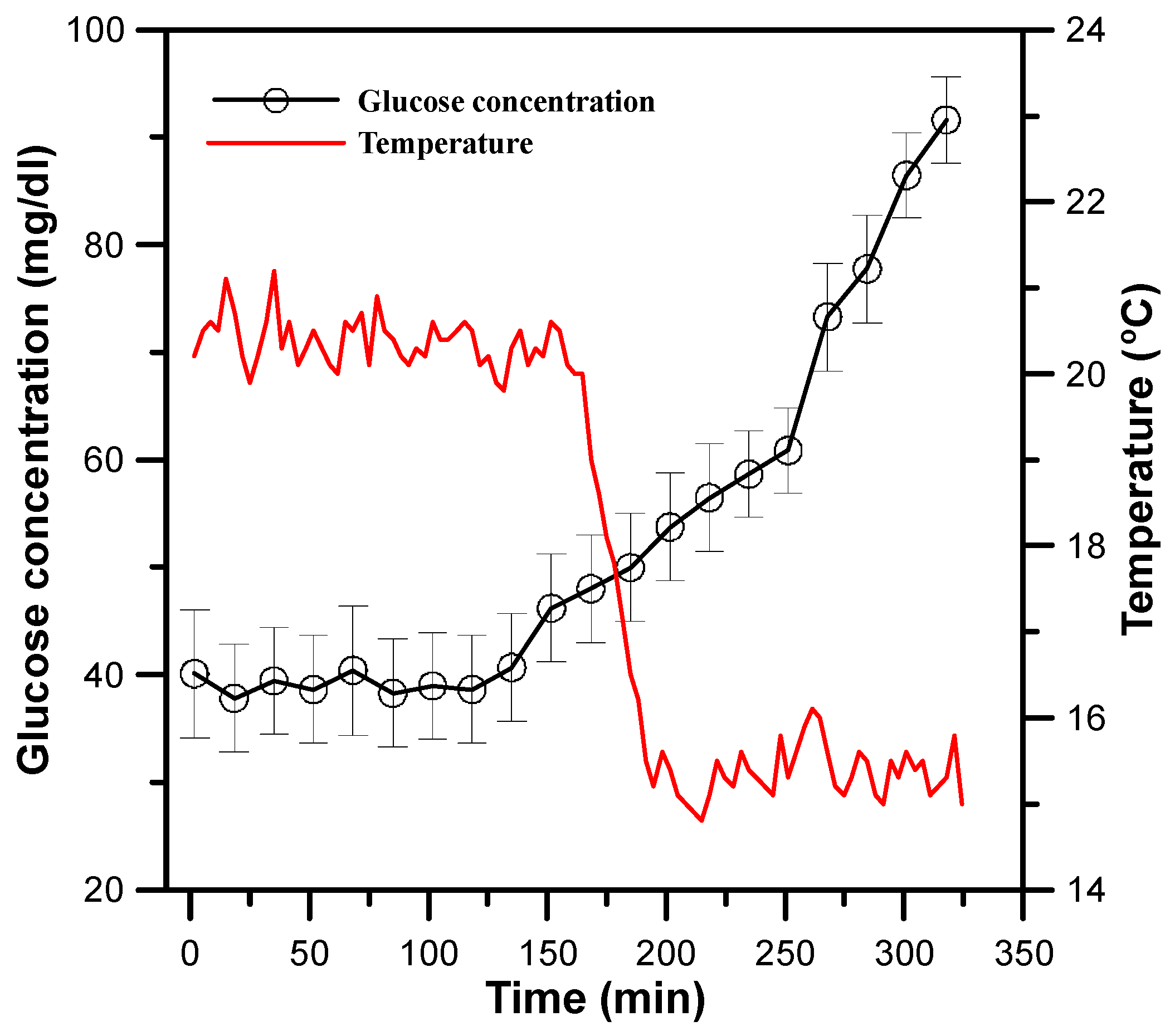
3.4. Monitoring of Physiological Status of a Free-Swimming Fish Treated with Cold Shock by SPGB
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Sopinka, N.M.; Donaldson, M.R.; O’Connor, C.M.; Suski, C.D.; Cooke, S.J. Stress indicators in fish. In Fish Physiology; Academic Press: Cambridge, MA, USA, 2016; Volume 35, pp. 405–462. [Google Scholar]
- El-Khaldi, A.T. Effect of different stress factors on some physiological parameters of nile tilapia (oreochromis niloticus). Saudi J. Biol. Sci. 2010, 17, 241–246. [Google Scholar] [CrossRef]
- Takase, M.; Yoneyama, Y.; Murata, M.; Hibi, K.; Ren, H.; Endo, H. Carbon nanotube enhanced mediator-type biosensor for real-time monitoring of glucose concentrations in fish. Anal. Bioanal. Chem. 2012, 403, 1187–1190. [Google Scholar] [CrossRef]
- Takase, M.; Yoneyama, Y.; Murata, M.; Hibi, K.; Ren, H.; Endo, H. Mediator-type biosensor for real-time wireless monitoring of blood glucose concentrations in fish. Fish. Sci. 2012, 78, 691–698. [Google Scholar] [CrossRef]
- Yoneyama, Y.; Yonemori, Y.; Murata, M.; Ohnuki, H.; Hibi, K.; Hayashi, T.; Ren, H.; Endo, H. Wireless biosensor system for real-time cholesterol monitoring in fish “nile tilapia”. Talanta 2009, 80, 909–915. [Google Scholar] [CrossRef]
- Hibi, K.; Hatanaka, K.; Takase, M.; Ren, H.; Endo, H. Wireless biosensor system for real-time l-lactic acid monitoring in fish. Sensors 2012, 12, 6269–6281. [Google Scholar] [CrossRef] [PubMed]
- Endo, H.; Yonemori, Y.; Hibi, K.; Ren, H.; Hayashi, T.; Tsugawa, W.; Sode, K. Wireless enzyme sensor system for real-time monitoring of blood glucose levels in fish. Biosens. Bioelectron. 2009, 24, 1417–1423. [Google Scholar] [CrossRef] [PubMed]
- Endo, H.; Takahashi, E.; Murata, M.; Ohnuki, H.; Ren, H.; Tsugawa, W.; Sode, K. Wireless monitoring of blood glucose levels in flatfish with a needle biosensor. Fish. Sci. 2010, 76, 687–694. [Google Scholar] [CrossRef]
- Gonzalez-Solino, C.; Lorenzo, M.D. Enzymatic fuel cells: Towards self-powered implantable and wearable diagnostics. Biosensors 2018, 8, 11. [Google Scholar] [CrossRef] [PubMed]
- Cosnier, S.; Le Goff, A.; Holzinger, M. Towards glucose biofuel cells implanted in human body for powering artificial organs: Review. Electrochem. Commun. 2014, 38, 19–23. [Google Scholar] [CrossRef]
- Castorena-Gonzalez, J.A.; Foote, C.; MacVittie, K.; Halámek, J.; Halámková, L.; Martinez-Lemus, L.A.; Katz, E. Biofuel cell operating in vivo in rat. Electroanalysis 2013, 25, 1579–1584. [Google Scholar] [CrossRef]
- Cheng, H.; Yu, P.; Lu, X.; Lin, Y.; Ohsaka, T.; Mao, L. Biofuel cell-based self-powered biogenerators for online continuous monitoring of neurochemicals in rat brain. Analyst 2013, 138, 179–185. [Google Scholar] [CrossRef] [PubMed]
- Zebda, A.; Cosnier, S.; Alcaraz, J.P.; Holzinger, M.; Le Goff, A.; Gondran, C.; Boucher, F.; Giroud, F.; Gorgy, K.; Lamraoui, H.; et al. Single glucose biofuel cells implanted in rats power electronic devices. Sci. Rep. 2013, 3, 1516. [Google Scholar] [CrossRef] [PubMed]
- Miyake, T.; Haneda, K.; Nagai, N.; Yatagawa, Y.; Onami, H.; Yoshino, S.; Abe, T.; Nishizawa, M. Enzymatic biofuel cells designed for direct power generation from biofluids in living organisms. Energy Environ. Sci. 2011, 4, 5008. [Google Scholar] [CrossRef]
- Shoji, K.; Akiyama, Y.; Suzuki, M.; Nakamura, N.; Ohno, H.; Morishima, K. Biofuel cell backpacked insect and its application to wireless sensing. Biosens. Bioelectron. 2016, 78, 390–395. [Google Scholar] [CrossRef]
- Szczupak, A.; Halámek, J.; Halámková, L.; Bocharova, V.; Alfonta, L.; Katz, E. Living battery – biofuel cells operating in vivo in clams. Energy Environ. Sci. 2012, 5, 8891. [Google Scholar] [CrossRef]
- Halamkova, L.; Halamek, J.; Bocharova, V.; Szczupak, A.; Alfonta, L.; Katz, E. Implanted biofuel cell operating in a living snail. J. Am. Chem. Soc. 2012, 134, 5040–5043. [Google Scholar] [CrossRef]
- MacVittie, K.; Halamek, J.; Halamkova, L.; Southcott, M.; Jemison, W.D.; Lobeld, R.; Katz, E. From “cyborg” lobsters to a pacemaker powered by implantable biofuel cells. Energy Environ. Sci. 2013, 6, 81–86. [Google Scholar] [CrossRef]
- Schwefel, J.; Ritzmann, R.E.; Lee, I.N.; Pollack, A.; Weeman, W.; Garverick, S.; Willis, M.; Rasmussen, M.; Scherson, D. Wireless communication by an autonomous self-powered cyborg insect. J. Electrochem. Soc. 2014, 161, H3113–H3116. [Google Scholar] [CrossRef]
- Murata, K.; Kajiya, K.; Nakamura, N.; Ohno, H. Direct electrochemistry of bilirubin oxidase on three-dimensional gold nanoparticle electrodes and its application in a biofuel cell. Energy Environ. Sci. 2009, 2, 1280. [Google Scholar] [CrossRef]
- Panase, P.; Saenphet, S.; Saenphet, K. Biochemical and physiological responses of nile tilapia oreochromis niloticus lin subjected to cold shock of water temperature. Aquacult. Rep. 2018, 11, 17–23. [Google Scholar] [CrossRef]
- Hanashi, T.; Yamazaki, T.; Tsugawa, W.; Ferri, S.; Nakayama, D.; Tomiyama, M.; Ikebukuro, K.; Sode, K. Biocapacitor—A novel category of biosensor. Biosens. Bioelectron. 2009, 24, 1837–1842. [Google Scholar] [CrossRef] [PubMed]
- Slaughter, G.; Kulkarni, T. A self-powered glucose biosensing system. Biosens. Bioelectron. 2016, 78, 45–50. [Google Scholar] [CrossRef] [PubMed]
- Kuo, C.M.; Hsieh, S.L. Comparisons of physiological and biochemical responses between milkfish (chanos chanos) and grass carp (ctenopharyngodon idella) to cold shock. Aquaculture 2006, 251, 525–536. [Google Scholar] [CrossRef]



© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, S.-H.; Chen, W.-H.; Lin, Y.-C. A Self-Powered Glucose Biosensor Operated Underwater to Monitor Physiological Status of Free-Swimming Fish. Energies 2019, 12, 1827. https://doi.org/10.3390/en12101827
Huang S-H, Chen W-H, Lin Y-C. A Self-Powered Glucose Biosensor Operated Underwater to Monitor Physiological Status of Free-Swimming Fish. Energies. 2019; 12(10):1827. https://doi.org/10.3390/en12101827
Chicago/Turabian StyleHuang, Shih-Hao, Wei-Hung Chen, and Yu-Chen Lin. 2019. "A Self-Powered Glucose Biosensor Operated Underwater to Monitor Physiological Status of Free-Swimming Fish" Energies 12, no. 10: 1827. https://doi.org/10.3390/en12101827
APA StyleHuang, S.-H., Chen, W.-H., & Lin, Y.-C. (2019). A Self-Powered Glucose Biosensor Operated Underwater to Monitor Physiological Status of Free-Swimming Fish. Energies, 12(10), 1827. https://doi.org/10.3390/en12101827




