Evaluation of Biochemical Methane Potential and Kinetics on the Anaerobic Digestion of Vegetable Crop Residues
Abstract
1. Introduction
2. Materials and Methods
2.1. Feedstock and Inoculum Used
2.2. Test Setup and Design
2.3. Biogas Measurement and Calculations
2.4. Analytical Methods
2.5. Kinetic Models
2.5.1. First-Order Kinetic Model
2.5.2. Chen and Hashimoto Model
2.5.3. Modified Gompertz Model
3. Results and Discussion
3.1. Compositional Characteristics of VCR
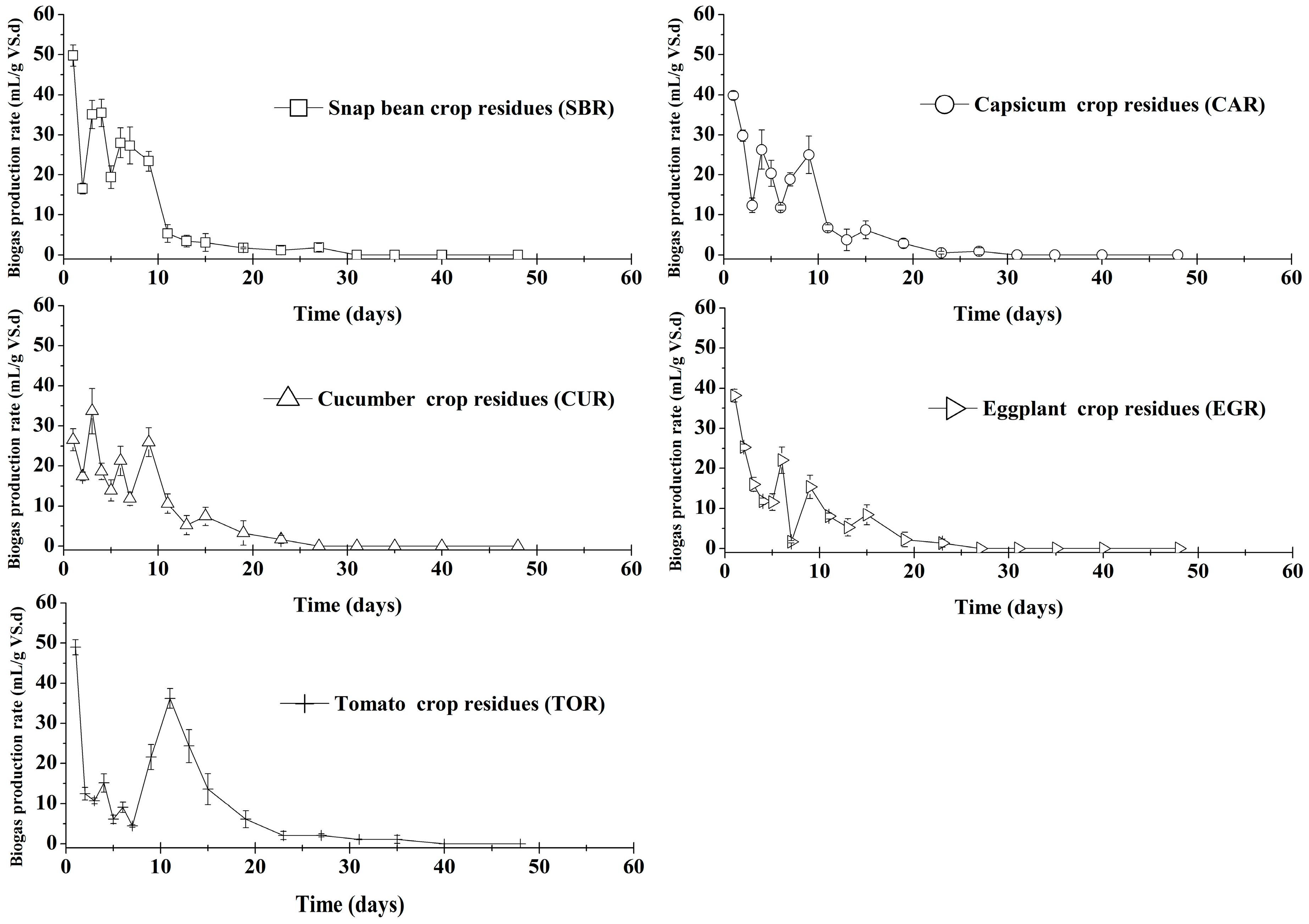
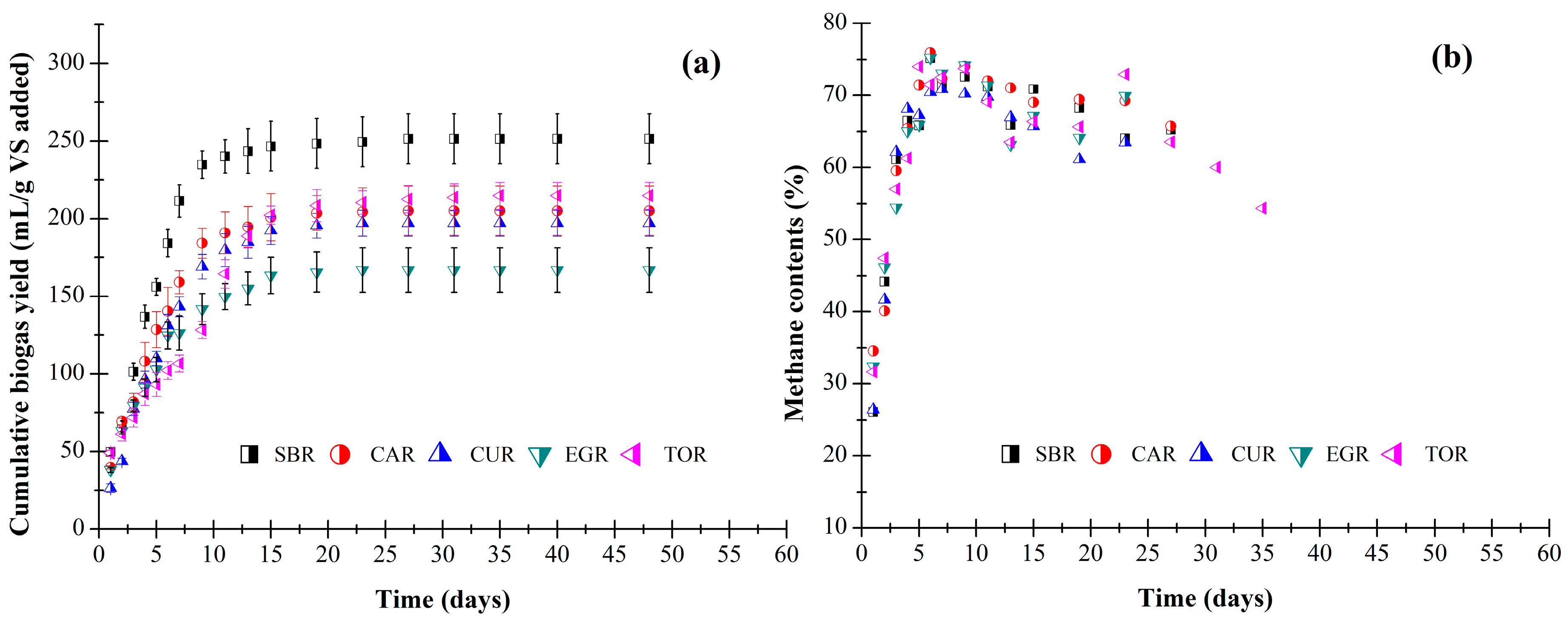
3.2. Batch Anaerobic Digestion Test Results
(R2 = 0.898, Adj. R2 = 0.864, p = 0.014)
3.3. Results of Linear Regression Analysis
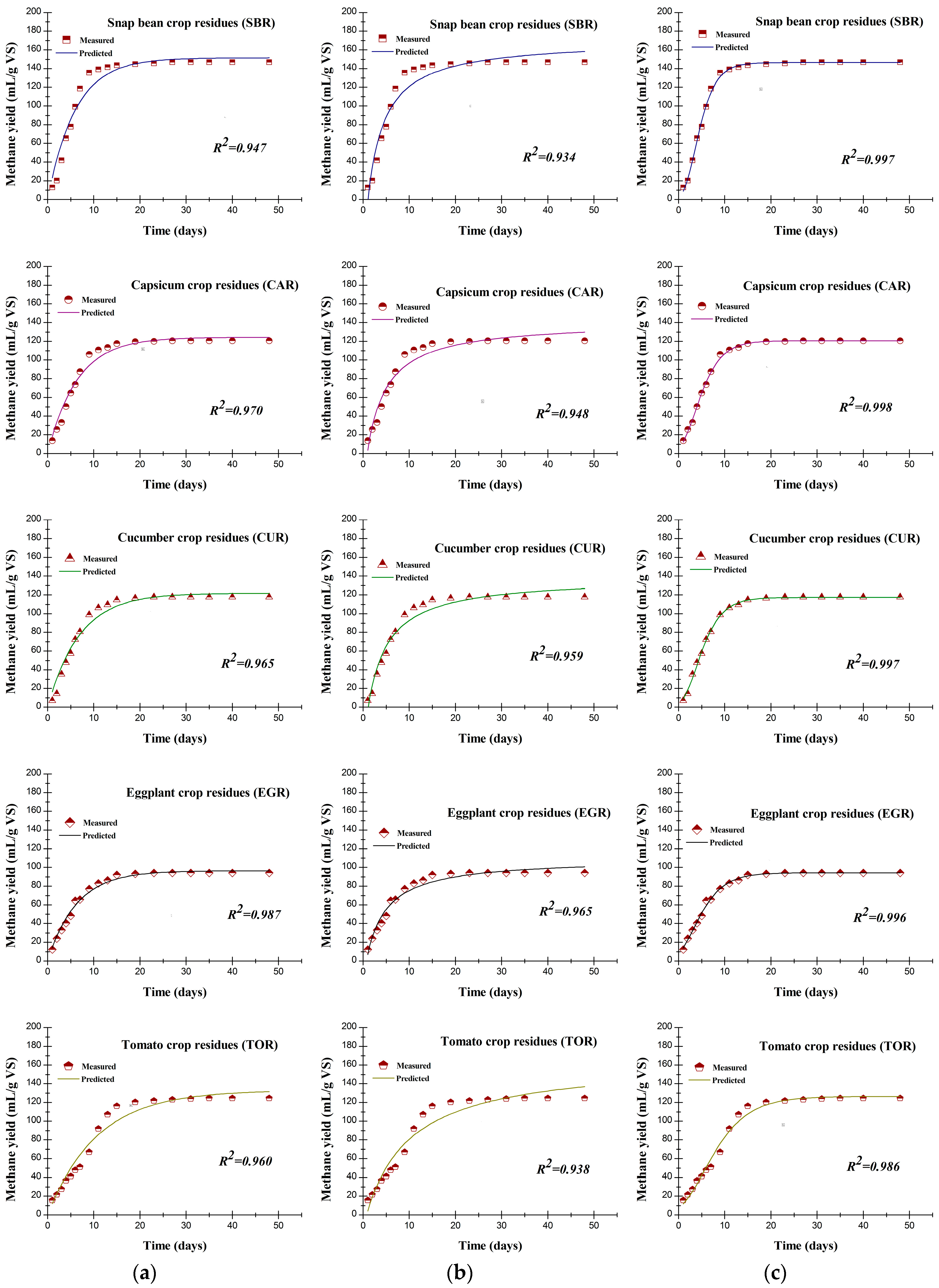
3.4. Results of Kinetic Analysis
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Lin, J.; Zuo, J.; Gan, L.; Li, P.; Liu, F.; Wang, K.; Chen, L.; Gan, H. Effects of mixture ratio on anaerobic co-digestion with fruit and vegetable waste and food waste of China. J. Environ. Sci. (China) 2011, 23, 1403–1408. [Google Scholar] [CrossRef]
- Majhi, B.K.; Jash, T. Two-phase anaerobic digestion of vegetable market waste fraction of municipal solid waste and development of improved technology for phase separation in two-phase reactor. Waste Manag. 2016, 58, 152–159. [Google Scholar] [CrossRef] [PubMed]
- Gunaseelan, V.N. Biochemical methane potential of fruits and vegetable solid waste feedstocks. Biomass Bioenergy 2004, 26, 389–399. [Google Scholar] [CrossRef]
- FAO. Agricultural and Food Organization of the United Nations. Available online: http://www.fao.org/faostat/en/#data/QC (accessed on 27 November 2018).
- Biswas, J.; Chowdhury, R.; Bhattacharya, P. Mathematical modeling for the prediction of biogas generation characteristics of an anaerobic digester based on food/vegetable residues. Biomass Bioenergy 2007, 31, 80–86. [Google Scholar] [CrossRef]
- Xue, H.; Chang, R.; Du, P.; Ji, L.; Li, Y. Straw Coefficient and Properties of Different Vegetable Wastes. J. Agric. Resour. Environ. 2015, 32, 377–382. [Google Scholar]
- Luo, J.; Tian, Y.; Chen, L.; Song, C.; Qi, Y.; Li, X.; Zhao, L. Biogas-producing characteristics of anaerobic digestion of protected horticulture residues. Trans. Chin. Soc. Agric. Eng. 2014, 30, 256–263. [Google Scholar]
- Sitorus, B.; Sukandar; Panjaitan, S.D. Biogas recovery from anaerobic digestion process of mixed fruit-vegetable wastes. Energy Procedia 2013, 32, 176–182. [Google Scholar] [CrossRef]
- Jiang, Y.; Heaven, S.; Banks, C.J. Strategies for stable anaerobic digestion of vegetable waste. Renew. Energy 2012, 44, 206–214. [Google Scholar] [CrossRef]
- Wei, L.; Wang, S.; Jin, Z.; Tong, X. Biochar influences the microbial community structure during tomato stalk composting with chicken manure. Bioresour. Technol. 2014, 154, 148–154. [Google Scholar] [CrossRef]
- Nabin, A.; Torben, K.; Fariza, A.; Pant, D.; Ottosen, L.D. An overview of microbial biogas enrichment. Bioresour. Technol. 2018, 264, 359–369. [Google Scholar]
- Milledge, J.; Nielsen, B.; Sadek, M.; Harvey, P. Effect of freshwater washing pretreatment on Sargassum muticum as a feedstock for biogas production. Energies 2018, 11, 1771. [Google Scholar] [CrossRef]
- Wang, Y.; Li, G.; Chi, M.; Sun, Y.; Zhang, J.; Jiang, S.; Cui, Z. Effects of co-digestion of cucumber residues to corn stover and pig manure ratio on methane production in solid state anaerobic digestion. Bioresour. Technol. 2018, 250, 328–336. [Google Scholar] [CrossRef] [PubMed]
- Bi, J.H.; Cheng, G.Y.; Chen, L.; Li, Y.; Hei, K.; Zhang, Y.; Huo, L.; Chang, Z. Chemical composition of different parts of chili stalks and their biogas production potentials during anaerobic fermentation. China Environ. Sci. 2016, 36, 2073–2078. [Google Scholar]
- Rincón, B.; Banks, C.J.; Heaven, S. Biochemical methane potential of winter wheat (Triticum aestivum L.): Influence of growth stage and storage practice. Bioresour. Technol. 2010, 101, 8179–8184. [Google Scholar] [CrossRef] [PubMed]
- Monlau, F.; Sambusiti, C.; Barakat, A.; Guo, X.M.; Latrille, E.; Trably, E.; Steyer, J.-P.; Carrere, H. Predictive models of biohydrogen and biomethane production based on the compositional and structural features of lignocellulosic materials. Environ. Sci. Technol. 2012, 46, 12217–12225. [Google Scholar] [CrossRef]
- Triolo, J.M.; Sommer, S.G.; Møller, H.B.; Weisbjerg, M.R.; Jiang, X.Y. A new algorithm to characterize biodegradability of biomass during anaerobic digestion: Influence of lignin concentration on methane production potential. Bioresour. Technol. 2011, 102, 9395–9402. [Google Scholar] [CrossRef] [PubMed]
- Kafle, G.K.; Chen, L. Comparison on batch anaerobic digestion of five different livestock manures and prediction of biochemical methane potential (bmp) using different statistical models. Waste Manag. 2016, 48, 492–502. [Google Scholar] [CrossRef]
- Gunaseelan, V.N. Regression models of ultimate methane yields of fruits and vegetable solid wastes, sorghum and napiergrass on chemical composition. Bioresour. Technol. 2007, 98, 1270–1277. [Google Scholar] [CrossRef]
- Wang, M.; Li, W.; Liu, S.; Liu, D.; Yin, L.; Yuan, H. Biogas production from chinese herb-extraction residues: Influence of biomass composition on methane yield. BioResources 2013, 8, 3732–3740. [Google Scholar] [CrossRef]
- Li, P.; Li, W.; Yang, F.; Yin, L. Predictive Model of Methane Production Based on the Compositional Features of Spent Edible Mushroom Substrate. J. Biobased Mater. Bioenergy 2017, 11, 291–297. [Google Scholar] [CrossRef]
- Kafle, G.K.; Kim, S.-H. Kinetic study of the anaerobic digestion of swine manure at mesophilic temperature: A lab scale batch operation. J. Biosyst. Eng. 2012, 37, 233–244. [Google Scholar] [CrossRef]
- Gioannis, G.D.; Muntoni, A.; Cappai, G.; Milia, S. Landfill gas generation after mechanical biological treatment of municipal solid waste. Estimation of gas generation rate constants. Waste Manag. 2009, 29, 1026–1034. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.W.; Viraraghavan, T. Analysis of the performance of an anaerobic digestion system at the Regina wastewater treatment plant. Bioresour. Technol. 2004, 95, 301–307. [Google Scholar] [CrossRef] [PubMed]
- Kafle, G.K.; Kim, S.H. Anaerobic treatment of apple waste with swine manure for biogas production: Batch and continuous operation. Appl. Energy 2013, 103, 61–72. [Google Scholar] [CrossRef]
- Gilcreas, F.W. Standard methods for the examination of water and waste water. Am. J. Public Health Nat. Health 1966, 56, 387–388. [Google Scholar] [CrossRef]
- Lane, J.H.; Eynon, L. Estimation of sugar in urine by means of Fehling’s solution with methylene blue as internal indicator. Analyst 1924, 49, 366–371. [Google Scholar] [CrossRef]
- Luque-García, J.L.; Castro, L.D. Ultrasound-assisted Soxhlet extraction: An expeditive approach for solid sample treatment. Application to the extraction of total fat from oleaginous seeds. J. Chromatogr. 2004, 1034, 237–242. [Google Scholar] [CrossRef]
- Van Soest, P.J.; Robertson, J.B.; Lewis, B.A. Methods for dietary fiber, neutral detergent fiber, and nonstarch polysaccharides in relation to animal nutrition. J. Dairy Sci. 1991, 74, 3583–3597. [Google Scholar] [CrossRef]
- Parameswaran, P.; Rittmann, B.E. Feasibility of anaerobic co-digestion of pig waste and paper sludge. Bioresour. Technol. 2012, 124, 163–168. [Google Scholar] [CrossRef]
- Wang, M.; Li, W.; Li, P.; Yan, S.; Zhang, Y. An alternative parameter to characterize biogas materials: Available carbon-nitrogen ratio. Waste Manag. 2017, 62, 76–83. [Google Scholar] [CrossRef]
- Romano, R.T.; Zhang, R. Co-digestion of onion juice and wastewater sludge using an anaerobic mixed biofilm reactor. Bioresour. Technol. 2008, 99, 631–637. [Google Scholar] [CrossRef] [PubMed]
- Mussatto, S.I.; Fernandes, M.; Milagres, A.M.F. Effect of hemicellulose and lignin on enzymatic hydrolysis of cellulose from brewer’s spent grain. Enzym. Microb. Technol. 2008, 43, 124–129. [Google Scholar] [CrossRef]
- Gunaseelan, V.N. Predicting ultimate methane yields of Jatropha curcus and Morus indica from their chemical composition. Bioresour. Technol. 2009, 100, 3426–3429. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Li, W.; Sun, M.; Li, Q.; Wang, M.; Sun, Y. Improved Buffering Capacity and Methane Production by Anaerobic Co-Digestion of Corn Stalk and Straw Depolymerization Wastewater. Energies 2018, 11, 1751. [Google Scholar] [CrossRef]
- İsmailTosun, M.; TalhaGönüllü, A. Anaerobic Digestion and Methane Generation Potential of Rose Residue in Batch Reactors. Environ. Lett. 2004, 39, 915–925. [Google Scholar]
| Feed | Units | Types of VCR | |||||
|---|---|---|---|---|---|---|---|
| SBR | CAR | CUR | EGR | TOR | Inoculum | ||
| Total solids (TS) | % | 21.08(0.08) | 20.35(<0.01) | 21.87(0.13) | 19.40(0.23) | 17.52(0.11) | 1.02(0.07) |
| Volatile soilds (VS) | % | 18.30(0.19) | 16.41(0.24) | 16.56(0.57) | 16.66(0.06) | 14.53(0.25) | 0.58(0.04) |
| pH | -- | 5.45(0.01) | 4.96(0.03) | 7.91(0.01) | 6.04(0.02) | 5.35(0.01) | 7.61(0.01) |
| Crude protein (CP) | %TS | 21.24(1.24) | 15.13(0.88) | 8.84(0.25) | 14.93(0.06) | 18.56(0.61) | - |
| Total fat (TF) | %TS | 3.40(0.13) | 1.67(0.10) | 2.02(0.12) | 3.54(0.24) | 2.44(0.05) | - |
| Total sugar (TSug) | %TS | 26.88(0.21) | 24.81(1.22) | 20.52(2.01) | 21.14(0.25) | 19.33(0.96) | - |
| Hemicellulose (Hem) | %TS | 10.27(0.91) | 18.63(1.22) | 11.60(0.59) | 16.25(1.01) | 16.91(0.15) | - |
| Cellulose (Cel) | %TS | 31.01(1.63) | 23.39(0.25) | 31.26(0.48) | 30.10(1.66) | 28.41(0.50) | - |
| Lignin (Lig) | %TS | 5.10(0.05) | 8.31(0.31) | 11.25(0.15) | 10.52(0.02) | 7.58(0.38) | - |
| C/N | -- | 11.65(1.01) | 15.45 (1.46) | 22.76(0.53) | 16.10 (1.27) | 11.99 (0.95) | - |
| Parameters | Units | VCR | ||||
|---|---|---|---|---|---|---|
| SBR | CAR | CUR | EGR | TOR | ||
| Biogas potential | mL g−1 VS | 251.3 (16.1) | 205.2 (15.8) | 197.2 (8.5) | 166.9 (14.3) | 214.8 (8.7) |
| Methane potential | mL g−1 VS | 146.8 (10.1) | 120.6 (9.7) | 117.5 (5.1) | 94.2 (8.7) | 124.4 (5.6) |
| Methane content | % | 58.4 (1.1) | 58.8 (0.8) | 59.6 (1.2) | 56.4 (0.4) | 57.9 (0.9) |
| TS degradation degree | % | 40.0 (0.7) | 37.2 (1.2) | 33.7 (0.6) | 31.7 (0.3) | 34.6 (0.4) |
| VS degradation degree | % | 49.9 (1.1) | 46.9 (0.8) | 47.2 (0.7) | 40.4 (0.5) | 47.1 (0.3) |
| ADF degradation degree | % | 42.8 (3.7) | 43.4 (1.8) | 31.2 (1.1) | 27.1 (2.2) | 25.9 (1.0) |
| Effluence pH | - | 7.68 (0.02) | 7.66 (0.01) | 7.56 (0.01) | 7.61 (0.00) | 7.48 (0.01) |
| T80 | days | 7 (1) | 7 (1) | 9 (2) | 9 (1) | 11 (1) |
| T90 | days | 9 (1) | 11 (1) | 11 (2) | 13 (2) | 15 (2) |
| Explanatory Variables | R2 | Adj. R2 | SE | p-Value | Equation |
|---|---|---|---|---|---|
| Simple Linear Regression | |||||
| CP | 0.319 | 0.093 | 17.9 | 0.321 | G0 = 84.92 + 2.275CP |
| TSug | 0.369 | 0.159 | 17.2 | 0.277 | G0 = 39.83 + 3.588TSug |
| Lig | 0.704 | 0.606 | 11.7 | 0.076 | G0 = 175.57 − 6.416Lig |
| Multiple Linear Regression | |||||
| CP, Lig | 0.976 | 0.903 | 5.8 | 0.048 | G0 = 326.56 − 4.936CP − 14.986Lig |
| TF, Lig | 0.776 | 0.552 | 12.6 | 0.224 | G0 = 196.666 − 6.28TF − 6.963Lig |
| TSug, Lig | 0.709 | 0.418 | 14.3 | 0.291 | G0 = 159.18 + 0.549TSug−5.946Lig |
| Hem, Lig | 0.832 | 0.664 | 10.9 | 0.168 | G0 = 198.886 − 5.874Hem − 1.897Lig |
| CP, TF, Hem | 0.997 | 0.990 | 1.9 | 0.065 | G0 = 169.173 + 3.91CP − 18.55TF − 4.18Hem |
| CP, TF, Lig | 0.980 | 0.843 | 7.4 | 0.251 | G0 = 343.18 − 5.81CP + 3.05TF − 16.25Lig |
| CP, TSug, Lig | 0.972 | 0.889 | 6.2 | 0.211 | G0 = 382.9 − 5.57CP − 1.24TSug − 17.15Lig |
| CP, Hem, Lig | 0.977 | 0.907 | 5.7 | 0.194 | G0 = 314.99 − 4.18CP − 0.93Hem − 13.41Lig |
| CP, Cel, Lig | 0.987 | 0.946 | 4.3 | 0.147 | G0 = 300.97 − 5.09CP − 15.4Lig + 1.09Cel |
| TF, Hem, Lig | 0.999 | 0.998 | 1.7 | 0.027 | G0 = 241.96 − 10.01TF − 2.64Hem − 6.54Lig |
| TF, Cel, Lig | 0.970 | 0.879 | 6.5 | 0.220 | G0 = 132.43 − 15.62TF + 3.45Cel − 8.24Lig |
| Hem, Cel, Lig | 0.916 | 0.663 | 10.9 | 0.364 | G0 = 315.18 − 4.76Lig − 3.13Cel − 4.31Hem |
| CP, TF, TSug, Hem | 0.999 | 0.999 | 0.5 | 0.000 | G0 = 160.15 − 4.05Hem + 0.38TSug − 18.13TF + 3.75CP |
| Equations and Parameters | Units | VCR | ||||
|---|---|---|---|---|---|---|
| SBR | CAR | CUR | EGR | TOR | ||
| First-order kinetic model | ||||||
| K | Day−1 | 0.167 | 0.157 | 0.146 | 0.161 | 0.094 |
| G0 | mL CH4 g−1 VS | 151.5 | 124.3 | 121.7 | 96.3 | 132.8 |
| Difference * | % | 3.2 | 3.0 | 3.5 | 2.3 | 5.1 |
| Chen and Hashimoto model | ||||||
| KCH | − | 3.5 | 4.7 | 3.9 | 5.8 | 12.8 |
| μm | Day−1 | 0.947 | 1.121 | 0.902 | 1.388 | 1.329 |
| HRTcritical | day | 1.056 | 0.892 | 1.108 | 0.721 | 0.752 |
| G0 | mL CH4 g−1 VS | 170.6 | 141.4 | 138.4 | 109.5 | 144.5 |
| Difference * | % | 16.2 | 17.2 | 17.8 | 16.4 | 12.1 |
| Modified Gompertz model | ||||||
| Rmax | mL CH4 g−1 VS day | 21.9 | 14.4 | 14.3 | 9.8 | 9.0 |
| λ | day | 1.109 | 0.512 | 0.845 | 0.000 | 0.330 |
| G0 | mL CH4 g−1 VS | 146.5 | 120.7 | 117.2 | 94.1 | 126.4 |
| Difference * | % | 0.2 | 0.0 | 0.3 | 0.0 | 0.0 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, P.; Li, W.; Sun, M.; Xu, X.; Zhang, B.; Sun, Y. Evaluation of Biochemical Methane Potential and Kinetics on the Anaerobic Digestion of Vegetable Crop Residues. Energies 2019, 12, 26. https://doi.org/10.3390/en12010026
Li P, Li W, Sun M, Xu X, Zhang B, Sun Y. Evaluation of Biochemical Methane Potential and Kinetics on the Anaerobic Digestion of Vegetable Crop Residues. Energies. 2019; 12(1):26. https://doi.org/10.3390/en12010026
Chicago/Turabian StyleLi, Pengfei, Wenzhe Li, Mingchao Sun, Xiang Xu, Bo Zhang, and Yong Sun. 2019. "Evaluation of Biochemical Methane Potential and Kinetics on the Anaerobic Digestion of Vegetable Crop Residues" Energies 12, no. 1: 26. https://doi.org/10.3390/en12010026
APA StyleLi, P., Li, W., Sun, M., Xu, X., Zhang, B., & Sun, Y. (2019). Evaluation of Biochemical Methane Potential and Kinetics on the Anaerobic Digestion of Vegetable Crop Residues. Energies, 12(1), 26. https://doi.org/10.3390/en12010026





