Combustion of Miscanthus: Composition of the Ash by Particle Size
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Particle Size of the Ash and the Ash Fractions Produced
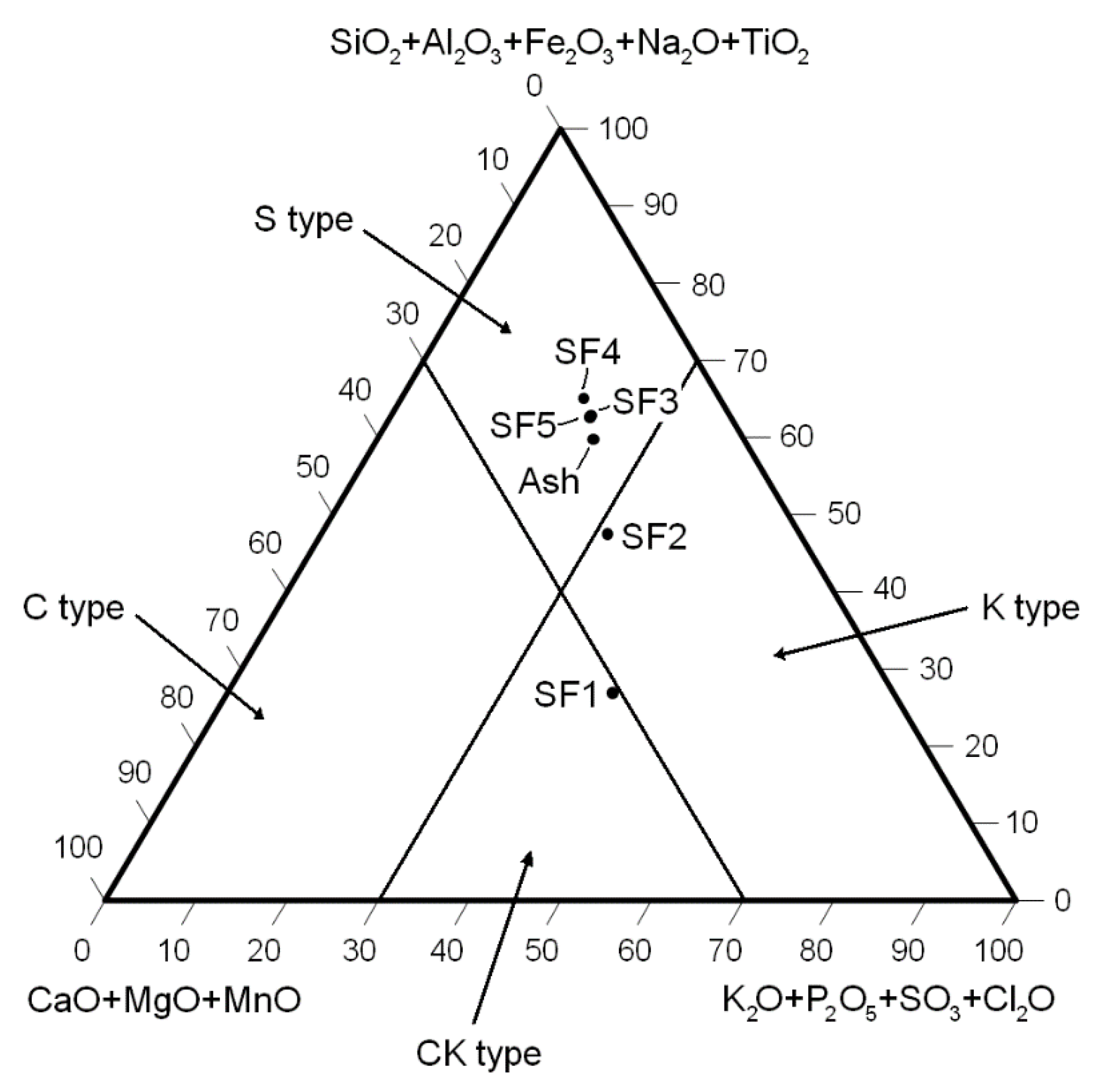
3.2. Chemical Analysis of the Ash Sample and the Size Fractions Produced
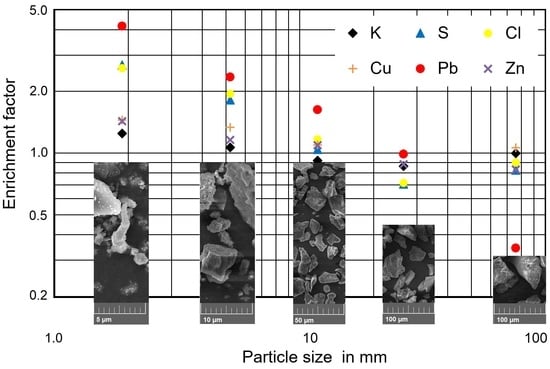
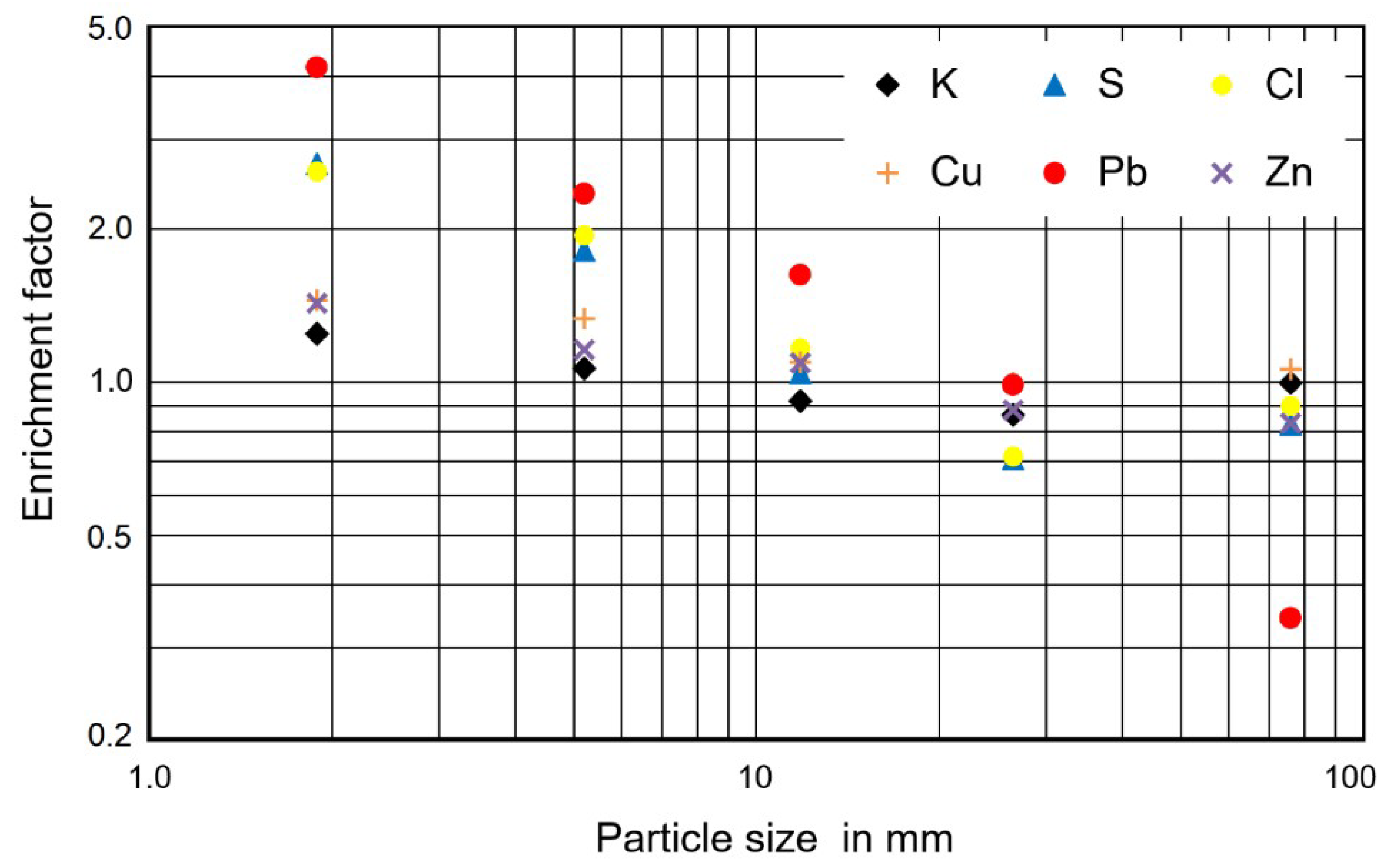
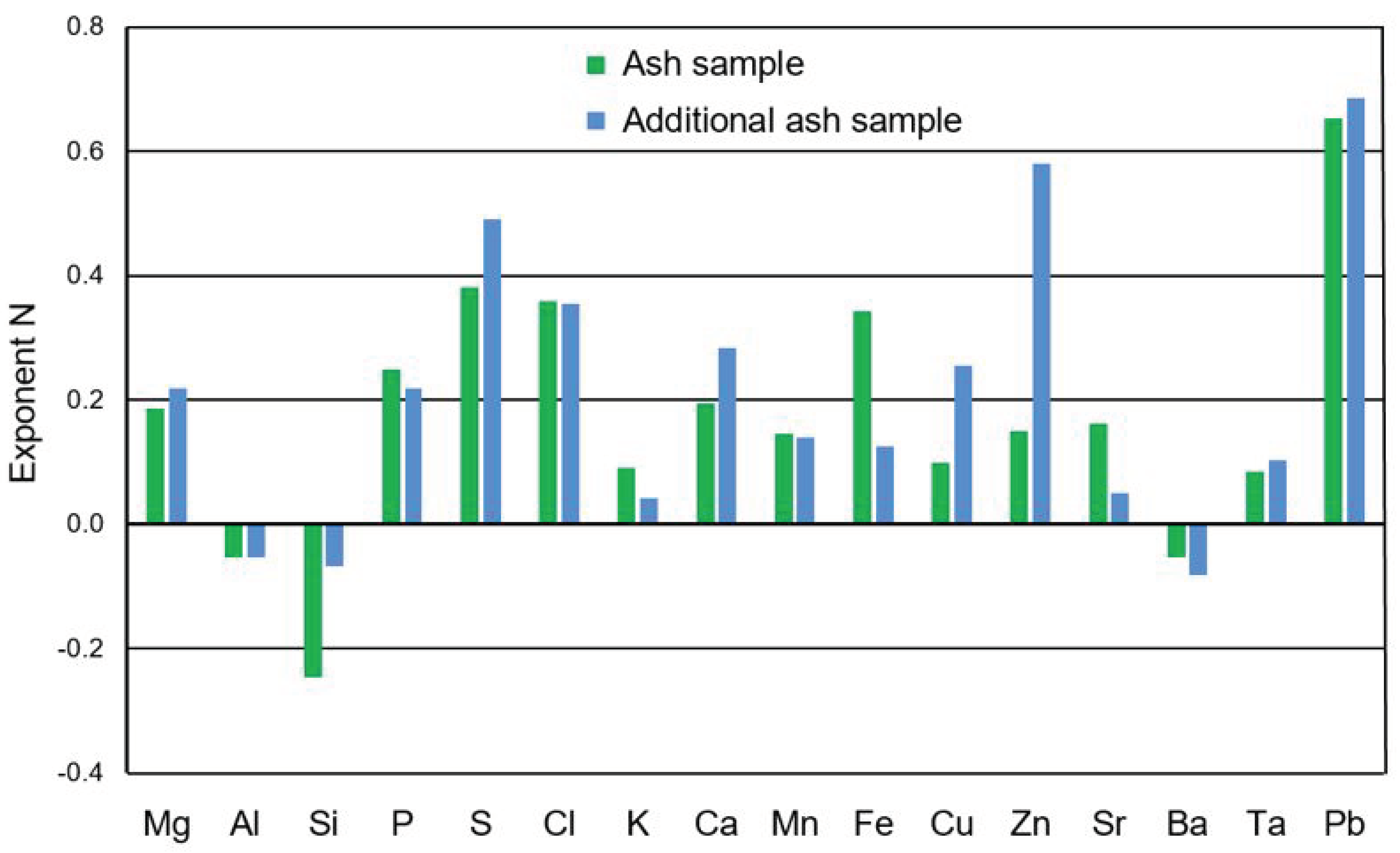
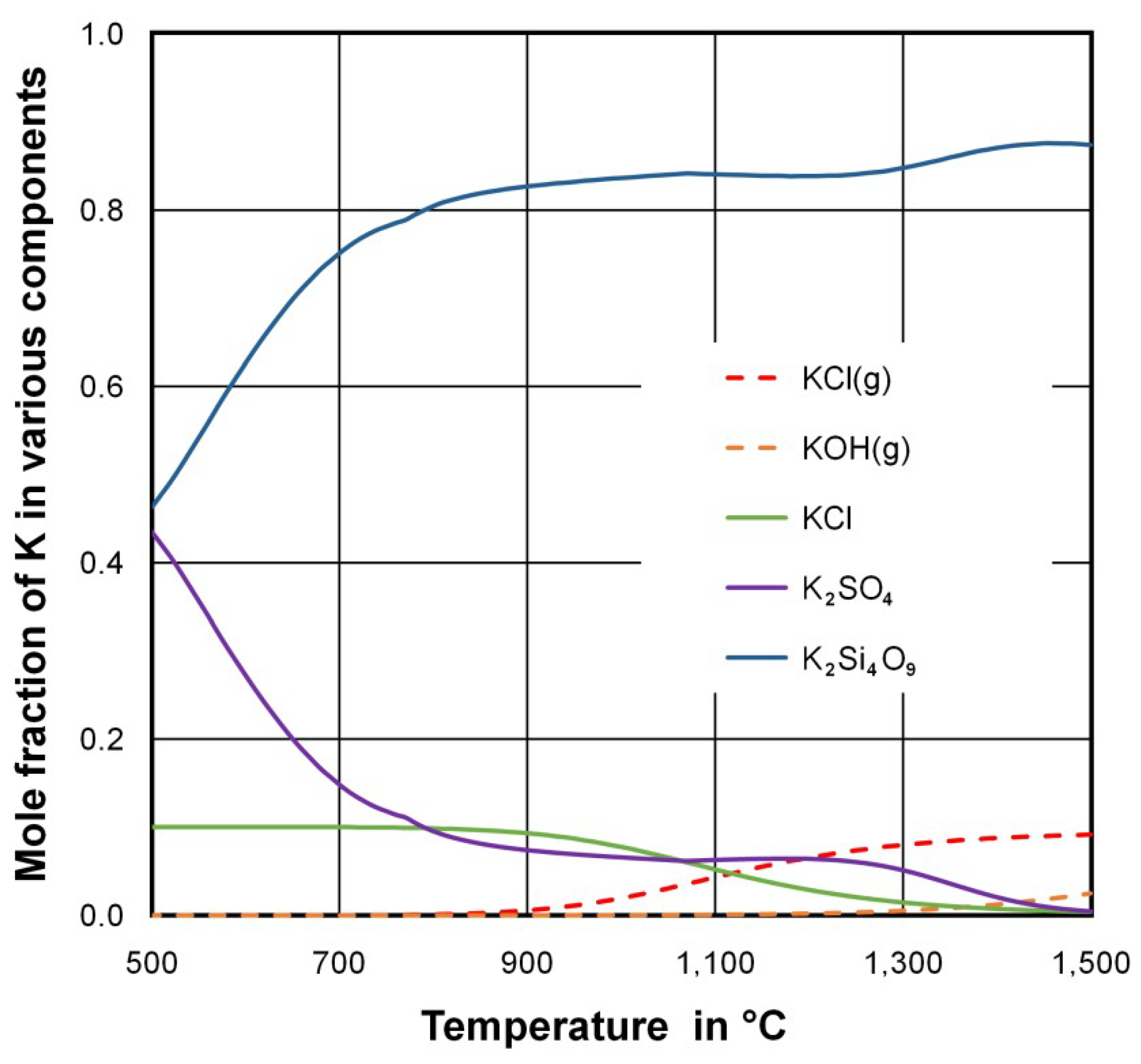
3.3. Reasons for the Low Enrichment Potassium in the Fine Size Fractions of the Ash
3.4. Consequences for Ash Utilization
4. Discussion
5. Conclusions
- (a)
- Ash from the combustion of miscanthus contains plant nutrients (K, P, Ca) and is relatively low contaminated with heavy metals. The use of the ash as soil conditioner on forest and agricultural land is feasible, especially on the land where the miscanthus was grown.
- (b)
- The production of a K-rich fraction out of the ash by classification as a source of K for fertilizer production is not applicable because of the low size dependence of the K concentration.
- (c)
- The reasons for the limited enrichment of K in the fine size fractions can be found in the ash chemistry, especially the low Cl content combined with the high Si content and in the low combustion temperature.
Funding
Acknowledgments
Conflicts of Interest
References
- Lewandowski, I.; Kicherer, A.; Vonier, P. CO2-Balance for the cultivation and combustion of Miscanthus. Biomass Bioenergy 1995, 8, 81–90. [Google Scholar] [CrossRef]
- Lewandowski, I.; Scurlock, J.M.O.; Lindvall, E.; Christou, M. The development and current status of perennial rhizomatous grasses as energy crops in the US and Europe. Biomass Bioenergy 2003, 25, 335–361. [Google Scholar] [CrossRef]
- Baxter, X.C.; Darvell, L.I.; Jones, J.M.; Barraclough, T.; Yates, N.E.; Shield, I. Miscanthus combustion properties and variations with Miscanthus agronomy. Fuel 2014, 117, 851–869. [Google Scholar] [CrossRef]
- Chou, C.-H. Miscanthus plants used as an alternative biofuel material: The basic studies on ecology and molecular evolution. Renew. Energy 2009, 34, 1908–1912. [Google Scholar] [CrossRef]
- Lewandowski, I.; Clifton-Brown, J.; Trindade, L.M.; van der Linden, G.C.; Schwarz, K.-U.; Müller-Sämann, K.; Anisimov, A.; Chen, C.-L.; Dolstra, O.; Donnison, I.S.; et al. Progress on Optimizing Miscanthus Biomass Production for the European Bioeconomy: Results of the EU FP7 Project OPTIMISC. Front. Plant Sci. 2016, 7, 1620. [Google Scholar] [CrossRef] [PubMed]
- Statistik Austria. 2016. Available online: http://www.statistik.at/web_de/statistiken/index.html (accessed on 8 August 2017).
- Frühwirth, P.; Liebhard, P. Miscanthus sinensis ‘Giganteus’. Produktion, Inhaltsstoffe und Verwertung; Landwirtschaftskammer Österreich, Bundes-LFI: Wien, Austria, 2006; pp. 7–47. [Google Scholar]
- Baxter, X.C.; Darvell, L.I.; Jones, J.M.; Barraclough, T.; Yates, N.E.; Shield, I. Study of Miscanthus x giganteus ash composition—Variation with agronomy and assessment method. Fuel 2012, 95, 50–62. [Google Scholar] [CrossRef]
- Michel, R.; Kaknics, J.; Bouchetou, M.L.; Gratuze, B.; Balland, M.; Hubert, J.; Poirier, J. Physicochemical changes in Miscanthus ash on agglomeration with fluidized bed material. Chem. Eng. J. 2012, 207–208, 497–503. [Google Scholar] [CrossRef]
- European Biomass Association. European Bioenergy Outlook 2013; Statistical Report; AEBIOM: Brussels, Belgium, 2013; pp. 108–109. [Google Scholar]
- Van der Weijde, T.; Kiesel, A.; Iqbal, Y.; Muylle, H.; Dolstra, O.; Visser, R.G.F.; Lewandowski, I.; Trindade, L.M. Evaluation of Miscanthus sinensis biomass quality as feedstock for conversion into different bioenergy products. GCB Bioenergy 2017, 9, 176–190. [Google Scholar] [CrossRef]
- Emilsson, S. International Handbook—From Extraction of Forest Fuels to Ash Recycling; Swedish Forest Agency: Jönköping, Sweden, 2006. [Google Scholar]
- Bundesministerium für Land- und Forstwirtschaft, Umwelt und Wasserwirtschaft Richtlinien für den sachgerechten Einsatz von Pflanzenaschen zur Verwertung auf land- und forstwirtschaftlich genutzten Flächen; Bundesministerium für Land- und Forstwirtschaft, Umwelt und Wasserwirtschaft: Vienna, Austria, 2011.
- Nurmesniemi, H.; Mäkelä, M.; Pöykiö, R.; Manskinen, K.; Dahl, O. Comparison of the forest fertilizer properties of ash fractions from two power plants of pulp and paper mills incinerating biomass-based fuels. Fuel Process. Technol. 2012, 104, 1–6. [Google Scholar] [CrossRef]
- Von Wilpert, K.; Bösch, B.; Puhlmann, H.; Zirlewagen, D. Wood ash recycling–an appropriate measure to close nutrient cycles in forests. In Proceedings of the 4th Central European Biomass Conference, Graz, Austria, 15–18 January 2014; Österreichischer Biomasse Verband: Vienna, Austria, 2014; p. 61. [Google Scholar]
- Saletnik, B.; Zagula, G.; Bajcar, M.; Czernicka, M.; Puchalski, C. Biochar and Biomass Ash as a Soil Ameliorant: The Effect on Selected Soil Properties and Yield of Giant Miscanthus (Miscanthus x giganteus). Energies 2018, 11, 2535. [Google Scholar] [CrossRef]
- Maeda, N.; Katakura, T.; Fukasawa, T.; Huang, A.-N.; Kawano, T.; Fukui, K. Morphology of woody biomass combustion ash and enrichment of potassium components by particle size classification. Fuel Process. Technol. 2017, 156, 1–8. [Google Scholar] [CrossRef]
- Obernberger, I.; Brunner, T.; Bärnthaler, G. Chemical properties of solid biofuels—Significance and impact. Biomass Bioenergy 2006, 30, 973–982. [Google Scholar] [CrossRef]
- Lanzerstorfer, C. Chemical composition and properties of ashes from combustion plants using Miscanthus as fuel. J. Environ. Sci. 2017, 54, 178–183. [Google Scholar] [CrossRef] [PubMed]
- Lanzerstorfer, C. Model based prediction of required cut size diameter for fractionation of fly ash from a grate-fired wood chips incineration plant. Fuel Process. Technol. 2011, 92, 1095–1100. [Google Scholar] [CrossRef]
- Lanzerstorfer, C. Cyclone fly ash from a grate-fired biomass combustion plant: Dependence of the concentration of various components on the particle size. Fuel Process. Technol. 2015, 131, 382–388. [Google Scholar] [CrossRef]
- Lanzerstorfer, C.; Kröppl, M. Air classification of blast furnace dust collected in a fabric filter for recycling to the sinter process. Resour. Conserv. Recycl. 2014, 86, 132–137. [Google Scholar] [CrossRef]
- Müller, G. Index of geo-accumulation in sediments of the Rhine River. Geochem. J. 1969, 2, 108–118. [Google Scholar]
- Taylor, S.R.; Mclennan, S.M. The geochemical evolution of the continental crust. Rev. Geophys. 1995, 33, 241–265. [Google Scholar] [CrossRef]
- Linak, W.P.; Wendt, J.O.L. Toxic metal emissions from incineration: Mechanisms and control. Prog. Energy Combust Sci. 1993, 119, 145–185. [Google Scholar] [CrossRef]
- Van Loo, S.; Koppejan, J. The Handbook of Biomass Combustion and Co-Firing; Earthscan: London, UK, 2008; pp. 350–353. ISBN 1-84407-249-5. [Google Scholar]
- Lanzerstorfer, C. Grate-fired biomass combustion plants using forest residues as fuel: Enrichment factors for components in the fly ash. Waste Biomass Valoriz. 2017, 8, 235–240. [Google Scholar] [CrossRef]
- Zevenhoven-Onderwater, M.; Backman, R.; Skrifvars, B.-J.; Hupa, M. The ash chemistry in fluidized bed gasification of biomass fuels. Part I: Predicting the chemistry of melting ashes and ash-bed material interaction. Fuel 2001, 80, 1489–1502. [Google Scholar] [CrossRef]
- Lanzerstorfer, C. Investigation of the contamination of a fly ash sample during sample preparation by classification. Int. J. Environ. Sci. Technol. 2015, 12, 1437–1442. [Google Scholar] [CrossRef]
- Vassilev, S.V.; Vassileva, C.G.; Baxter, D. Trace element concentrations and associations in some biomass ashes. Fuel 2014, 129, 292–313. [Google Scholar] [CrossRef]
- Niu, Y.; Tan, H.; Hui, S. Ash-related issues during biomass combustion: Alkali-induced slagging, silicate melt-induced slagging (ash fusion) agglomeration, corrosion, ash utilization, and related countermeasures. Prog. Energ. Combust. 2016, 52, 1–62. [Google Scholar] [CrossRef]
- Kaknics, J.; Michel, R.; Poirier, J. Miscanthus ash transformation and interaction with bed materials at high temperature. Fuel Process. Technol. 2016, 141, 178–184. [Google Scholar] [CrossRef]
- Steenari, B.-M.; Schelander, S.; Lindqvist, O. Chemical and leaching characteristics of ash from combustion of coal, peat and wood in a 12 MW CFB—A comparative study. Fuel 1999, 78, 249–258. [Google Scholar] [CrossRef]
- Knudsen, J.N.; Jensen, P.A.; Dam-Johansen, K. Transformation and release tot he gas phase of Cl, K and S during combustion of annual biomass. Energy Fuels 2004, 18, 1385–1399. [Google Scholar] [CrossRef]
- Steenari, B.-M.; Lindqvist, O. Stabilisation of biofuel ashes for recycling to forest soil. Biomass Bioenergy 1997, 13, 39–50. [Google Scholar] [CrossRef]
- Wei, X.; Schnell, U.; Hein, K.R.G. Behaviour of gaseous chlorine and alkali metals during biomass thermal utilization. Fuel 2005, 84, 841–848. [Google Scholar] [CrossRef]
- Budhathoki, R.; Väisänen, A. Particle size based recovery of phosphorus from combined peat and wood fly ash for forest fertilization. Fuel Process. Technol. 2016, 146, 85–89. [Google Scholar] [CrossRef]
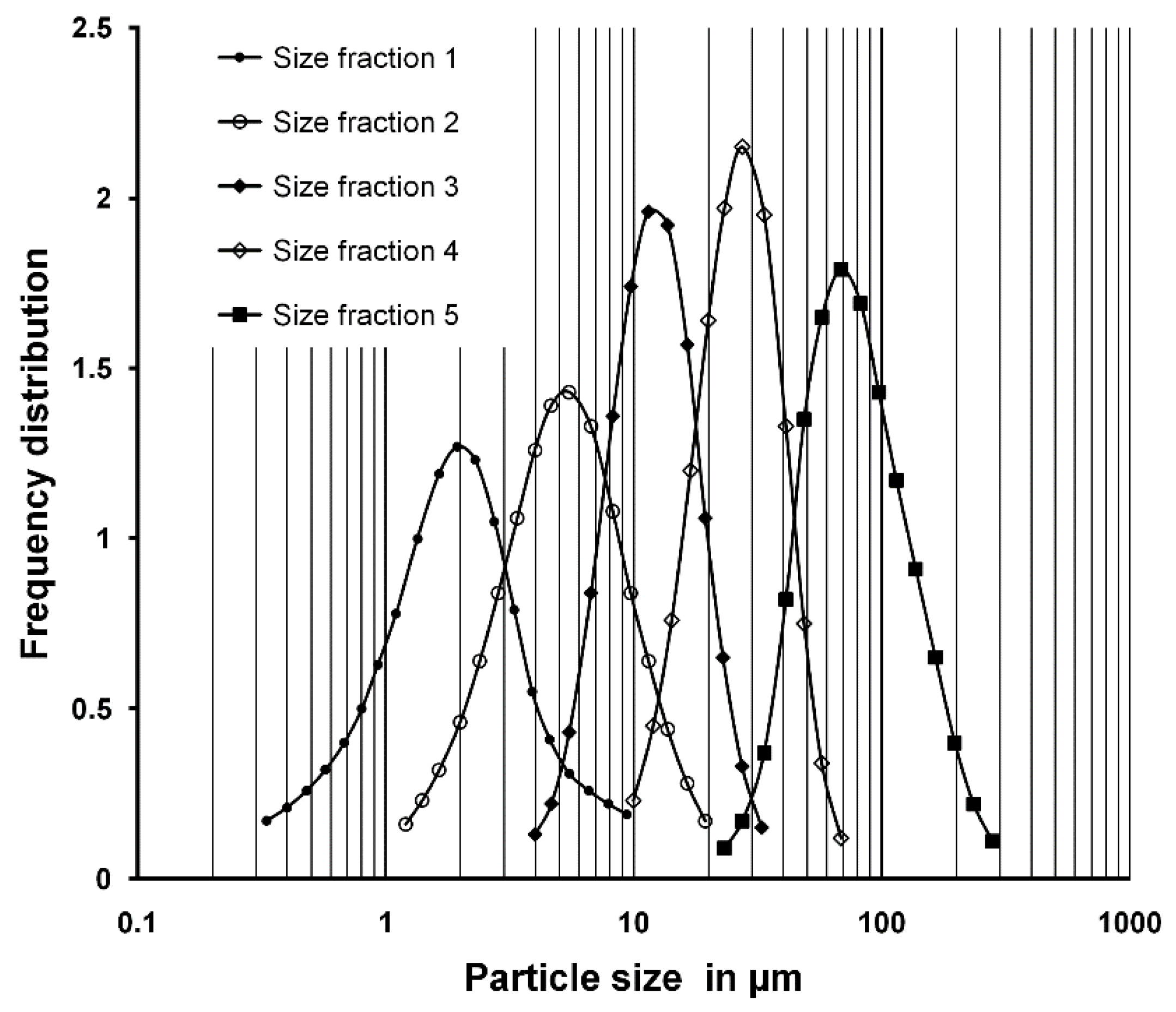
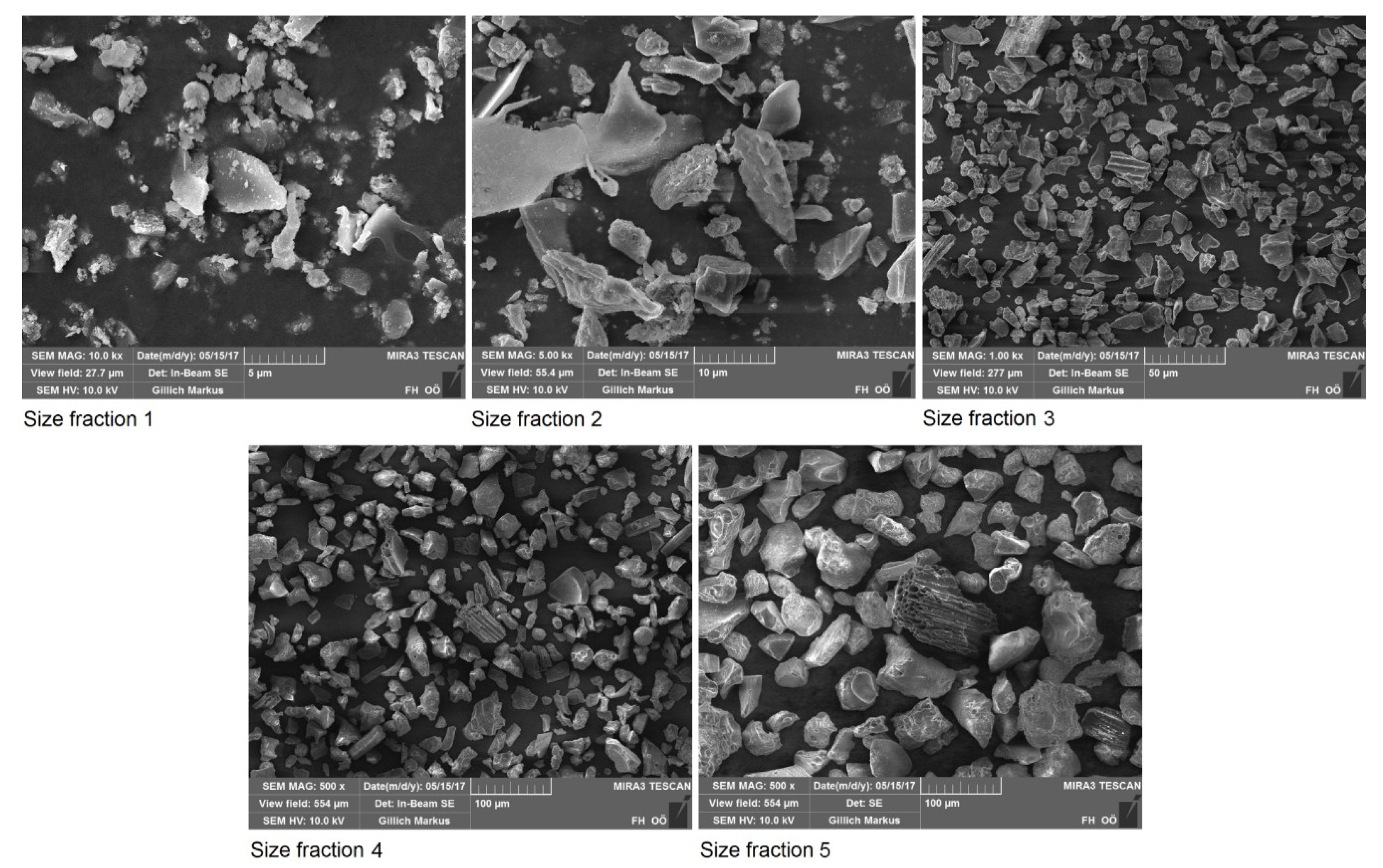
| Size Fraction | Mass Fraction in % | Mass Median Diameter d50 in µm | TC Content in % |
|---|---|---|---|
| SF 1 | 6.5 | 1.9 | 7.4 |
| SF 2 | 7.5 | 5.2 | 6.0 |
| SF 3 | 10.7 | 12 | 3.5 |
| SF 4 | 25.6 | 27 | 1.9 |
| SF 5 | 49.7 | 76 | 1.0 |
| Component | Ash | SF 1 | SF 2 | SF 3 | SF 4 | SF 5 | RR a | Nb | R2 c |
|---|---|---|---|---|---|---|---|---|---|
| Al2O3 | 0.90 | 0.77 | 1.02 | 0.99 | 0.99 | 0.99 | 1.08 | −0.05 | 0.43 |
| Fe2O3 | 1.00 | 2.08 | 2.83 | 1.80 | 1.02 | 0.72 | 1.16 | 0.34 | 0.78 |
| SiO2 | 57.9 | 24.2 | 43.8 | 60.2 | 63.1 | 61.0 | 1.00 | −0.24 | 0.74 |
| TiO2 | 0.06 | 0.04 | 0.06 | 0.06 | 0.06 | 0.06 | 1.02 | −0.12 | 0.55 |
| CaO | 11.1 | 19.7 | 14.5 | 10.1 | 9.83 | 9.95 | 0.98 | 0.19 | 0.80 |
| MgO | 5.07 | 10.6 | 6.20 | 4.71 | 4.78 | 5.21 | 1.08 | 0.19 | 0.62 |
| MnO | 0.38 | 0.58 | 0.43 | 0.32 | 0.32 | 0.35 | 0.94 | 0.15 | 0.66 |
| K2O | 14.1 | 18.7 | 16.3 | 13.0 | 12.8 | 13.9 | 1.00 | 0.09 | 0.65 |
| P2O5 | 4.75 | 9.86 | 5.41 | 3.68 | 3.50 | 3.95 | 0.90 | 0.25 | 0.69 |
| SO3 | 3.75 | 10.8 | 7.39 | 3.93 | 2.81 | 3.06 | 1.04 | 0.38 | 0.86 |
| Cl | 0.96 | 2.65 | 2.03 | 1.13 | 0.73 | 0.85 | 1.10 | 0.36 | 0.85 |
| Component | Ash | SF 1 | SF 2 | SF 3 | SF 4 | SF 5 | RR a | Nb | r2 c |
|---|---|---|---|---|---|---|---|---|---|
| Ba | 249 | 265 | 205 | 237 | 289 | 287 | 1.10 | −0.052 | 0.27 |
| Br | 70.2 | 233 | 191 | 113 | 58.1 | 65.2 | 1.26 | 0.407 | 0.88 |
| Cu | 92.8 | 135 | 124 | 102 | 92.4 | 98.6 | 1.09 | 0.099 | 0.78 |
| Ga | 5.6 | 4.1 | 4.1 | 1.9 | 5.6 | 5.1 | 0.85 | −0.081 | 0.07 |
| Ge | 1.1 | 2.7 | 0.9 | 0.2 | 1.2 | 0.9 | 0.93 | 0.218 | 0.11 |
| Pb | 15.4 | 64.1 | 36.2 | 25.1 | 15.2 | 5.3 | 1.04 | 0.653 | 0.97 |
| Rb | 66.9 | 45.5 | 54 | 61 | 64.6 | 66.5 | 0.94 | −0.104 | 0.91 |
| Se | 2.1 | 6.6 | 4.7 | 2.3 | 1.7 | 1.5 | 1.05 | 0.437 | 0.93 |
| Sr | 195 | 315 | 222 | 176 | 160 | 178 | 0.95 | 0.161 | 0.72 |
| Ta | 27.1 | 28.5 | 28 | 29.7 | 20.8 | 22.4 | 0.87 | 0.084 | 0.56 |
| Th | 4.5 | 5.8 | 5.1 | 4.5 | 3.9 | 4.7 | 1.02 | 0.074 | 0.51 |
| Tl | 1.0 | 2.3 | 1.7 | 1.6 | 0.5 | 1.2 | 1.17 | 0.268 | 0.43 |
| V | 32.9 | 49.9 | 53 | 37.1 | 18.9 | 33.4 | 0.99 | 0.193 | 0.45 |
| Y | 3.3 | 2 | 2.1 | 2.3 | 2 | 3.6 | 0.86 | −0.129 | 0.55 |
| Zn | 617 | 882 | 715 | 674 | 543 | 512 | 0.94 | 0.151 | 0.96 |
© 2019 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lanzerstorfer, C. Combustion of Miscanthus: Composition of the Ash by Particle Size. Energies 2019, 12, 178. https://doi.org/10.3390/en12010178
Lanzerstorfer C. Combustion of Miscanthus: Composition of the Ash by Particle Size. Energies. 2019; 12(1):178. https://doi.org/10.3390/en12010178
Chicago/Turabian StyleLanzerstorfer, Christof. 2019. "Combustion of Miscanthus: Composition of the Ash by Particle Size" Energies 12, no. 1: 178. https://doi.org/10.3390/en12010178
APA StyleLanzerstorfer, C. (2019). Combustion of Miscanthus: Composition of the Ash by Particle Size. Energies, 12(1), 178. https://doi.org/10.3390/en12010178