Design and Simulation of a Powertrain System for a Fuel Cell Extended Range Electric Golf Car
Abstract
1. Introduction
2. Methodology
2.1. Design the Traction System
- = Gross mass and is the sum of mv + mBAT + mPAS + mBOP + mH-T + mequi
- = longitudinal acceleration
- = Front traction effort
- = Rear traction effort
- = Front rolling resistance
- = Rear rolling resistance
- = Aerodynamic drag
- = Weight of the vehicle, is the product of the mass and gravity
- = Slope angle to be overcome
- = Mass factor
- = Moment of inertia of the masses that turn with the wheels with respect to their rotary axes
- = Moment of inertia of the transmission components
- = kinematic radius equivalent to the radius of the wheel under a load
- = Transmission ratio respect to the wheels
- = Tractive effort developed by a traction motor on driven wheels
- = Torque of the electric motor
- = Ratio to the bevel gear
- = Ratio of the gearbox
- = Efficiency of the transmission
- = Radius under a load
2.1.1. Golf Car Performance Criteria
Power Required for Reaching Maximum Speed
- = Power rating for reaching maximum speed
- = Gravity
- = Rolling resistance coefficient
- = Aerodynamic drag coefficient
- = Air density
- = Frontal area
- = Maximum speed
Power Required for Acceleration
- = Maximum Power of the electric motor
- = Base Speed
- V = Vehicle Speed
- Acceleration time from 0 to 20 km/h
- = Final Speed
- = Power consumed for vehicle acceleration
Traction Power Required for the Maximum Slope
Torque Required by the Electric Motor
- = Output torque
- = Output power
- = Angular speed
- = Torque on the wheel
- = Final transmission ratio
Determining the Size of the Batteries
- = Energy of the vehicle’s storage system
- = Energy consumed during the cycle
- = Top state of charge
- = Bottom Minimum state of charge
- = Capacity of batteries
- = Nominal voltage of the system
- = Battery efficiency
- = Charge factor
State of Charge of Batteries
- = State of charge
- = battery discharge power
- = battery charge power from regenerated kinetic energy
- = battery charge power from Fuel cell system
- = battery’s energy storage capacity, which is obtained by the multiplication of the capacity of the battery and the nominal voltage.
2.2. Energy Management Proposal with Fuel Cell
Fuel Cell Model
2.3. Efficiency of the System
- = Total efficiency
- = Fuel cell System efficiency
- = Efficiency of the DC-DC converter
- = Battery efficiency
- = Efficiency of electric machine
- = Drivetrain efficiency
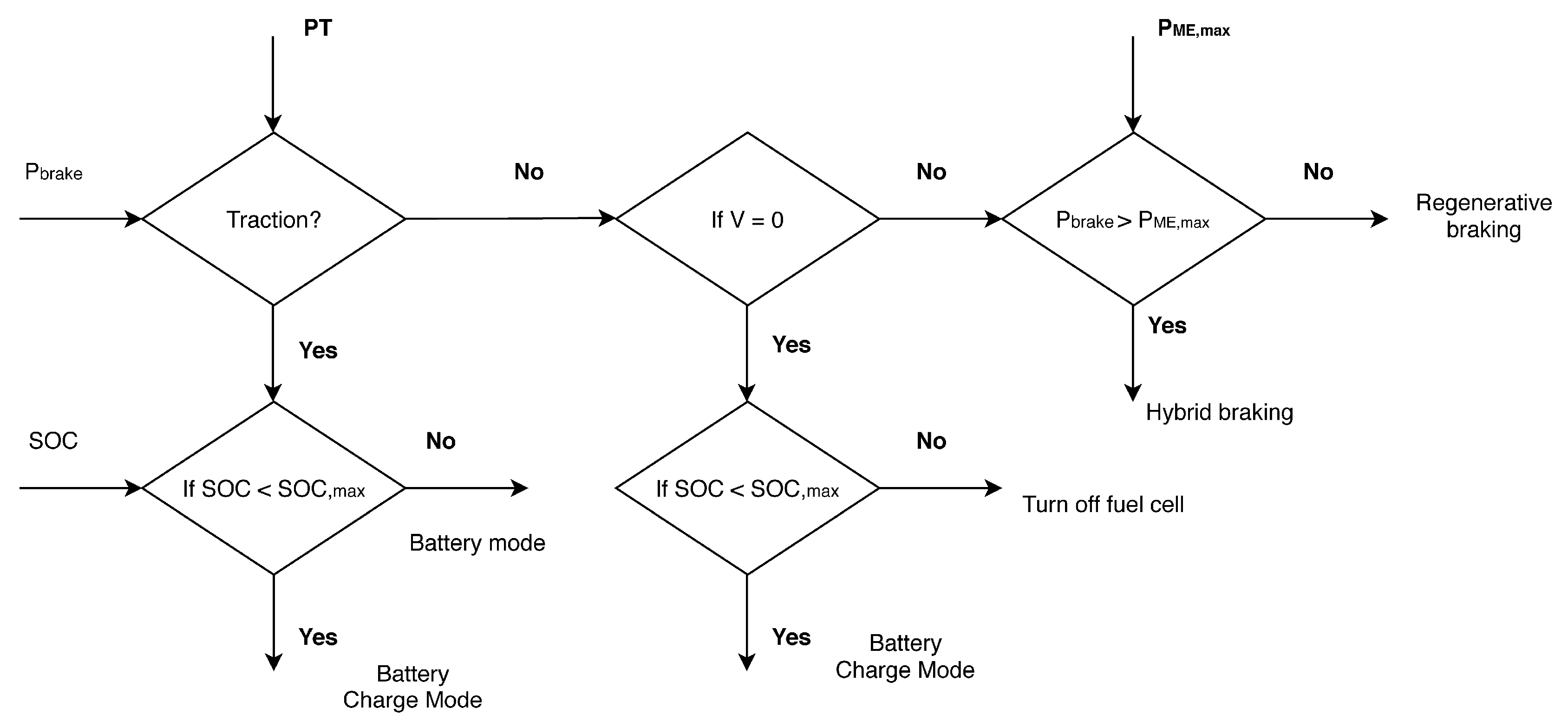
2.4. Control Strategy—Max. SOC of Battery
3. Results and Discussion
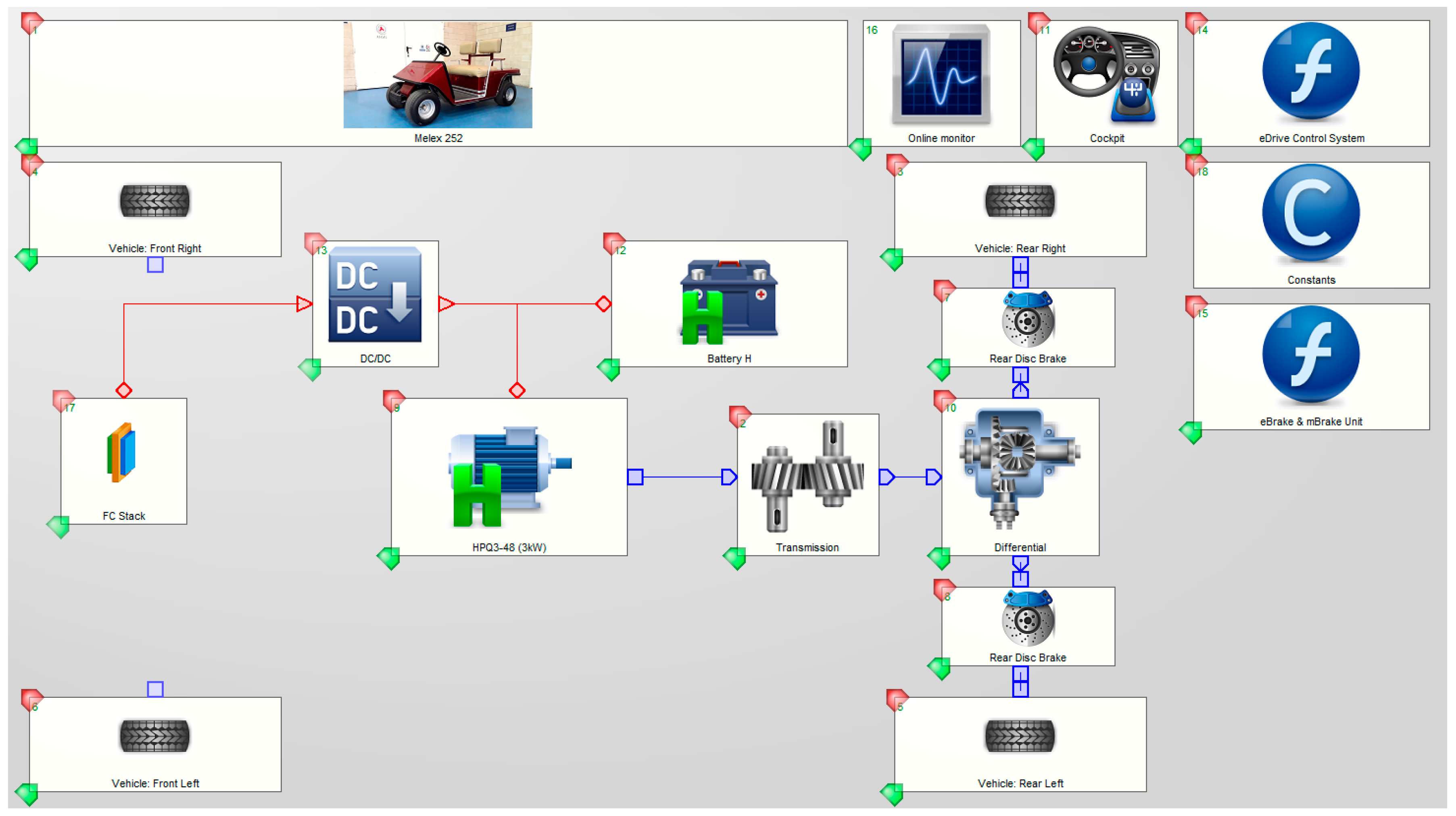
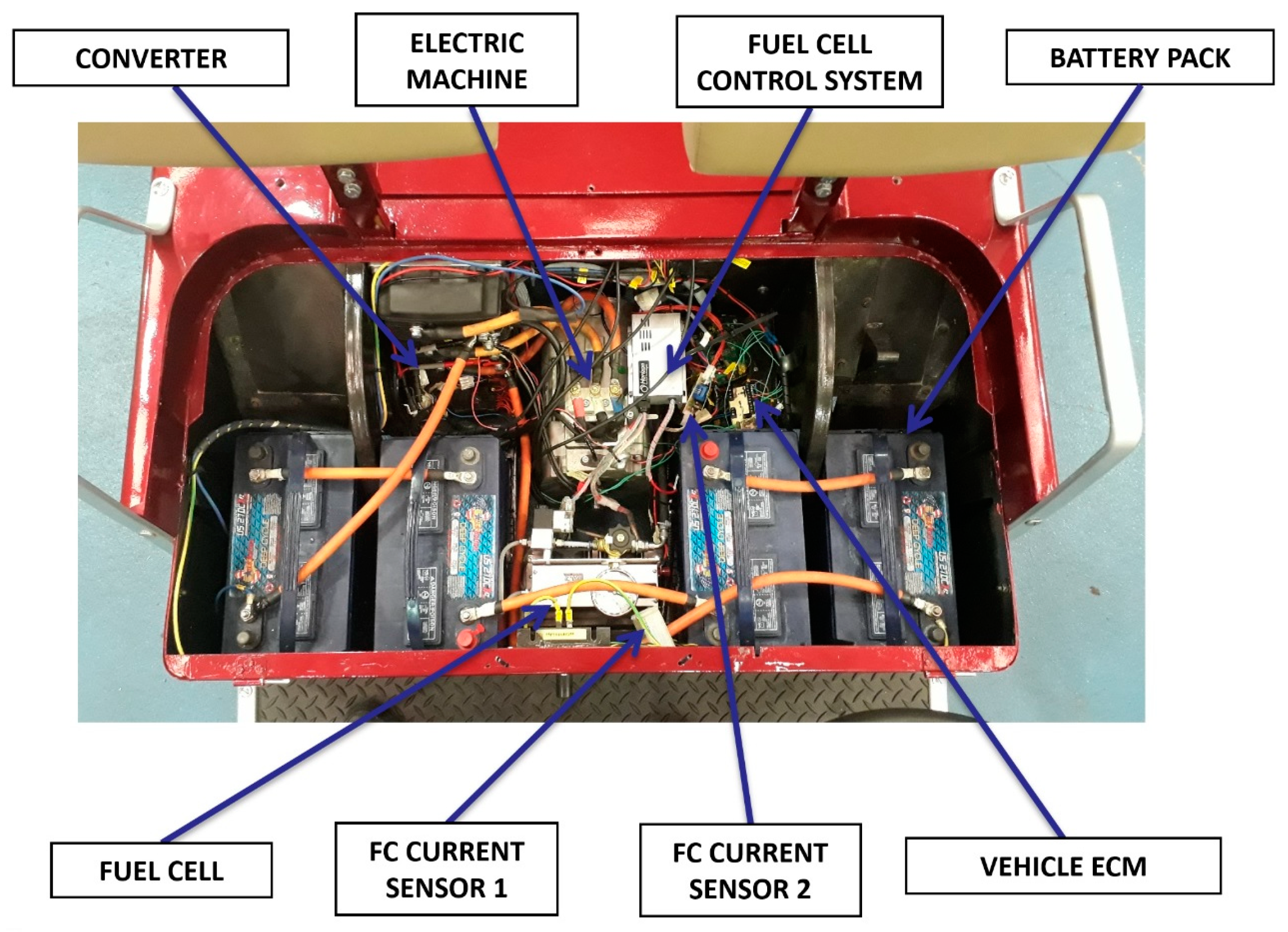
3.1. The Simulation Tool
3.2. Model Simulation and Validation
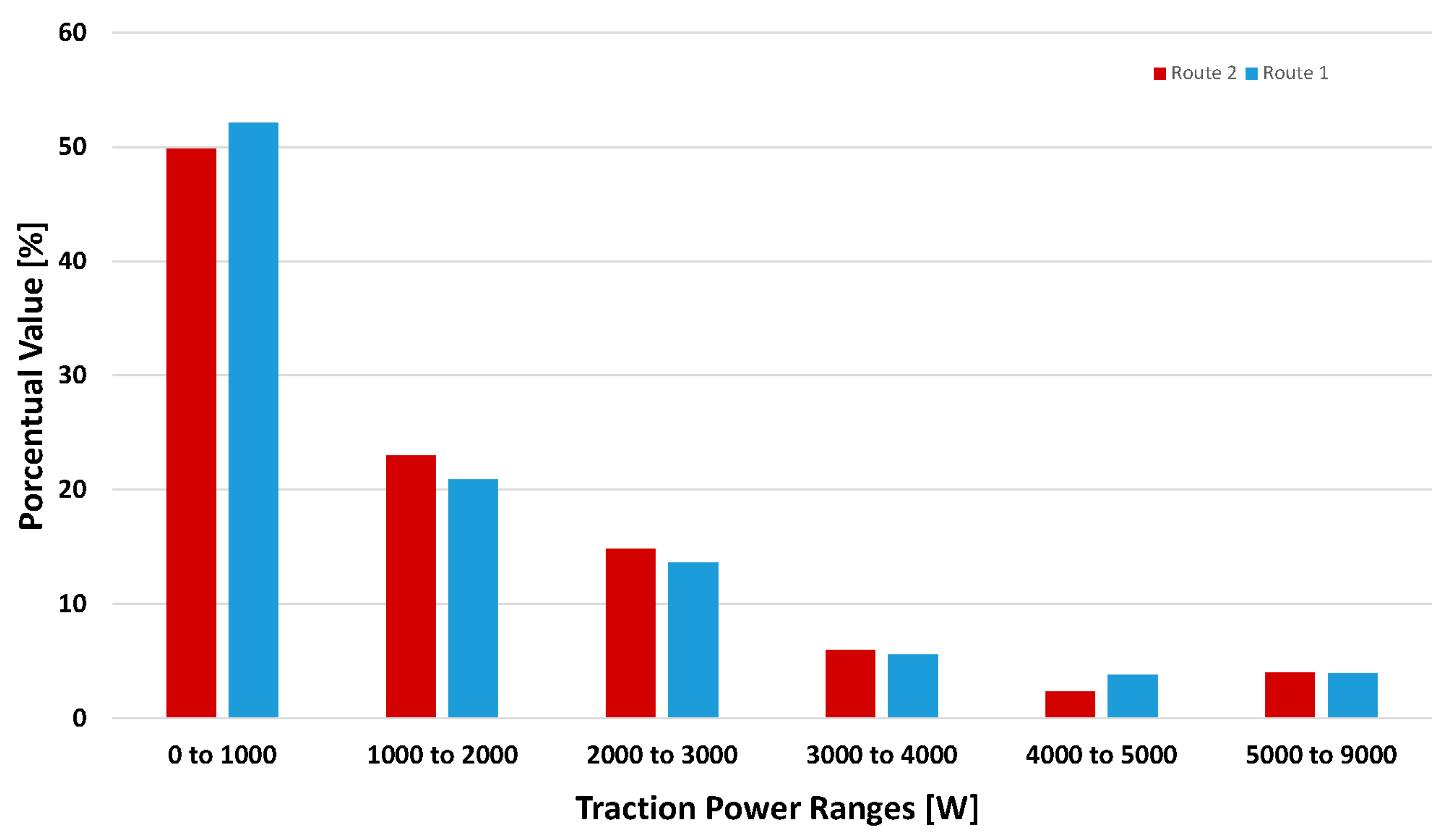
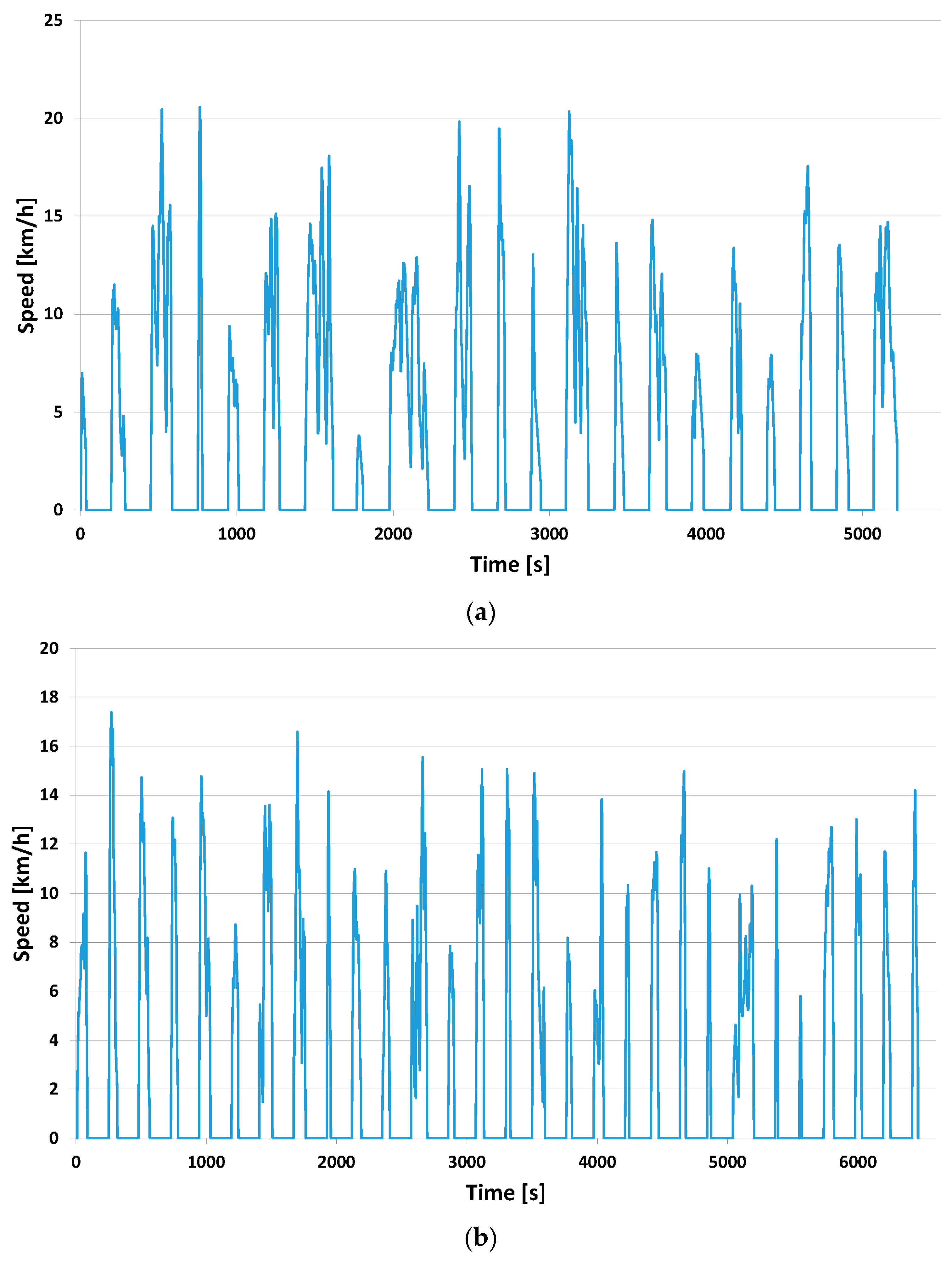
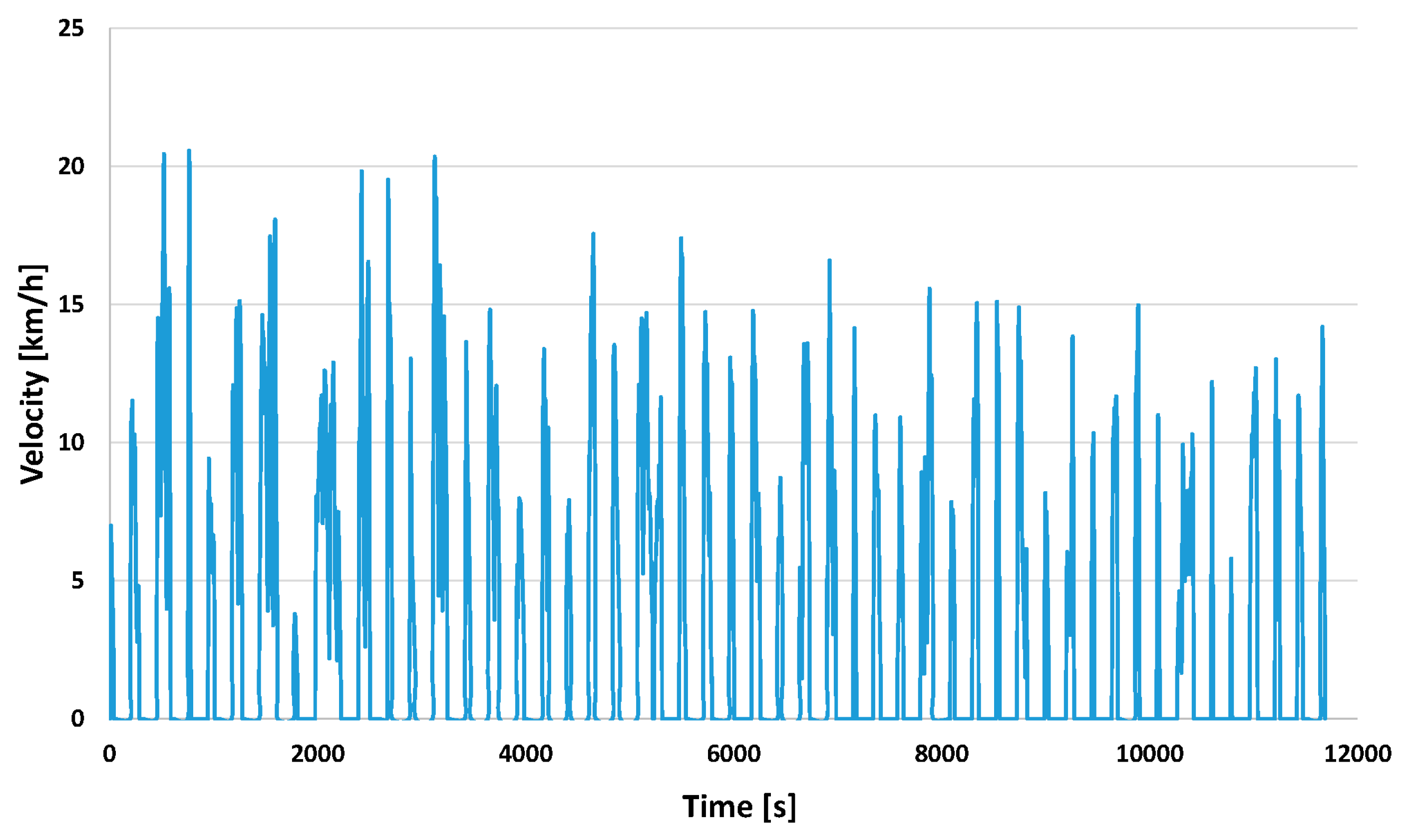
3.2.1. Tractive Effort throughout the Course
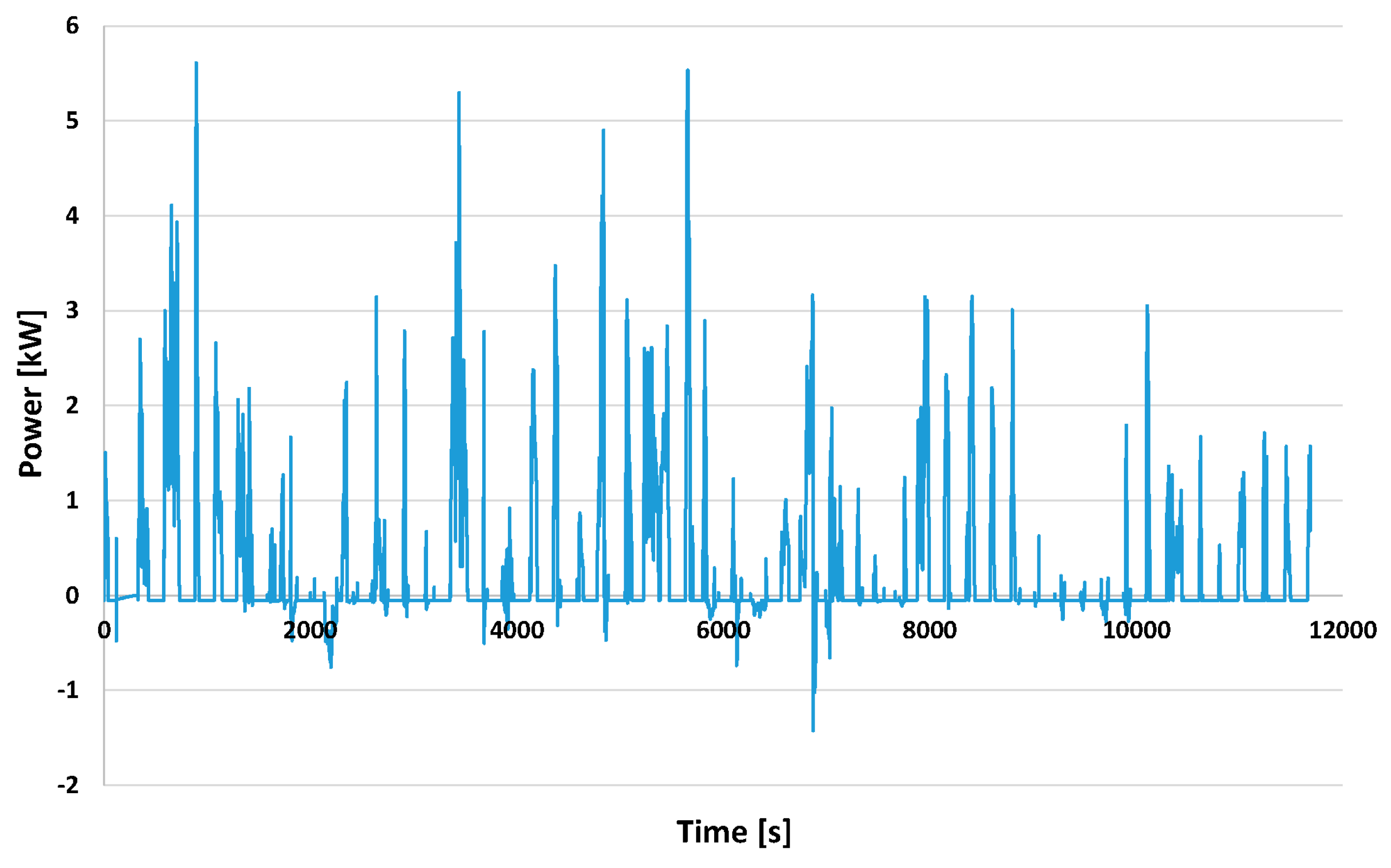
3.2.2. Managing the Power and Energy of the Drive System
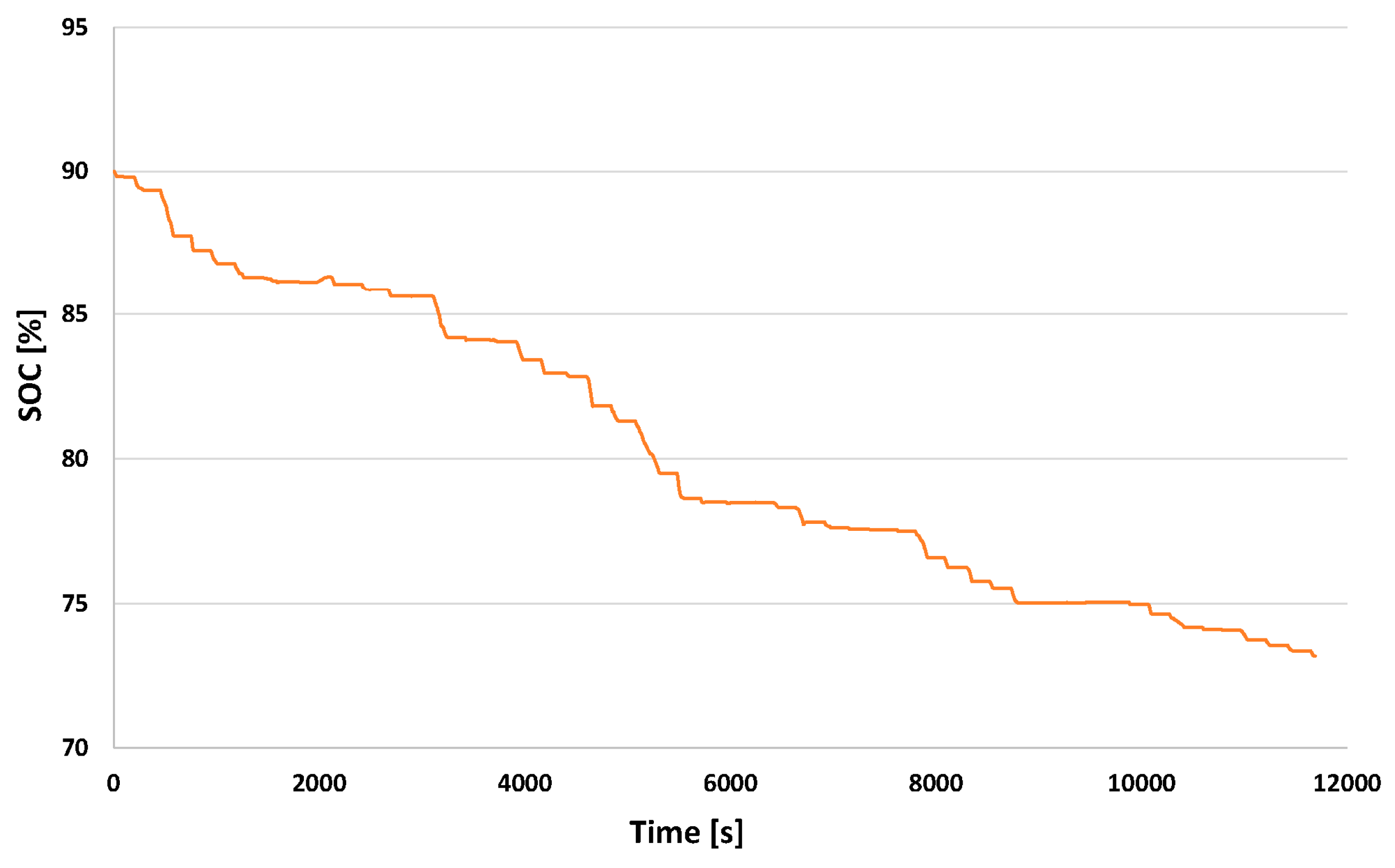
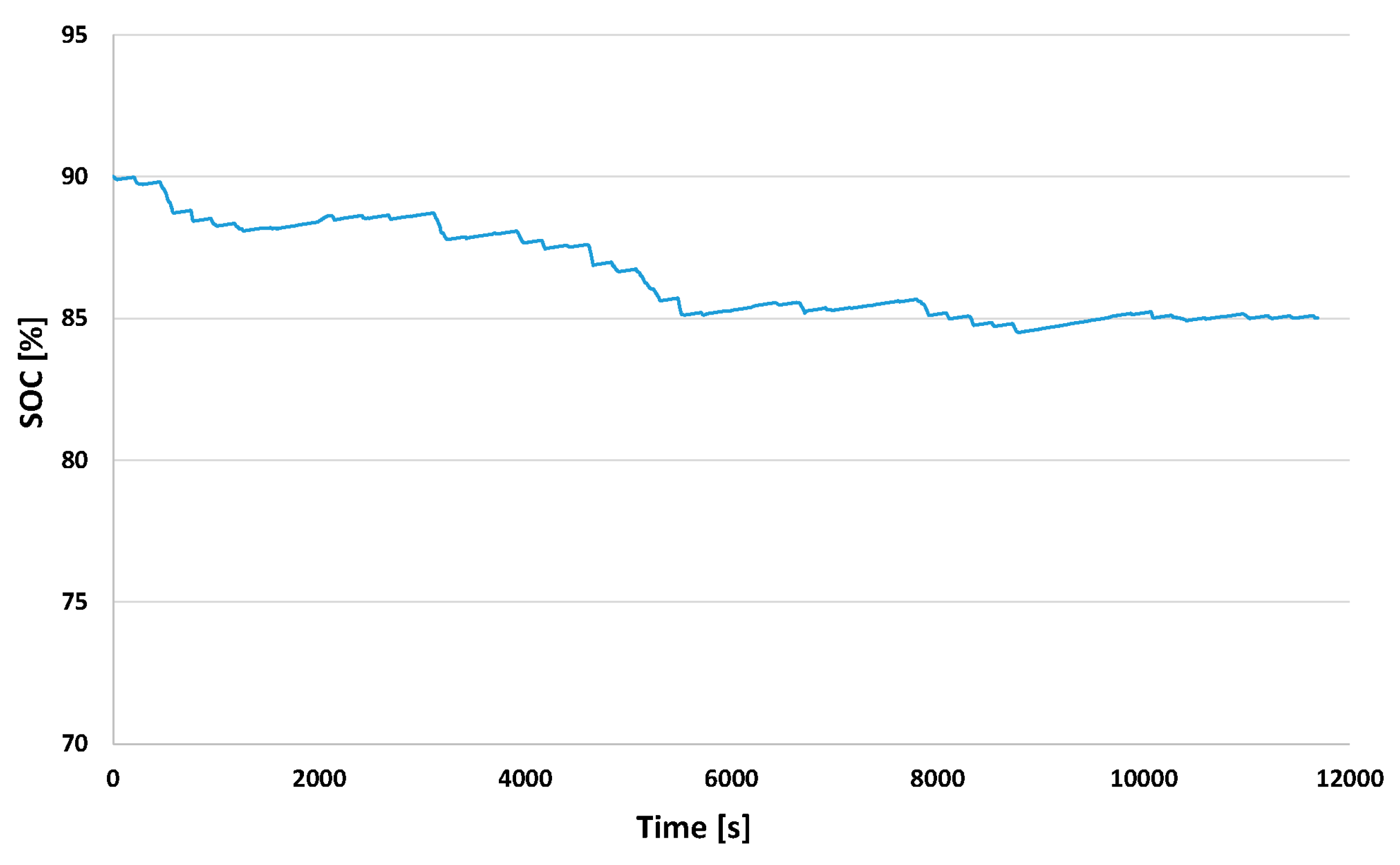
3.2.3. State of Charge in EV and in FCEREV
3.2.4. Comparative Analysis with Others Battery Technologies
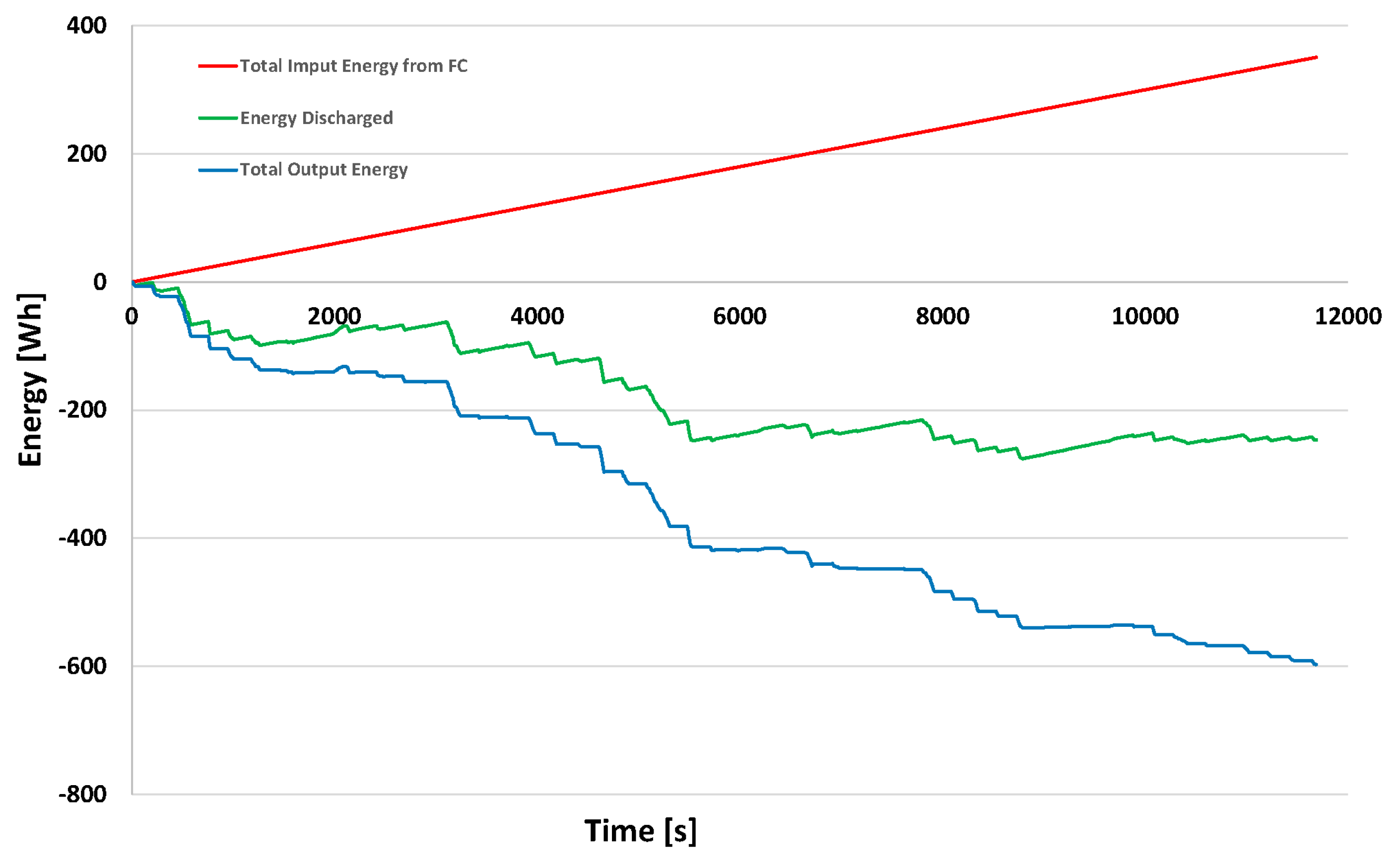
3.2.5. Sankey Energy Diagram during the Trip
3.2.6. Comparison between Experimental and Simulated Results
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- IEA (International Energy Agency). Energy Efficiency Indicators. Available online: http://www.iea.org/Sankey/#?c=World&s=Final (accessed on 3 July 2018).
- IEA (International Energy Agency). Key World Energy Statistics; IEA: Paris, France, 2017; pp. 1–97. [Google Scholar] [CrossRef]
- Ligen, Y.; Vrubel, H.; Girault, H. Mobility from renewable electricity: Infrastructure comparison for battery and hydrogen fuel cell vehicles. World Electr. Veh. J. 2018, 9, 3. [Google Scholar] [CrossRef]
- Messagie, M.; Boureima, F.S.; Coosemans, T.; Macharis, C.; Van Mierlo, J. A range-based vehicle life cycle assessment incorporating variability in the environmental assessment of different vehicle technologies and fuels. Energies 2014, 7, 1467–1482. [Google Scholar] [CrossRef]
- Lombardi, L.; Tribioli, L.; Cozzolino, R.; Bella, G. Comparative environmental assessment of conventional, electric, hybrid, and fuel cell powertrains based on LCA. Int. J. Life Cycle Assess. 2017, 22, 1989–2006. [Google Scholar] [CrossRef]
- IPCC (Intergovernmental Panel on Climate Change). Climate Change 2007 Synthesis Report; IPCC: Geneva, Switzerland, 2007; ISBN 9291691224. [Google Scholar]
- IPCC (Intergovernmental Panel on Climate Change). Summary for Policymakers; IPCC: Geneva, Switzerland, 2014; ISBN 9789291691432. [Google Scholar]
- IEA (International Energy Agency). CO2 Emissions from Fuel Combustion; OECD/IEA: Paris, France, 2016; pp. 1–155. [Google Scholar] [CrossRef]
- Un-Noor, F.; Padmanaban, S.; Mihet-Popa, L.; Mollah, M.N.; Hossain, E. A comprehensive study of key electric vehicle (EV) components, technologies, challenges, impacts, and future direction of development. Energies 2017, 10, 1217. [Google Scholar] [CrossRef]
- Hutchinson, T.; Burgess, S.; Herrmann, G. Current hybrid-electric powertrain architectures: Applying empirical design data to life cycle assessment and whole-life cost analysis. Appl. Energy 2014, 119, 314–329. [Google Scholar] [CrossRef]
- Cheng, S.; Xu, L.; Li, J.; Fang, C.; Hu, J.; Ouyang, M. Development of a PEM fuel cell city bus with a hierarchical control system. Energies 2016, 9, 417. [Google Scholar] [CrossRef]
- Nikolaidis, P.; Poullikkas, A. A comparative overview of hydrogen production processes. Renew. Sustain. Energy Rev. 2017, 67, 597–611. [Google Scholar] [CrossRef]
- Mazloomi, K.; Gomes, C. Hydrogen as an energy carrier: Prospects and challenges. Renew. Sustain. Energy Rev. 2012, 16, 3024–3033. [Google Scholar] [CrossRef]
- Astbury, G.R. A review of the properties and hazards of some alternative fuels. Process Saf. Environ. Prot. 2008, 86, 397–414. [Google Scholar] [CrossRef]
- Cengel, Y.; Boles, M. Termodinámica, 8th ed.; McGraw Hill: Mexico City, Mexico, 2015; ISBN 978-607-15-1281-9. [Google Scholar]
- Dincer, I.; Acar, C. Review and evaluation of hydrogen production methods for better sustainability. Int. J. Hydrogen Energy 2014, 40, 11094–11111. [Google Scholar] [CrossRef]
- Midilli, A.; Dincer, I. Key strategies of hydrogen energy systems for sustainability. Int. J. Hydrogen Energy 2007, 32, 511–524. [Google Scholar] [CrossRef]
- Sharma, S.; Ghoshal, S.K. Hydrogen the future transportation fuel: From production to applications. Renew. Sustain. Energy Rev. 2015, 43, 1151–1158. [Google Scholar] [CrossRef]
- Zeng, K.; Zhang, D. Recent progress in alkaline water electrolysis for hydrogen production and applications. Prog. Energy Combust. Sci. 2010, 36, 307–326. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, K.S.; Mishler, J.; Cho, S.C.; Adroher, X.C. A review of polymer electrolyte membrane fuel cells: Technology, applications, and needs on fundamental research. Appl. Energy 2011, 88, 981–1007. [Google Scholar] [CrossRef]
- Department of Energy. Fuel Cells. Available online: https://www.energy.gov/eere/fuelcells/fuel-cells (accessed on 22 June 2018).
- Tribioli, L.; Iora, P.; Cozzolino, R.; Chiappini, D. Influence of Fuel Type on the Performance of a Plug-In Fuel Cell/Battery Hybrid Vehicle with On-Board Fuel Processing. In Proceedings of the SAE 13th International Conference on Engines and Vehicles, Capri, Italy, 10–14 September 2017. [Google Scholar]
- Tribioli, L.; Cozzolino, R.; Chiappini, D.; Iora, P. Energy management of a plug-in fuel cell/battery hybrid vehicle with on-board fuel processing. Appl. Energy 2016, 184, 140–154. [Google Scholar] [CrossRef]
- Tribioli, L.; Cozzolino, R.; Chiappini, D. Technical Assessment of Different Operating Conditions of an On-Board Autothermal Reformer for Fuel Cell Vehicles. Energies 2017, 10, 839. [Google Scholar] [CrossRef]
- Tribioli, L.; Cozzolino, R.; Barbieri, M. Optimal control of a repowered vehicle: Plug-in fuel cell against plug-in hybrid electric powertrain. AIP Conf. Proc. 2015, 1648, 570014. [Google Scholar]
- Ehsani, M.; Gao, Y.; Emadi, A. Modern Electric, Hybrid Electric, and Fuel Cell Vehicles: Fundamentals, Theory, and Design; CRC Press: Boca Raton, FL, USA, 2017; ISBN 1420054007. [Google Scholar]
- Das, H.S.; Tan, C.W.; Yatim, A.H.M. Fuel cell hybrid electric vehicles: A review on power conditioning units and topologies. Renew. Sustain. Energy Rev. 2017, 76, 268–291. [Google Scholar] [CrossRef]
- Seluga, K.J.; Baker, L.L.; Ojalvo, I.U. A parametric study of golf car and personal transport vehicle braking stability and their deficiencies. Accid. Anal. Prev. 2009, 41, 839–848. [Google Scholar] [CrossRef] [PubMed]
- Rahman, A.; Mohiuddin, A.K.M.; Ihsan, S.I. A study on automated traction control system of an electrical golf car. Int. J. Electr. Hybrid Veh. 2011, 3, 47–61. [Google Scholar] [CrossRef]
- Aparicio, F.; Vera, C.; Díaz, V. Teoría de los Vehículos Automóviles; Sección Publicaciones la ETSII-UPM; ETSII-UPM: Madrid, Spain, 1995. [Google Scholar]
- Engineers, M.E.A.F.; Tyler, J.; Store, A. Golf Cars—Safety and Performance Specifications; American National Standards Institute (ANSI): Washington, DC, USA, 2004. [Google Scholar]
- Vasebi, A.; Bathaee, S.M.T.; Partovibakhsh, M. Predicting state of charge of lead-acid batteries for hybrid electric vehicles by extended Kalman filter. Energy Convers. Manag. 2008, 49, 75–82. [Google Scholar] [CrossRef]
- Stevens, J.W.; Corey, G.P. A study of lead-acid battery efficiency near top-of-charge and the impact on PV system design. In Proceedings of the Twenty Fifth IEEE Photovoltaic Specialists Conference, Washington, DC, USA, 13–17 May 1996; pp. 1485–1488. [Google Scholar] [CrossRef]
- Alvarez, R.; López, A.; De La Torre, N. Evaluating the effect of a driver’s behaviour on the range of a battery electric vehicle. Proc. Inst. Mech. Eng. Part D J. Automob. Eng. 2015, 229, 1379–1391. [Google Scholar] [CrossRef]
- Gao, Z.; Lin, Z.; LaClair, T.J.; Liu, C.; Li, J.M.; Birky, A.K.; Ward, J. Battery capacity and recharging needs for electric buses in city transit service. Energy 2017, 122, 588–600. [Google Scholar] [CrossRef]
- Montenegro, D.; Rodríguez, S.; Fuelagán, J.R.; Jimenéz, J.B. An estimation method of state of charge and lifetime for lead-acid batteries in smart grid scenario. In Proceedings of the 2015 IEEE PES Innovation Smart Grid Technologies Latin America (ISGT LATAM 2015), Montevideo, Uruguay, 5–7 October 2015; pp. 564–569. [Google Scholar] [CrossRef]
- H-200 Fuel Cell Stack User Manual. Available online: https://www.horizonfuelcell.com/h-series-stacks (accessed on 6 July 2017).
- Khanungkhid, P.; Piumsomboon, P. 200W PEM Fuel Cell Stack with Online Model-Based Monitoring System. Eng. J. 2014, 18, 13–26. [Google Scholar] [CrossRef]
- Kulikovsky, A.A. A Physically-Based Analytical Polarization Curve of a PEM Fuel Cell. Available online: http://jes.ecsdl.org/cgi/doi/10.1149/2.028403jes (accessed on 3 July 2018).
- Eikerling, M.; Kulikovsky, A. Polymer Electrolyte Fuel Cells: Physical Principles of Materials and Operation; CRC Press: Boca Raton, FL, USA, 2014; ISBN 9781439854068. [Google Scholar]
- López Martínez, J.M. Vehículos Híbridos y Eléctricos, 2nd ed.; Dextra: Madrid, Spain, 2016; ISBN 978-84-16277-42-1. [Google Scholar]
- AVL CRUISETM—avl.com. Available online: https://www.avl.com/cruise (accessed on 22 June 2018).
- Miller, J.M. Energy storage system technology challenges facing strong hybrid, plug-in and battery electric vehicles. In Proceedings of the 2009 IEEE Vehicle Power and Propulsion Conference, Dearborn, MI, USA, 7–10 September 2009; pp. 4–10. [Google Scholar] [CrossRef]
- Burke, A.; Jungers, B.; Yang, C.; Ogden, J. Battery Electric Vehicles: An Assessment of the Technology and Factors Influencing Market Readiness. Available online: http://samersanaat.com/en/wp-content/uploads/2012/07/AEP_Tech_AssessmentBEVs.pdf (accessed on 5 July 2018).
- Mahmoudzadeh Andwari, A.; Pesiridis, A.; Rajoo, S.; Martinez-Botas, R.; Esfahanian, V. A review of Battery Electric Vehicle technology and readiness levels. Renew. Sustain. Energy Rev. 2017, 78, 414–430. [Google Scholar] [CrossRef]
- Dixon, J.; Nakashima, I.; Arcos, E.F.; Ortúzar, M. Electric vehicle using a combination of ultracapacitors and ZEBRA battery. IEEE Trans. Ind. Electron. 2010, 57, 943–949. [Google Scholar] [CrossRef]

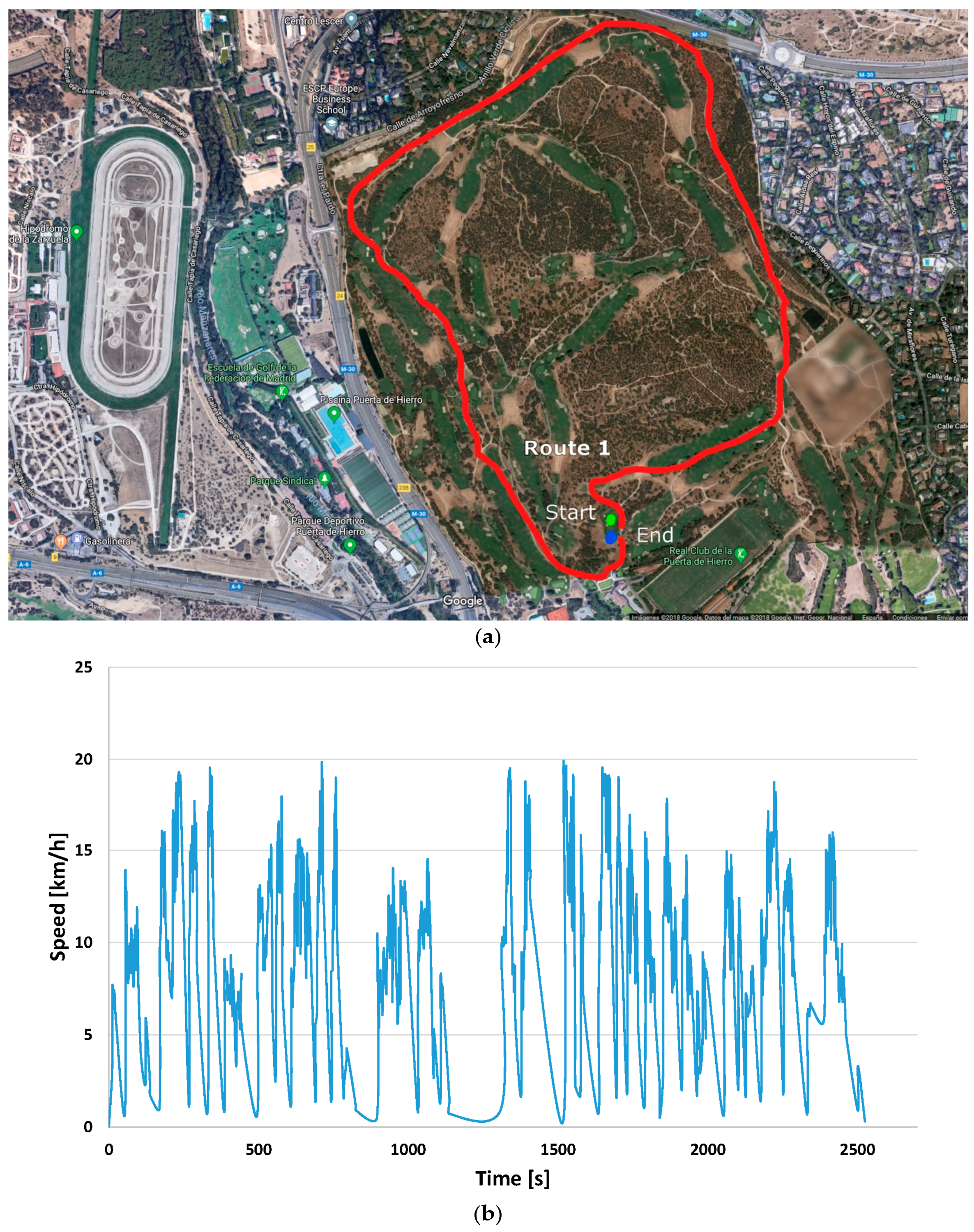

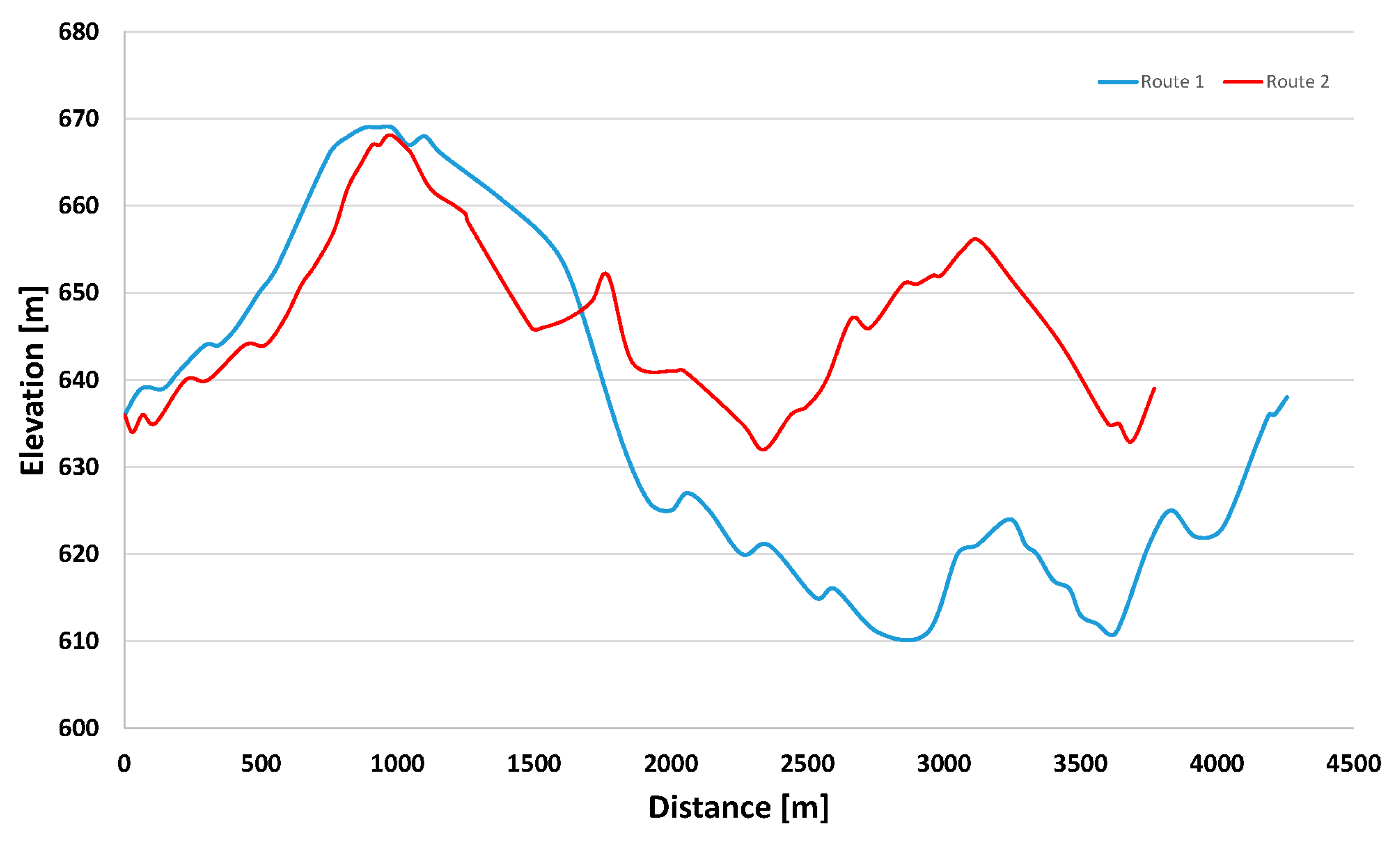
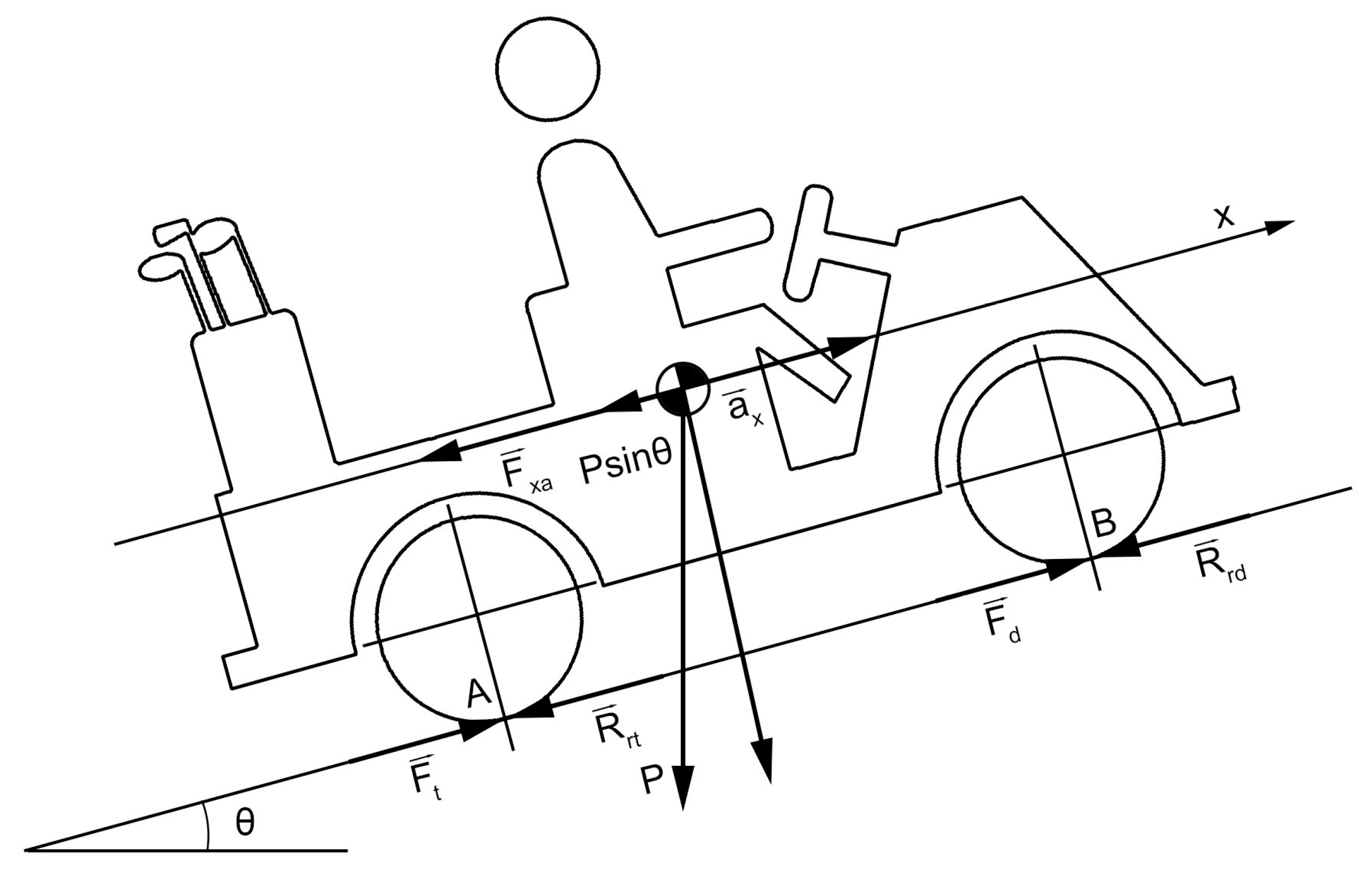
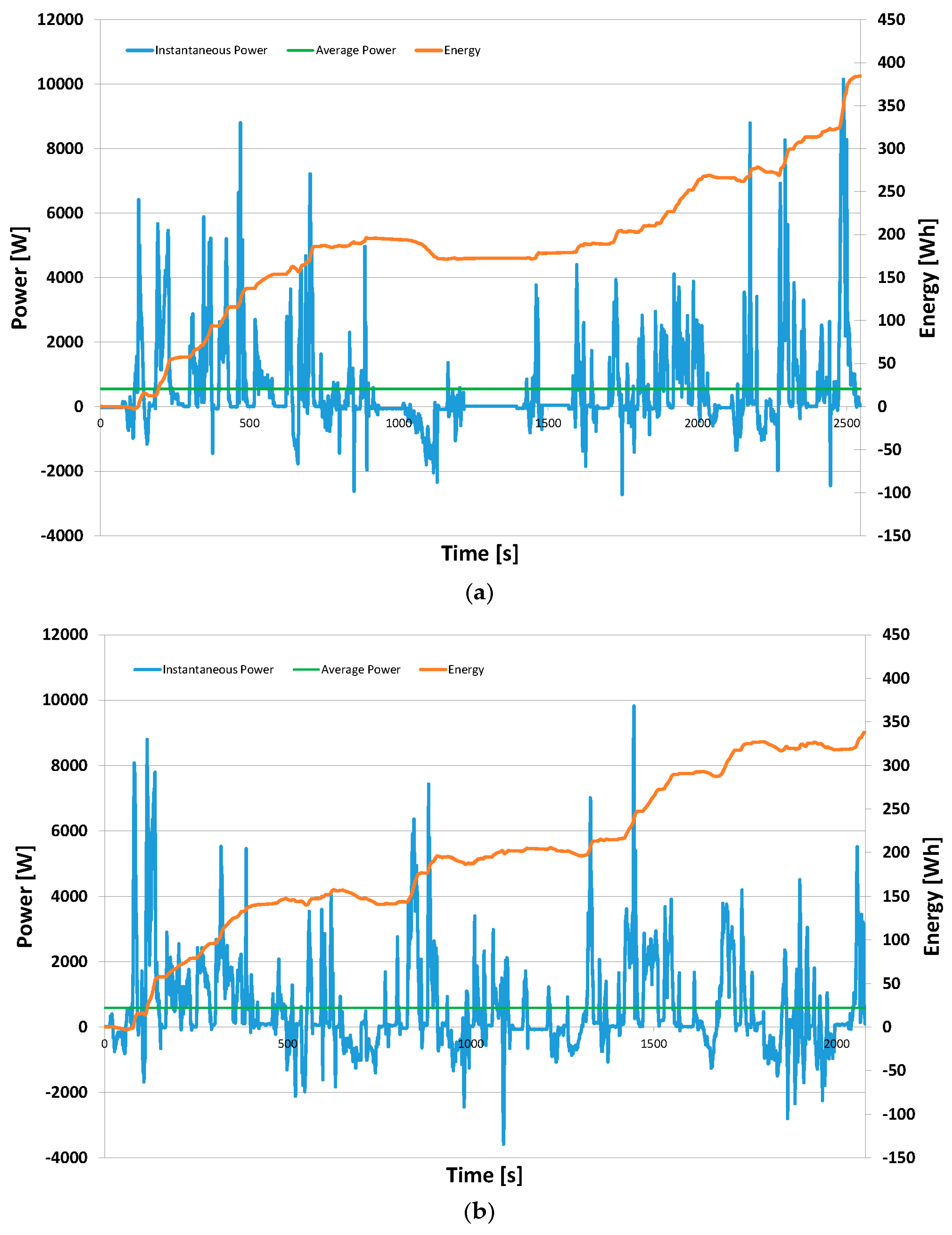






| Transport | Value (Mtoe) | Product |
|---|---|---|
| Road | 2026 | Oil Products, natural gas, bio fuels and waste |
| Electricity Road | 11 | Electricity |
| World Aviation bunkers | 177 | Oil Products |
| Domestic Aviation | 113 | Oil Products |
| Rail | 51 | Oil products, Coal, electricity |
| Pipeline Transport | 59 | Natural gas, Electricity |
| World Marine bunkers | 205 | Oil Products |
| Domestic Navigation | 51 | Oil Products |
| Others | 11 | Oil products, Electricity |
| Fuel | High Heat Value (mJ/kg) | Low Heat Value (mJ/kg) | Energy Per Litter (mJ/L) |
|---|---|---|---|
| Hydrogen (liquid) | 141.9 | 119.9 | 10.1 |
| Hydrogen gas (compressed, 700 bar) | 141.9 | 119.9 | 5.6 |
| Hydrogen (ambient pressure) | 141.9 | 119.9 | 0.0107 |
| Gasoline | 47.5 | 44.5 | 34.2 |
| Diesel | 44.8 | 42.5 | 34.6 |
| Natural Gas (ambient pressure) | 55.5 | 50 | 0.0378 |
| Ethanol | 29.73 | 26.81 | 23.66 |
| Methanol | 22.72 | 18.1 | 18.08 |
| LPG (Propane) | 49.6 | 46.35 | 25.3 |
| Specification | Value | Specification | Value |
|---|---|---|---|
| Vehicle mass (mv) | 342 kg | Mass factor (γm) | 1.042 |
| Battery Pack (mBAT) | 106.4 kg | Maximum slope (θ) | 25% |
| Rate load (mPAS) | 154 kg | Maximum speed (vmax) | 20 km/h |
| BOP mass (mBOP) | 3 kg | Base Speed (Vb) | 6 km/h |
| Mass of the hydrogen fuel tank (mH-T) | 6.5 kg | Radius Wheel (re) | 0.223m |
| Mass of golf equipment (mequi) | 60 kg | Acceleration time from 0 to 20 km/h (ta) | 10 s |
| Frontal area (Af) | 1.72 m2 | Drivetrain efficiency (ηj) | 99% |
| Aerodynamic drag coefficient (Cx) | 0.45 | Ratio of the differential () | 12.25 |
| Air density () | 1.225 kg/m3 | Ratio of the gearbox () | 1 |
| Gravity acceleration (g) | 9.81 m/s2 | Maximum efficiency of the E.M (ηME) | 92% |
| Rolling resistance coefficient (fr) | 0.02 | Efficiency of the DC-DC converter (ηDC-DC) | 90% |
| Specification | Values |
|---|---|
| Drive Motor | 48 VDC, 3 kW @ 3000 rpm |
| Peak Power | 7.82 kW @ 1248 rpm |
| Torque | 10.4 Nm @ 3000 rpm (Nominal Torque) 40.71 Nm @ 1248 rpm (Maximum Torque) |
| Batteries | Four 12 V, 77 min @ 56 A |
| Specification | Value | Specification | Value |
|---|---|---|---|
| Number of cells | 40 | Max stack temperature | 65 (°C) |
| Cell active area | 19 (cm2) | H2 Pressure | 0.45–0.55 (bar) |
| Current density | 0.437 (A·cm−2) | Hydrogen purity | ≥99.995 H2 |
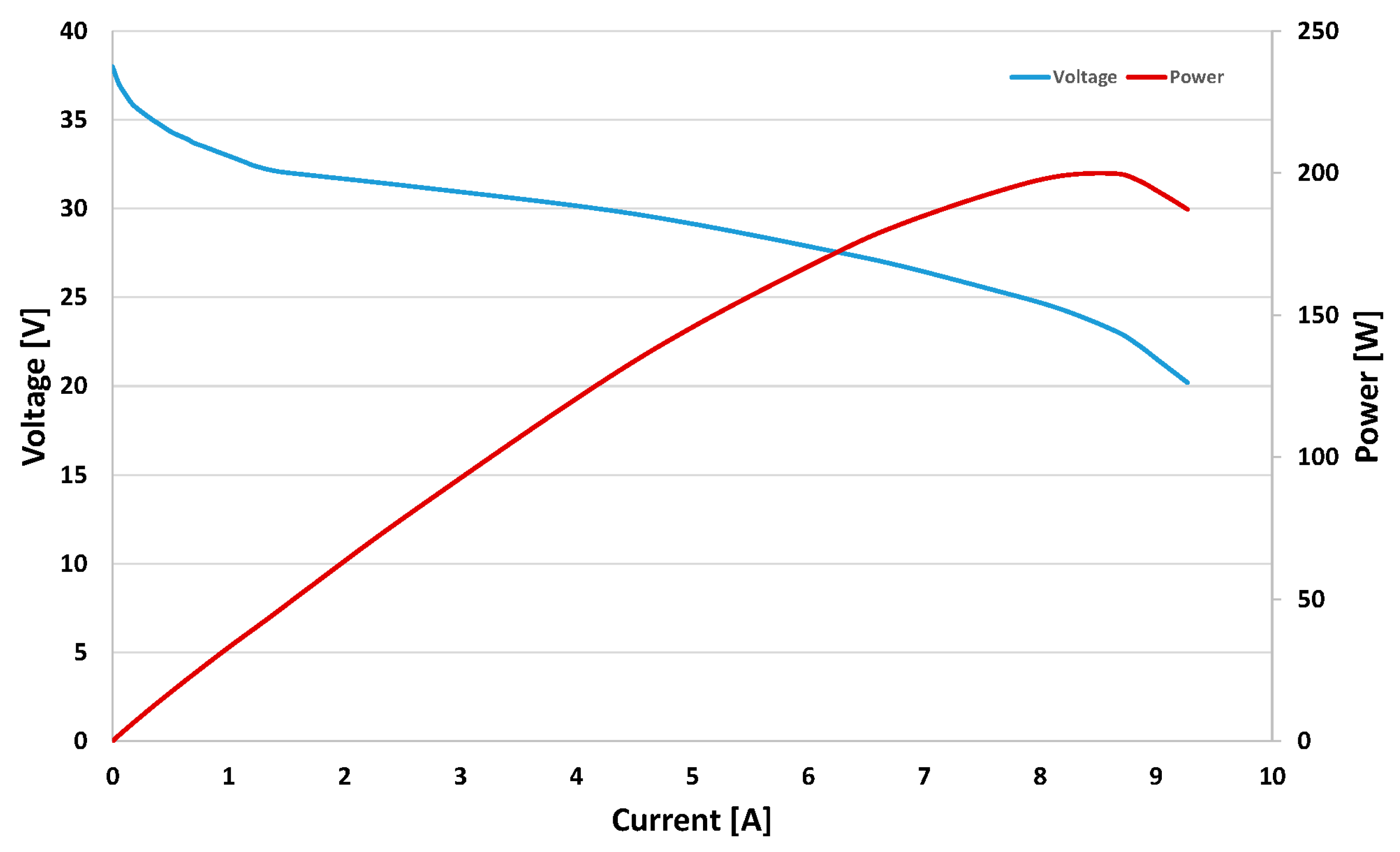
| Rated Power | 200 (W) | Efficiency of stack | 40% @ 24 V |
| Voltage in the maximum power point | 24 (V) | Flow rate at max output | 2.6 (L/min) |
| Current in the maximum power point | 8.3 (A) | Stack weight (with fan, casing and Controller) | 2.63 (kg) |
| Open circuit voltage (VOC) | 38 (V) | Size | 11.8 × 18.3 × 9.4 (cm) |
| Specification | Value | Specification | Value |
|---|---|---|---|
| Open circuit voltage (VOC) | 0.95 (V) | Cell current density (j0) | 0.437 (A·cm−2) |
| Tafel slope (b) | 0.03 (V) | Faraday constant (F) | 96,485 (As·mol−1) |
| Current density parameter (jσ) | 1.212 × 10−3 (A·cm−2) | Oxygen diffusion coefficient in the CCL () | 1.36 × 10−4 (cm2·s−1) |
| Scalar parameter of current density () | 0.9 (A·cm−2) | Dimensionless parameter () | 1311 |
| Volumetric exchange current density (i∗) | 8.17 × 10−4 (A·cm−3) | Limiting current density due to oxygen transport in the GDL () | 2958 (A·cm−2) |
| Catalyst layer thickness | 0.001 (cm) | Oxygen diffusion coefficient in the GDL () | 0.0259 (cm2·s−1) |
| Oxygen concentration in the channel () | 7.4 × 10−6 (mol·cm−3) | Ohmic Resistance | 0.126 (Ω·cm−2) |
| Oxygen concentration at the channel inlet () | 7.36 × 10−6 (mol·cm−3) | GDL thickness () | 0.025 (cm) |
| Reference oxygen molar concentration () | 7.36 × 10−6 (mol·cm−3) | Dimensionless current density () | 1 |
| CCL ionic conductivity (σt) | 0.03 (S/cm) | Limiting current density due to oxygen transport in the GDL () | 2.95 (A·cm−2) |
| Parameter | Mode EV | Mode ERFCEV | Difference |
|---|---|---|---|
| Autonomy (km) | 30.8 | 42.5 | 11.7 |
| H2 Consumption (kg/100 km) | - | 0.200 | - |
| Chargeini (Ah) | 94.5 | 94.5 | - |
| Chargefin a (Ah) | 76.83 | 89.26 | 12.43 |
| SOCini (%) | 90.0 | 90.0 | - |
| SOCfin a (%) | 73.17 | 85.0 | 11.83 |
| SOCfin b (%) | 56.34 | 80.0 | 23.66 |
| SOCfin c (%) | 39.51 | 75.0 | 35.49 |
| Battery Technologies | Energy Density (Wh/kg) | Implementation Technology Cost ($) | Total Output Energy (kWh) | ΔE (%) | SOC (%) | ΔSOC (%) |
|---|---|---|---|---|---|---|
| Lead acid battery | 47.3 | 504 | 0.597 | 0 | 85.0 | 0 |
| Nickel Metal Hydride battery (Ni-MH) | 70.0 | 3528 | 0.5829 | −2.36 | 85.67 | 0.79 |
| Lithium-ion battery (Li-Ion) | 100 | 3780 | 0.5722 | −4.15 | 85.78 | 0.92 |
| Sodium Nickel Chloride (Na/NiCl2, Zebra) battery | 100 | 3780 | 0,6539 | 9.53 | 84.71 | −0.58 |
| Parameter | Measure | Simulated | Error |
|---|---|---|---|
| H2 Consumption (kg/100 km) | 0.132 | 0.13 | 1.5% |
| Overall Energy Consumption (kWh) | 45.80 | 43.2 | −5.6% |
| Energy consumption per km (kWh/km) | 87.68 | 82.69 | −5.7% |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grijalva, E.R.; López Martínez, J.M.; Flores, M.N.; Del Pozo, V. Design and Simulation of a Powertrain System for a Fuel Cell Extended Range Electric Golf Car. Energies 2018, 11, 1766. https://doi.org/10.3390/en11071766
Grijalva ER, López Martínez JM, Flores MN, Del Pozo V. Design and Simulation of a Powertrain System for a Fuel Cell Extended Range Electric Golf Car. Energies. 2018; 11(7):1766. https://doi.org/10.3390/en11071766
Chicago/Turabian StyleGrijalva, Edwin R., José María López Martínez, M. Nuria Flores, and Víctor Del Pozo. 2018. "Design and Simulation of a Powertrain System for a Fuel Cell Extended Range Electric Golf Car" Energies 11, no. 7: 1766. https://doi.org/10.3390/en11071766
APA StyleGrijalva, E. R., López Martínez, J. M., Flores, M. N., & Del Pozo, V. (2018). Design and Simulation of a Powertrain System for a Fuel Cell Extended Range Electric Golf Car. Energies, 11(7), 1766. https://doi.org/10.3390/en11071766






