Verification of Heat and Mass Transfer Closures in Industrial Scale Packed Bed Reactor Simulations
Abstract
:1. Introduction
2. Methodology
2.1. Thiele Modulus and Effectiveness Factor
2.2. Conservation Equations
2.3. PR-DNS Simulation Setup
2.3.1. Geometry and Mesh Development
2.3.2. Boundary Conditions and Simulation Parameters
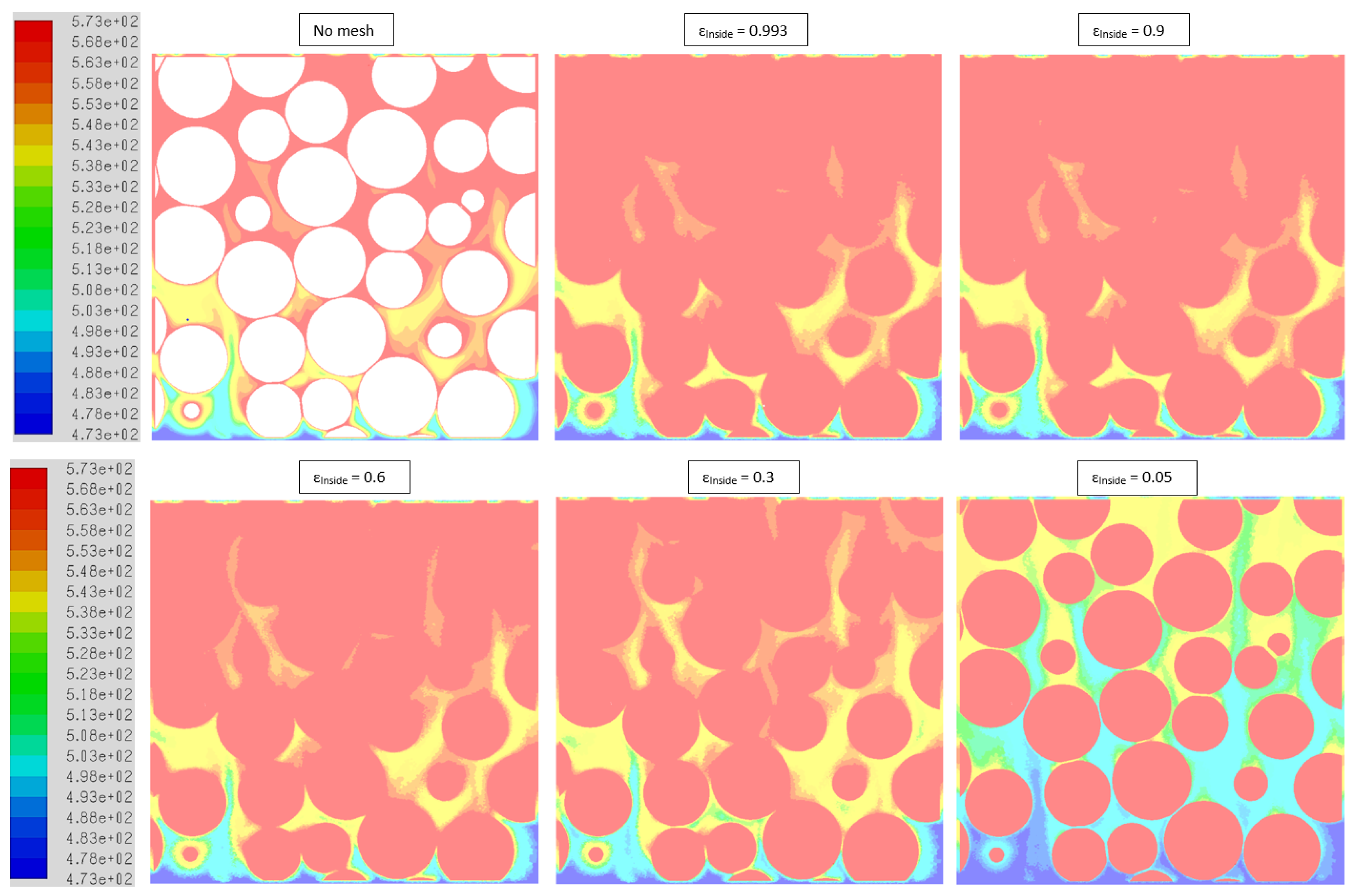
2.3.3. Mesh Dependence
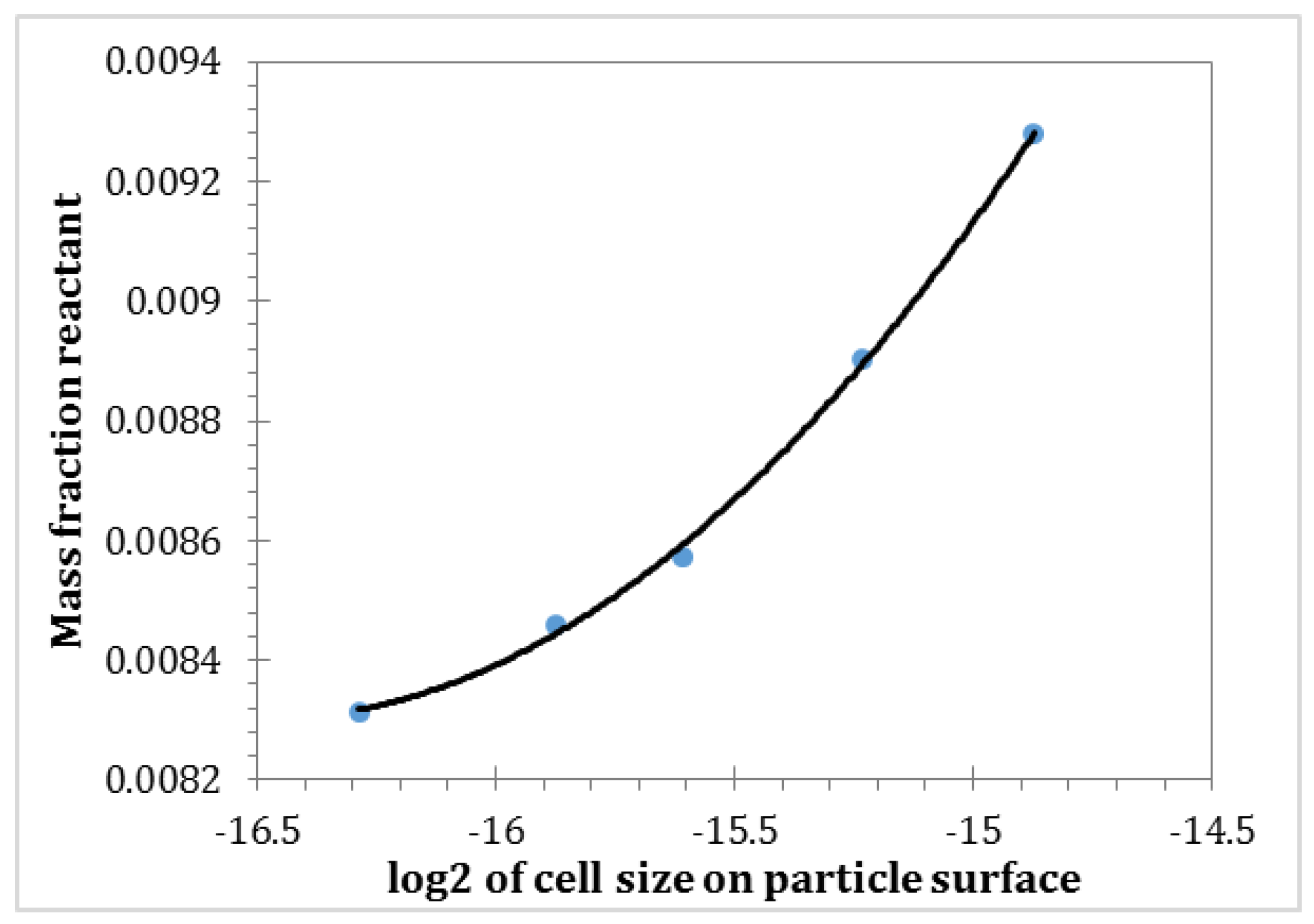
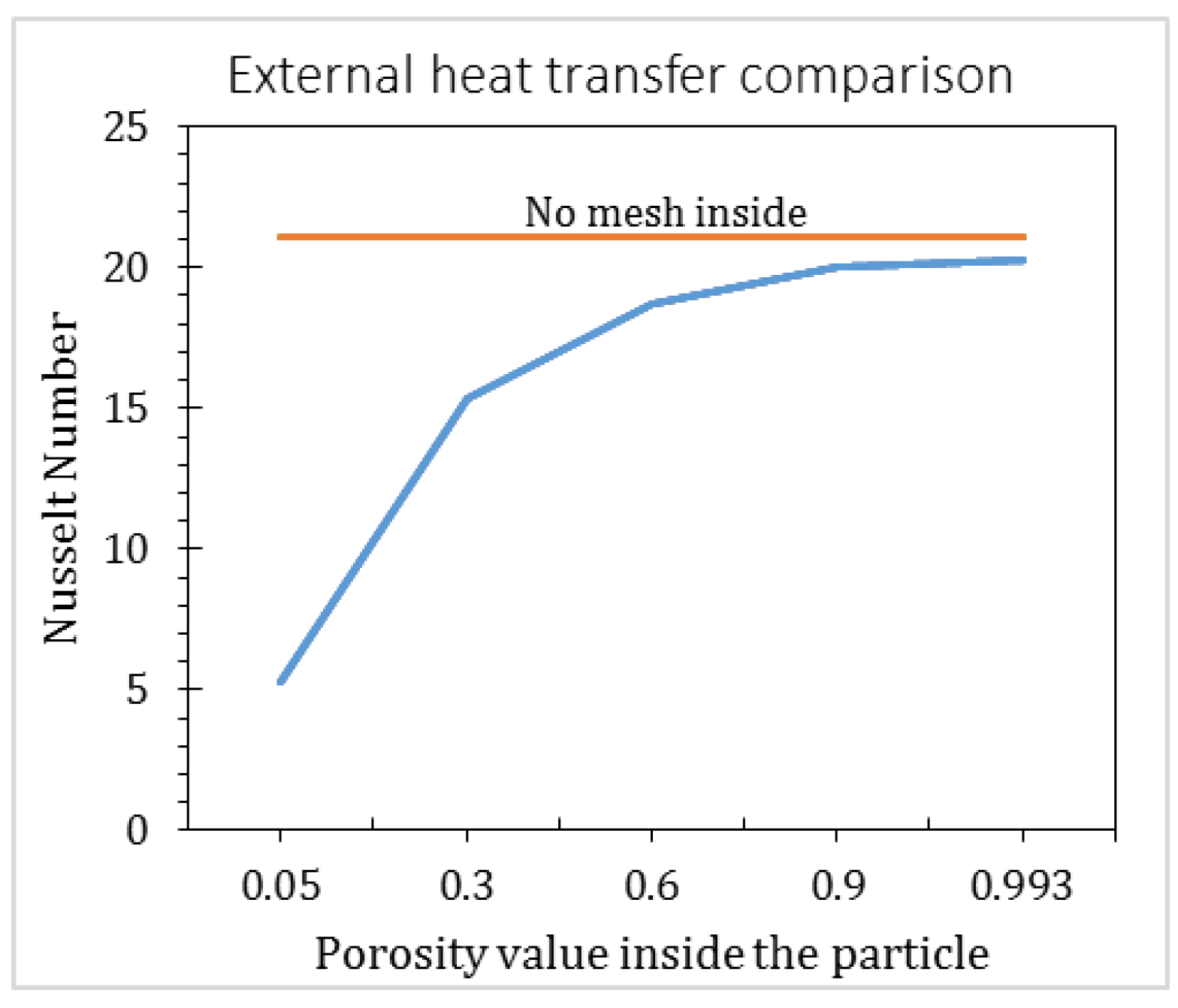
2.3.4. Void Fraction inside the Particle
2.4. 1D Packed Bed Model
3. Results and Discussion
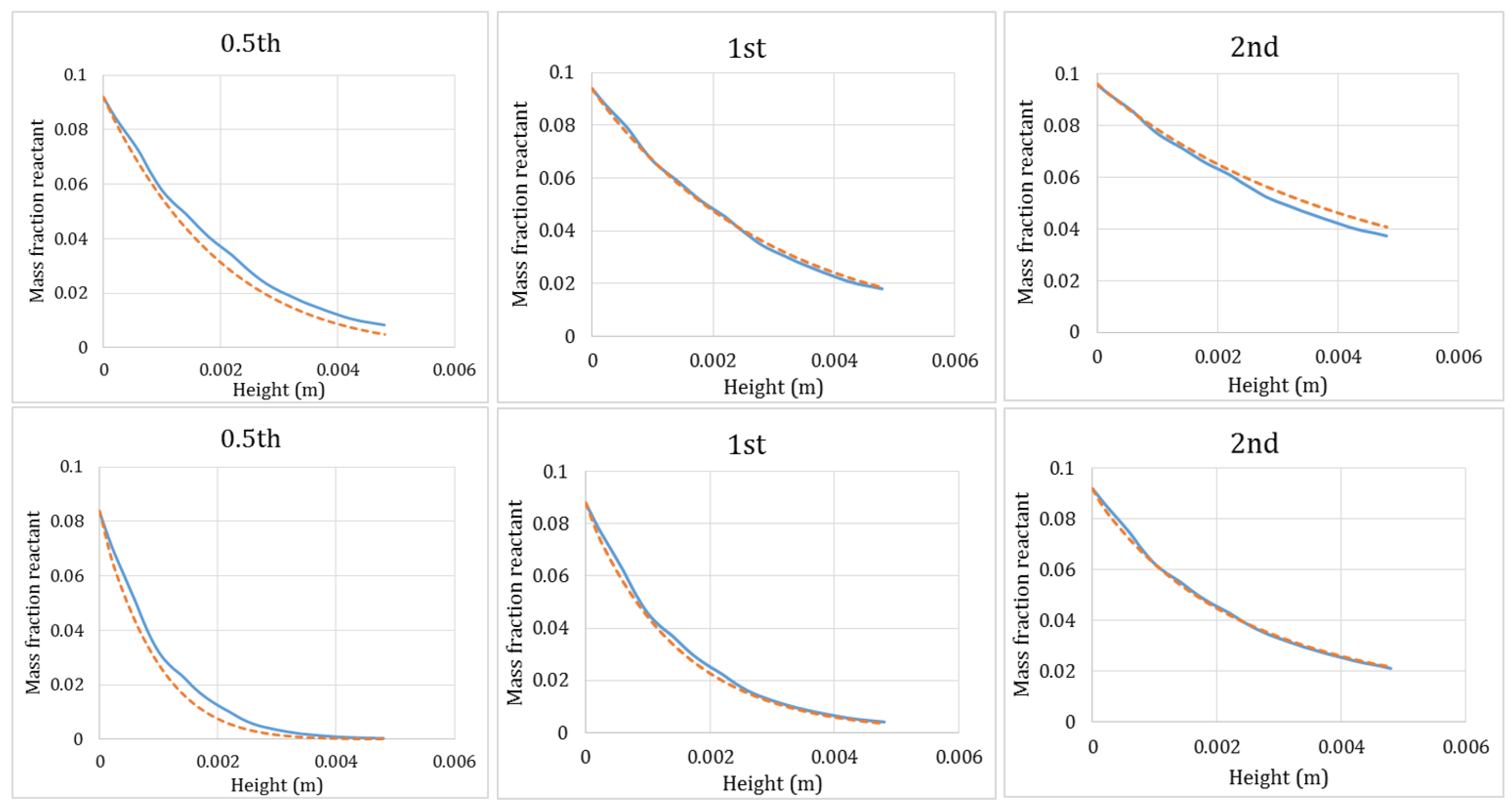
3.1. Single Isothermal Reaction with Different Reaction Orders
3.2. Multiple Isothermal Reactions
- single catalytic reaction with two reactants (Case 1, Equation (19))
- Two reactions with one independent reactant each (Case 2, Equation (20))
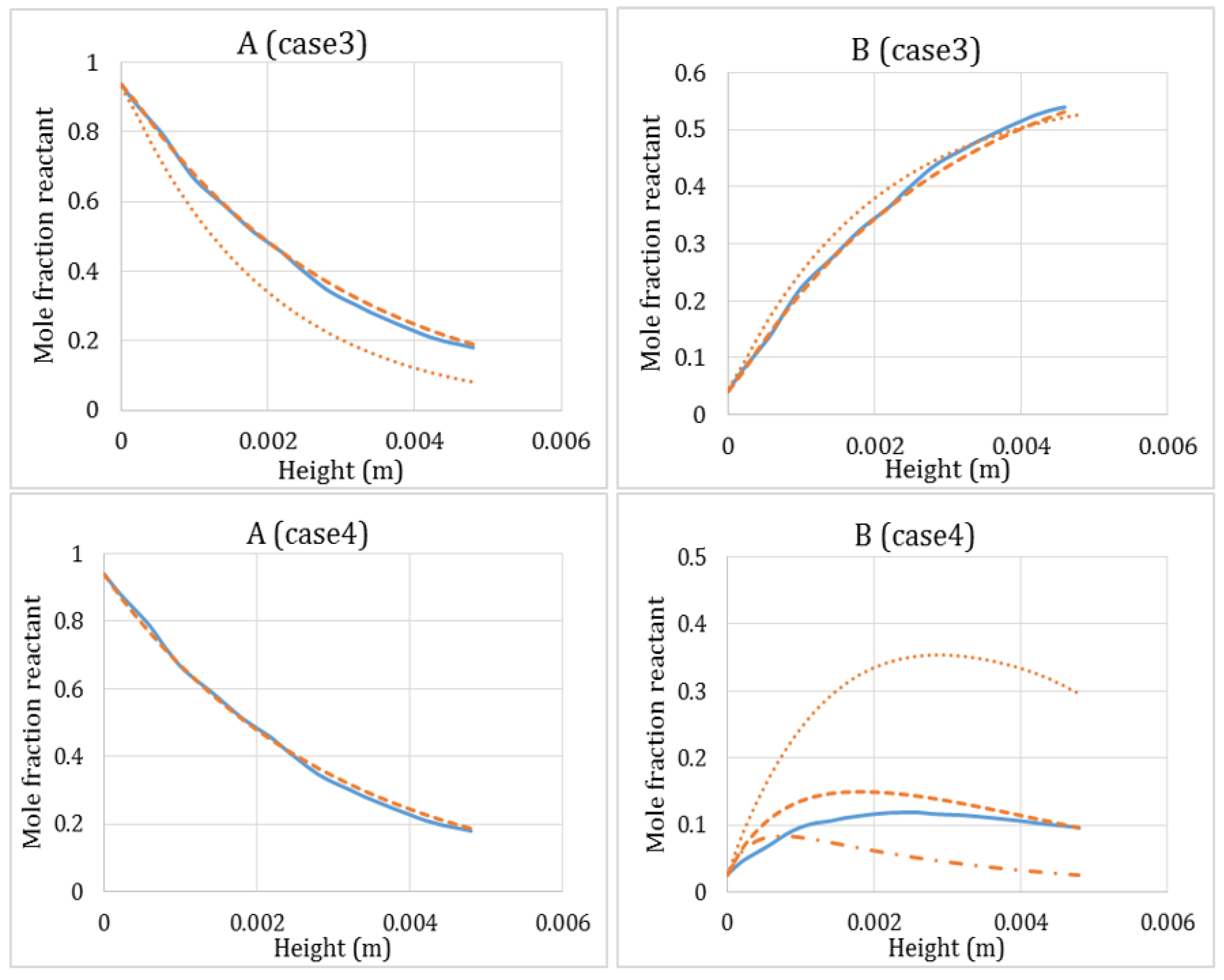
- Two reactions consuming the same reactant at different rates (Case 3, Equation (21), parallel reactions)
- Two reactions where the product of the first reaction is the reactant of the second (Case 4, Equation (22), sequential reactions)
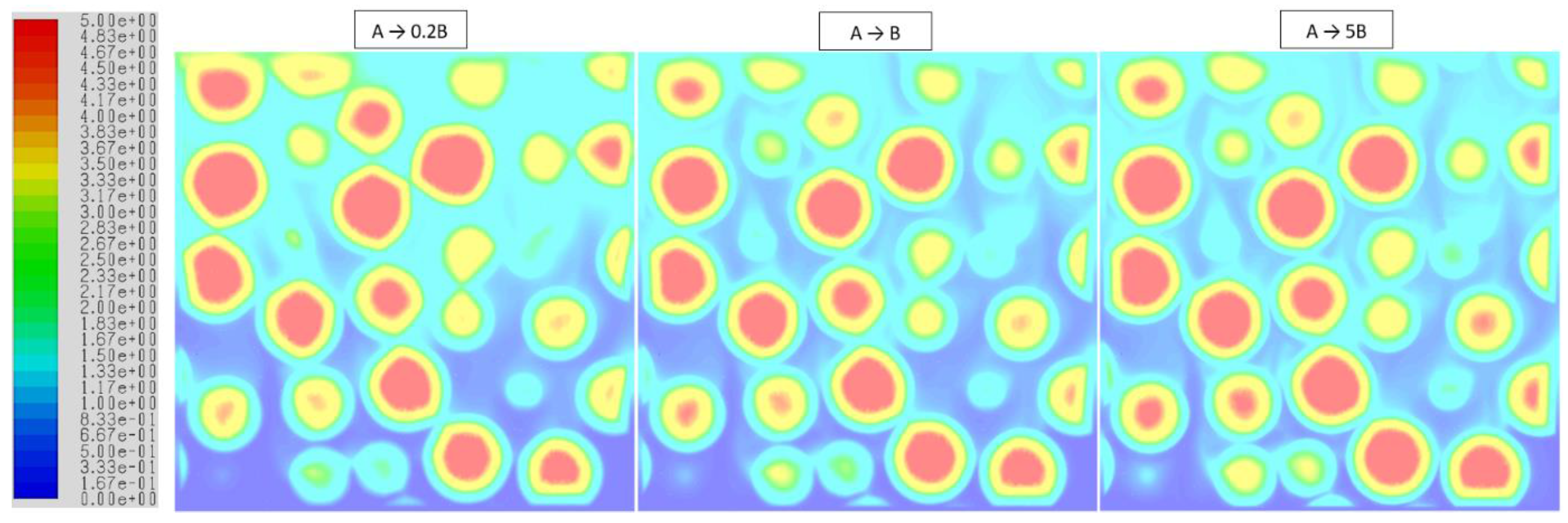
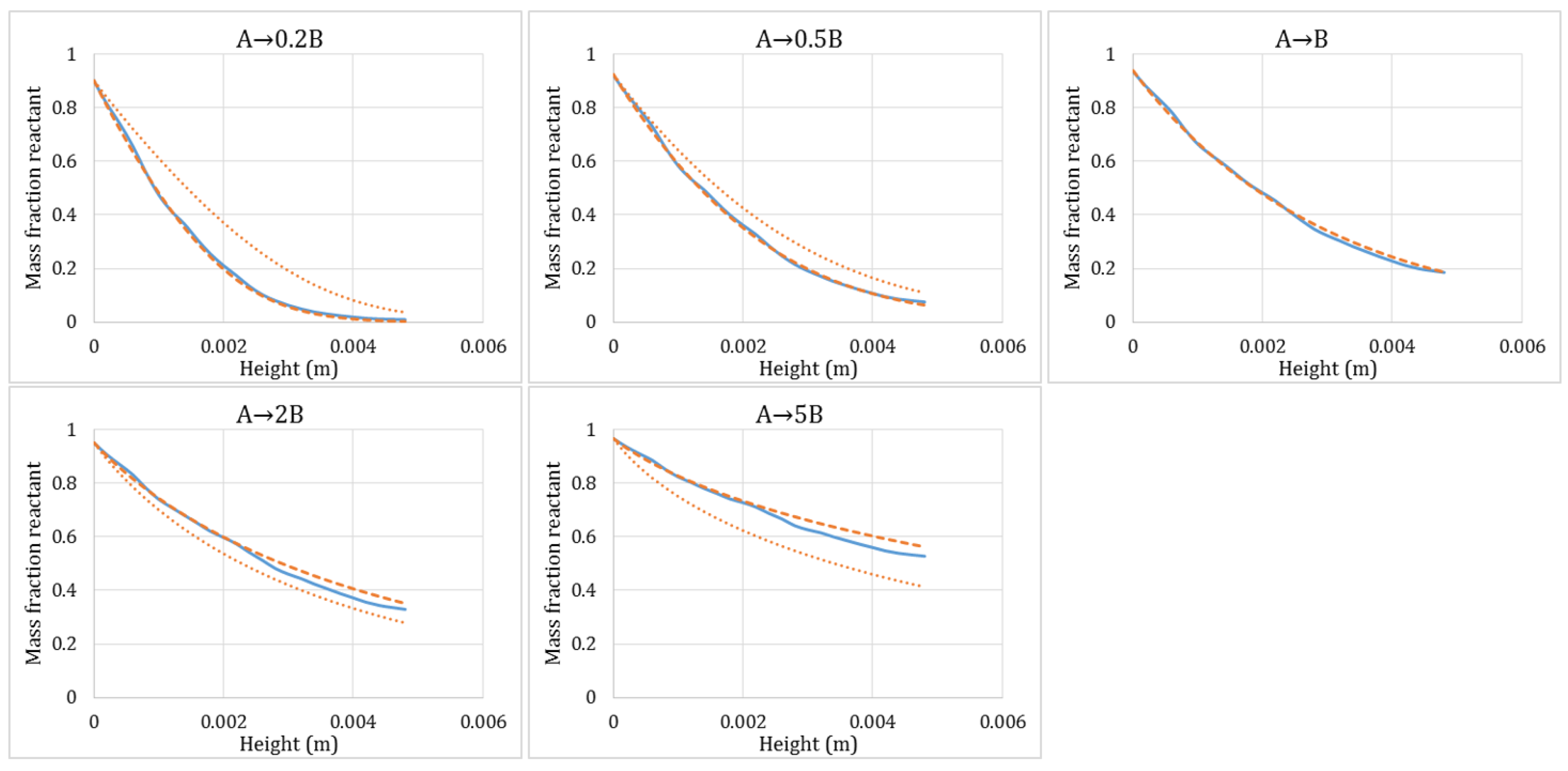
3.3. Isothermal Reactions with Gas Volume Generation/Consumption
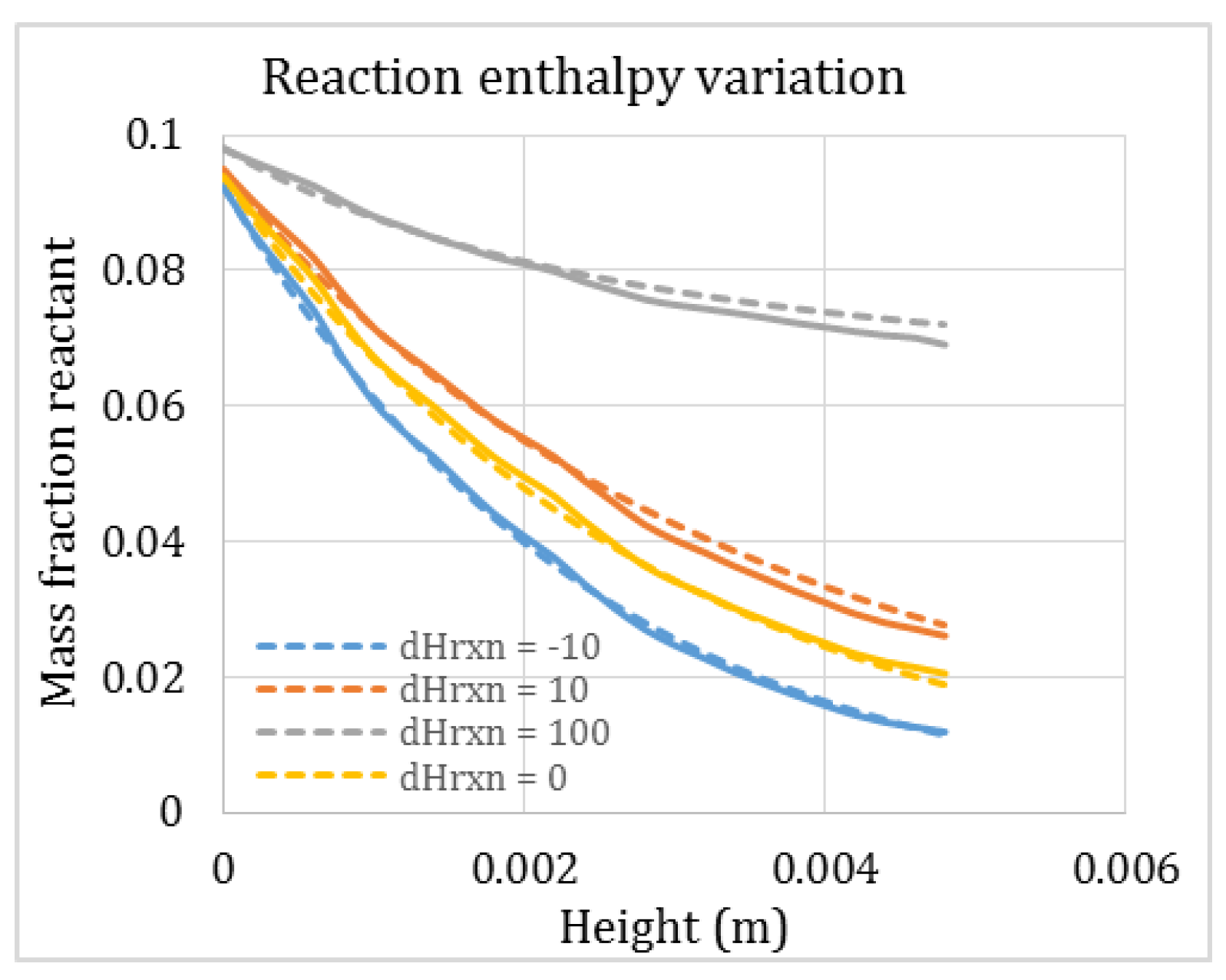
3.4. Combined Heat and Mass Transfer Resistance
3.5. Steam Methane Reforming Reactions
- Inclusion of all available model adjustments including multiple reactants (Equation (19)), reactions consuming the same reactant (Equation (22)), reactions consuming the products of other reactions (Equation (23)), and the effect of a varying reaction rate constant (Equation (26)).
- Deactivation of the adjustment for the varying reaction rate constant relative to Case 1 (Equation (26)).
- Deactivation of the adjustment for reactions consuming the products of other reactions relative to Case 2 (Equation (23)).
- Deactivation of the adjustment for reactions consuming the same reactant relative to Case 3 (Equation (22)).
- Deactivation of the adjustment for multiple reactants relative to Case 4 (Equation (19)).
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Nomenclature
| Symbols | |
| Characteristic length of spherical particle (dp/6) | |
| Spherical particle-fluid heat transfer surface per unit volume (ap = 6 ∙ (1 − ε)/dp) (1/m) | |
| Concentration of specie A (kmol/m3) | |
| Cp | Specific heat capacity of fluid (J/kg K) |
| dp | Diameter of the spherical particle (m) |
| dA | Area of the axial plane (m2) |
| D | Molecular diffusivity of gas (m2/s) |
| De | Effective diffusivity of gas (m2/s) |
| Vector in z-direction | |
| Activation energy (J/mol) | |
| g | Gravity (m/s2) |
| Enthalpy (J/kg) | |
| Heat transfer coefficient (W/m2K) | |
| J | Diffusive flux (kg/(m2 s)) |
| Reaction rate constant (mol1−n m3n−2/s) | |
| Arrhenius constant (1/s) | |
| K | Thermal conductivity of gas (W/m K) |
| Adsorption constant for specie (x = CH4, CO, H2,H2O) | |
| Interphase momentum exchange coefficient (kg/(m3·s)) | |
| mp | Mass of the particle (kg) |
| N | Ratio of diameter of reactor to diameter of the particle |
| Nu | Nusselt number |
| Partial pressure of the specie (bar) | |
| P | Pressure (Pa) |
| Pr | Prandtl number () |
| r | Reaction rate (mol/m3·s) |
| R | Gas constant (8.314 J/mol/K) |
| Re | Reynolds number () |
| S | Source term |
| Sc | Schmidt number () |
| t | Tortuosity |
| Static temperature of the fluid (K) | |
| Particle surface temperature (K) | |
| Bulk fluid temperature (K) | |
| Superficial velocity of the fluid (m/s) | |
| Velocity of the fluid in Z-direction (m/s) | |
| v | Velocity of the particles for Dem (m/s) |
| Volume in the region of interest (m3) | |
| Y | Specie concentration (mass or molar fraction) |
| y | Specie mole fraction |
| x | Specie mass fraction |
| Greek | |
| α | Volume fraction |
| ε | Porosity (void fraction) |
| Thiele modulus | |
| Density (kg/m3) | |
| Effectiveness factor | |
| Stress tensor (kg/(m·s2)) | |
| μ | Viscosity of fluid (kg/m s) |
| Vectors | |
| velocity (m/s) | |
| Gravity (m/s2) | |
| Subscripts | |
| p | particle |
| g | gas |
| e | effective |
| n | reaction order |
| i | specie i |
| j | Reaction and particle index |
| Operators | |
| Gradient operator (m−1) | |
| Divergence operator (m−1) | |
| time derivative (s−1) | |
Appendix A
| Parameters | Value |
|---|---|
| Particle diameter (dp) (m) | 0.005 |
| Packed bed voidage (ε) | 0.352 |
| Particle volume fraction (αp) | 0.007 |
| Density (solid) (kg/m3) | 2500 |
| Fluid velocity (m/s) | 0.5 |
| Inlet mole fraction ratio (CH4:H2O) | 1:2 |
| Specific heat capacity (Cp) (solid) (J/kg/k) | 1200 |
| Inlet temperature (°C) | 1100 |
| Operating pressure (bar) | 20 |
| Reactions | Kinetic Parameters | Equilibrium Parameters | ||
|---|---|---|---|---|
| k0,j | Ea | K0,j | Ea | |
| Steam methane reforming (R × 1) | 5.83 × 1011 | 218.55 × 103 | 1.2 × 1013 | 223.08 × 103 |
| Water gas shift (R × 2) | 2.51 × 104 | 73.523 × 103 | 1.77 × 10−2 | −36.58 × 103 |
| Overall steam methane reforming (R × 3) | 4.67 × 1013 | 236.85 × 103 | 2.124 × 1011 | 186 × 103 |
| Adsorption Parameters | ||
|---|---|---|
| Specie | k0,i | Ea |
| CH4 | 6.66 × 10−4 | −38.28 × 103 |
| CO | 8.25 × 10−5 | −70.65 × 103 |
| H2 | 6.15 × 10−9 | −82.90 × 103 |
| H2O | 1.77 × 105 | 88.68 × 103 |
References
- Dixon, A.G.; Nijemeisland, M. CFD as a design tool for fixed-bed reactors. Ind. Eng. Chem. Res. 2001, 40, 5246–5254. [Google Scholar] [CrossRef]
- Eppinger, T.; Wehinger, G.D.; Jurtz, N.; Aglave, R.; Kraume, M. A numerical optimization study on the catalytic dry reforming of methane in a spatially resolved fixed-bed reactor. Chem. Eng. Res. Des. 2016, 115, 374–381. [Google Scholar] [CrossRef]
- Karthik, G.M.; Buwa, V.V. Effect of particle shape on fluid flow and heat transfer for methane steam reforming reactions in a packed bed. AIChE J. 2017, 63, 366–377. [Google Scholar] [CrossRef]
- Maestri, M.; Cuoci, A. Coupling CFD with detailed microkinetic modeling in heterogeneous catalysis. Chem. Eng. Sci. 2013, 96, 106–117. [Google Scholar] [CrossRef]
- Nijemeisland, M.; Dixon, A.G.; Hugh Stitt, E. Catalyst design by CFD for heat transfer and reaction in steam reforming. Chem. Eng. Sci. 2004, 59, 5185–5191. [Google Scholar] [CrossRef]
- Partopour, B.; Dixon, A.G. Resolved-particle fixed bed CFD with microkinetics for ethylene oxidation. AIChE J. 2017, 63, 87–94. [Google Scholar] [CrossRef]
- Wehinger, G.D.; Fütterer, C.; Kraume, M. Contact modifications for CFD simulations of fixed-bed reactors: Cylindrical particles. Ind. Eng. Chem. Res. 2017, 56, 87–99. [Google Scholar] [CrossRef]
- Mirhashemi, F.S.; Hashemabadi, S.H. Experimental and CFD study of wall effects on orderly stacked cylindrical particles heat transfer in a tube channel. Int. Commun. Heat Mass Transf. 2012, 39, 449–455. [Google Scholar] [CrossRef]
- Mirhashemi, F.S.; Hashemabadi, S.H.; Noroozi, S. CFD simulation and experimental validation for wall effects on heat transfer of finite cylindrical catalyst. Int. Commun. Heat Mass Transf. 2011, 38, 1148–1155. [Google Scholar] [CrossRef]
- Motlagh, A.H.A.; Hashemabadi, S.H. 3D CFD simulation and experimental validation of particle-to-fluid heat transfer in a randomly packed bed of cylindrical particles. Int. Commun. Heat Mass Transf. 2008, 35, 1183–1189. [Google Scholar] [CrossRef]
- Zare, M.; Hashemabadi, S.H. Experimental study and CFD simulation of wall effects on heat transfer of an extrudate multi-lobe particle. Int. Commun. Heat Mass Transf. 2013, 43, 122–130. [Google Scholar] [CrossRef]
- Atmakidis, T.; Kenig, E.Y. CFD-based analysis of the wall effect on the pressure drop in packed beds with moderate tube/particle diameter ratios in the laminar flow regime. Chem. Eng. J. 2009, 155, 404–410. [Google Scholar] [CrossRef]
- Bai, H.; Theuerkauf, J.; Gillis, P.A.; Witt, P.M. A coupled DEM and CFD simulation of flow field and pressure drop in fixed bed reactor with randomly packed catalyst particles. Ind. Eng. Chem. Res. 2009, 48, 4060–4074. [Google Scholar] [CrossRef]
- Eppinger, T.; Seidler, K.; Kraume, M. DEM-CFD simulations of fixed bed reactors with small tube to particle diameter ratios. Chem. Eng. J. 2011, 166, 324–331. [Google Scholar] [CrossRef]
- Lee, J.J.; Yoon, S.J.; Park, G.C.; Lee, W.J. Turbulence-induced heat transfer in pbmr core using les and rans. J. Nucl. Sci. Technol. 2007, 44, 985–996. [Google Scholar] [CrossRef]
- Romkes, S.J.P.; Dautzenberg, F.M.; van den Bleek, C.M.; Calis, H.P.A. CFD modelling and experimental validation of particle-to-fluid mass and heat transfer in a packed bed at very low channel to particle diameter ratio. Chem. Eng. J. 2003, 96, 3–13. [Google Scholar] [CrossRef]
- Jafari, A.; Zamankhan, P.; Mousavi, S.M.; Pietarinen, K. Modeling and CFD simulation of flow behavior and dispersivity through randomly packed bed reactors. Chem. Eng. J. 2008, 144, 476–482. [Google Scholar] [CrossRef]
- Deen, N.G.; Peters, E.A.J.F.; Padding, J.T.; Kuipers, J.A.M. Review of direct numerical simulation of fluid–particle mass, momentum and heat transfer in dense gas-solid flows. Chem. Eng. Sci. 2014, 116, 710–724. [Google Scholar] [CrossRef]
- Singhal, A.; Cloete, S.; Radl, S.; Quinta-Ferreira, R.; Amini, S. Heat transfer to a gas from densely packed beds of monodisperse spherical particles. Chem. Eng. J. 2017, 314, 27–37. [Google Scholar] [CrossRef]
- Singhal, A.; Cloete, S.; Radl, S.; Quinta-Ferreira, R.; Amini, S. Heat transfer to a gas from densely packed beds of cylindrical particles. Chem. Eng. Sci. 2017, 172, 1–12. [Google Scholar] [CrossRef]
- Sun, B.; Tenneti, S.; Subramaniam, S. Modeling average gas-solid heat transfer using particle-resolved direct numerical simulation. Int. J. Heat Mass Transf. 2015, 86, 898–913. [Google Scholar] [CrossRef]
- Tavassoli, H.; Peters, E.A.J.F.; Kuipers, J.A.M. Direct numerical simulation of fluid–particle heat transfer in fixed random arrays of non-spherical particles. Chem. Eng. Sci. 2015, 129, 42–48. [Google Scholar] [CrossRef]
- Augier, F.; Idoux, F.; Delenne, J.Y. Numerical simulations of transfer and transport properties inside packed beds of spherical particles. Chem. Eng. Sci. 2010, 65, 1055–1064. [Google Scholar] [CrossRef]
- Dixon, A.G.; Nijemeisland, M.; Stitt, H. CFD simulation of reaction and heat transfer near the wall of a fixed bed. Int. J. Chem. React. Eng. 2003, 1. [Google Scholar] [CrossRef]
- Wehinger, G.D.; Kraume, M.; Berg, V.; Korup, O.; Mette, K.; Schlögl, R.; Behrens, M.; Horn, R. Investigating dry reforming of methane with spatial reactor profiles and particle-resolved CFD simulations. AIChE J. 2016, 62, 4436–4452. [Google Scholar] [CrossRef]
- Singhal, A.; Cloete, S.; Radl, S.; Quinta-Ferreira, R.; Amini, S. Multiscale modelling of heat transfer from arrays of spherical particles. In Proceedings of the 9th International Conference on Multiphase Flow (ICMF), Firenze, Italy, 22–27 May 2016; ICMF: Firenze, Italy, 2016. [Google Scholar]
- Dixon, A.G. Local transport and reaction rates in a fixed bed reactor tube: Endothermic steam methane reforming. Chem. Eng. Sci. 2017, 168, 156–177. [Google Scholar] [CrossRef]
- Mousazadeh, F.; van Den Akker, H.E.A.; Mudde, R.F. Direct numerical simulation of an exothermic gas-phase reaction in a packed bed with random particle distribution. Chem. Eng. Sci. 2013, 100, 259–265. [Google Scholar] [CrossRef]
- Zhou, X.; Duan, Y.; Huai, X.; Li, X. 3D CFD modeling of acetone hydrogenation in fixed bed reactor with spherical particles. Particuology 2013, 11, 715–722. [Google Scholar] [CrossRef]
- Dixon, A.G.; Taskin, M.E.; Nijemeisland, M.; Stitt, E.H. CFD method to couple three-dimensional transport and reaction inside catalyst particles to the fixed bed flow field. Ind. Eng. Chem. Res. 2010, 49, 9012–9025. [Google Scholar] [CrossRef]
- Dixon, A.G.; Boudreau, J.; Rocheleau, A.; Troupel, A.; Taskin, M.E.; Nijemeisland, M.; Stitt, E.H. Flow, transport, and reaction interactions in shaped cylindrical particles for steam methane reforming. Ind. Eng. Chem. Res. 2012, 51, 15839–15854. [Google Scholar] [CrossRef]
- Singhal, A.; Cloete, S.; Quinta-Ferreira, R.; Amini, S. Multiscale modelling of packed bed chemical looping reforming. Energy Procedia 2017, 136, 349–355. [Google Scholar] [CrossRef]
- Singhal, A.; Cloete, S.; Radl, S.; Quinta-Ferreira, R.; Amini, S. Comparison of particle-resolved direct numerical simulation and 1d modelling of catalytic reactions in a packed bed. In Proceedings of the 12th International Conference on CFD in Oil & Gas, Metallurgical and Process Industries, Trondheim, Norway, 30 May–1 June 2017; Progress in Applied CFD—CFD2017: Trondheim, Norway, 2017; pp. 667–674. [Google Scholar]
- Singhal, A.; Cloete, S.; Quinta-Ferreira, R.; Amini, S. Comparison of particle-resolved direct numerical simulation and 1d modelling of catalytic reactions in a cylindrical particle bed. In Proceedings of the Vth International Conference on Particle-Based Methods—Fundamentals and Applications, Hannover, Germany, 26–28 September 2017; International Center for Numerical Methods in Engineering (CIMNE): Hannover, Germany, 2017; pp. 802–812. [Google Scholar]
- Rawlings, J.B.A.E.; John, G. Chapter 7. In Chemical Reactor Analysis and Design Fundamentals; Nob Hill Publishing: Madison, WI, USA, 2002. [Google Scholar]
- Yang, W.; Cloete, S.; Morud, J.; Amini, S. An effective reaction rate model for gas-solid reactions with high intra-particle diffusion resistance. Int. J. Chem. React. Eng. 2016, 14, 331–342. [Google Scholar] [CrossRef]
- Ishida, M.; Wen, C.Y. Comparison of kinetic and diffusional models for solid-gas reactions. AIChE J. 1968, 14, 311–317. [Google Scholar] [CrossRef]
- Levenspiel, O. Chemical Reaction Engineering, 3rd ed.; John Wiley & Sons: New York, NY, USA, 1999; Volume 3. [Google Scholar]
- Thiele, E.W. Relation between catalytic activity and size of particle. Ind. Eng. Chem. 1939, 31, 916–920. [Google Scholar] [CrossRef]
- Ergun, S. Fluid flow through packed columns. Chem. Eng. Prog. 1952, 48, 89–94. [Google Scholar]
- Szekely, J.; Evans, J.W.; Sohn, H.Y. Gas-Solid Reactions; Academic Press: New York, NY, USA, 1976; p. 612. [Google Scholar]
- Deen, N.G.; Kriebitzsch, S.H.L.; van der Hoef, M.A.; Kuipers, J.A.M. Direct numerical simulation of flow and heat transfer in dense fluid–particle systems. Chem. Eng. Sci. 2012, 81, 329–344. [Google Scholar] [CrossRef]
- Tenneti, S.; Sun, B.; Garg, R.; Subramaniam, S. Role of fluid heating in dense gas-solid flow as revealed by particle-resolved direct numerical simulation. Int. J. Heat Mass Transf. 2013, 58, 471–479. [Google Scholar] [CrossRef]
- Cloete, S.; Gallucci, F.; van Sint Annaland, M.; Amini, S. Gas switching as a practical alternative for scaleup of chemical looping combustion. Energy Technol. 2016, 4, 1286–1298. [Google Scholar] [CrossRef]
- Singhal, A.; Cloete, S.; Quinta-Ferreira, R.; Amini, S. Multiscale modeling of a packed bed chemical looping reforming (pbclr) reactor. Energies 2017, 10, 2056. [Google Scholar] [CrossRef]
- Xu, J.; Froment, G.F. Methane steam reforming, methanation and water-gas shift: I. Intrinsic kinetics. AIChE J. 1989, 35, 88–96. [Google Scholar] [CrossRef]
- Oliveira, E.L.G.; Grande, C.A.; Rodrigues, A.E. Methane steam reforming in large pore catalyst. Chem. Eng. Sci. 2010, 65, 1539–1550. [Google Scholar] [CrossRef]
- Francisco Morgado, J.; Cloete, S.; Morud, J.; Gurker, T.; Amini, S. Modelling study of two chemical looping reforming reactor configurations: Looping vs. Switching. Powder Technol. 2016, 316, 599–613. [Google Scholar] [CrossRef]
- Ortiz, M.; de Diego, L.F.; Abad, A.; García-Labiano, F.; Gayán, P.; Adánez, J. Catalytic activity of ni-based oxygen-carriers for steam methane reforming in chemical-looping processes. Energy Fuels 2012, 26, 791–800. [Google Scholar] [CrossRef]
- Zhou, Z.; Han, L.; Bollas, G.M. Model-based analysis of bench-scale fixed-bed units for chemical-looping combustion. Chem. Eng. J. 2013, 233, 331–348. [Google Scholar] [CrossRef]





| Parameter | Value |
|---|---|
| Particle size (m) | 1 × 10−3 |
| Bed porosity (ε) | 0.352 |
| Particle inside volume fraction (αp) | 0.007 |
| Number of particles | 107 |
| Diameter of the geometry (m) | 4.8 × 10−3 |
| Height of the geometry (m) | 4.8 × 10−3 |
| Mesh resolution (particle surface) | dp/60 |
| Pre-exponential factor (k0) (1/s) | 1.674 × 109 |
| Activation energy (J/mol) | 100,000 |
| Thermal conductivity of solid (W/m K) | 500 |
| Parameter | Value | |
| Prandtl number | 1 | |
| Reynolds number | 100 | |
| Thiele modulus | 10; 5 | |
| Temperature of Inlet (K) | 1000 | |
| Inlet mass fraction (specie A) | 0.1 | |
| Heat of reaction (kJ/mol) | 0 | |
| Operating pressure (bar) | 1 | |
| Thiele modulus | = 10 | = 5 |
| Molecular diffusivity (m2/s) | 1.38889 × 10−5 | 5.55556 × 10−5 |
| Reaction rate constants | ||
| 0.5th order | 4.22 × 104 | |
| 1st order | 1.00 × 104 | |
| 2nd order | 6.67 × 102 | |
| Parameter | Gases | Particles |
| Dynamic Viscosity (µ) (kg/m·s) | 1 × 10−5 | - |
| Density (ρ) (kg/m3) | 1 | 2500 |
| Thermal conductivity (W/m·K) | 0.01 | 500 |
| Molecular weight (kg/kg·mol) | 10 | 10 |
| Specific heat (Cp) (J/kg·K) | 1000 | 1000 |
| Reactions | Molecular Weight (kg/kmol) | Density (kg/m3) | ||
|---|---|---|---|---|
| Analogous Cases | A | B | A | B |
| (i) (a) A + solid → 0.2B + solid | 10 | 50 | 1 | 5 |
| (b) A + solid → 5B + solid | 10 | 2 | 1 | 0.2 |
| (ii) A + solid → B + solid | 10 | 10 | 1 | 1 |
| (iii) (a) A + solid → 0.5B + solid | 10 | 20 | 1 | 2 |
| (b) A + solid → 2B + solid | 10 | 5 | 1 | 0.5 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Singhal, A.; Cloete, S.; Quinta-Ferreira, R.; Amini, S. Verification of Heat and Mass Transfer Closures in Industrial Scale Packed Bed Reactor Simulations. Energies 2018, 11, 805. https://doi.org/10.3390/en11040805
Singhal A, Cloete S, Quinta-Ferreira R, Amini S. Verification of Heat and Mass Transfer Closures in Industrial Scale Packed Bed Reactor Simulations. Energies. 2018; 11(4):805. https://doi.org/10.3390/en11040805
Chicago/Turabian StyleSinghal, Arpit, Schalk Cloete, Rosa Quinta-Ferreira, and Shahriar Amini. 2018. "Verification of Heat and Mass Transfer Closures in Industrial Scale Packed Bed Reactor Simulations" Energies 11, no. 4: 805. https://doi.org/10.3390/en11040805
APA StyleSinghal, A., Cloete, S., Quinta-Ferreira, R., & Amini, S. (2018). Verification of Heat and Mass Transfer Closures in Industrial Scale Packed Bed Reactor Simulations. Energies, 11(4), 805. https://doi.org/10.3390/en11040805




