Algal Biomass from Wastewater and Flue Gases as a Source of Bioenergy
Abstract
:1. Introduction
2. The Composition of Wastewater and Flue Gases
3. Microalgal Wastewater Reclamation and Biomass Production
3.1. Algal Growing Regimes and Physiology to Increase Target Macromolecules
3.2. Microalgae Strains Used for Wastewater Reclamation
3.3. Monoculture Versus Community
3.4. Control of Grazers
3.5. Enhanced Lipid Production During Wastewater Treatment
3.6. Microalgae Used in Wastewater and Flue-Gas Treatment of Different Origin
3.7. Removal of Pharmaceuticals
3.8. Heavy-Metal Contamination in Wastewater
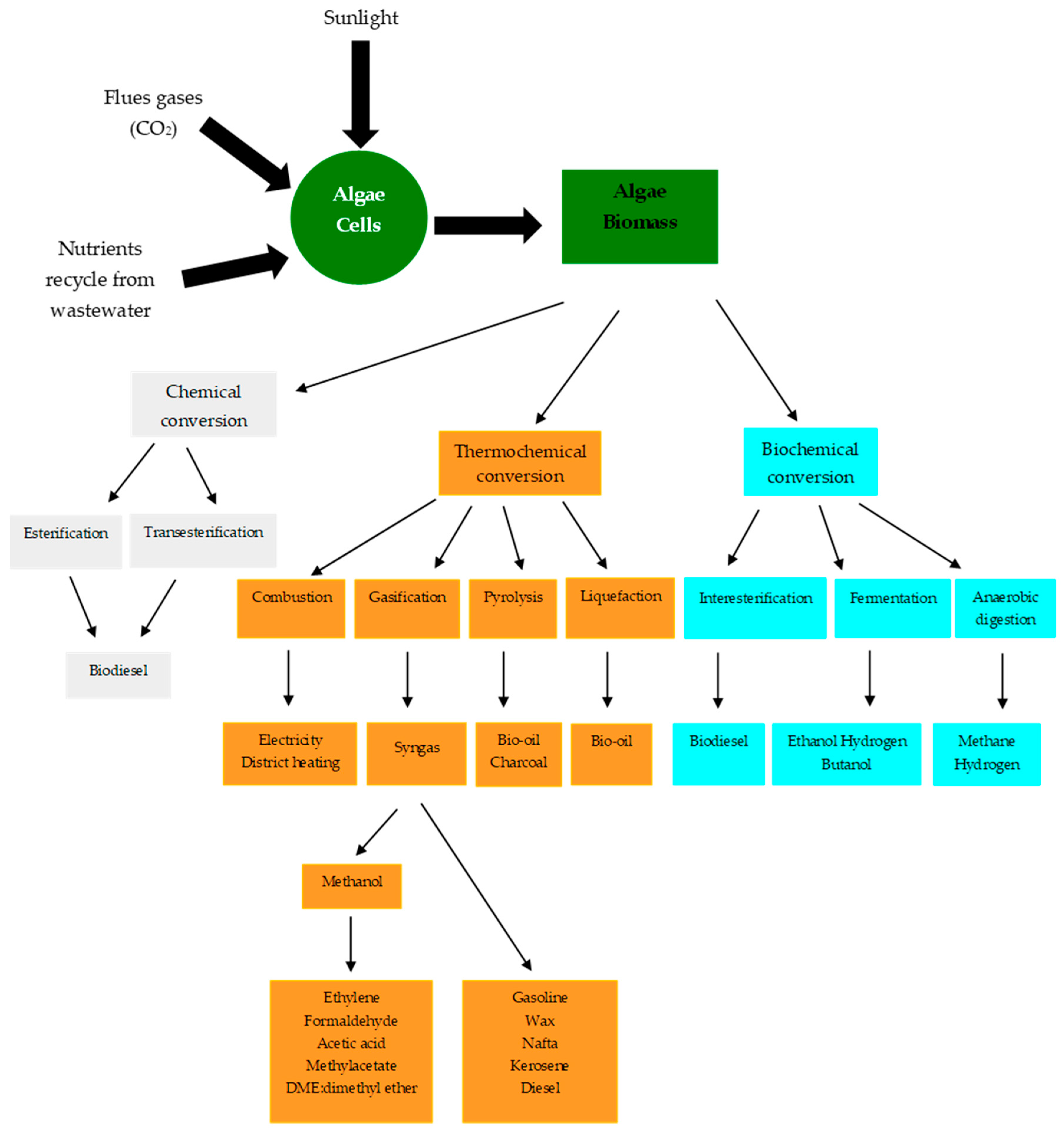
4. The Use of Algal Biomass
4.1. Chemical Conversion of Algal Lipids
4.1.1. Esterification
4.1.2. Transesterification
4.2. Thermochemical Conversion
4.2.1. Combustion
4.2.2. Pyrolysis
4.2.3. Liquefaction
4.2.4. Gasification
4.3. Biochemical Conversion
4.3.1. Interesterification
4.3.2. Anaerobic Digestion
4.3.3. Fermentation
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- United Nations. World Population Prospects: The 2006 Revision, Highlights; Working Paper No. Esa/p/wp. 202; Department of Economic and Social Affairs, Population Division, United Nations: New York, NY, USA, 2007. [Google Scholar]
- International Energy Agency. Key World Energy Statistics; International Energy Agency Publications: Paris, France, 2017. [Google Scholar]
- Hill, J.; Nelson, E.; Tilman, D.; Polasky, S.; Tiffany, D. Environmental, economic, and energetic costs and benefits of biodiesel and ethanol biofuels. Proc. Natl. Acad. Sci. USA 2006, 103, 11206–11210. [Google Scholar] [CrossRef] [PubMed]
- Ormerod, W.G.; Freund, P.; Smith, A.; Davison, J. Ocean Storage of CO2; International Energy Agency Greenhouse Gas R&D Programme: Cheltenham, UK, 2002. [Google Scholar]
- European Council Directive 2009/28/EC on the Promotion of the Use of Energy from Renewable Sources and Amending and Subsequently Repealing Directives 2001/77/EC and 2003/30/EC; European Council: Brussels, Belgium, 2009.
- Beurskens, L.W.M.; Hekkenberg, M. Renewable Energy Projections as Published in the National Renewable Energy Action Plans of the European Member States; Energy Research Centre of the Netherlands and European Environment Agency: Petten, The Netherlands, 2011. [Google Scholar]
- European Commission. Energy 2020—A Strategy for Competitive, Sustainable and Secure Energy; European Commission: Brussels, Belgium, 2010. [Google Scholar]
- Food and Agricultural Organization (FAO). Introducing the International Bio-Energy Platform; FAO: Rome, Italy, 2006. [Google Scholar]
- Hay, R.K.M.; Porter, J.R. The Physiology of Crop Yield; Blackwell Publishing: Oxford, UK, 2006. [Google Scholar]
- Zhang, Q.; Chang, J.; Wang, T.; Xu, Y. Review of biomass pyrolysis oil properties and upgrading research. Energy Convers. Manag. 2007, 48, 87–92. [Google Scholar] [CrossRef]
- Olsson, G. Water and Energy: Threats and Opportunities; IWA Publishing: London, UK, 2015. [Google Scholar]
- Fargione, J.E.; Plevin, R.J.; Hill, J.D. The ecological impact of biofuels. Annu. Rev. Ecol. Evol. Syst. 2010, 41, 351–377. [Google Scholar] [CrossRef]
- McKendry, P. Energy production from biomass (part 1): Overview of biomass. Bioresour. Technol. 2002, 83, 37–46. [Google Scholar] [CrossRef]
- European Commission. The Impact of Biofuels on Transport and the Environment, and Their Connection with Agricultural Development in Europe; Directorate General for the Internal Policies Policy Department B: Structural and Cohesion Policies—Transport and Tourism; European Commission: Brussels, Belgium, 2015. [Google Scholar]
- Gibbs, H.K.; Brown, S.; Niles, J.O.; Foley, J.A. Monitoring and estimating tropical forest carbon stocks: Making redd a reality. Environ. Res. Lett. 2007, 2, 045023. [Google Scholar] [CrossRef]
- Fargione, J.; Hill, J.; Tilman, D.; Polasky, S.; Hawthorne, P. Land clearing and the biofuel carbon debt. Science 2008, 319, 1235–1238. [Google Scholar] [CrossRef] [PubMed]
- Larson, E.D. A review of life-cycle analysis studies on liquid biofuel systems for the transport sector. Energy Sustain. Dev. 2006, 10, 109–126. [Google Scholar] [CrossRef]
- Dominguez-Faus, R.; Powers, S.E.; Burken, J.G.; Alvarez, P.J. The water footprint of biofuels: A drink or drive issue? Environ. Sci. Technol. 2009, 43, 3005–3010. [Google Scholar] [CrossRef] [PubMed]
- Immerzeel, D.J.; Verweij, P.A.; van der Hilst, F.; Faaij, A.P.C. Biodiversity impacts of bioenergy crop production: A state-of-the-art review. GCB Bioenergy 2014, 6, 183–209. [Google Scholar] [CrossRef]
- Mitchell, D. A Note on Rising Food Prices; Police Research Working Paper; World Bank: Washington, DC, USA, 2008; Volume 4682. [Google Scholar]
- Eisberg, N. Harvesting Energy: Neil Eisberg Catches Up on the Latest Developments in Second-Generation Bioethanol Production.(Biofuels); Ten Alps Publishing: London, UK, 2006. [Google Scholar]
- Zhang, J.; Osmani, A.; Awudu, I.; Gonela, V. An integrated optimization model for switchgrass-based bioethanol supply chain. Appl. Energy 2013, 102, 1205–1217. [Google Scholar] [CrossRef]
- Banse, M.; Meijl, H.V.; Tabeau, A.; Woltjer, G.; Hellmann, F.; Verburg, P.H. Impact of eu biofuel policies on world agricultural production and land use. Biomass Bioenergy 2011, 35, 2385–2390. [Google Scholar] [CrossRef]
- Secchi, S.; Kurkalova, L.; Gassman, P.W.; Hart, C. Land Use Change in a Biofuels Hotspot: The Case of Iowa, Usa; Department of Economics, Iowa State University: Ames, IA, USA, 2011. [Google Scholar]
- Chisti, Y. Biodiesel from microalgae. Biotechnol. Adv. 2007, 25, 294–306. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Horsman, M.; Wu, N.; Lan, C.Q.; Dubois-Calero, N. Biofuels from microalgae. Biotechnol. Prog. 2008, 24, 815–820. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Horsman, M.; Wang, B.; Wu, N.; Lan, C.Q. Effects of nitrogen sources on cell growth and lipid accumulation of green alga Neochloris oleoabundans. Appl. Microbiol. Biotechnol. 2008, 81, 629–636. [Google Scholar] [CrossRef] [PubMed]
- Metting, F.B. Biodiversity and application of microalgae. J. Ind. Microbiol. 1996, 17, 477–489. [Google Scholar] [CrossRef]
- Schenk, P.M.; Thomas-Hall, S.R.; Stephens, E.; Marx, U.C.; Mussgnug, J.H.; Posten, C.; Kruse, O.; Hankamer, B. Second generation biofuels: High-efficiency microalgae for biodiesel production. Bioenergy Res. 2008, 1, 20–43. [Google Scholar] [CrossRef]
- Griffiths, M.J.; Harrison, S.T.L. Lipid productivity as a key characteristic for choosing algal species for biodiesel production. J. Appl. Phycol. 2009, 21, 493–507. [Google Scholar] [CrossRef]
- Ullah, K.; Ahmad, M.; Sofia; Sharma, V.K.; Lu, P.; Harvey, A.; Zafar, M.; Sultana, S.; Anyanwu, C.N. Algal biomass as a global source of transport fuels: Overview and development perspectives. Prog. Nat. Sci. Mater. 2014, 24, 329–339. [Google Scholar] [CrossRef]
- Rodolfi, L.; Chini Zittelli, G.; Bassi, N.; Padovani, G.; Biondi, N.; Bonini, G.; Tredici, M.R. Microalgae for oil: Strain selection, induction of lipid synthesis and outdoor mass cultivation in a low-cost photobioreactor. Biotechnol. Bioeng. 2009, 102, 100–112. [Google Scholar] [CrossRef] [PubMed]
- Mata, T.M.; Martins, A.A.; Caetano, N.S. Microalgae for biodiesel production and other applications: A review. Renew. Sust. Energy Rev. 2010, 14, 217–232. [Google Scholar] [CrossRef]
- Delucchi, M. A Lifecycle Emissions Model (LEM): Lifecycle Emissions from Transportation Fuels, Motor Vehicles, Transportation Modes, Electricity Use, Heating and Cooking Fuels, and Materials; Institute of Transportation Studies, University of California: Davis, CA, USA, 2003. [Google Scholar]
- Pittman, J.K.; Dean, A.P.; Osundeko, O. The potential of sustainable algal biofuel production using wastewater resources. Bioresour. Technol. 2011, 102, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Li, Y.; Wu, N.; Lan, C.Q. CO2 bio-mitigation using microalgae. Appl. Microbiol. Biotechnol. 2008, 79, 707–718. [Google Scholar] [CrossRef] [PubMed]
- Lundquist, T.J.; Woertz, I.C.; Quinn, N.; Benemann, J.R. A Realistic Technology and Engineering Assessment of Algae Biofuel Production; Energy Biosciences Institute: Berkeley, CA, USA, 2010. [Google Scholar]
- Norsker, N.-H.; Barbosa, M.J.; Vermuë, M.H.; Wijffels, R.H. Microalgal production—A close look at the economics. Biotechnol. Adv. 2011, 29, 24–27. [Google Scholar] [CrossRef] [PubMed]
- Quinn, J.C.; Yates, T.; Douglas, N.; Weyer, K.; Butler, J.; Bradley, T.H.; Lammers, P.J. Nannochloropsis production metrics in a scalable outdoor photobioreactor for commercial applications. Bioresour. Technol. 2012, 117, 164–171. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Xu, M.; Zhang, X.; Hu, Q.; Sommerfeld, M.; Chen, Y. Life-cycle analysis on biodiesel production from microalgae: Water footprint and nutrients balance. Bioresour. Technol. 2011, 102, 159–165. [Google Scholar] [CrossRef] [PubMed]
- Oswald, W.J.; Gotaas, H.B. Photosynthesis in sewage treatment. Trans. Am. Soc. Civ. Eng. 1957, 122, 73–105. [Google Scholar]
- Vitousek, P.M.; Aber, J.D.; Howarth, R.W.; Likens, G.E.; Matson, P.A.; Schindler, D.W.; Schlesinger, W.H.; Tilman, D.G. Human alteration of the global nitrogen cycle: Sources and consequences. Ecol. Appl. 1997, 7, 737–750. [Google Scholar] [CrossRef]
- Carpenter, S.R.; Caraco, N.F.; Correll, D.L.; Howarth, R.W.; Sharpley, A.N.; Smith, V.H. Nonpoint pollution of surface waters with phosphorus and nitrogen. Ecol. Appl. 1998, 8, 559–568. [Google Scholar] [CrossRef]
- De la Noüe, J.; Laliberté, G.; Proulx, D. Algae and waste water. J. Appl. Phycol. 1992, 4, 247–254. [Google Scholar] [CrossRef]
- European Commission on Environmnet. Heavy Metals in Wastes; COWI A/S: Copenhagen, Denmark, 2002. [Google Scholar]
- Chinnasamy, S.; Bhatnagar, A.; Hunt, R.W.; Das, K.C. Microalgae cultivation in a wastewater dominated by carpet mill effluents for biofuel applications. Bioresour. Technol. 2010, 101, 3097–3105. [Google Scholar] [CrossRef] [PubMed]
- Henze, M.; Harremoes, P.; la Cour Jansen, J.; Arvin, E. Wastewater Treatment: Biological and Chemical Processes; Springer Science & Business Media: Berlin, Germany, 2001. [Google Scholar]
- Westholm, L.J. Substrates for phosphorus removal—Potential benefits for on-site wastewater treatment? Water Res. 2006, 40, 23–36. [Google Scholar] [CrossRef] [PubMed]
- De-Bashan, L.E.; Bashan, Y. Recent advances in removing phosphorus from wastewater and its future use as fertilizer (1997–2003). Water Res. 2004, 38, 4222–4246. [Google Scholar] [CrossRef] [PubMed]
- Sutton, M.A.; Oenema, O.; Erisman, J.W.; Leip, A.; van Grinsven, H.; Winiwarter, W. Too much of a good thing. Nature 2011, 472, 159–161. [Google Scholar] [CrossRef] [PubMed]
- Cordell, D.; Drangert, J.-O.; White, S. The story of phosphorus: Global food security and food for thought. Glob. Environ. Chang. 2009, 19, 292–305. [Google Scholar] [CrossRef]
- Clarens, A.F.; Resurreccion, E.P.; White, M.A.; Colosi, L.M. Environmental life cycle comparison of algae to other bioenergy feedstocks. Environ. Sci. Technol. 2010, 44, 1813–1819. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Min, M.; Li, Y.; Chen, P.; Chen, Y.; Liu, Y.; Wang, Y.; Ruan, R. Cultivation of green algae Chlorella sp. In different wastewaters from municipal wastewater treatment plant. Appl. Biochem. Biotechnol. 2010, 162, 1174–1186. [Google Scholar] [CrossRef] [PubMed]
- McGinn, P.J.; Dickinson, K.E.; Bhatti, S.; Frigon, J.C.; Guiot, S.R.; O’Leary, S.J. Integration of microalgae cultivation with industrial waste remediation for biofuel and bioenergy production: Opportunities and limitations. Photosynth. Res. 2011, 109, 231–247. [Google Scholar] [CrossRef] [PubMed]
- Christenson, L.; Sims, R. Production and harvesting of microalgae for wastewater treatment, biofuels, and bioproducts. Biotechnol. Adv. 2011, 29, 686–702. [Google Scholar] [CrossRef] [PubMed]
- Larsdotter, K. Wastewater treatment with microalgae-a literature review. Vatten 2006, 62, 31. [Google Scholar]
- Park, J.; Craggs, R.; Shilton, A. Wastewater treatment high rate algal ponds for biofuel production. Bioresour. Technol. 2011, 102, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Cai, T.; Park, S.Y.; Li, Y. Nutrient recovery from wastewater streams by microalgae: Status and prospects. Renew. Sust. Energy Rev. 2013, 19, 360–369. [Google Scholar] [CrossRef]
- Stumm, W.; Morgan, J.J. Aquatic Chemistry; an Introduction Emphasizing Chemical Equilibria in Natural Waters; Willey-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 1970. [Google Scholar]
- Chisti, Y. Constraints to commercialization of algal fuels. J. Biotechnol. 2013, 167, 201–214. [Google Scholar] [CrossRef] [PubMed]
- Powell, N.; Shilton, A.; Chisti, Y.; Pratt, S. Towards a luxury uptake process via microalgae—Defining the polyphosphate dynamics. Water Res. 2009, 43, 4207–4213. [Google Scholar] [CrossRef] [PubMed]
- Benvenuti, G.; Bosma, R.; Cuaresma, M.; Janssen, M.; Barbosa, M.J.; Wijffels, R.H. Selecting microalgae with high lipid productivity and photosynthetic activity under nitrogen starvation. J. Appl. Phycol. 2015, 27, 1425–1431. [Google Scholar] [CrossRef]
- Breuer, G.; Martens, D.E.; Draaisma, R.B.; Wijffels, R.H.; Lamers, P.P. Photosynthetic efficiency and carbon partitioning in nitrogen-starved Scenedesmus obliquus. Algal Res. 2015, 9, 254–262. [Google Scholar] [CrossRef]
- Cheah, W.Y.; Show, P.L.; Chang, J.-S.; Ling, T.C.; Juan, J.C. Biosequestration of atmospheric CO2 and flue gas-containing CO2 by microalgae. Bioresour. Technol. 2015, 184, 190–201. [Google Scholar] [CrossRef] [PubMed]
- Van Den Hende, S.; Vervaeren, H.; Boon, N. Flue gas compounds and microalgae: (Bio-)chemical interactions leading to biotechnological opportunities. Biotechnol. Adv. 2012, 30, 1405–1424. [Google Scholar] [CrossRef] [PubMed]
- Steeneveldt, R.; Berger, B.; Torp, T. CO2 capture and storage: Closing the knowing–doing gap. Chem. Eng. Res. Des. 2006, 84, 739–763. [Google Scholar] [CrossRef]
- Kanniche, M.; Bouallou, C. CO2 capture study in advanced integrated gasification combined cycle. Appl. Therm. Eng. 2007, 27, 2693–2702. [Google Scholar] [CrossRef] [Green Version]
- Svensson, R.; Odenberger, M.; Johnsson, F.; Strömberg, L. Transportation systems for CO2––Application to carbon capture and storage. Energy Convers. Manag. 2004, 45, 2343–2353. [Google Scholar] [CrossRef]
- Berberoglu, H.; Gomez, P.S.; Pilon, L. Radiation characteristics of Botryococcus braunii, Chlorococcum littorale, and Chlorella sp. Used for CO2 fixation and biofuel production. J. Quant. Spectrosc. Radiat. Transf. 2009, 110, 1879–1893. [Google Scholar] [CrossRef]
- Yamasaki, A. An overview of CO2 mitigation options for global warming—Emphasizing CO2 sequestration options. J. Chem. Eng. Japan 2003, 36, 361–375. [Google Scholar] [CrossRef]
- Richmond, A. Handbook of Microalgal Culture: Biotechnology and Applied Phycology; John Wiley & Sons: Oxford, UK, 2008. [Google Scholar]
- Chojnacka, K.; Marquez-Rocha, F.-J. Kinetic and stoichiometric relationships of the energy and carbon metabolism in the culture of microalgae. Biotechnology 2004, 3, 21–34. [Google Scholar]
- Markou, G.; Angelidaki, I.; Georgakakis, D. Microalgal carbohydrates: An overview of the factors influencing carbohydrates production, and of main bioconversion technologies for production of biofuels. Appl. Microbiol. Biotechnol. 2012, 96, 631–645. [Google Scholar] [CrossRef] [PubMed]
- Cheirsilp, B.; Torpee, S. Enhanced growth and lipid production of microalgae under mixotrophic culture condition: Effect of light intensity, glucose concentration and fed-batch cultivation. Bioresour. Technol. 2012, 110, 510–516. [Google Scholar] [CrossRef] [PubMed]
- Zhan, J.; Rong, J.F.; Wang, Q. Mixotrophic cultivation, a preferable microalgae cultivation mode for biomass/bioenergy production, and bioremediation, advances and prospect. Int. J. Hydrog. Energy 2017, 42, 8505–8517. [Google Scholar] [CrossRef]
- Izumo, A.; Fujiwara, S.; Oyama, Y.; Satoh, A.; Fujita, N.; Nakamura, Y.; Tsuzuki, M. Physicochemical properties of starch in chlorella change depending on the CO2 concentration during growth: Comparison of structure and properties of pyrenoid and stroma starch. Plant Sci. 2007, 172, 1138–1147. [Google Scholar] [CrossRef]
- Brányiková, I.; Maršálková, B.; Doucha, J.; Brányik, T.; Bišová, K.; Zachleder, V.; Vítová, M. Microalgae—Novel highly efficient starch producers. Biotechnol. Bioeng. 2011, 108, 766–776. [Google Scholar] [CrossRef] [PubMed]
- El-Sheekh, M.; Abomohra, A.E.-F.; El-Azim, M.A.; Abou-Shanab, R. Effect of temperature on growth and fatty acids profile of the biodiesel producing microalga Scenedesmus acutus. Biotechnol. Agron. Soc. Environ. 2017, 21, 233–239. [Google Scholar]
- Kessler, E. Comparative physiology, biochemistry, and the taxonomy of Chlorella (chlorophyceae). Plant Syst. Evol. 1976, 125, 129–138. [Google Scholar] [CrossRef]
- Beherepatil, K.; Deore, L. Genus Scenedesmus from different habitats of nashik and it’s environs (ms) india. Int. J. Bioassays 2013, 2, 727–734. [Google Scholar]
- De Godos, I.; Arbib, Z.; Lara, E.; Rogalla, F. Evaluation of high rate algae ponds for treatment of anaerobically digested wastewater: Effect of CO2 addition and modification of dilution rate. Bioresour. Technol. 2016, 220, 253–261. [Google Scholar] [CrossRef] [PubMed]
- Novoveská, L.; Zapata, A.K.; Zabolotney, J.B.; Atwood, M.C.; Sundstrom, E.R. Optimizing microalgae cultivation and wastewater treatment in large-scale offshore photobioreactors. Algal Res. 2016, 18, 86–94. [Google Scholar] [CrossRef]
- Shen, L.; Ndayambaje, J.D.; Murwanashyaka, T.; Cui, W.; Manirafasha, E.; Chen, C.; Wang, Y.; Lu, Y. Assessment upon heterotrophic microalgae screened from wastewater microbiota for concurrent pollutants removal and biofuel production. Bioresour. Technol. 2017, 245, 386–393. [Google Scholar] [CrossRef] [PubMed]
- Razzak, S.A.; Hossain, M.M.; Lucky, R.A.; Bassi, A.S.; de Lasa, H. Integrated CO2 capture, wastewater treatment and biofuel production by microalgae culturing—A review. Renew. Sust. Energy Rev. 2013, 27, 622–653. [Google Scholar] [CrossRef]
- Vasudevan, P.T.; Briggs, M. Biodiesel production—Current state of the art and challenges. J. Ind. Microbiol. Biotechnol. 2008, 35, 421. [Google Scholar] [CrossRef] [PubMed]
- Jimenez-Perez, M.; Sanchez-Castillo, P.; Romera, O.; Fernandez-Moreno, D.; Pérez-Martınez, C. Growth and nutrient removal in free and immobilized planktonic green algae isolated from pig manure. Enzyme Microb. Technol. 2004, 34, 392–398. [Google Scholar] [CrossRef]
- Zhou, W.; Chen, P.; Min, M.; Ma, X.; Wang, J.; Griffith, R.; Hussain, F.; Peng, P.; Xie, Q.; Li, Y. Environment-enhancing algal biofuel production using wastewaters. Renew. Sust. Energy Rev. 2014, 36, 256–269. [Google Scholar] [CrossRef]
- Moreno-Garrido, I.; Canavate, J. Assessing chemical compounds for controlling predator ciliates in outdoor mass cultures of the green algae Dunaliella salina. Aquacult. Eng. 2001, 24, 107–114. [Google Scholar] [CrossRef]
- Richardson, J.W.; Johnson, M.D.; Zhang, X.; Zemke, P.; Chen, W.; Hu, Q. A financial assessment of two alternative cultivation systems and their contributions to algae biofuel economic viability. Algal Res. 2014, 4, 96–104. [Google Scholar] [CrossRef]
- Chen, G.; Zhao, L.; Qi, Y. Enhancing the productivity of microalgae cultivated in wastewater toward biofuel production: A critical review. Appl. Energy 2015, 137, 282–291. [Google Scholar] [CrossRef]
- Ren, H.; Tuo, J.; Addy, M.M.; Zhang, R.; Lu, Q.; Anderson, E.; Chen, P.; Ruan, R. Cultivation of Chlorella vulgaris in a pilot-scale photobioreactor using real centrate wastewater with waste glycerol for improving microalgae biomass production and wastewater nutrients removal. Bioresour. Technol. 2017, 245, 1130–1138. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Linares, L.C.; Barajas, C.G.; Páramo, E.D.; Corona, J.A.B. Assessment of Chlorella vulgaris and indigenous microalgae biomass with treated wastewater as growth culture medium. Bioresour. Technol. 2017, 244, 400–406. [Google Scholar] [CrossRef] [PubMed]
- Benvenuti, G.; Bosma, R.; Klok, A.J.; Ji, F.; Lamers, P.P.; Barbosa, M.J.; Wijffels, R.H. Microalgal triacylglycerides production in outdoor batch-operated tubular pbrs. Biotechnol. Biofuels 2015, 8, 100. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, J.-Y.; Lay, C.-H.; Chen, C.-C.; Wu, S.-Y. Lipid accumulating microalgae cultivation in textile wastewater: Environmental parameters optimization. J. Taiwan Inst. Chem. Eng. 2017, 79, 1–6. [Google Scholar] [CrossRef]
- Álvarez-Díaz, P.; Ruiz, J.; Arbib, Z.; Barragán, J.; Garrido-Pérez, M.; Perales, J. Freshwater microalgae selection for simultaneous wastewater nutrient removal and lipid production. Algal Res. 2017, 24, 477–485. [Google Scholar] [CrossRef]
- Rinna, F.; Buono, S.; Cabanelas, I.T.D.; Nascimento, I.A.; Sansone, G.; Barone, C.M.A. Wastewater treatment by microalgae can generate high quality biodiesel feedstock. J. Water Process. Eng. 2017, 18, 144–149. [Google Scholar] [CrossRef]
- Lau, P.; Tam, N.; Wong, Y. Effect of algal density on nutrient removal from primary settled wastewater. Environ. Pollut. 1995, 89, 59–66. [Google Scholar] [CrossRef]
- Martınez, M.; Sánchez, S.; Jimenez, J.; El Yousfi, F.; Munoz, L. Nitrogen and phosphorus removal from urban wastewater by the microalga Scenedesmus obliquus. Bioresour. Technol. 2000, 73, 263–272. [Google Scholar] [CrossRef]
- Ruiz-Marin, A.; Mendoza-Espinosa, L.G.; Stephenson, T. Growth and nutrient removal in free and immobilized green algae in batch and semi-continuous cultures treating real wastewater. Bioresour. Technol. 2010, 101, 58–64. [Google Scholar] [CrossRef] [PubMed]
- Di Termini, I.; Prassone, A.; Cattaneo, C.; Rovatti, M. On the nitrogen and phosphorus removal in algal photobioreactors. Ecol. Eng. 2011, 37, 976–980. [Google Scholar] [CrossRef]
- Gentili, F.G. Microalgal biomass and lipid production in mixed municipal, dairy, pulp and paper wastewater together with added flue gases. Bioresour. Technol. 2014, 169, 27–32. [Google Scholar] [CrossRef] [PubMed]
- Olguín, E.J.; Galicia, S.; Mercado, G.; Pérez, T. Annual productivity of spirulina (arthrospira) and nutrient removal in a pig wastewater recycling process under tropical conditions. J. Appl. Phycol. 2003, 15, 249–257. [Google Scholar] [CrossRef]
- An, J.-Y.; Sim, S.-J.; Lee, J.S.; Kim, B.W. Hydrocarbon production from secondarily treated piggery wastewater by the green alga Botryococcus braunii. J. Appl. Phycol. 2003, 15, 185–191. [Google Scholar] [CrossRef]
- Dumas, A.; Laliberte, G.; Lessard, P.; De La Noüe, J. Biotreatment of fish farm effluents using the cyanobacterium Phormidium bohneri. Aquacult. Eng. 1998, 17, 57–68. [Google Scholar] [CrossRef]
- Yewalkar-Kulkarni, S.; Gera, G.; Nene, S.; Pandare, K.; Kulkarni, B.; Kamble, S. Exploiting phosphate-starved cells of Scenedesmus sp. For the treatment of raw sewage. Indian J. Microbiol. 2017, 57, 241–249. [Google Scholar] [CrossRef] [PubMed]
- Úbeda, B.; Gálvez, J.Á.; Michel, M.; Bartual, A. Microalgae cultivation in urban wastewater: Coelastrum cf. pseudomicroporum as a novel carotenoid source and a potential microalgae harvesting tool. Bioresour. Technol. 2017, 228, 210–217. [Google Scholar] [CrossRef] [PubMed]
- De Godos, I.; Blanco, S.; García-Encina, P.A.; Becares, E.; Muñoz, R. Influence of flue gas sparging on the performance of high rate algae ponds treating agro-industrial wastewaters. J. Hazard. Mater. 2010, 179, 1049–1054. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Yuan, W.; Pei, Z.; Mao, E. Culture of microalga Botryococcus in livestock wastewater. Trans. ASABE 2008, 51, 1395–1400. [Google Scholar] [CrossRef]
- Kothari, R.; Pathak, V.V.; Kumar, V.; Singh, D. Experimental study for growth potential of unicellular alga Chlorella pyrenoidosa on dairy waste water: An integrated approach for treatment and biofuel production. Bioresour. Technol. 2012, 116, 466–470. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, C.; Marciniak, J.; Villaverde, S.; Leon, C.; Garcia, P.A.; Munoz, R. Efficient nutrient removal from swine manure in a tubular biofilm photo-bioreactor using algae-bacteria consortia. Water Sci. Technol. 2008, 58, 95–102. [Google Scholar] [CrossRef] [PubMed]
- Qi, W.; Chen, T.; Wang, L.; Wu, M.; Zhao, Q.; Wei, W. High-strength fermentable wastewater reclamation through a sequential process of anaerobic fermentation followed by microalgae cultivation. Bioresour. Technol. 2017, 227, 317–323. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Lu, Q.; Wang, Q.; Liu, W.; Wei, Q.; Ren, H.; Ming, C.; Min, M.; Chen, P.; Ruan, R. Isolation of a bacterial strain, Acinetobacter sp. From centrate wastewater and study of its cooperation with algae in nutrients removal. Bioresour. Technol. 2017, 235, 59–69. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.-Y.; Nguyen, M.-L.T.; Lay, C.-H. Starch-containing textile wastewater treatment for biogas and microalgae biomass production. J. Clean. Prod. 2017, 168, 331–337. [Google Scholar] [CrossRef]
- Da Fontoura, J.T.; Rolim, G.S.; Farenzena, M.; Gutterres, M. Influence of light intensity and tannery wastewater concentration on biomass production and nutrient removal by microalgae Scenedesmus sp. Process. Saf. Environ. 2017, 111, 355–362. [Google Scholar] [CrossRef]
- Abid, A.; Saidane, F.; Hamdi, M. Feasibility of carbon dioxide sequestration by Spongiochloris sp. microalgae during petroleum wastewater treatment in airlift bioreactor. Bioresour. Technol. 2017, 234, 297–302. [Google Scholar] [CrossRef] [PubMed]
- Reyimu, Z.; Özçimen, D. Batch cultivation of marine microalgae Nannochloropsis oculata and Tetraselmis suecica in treated municipal wastewater toward bioethanol production. J. Clean. Prod. 2017, 150, 40–46. [Google Scholar] [CrossRef]
- Brennan, L.; Owende, P. Biofuels from microalgae—A review of technologies for production, processing, and extractions of biofuels and co-products. Renew. Sust. Energy Rev. 2010, 14, 557–577. [Google Scholar] [CrossRef]
- Sutherland, D.L. Enhancing the Performance of Wastewater Microalgae through Chemical and Physical Modifications in High Rate Algal Ponds. Ph.D. Thesis, University of Canterbury, Christchurch, New Zealand, 2015. [Google Scholar]
- Becker, E.W. Microalgae: Biotechnology and Microbiology; Cambridge University Press: Cambridge, UK, 1994; Volume 10. [Google Scholar]
- Pulz, O. Photobioreactors: Production systems for phototrophic microorganisms. Appl. Microbiol. Biotechnol. 2001, 57, 287–293. [Google Scholar] [PubMed]
- Acién Fernández, F.G.; González-López, C.V.; Fernández Sevilla, J.M.; Molina Grima, E. Conversion of CO2 into biomass by microalgae: How realistic a contribution may it be to significant CO2 removal? Appl. Microbiol. Biotechnol. 2012, 96, 577–586. [Google Scholar] [CrossRef] [PubMed]
- Trozzi, C.; Rentz, O.; Oertel, D.; Woodfield, M.; Stewart, R. Energy Industries. Combustion in Energy and Transformation Industries. Air Pollutant Emission Inventory Guidebook; European Environment Agency: Copenhagen, Denmark, 2010. [Google Scholar]
- Niessen, W.R. Combustion and Incineration Processes: Applications in Environmental Engineering; CRC Press Taylor & Francis Group: Boca Raton, FL, USA, 2010. [Google Scholar]
- Ji, M.-K.; Yun, H.-S.; Park, Y.-T.; Kabra, A.N.; Oh, I.-H.; Choi, J. Mixotrophic cultivation of a microalga Scenedesmus obliquus in municipal wastewater supplemented with food wastewater and flue gas CO2 for biomass production. J. Environ. Manag. 2015, 159, 115–120. [Google Scholar] [CrossRef] [PubMed]
- Riebesell, U.; Wolf-Gladrow, D.A.; Smetacek, V. Carbon dioxide limitation of marine phytoplankton growth rates. Nature 1993, 361, 249. [Google Scholar] [CrossRef]
- He, L.; Subramanian, V.R.; Tang, Y.J. Experimental analysis and model-based optimization of microalgae growth in photo-bioreactors using flue gas. Biomass Bioenergy 2012, 41, 131–138. [Google Scholar] [CrossRef]
- Jiang, Y.; Zhang, W.; Wang, J.; Chen, Y.; Shen, S.; Liu, T. Utilization of simulated flue gas for cultivation of Scenedesmus dimorphus. Bioresour. Technol. 2013, 128, 359–364. [Google Scholar] [CrossRef] [PubMed]
- Olaizola, M. Microalgal removal of CO2 from flue gases: Changes in medium ph and flue gas composition do not appear to affect the photochemical yield of microalgal cultures. Biotechnol. Bioprocess. Eng. 2003, 8, 360–367. [Google Scholar] [CrossRef]
- Chiu, S.-Y.; Kao, C.-Y.; Chen, C.-H.; Kuan, T.-C.; Ong, S.-C.; Lin, C.-S. Reduction of CO2 by a high-density culture of Chlorella sp. in a semicontinuous photobioreactor. Bioresour. Technol. 2008, 99, 3389–3396. [Google Scholar] [CrossRef] [PubMed]
- Tang, D.; Han, W.; Li, P.; Miao, X.; Zhong, J. CO2 biofixation and fatty acid composition of Scenedesmus obliquus and Chlorella pyrenoidosa in response to different CO2 levels. Bioresour. Technol. 2011, 102, 3071–3076. [Google Scholar] [CrossRef] [PubMed]
- Douskova, I.; Doucha, J.; Livansky, K.; Machat, J.; Novak, P.; Umysova, D.; Zachleder, V.; Vitova, M. Simultaneous flue gas bioremediation and reduction of microalgal biomass production costs. Appl. Microbiol. Biotechnol. 2009, 82, 179–185. [Google Scholar] [CrossRef] [PubMed]
- La Rocca, N.; Andreoli, C.; Giacometti, G.M.; Rascio, N.; Moro, I. Responses of the antarctic microalga Koliella antarctica (trebouxiophyceae, chlorophyta) to cadmium contamination. Photosynthetica 2009, 47, 471–479. [Google Scholar] [CrossRef]
- Hess, D.; Napan, K.; McNeil, B.T.; Torres, E.M.; Guy, T.; McLean, J.E.; Quinn, J.C. Quantification of effects of flue gas derived inorganic contaminants on microalgae growth system and end fate of contaminants. Algal Res. 2017, 25, 68–75. [Google Scholar] [CrossRef]
- Greenwell, H.C.; Laurens, L.M.L.; Shields, R.J.; Lovitt, R.W.; Flynn, K.J. Placing microalgae on the biofuels priority list: A review of the technological challenges. J. R. Soc. Interface 2010, 7, 703–726. [Google Scholar] [CrossRef] [PubMed]
- Yoo, C.; Jun, S.-Y.; Lee, J.-Y.; Ahn, C.-Y.; Oh, H.-M. Selection of microalgae for lipid production under high levels carbon dioxide. Bioresour. Technol. 2010, 101, S71–S74. [Google Scholar] [CrossRef] [PubMed]
- Morais, M.G.; Costa, J.A.V. Isolation and selection of microalgae from coal fired thermoelectric power plant for biofixation of carbon dioxide. Energy Convers. Manag. 2007, 48, 2169–2173. [Google Scholar] [CrossRef]
- Yun, Y.S.; Lee, S.B.; Park, J.M.; Lee, C.I.; Yang, J.W. Carbon dioxide fixation by algal cultivation using wastewater nutrients. J. Chem. Technol. Biotechnol. 1997, 69, 451–455. [Google Scholar] [CrossRef]
- Nayak, M.; Karemore, A.; Sen, R. Sustainable valorization of flue gas co2 and wastewater for the production of microalgal biomass as a biofuel feedstock in closed and open reactor systems. RSC Adv. 2016, 6, 91111–91120. [Google Scholar] [CrossRef]
- Rivera-Utrilla, J.; Sánchez-Polo, M.; Ferro-García, M.Á.; Prados-Joya, G.; Ocampo-Pérez, R. Pharmaceuticals as emerging contaminants and their removal from water. A review. Chemosphere 2013, 93, 1268–1287. [Google Scholar] [CrossRef] [PubMed]
- Solé, A.; Matamoros, V. Removal of endocrine disrupting compounds from wastewater by microalgae co-immobilized in alginate beads. Chemosphere 2016, 164, 516–523. [Google Scholar] [CrossRef] [PubMed]
- Guo, W.-Q.; Zheng, H.-S.; Li, S.; Du, J.-S.; Feng, X.-C.; Yin, R.-L.; Wu, Q.-L.; Ren, N.-Q.; Chang, J.-S. Removal of cephalosporin antibiotics 7-aca from wastewater during the cultivation of lipid-accumulating microalgae. Bioresour. Technol. 2016, 221, 284–290. [Google Scholar] [CrossRef] [PubMed]
- Geiger, E.; Hornek-Gausterer, R.; Saçan, M.T. Single and mixture toxicity of pharmaceuticals and chlorophenols to freshwater algae Chlorella vulgaris. Ecotox Environ. Saf. 2016, 129, 189–198. [Google Scholar] [CrossRef] [PubMed]
- Bai, X.; Acharya, K. Removal of trimethoprim, sulfamethoxazole, and triclosan by the green alga Nannochloris sp. J. Hazard. Mater. 2016, 315, 70–75. [Google Scholar] [CrossRef] [PubMed]
- Peng, F.-Q.; Ying, G.-G.; Yang, B.; Liu, S.; Lai, H.-J.; Liu, Y.-S.; Chen, Z.-F.; Zhou, G.-J. Biotransformation of progesterone and norgestrel by two freshwater microalgae (Scenedesmus obliquus and Chlorella pyrenoidosa): Transformation kinetics and products identification. Chemosphere 2014, 95, 581–588. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Wang, X.; Poon, K.; Wang, Y.; Li, S.; Liu, H.; Lin, S.; Cai, Z. Removal and reductive dechlorination of triclosan by Chlorella pyrenoidosa. Chemosphere 2013, 92, 1498–1505. [Google Scholar] [CrossRef] [PubMed]
- Escapa, C.; Coimbra, R.; Paniagua, S.; García, A.; Otero, M. Nutrients and pharmaceuticals removal from wastewater by culture and harvesting of Chlorella sorokiniana. Bioresour. Technol. 2015, 185, 276–284. [Google Scholar] [CrossRef] [PubMed]
- Gentili, F.G.; Fick, J. Algal cultivation in urban wastewater: An efficient way to reduce pharmaceutical pollutants. J. Appl. Phycol. 2017, 29, 255–262. [Google Scholar] [CrossRef] [PubMed]
- Lima, S.A.; Raposo, M.F.J.; Castro, P.M.; Morais, R.M. Biodegradation of p-chlorophenol by a microalgae consortium. Water Res. 2004, 38, 97–102. [Google Scholar] [CrossRef] [PubMed]
- Papazi, A.; Kotzabasis, K. “Rational” management of dichlorophenols biodegradation by the microalga Scenedesmus obliquus. PLoS ONE 2013, 8, e61682. [Google Scholar] [CrossRef]
- Wilde, E.W.; Benemann, J.R. Bioremoval of heavy-metals by the use of microalgae. Biotechnol. Adv. 1993, 11, 781–812. [Google Scholar] [CrossRef]
- Srivastava, N.; Majumder, C. Novel biofiltration methods for the treatment of heavy metals from industrial wastewater. J. Hazard. Mater. 2008, 151, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Gong, R.; Ding, Y.; Liu, H.; Chen, Q.; Liu, Z. Lead biosorption and desorption by intact and pretreated Spirulina maxima biomass. Chemosphere 2005, 58, 125–130. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Raouf, N.; Al-Homaidan, A.; Ibraheem, I. Microalgae and wastewater treatment. Saudi J. Biol. Sci. 2012, 19, 257–275. [Google Scholar] [CrossRef] [PubMed]
- Abbas, S.H.; Ismail, I.M.; Mostafa, T.M.; Sulaymon, A.H. Biosorption of heavy metals: A review. J. Chem. Sci. Technol. 2014, 3, 74–102. [Google Scholar]
- Muñoz, R.; Guieysse, B. Algal–bacterial processes for the treatment of hazardous contaminants: A review. Water Res. 2006, 40, 2799–2815. [Google Scholar] [CrossRef] [PubMed]
- Moye, H.A.; Miles, C.J.; Phlips, E.J.; Sargent, B.; Merritt, K.K. Kinetics and uptake mechanisms for monomethylmercury between freshwater algae and water. Environ. Sci. Technol. 2002, 36, 3550–3555. [Google Scholar] [CrossRef] [PubMed]
- Vilchez, C.; Garbayo, I.; Lobato, M.V.; Vega, J. Microalgae-mediated chemicals production and wastes removal. Enzyme Microb. Technol. 1997, 20, 562–572. [Google Scholar] [CrossRef]
- Geoffroy, L.; Gilbin, R.; Simon, O.; Floriani, M.; Adam, C.; Pradines, C.; Cournac, L.; Garnier-Laplace, J. Effect of selenate on growth and photosynthesis of Chlamydomonas reinhardtii. Aquat. Toxicol 2007, 83, 149–158. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Wang, W.-X. Accumulation, subcellular distribution and toxicity of inorganic mercury and methylmercury in marine phytoplankton. Environ. Pollut. 2011, 159, 3097–3105. [Google Scholar] [CrossRef] [PubMed]
- Rodgher, S.; Espíndola, E.L.G.; Simões, F.C.F.; Tonietto, A.E. Cadmium and chromium toxicity to Pseudokirchneriella subcapitata and Microcystis aeruginosa. Braz. Arch. Biol. Technol. 2012, 55, 161–169. [Google Scholar] [CrossRef]
- Carfagna, S.; Lanza, N.; Salbitani, G.; Basile, A.; Sorbo, S.; Vona, V. Physiological and morphological responses of lead or cadmium exposed Chlorella sorokiniana 211-8k (chlorophyceae). SpringerPlus 2013, 2, 147. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Jacinto, V.; García-Barrera, T.; Gómez-Ariza, J.L.; Garbayo-Nores, I.; Vílchez-Lobato, C. Elucidation of the defence mechanism in microalgae Chlorella sorokiniana under mercury exposure. Identification of hg–phytochelatins. Chem. Biol. Interact. 2015, 238, 82–90. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.-X.; Fu, Q.; Huang, Y.; Xia, A.; Liao, Q.; Zhu, X.; Zheng, Y.-P.; Sun, C.-H. An annular photobioreactor with ion-exchange-membrane for non-touch microalgae cultivation with wastewater. Bioresour. Technol. 2016, 219, 668–676. [Google Scholar] [CrossRef] [PubMed]
- Katiyar, R.; Gurjar, B.; Biswas, S.; Pruthi, V.; Kumar, N.; Kumar, P. Microalgae: An emerging source of energy based bio-products and a solution for environmental issues. Renew. Sust. Energy Rev. 2017, 72, 1083–1093. [Google Scholar] [CrossRef]
- Da Silva, M.J.; Cardoso, A.L. Heterogeneous tin catalysts applied to the esterification and transesterification reactions. J. Catal. 2013, 2013, 510509. [Google Scholar] [CrossRef]
- Veillette, M.; Giroir-Fendler, A.; Faucheux, N.; Heitz, M. Esterification of free fatty acids with methanol to biodiesel using heterogeneous catalysts: From model acid oil to microalgae lipids. Chem. Eng. J. 2017, 308, 101–109. [Google Scholar] [CrossRef]
- Orr, V.C.; Plechkova, N.V.; Seddon, K.R.; Rehmann, L. Disruption and wet extraction of the microalgae Chlorella vulgaris using room-temperature ionic liquids. ACS Sustain. Chem. Eng. 2016, 4, 591–600. [Google Scholar] [CrossRef]
- Graboski, M.S.; McCormick, R.L. Combustion of fat and vegetable oil derived fuels in diesel engines. Prog. Energy Combust. Sci. 1998, 24, 125–164. [Google Scholar] [CrossRef]
- Szybist, J.P.; Kirby, S.R.; Boehman, A.L. No x emissions of alternative diesel fuels: A comparative analysis of biodiesel and ft diesel. Energy Fuels 2005, 19, 1484–1492. [Google Scholar] [CrossRef]
- Ji, M.-K.; Abou-Shanab, R.A.; Kim, S.-H.; Salama, E.-S.; Lee, S.-H.; Kabra, A.N.; Lee, Y.-S.; Hong, S.; Jeon, B.-H. Cultivation of microalgae species in tertiary municipal wastewater supplemented with CO2 for nutrient removal and biomass production. Ecol. Eng. 2013, 58, 142–148. [Google Scholar] [CrossRef]
- Raheem, A.; Azlina, W.W.; Yap, Y.T.; Danquah, M.K.; Harun, R. Thermochemical conversion of microalgal biomass for biofuel production. Renew. Sust. Energy Rev. 2015, 49, 990–999. [Google Scholar] [CrossRef]
- Selvaratnam, T.; Pegallapati, A.; Montelya, F.; Rodriguez, G.; Nirmalakhandan, N.; Lammers, P.J.; Van Voorhies, W. Feasibility of algal systems for sustainable wastewater treatment. Renew. Energy 2015, 82, 71–76. [Google Scholar] [CrossRef]
- Asomaning, J.; Omidghane, M.; Chae, M.; Bressler, D.C. Thermal processing of algal biomass for biofuel production. Curr. Opin. Green Sustain. Chem. 2016, 2, 1–5. [Google Scholar] [CrossRef]
- Hallenbeck, P.; Grogger, M.; Mraz, M.; Veverka, D. Solar biofuels production with microalgae. Appl. Energy 2016, 179, 136–145. [Google Scholar] [CrossRef]
- Zhu, Y.; Piotrowska, P.; van Eyk, P.J.; Boström, D.; Kwong, C.W.; Wang, D.; Cole, A.J.; de Nys, R.; Gentili, F.G.; Ashman, P.J. Cogasification of australian brown coal with algae in a fluidized bed reactor. Energy Fuels 2015, 29, 1686–1700. [Google Scholar] [CrossRef]
- Chen, C.; Ma, X.; Liu, K. Thermogravimetric analysis of microalgae combustion under different oxygen supply concentrations. Appl. Energy 2011, 88, 3189–3196. [Google Scholar] [CrossRef]
- Vassilev, S.V.; Vassileva, C.G. Composition, properties and challenges of algae biomass for biofuel application: An overview. Fuel 2016, 181, 1–33. [Google Scholar] [CrossRef]
- Mu, D.; Min, M.; Krohn, B.; Mullins, K.A.; Ruan, R.; Hill, J. Life cycle environmental impacts of wastewater-based algal biofuels. Environ. Sci. Technol. 2014, 48, 11696–11704. [Google Scholar] [CrossRef] [PubMed]
- Mehrabadi, A.; Craggs, R.; Farid, M.M. Pyrolysis of wastewater treatment high rate algal pond (wwt hrap) biomass. Algal Res. 2017, 24, 509–519. [Google Scholar] [CrossRef]
- Grierson, S.; Strezov, V.; Shah, P. Properties of oil and char derived from slow pyrolysis of tetraselmis chui. Bioresour. Technol. 2011, 102, 8232–8240. [Google Scholar] [CrossRef] [PubMed]
- Azizi, K.; Keshavarz Moraveji, M.; Abedini Najafabadi, H. A review on bio-fuel production from microalgal biomass by using pyrolysis method. Renew. Sust. Energy Rev. 2018, 82, 3046–3059. [Google Scholar] [CrossRef]
- Ferreira, A.; Ribeiro, B.; Marques, P.A.; Ferreira, A.F.; Dias, A.P.; Pinheiro, H.M.; Reis, A.; Gouveia, L. Scenedesmus obliquus mediated brewery wastewater remediation and CO2 biofixation for green energy purposes. J. Clean. Prod. 2017, 165, 1316–1327. [Google Scholar] [CrossRef]
- Grierson, S.; Strezov, V.; Ellem, G.; Mcgregor, R.; Herbertson, J. Thermal characterisation of microalgae under slow pyrolysis conditions. J. Anal. Appl. Pyrolysis 2009, 85, 118–123. [Google Scholar] [CrossRef]
- Biller, P.; Ross, A.B. Hydrothermal processing of algal biomass for the production of biofuels and chemicals. Biofuels 2012, 3, 603–623. [Google Scholar] [CrossRef]
- Gollakota, A.; Kishore, N.; Gu, S. A review on hydrothermal liquefaction of biomass. Renew. Sust. Energy Rev. 2017. [CrossRef]
- Yoo, G.; Park, M.S.; Yang, J.-W.; Choi, M. Lipid content in microalgae determines the quality of biocrude and energy return on investment of hydrothermal liquefaction. Appl. Energy 2015, 156, 354–361. [Google Scholar] [CrossRef]
- Chen, W.-T.; Qian, W.; Zhang, Y.; Mazur, Z.; Kuo, C.-T.; Scheppe, K.; Schideman, L.C.; Sharma, B.K. Effect of ash on hydrothermal liquefaction of high-ash content algal biomass. Algal Res. 2017, 25, 297–306. [Google Scholar] [CrossRef]
- Biller, P.; Sharma, B.K.; Kunwar, B.; Ross, A.B. Hydroprocessing of bio-crude from continuous hydrothermal liquefaction of microalgae. Fuel 2015, 159, 197–205. [Google Scholar] [CrossRef]
- Cheng, F.; Cui, Z.; Chen, L.; Jarvis, J.; Paz, N.; Schaub, T.; Nirmalakhandan, N.; Brewer, C.E. Hydrothermal liquefaction of high-and low-lipid algae: Bio-crude oil chemistry. Appl. Energy 2017, 206, 278–292. [Google Scholar] [CrossRef]
- Jazrawi, C.; Biller, P.; He, Y.; Montoya, A.; Ross, A.B.; Maschmeyer, T.; Haynes, B.S. Two-stage hydrothermal liquefaction of a high-protein microalga. Algal Res. 2015, 8, 15–22. [Google Scholar] [CrossRef]
- Cantrell, K.B.; Ducey, T.; Ro, K.S.; Hunt, P.G. Livestock waste-to-bioenergy generation opportunities. Bioresour. Technol. 2008, 99, 7941–7953. [Google Scholar] [CrossRef] [PubMed]
- Show, P.L.; Tang, M.S.; Nagarajan, D.; Ling, T.C.; Ooi, C.-W.; Chang, J.-S. A holistic approach to managing microalgae for biofuel applications. Int. J. Mol. Sci. 2017, 18, 215. [Google Scholar] [CrossRef] [PubMed]
- Abdelaziz, A.E.; Leite, G.B.; Hallenbeck, P.C. Addressing the challenges for sustainable production of algal biofuels: II. Harvesting and conversion to biofuels. Environ. Technol. 2013, 34, 1807–1836. [Google Scholar] [CrossRef] [PubMed]
- Bahadar, A.; Khan, M.B. Progress in energy from microalgae: A review. Renew. Sust. Energy Rev. 2013, 27, 128–148. [Google Scholar] [CrossRef]
- Patzelt, D.J.; Hindersin, S.; Elsayed, S.; Boukis, N.; Kerner, M.; Hanelt, D. Hydrothermal gasification of Acutodesmus obliquus for renewable energy production and nutrient recycling of microalgal mass cultures. J. Appl. Phycol. 2015, 27, 2239–2250. [Google Scholar] [CrossRef]
- Kodali, D.R. Trans Fats Replacement Solutions; AOCS Press, Elsevier: Urbana, IL, USA, 2014. [Google Scholar]
- Van Damme, S.; Bram, S.; Contino, F. Comparison of biodiesel production scenarios with coproduction of triacetin according to energy and ghg emissions. Energy Procedia 2014, 61, 1852–1859. [Google Scholar] [CrossRef]
- Samson, R.; Leduy, A. Biogas production from anaerobic digestion of Spirulina maxima algal biomass. Biotechnol. Bioeng. 1982, 24, 1919–1924. [Google Scholar] [CrossRef] [PubMed]
- Golueke, C.G.; Oswald, W.J.; Gotaas, H.B. Anaerobic digestion of algae. Appl. Microbiol. 1957, 5, 47. [Google Scholar] [PubMed]
- Prajapati, S.K.; Kaushik, P.; Malik, A.; Vijay, V.K. Phycoremediation coupled production of algal biomass, harvesting and anaerobic digestion: Possibilities and challenges. Biotechnol. Adv. 2013, 31, 1408–1425. [Google Scholar] [CrossRef] [PubMed]
- Angelidaki, I.; Ahring, B. Thermophilic anaerobic digestion of livestock waste: The effect of ammonia. Appl. Microbiol. Biotechnol. 1993, 38, 560–564. [Google Scholar] [CrossRef]
- Chen, Y.; Cheng, J.J.; Creamer, K.S. Inhibition of anaerobic digestion process: A review. Bioresour. Technol. 2008, 99, 4044–4064. [Google Scholar] [CrossRef] [PubMed]
- Sialve, B.; Bernet, N.; Bernard, O. Anaerobic digestion of microalgae as a necessary step to make microalgal biodiesel sustainable. Biotechnol. Adv. 2009, 27, 409–416. [Google Scholar] [CrossRef] [PubMed]
- Yenigün, O.; Demirel, B. Ammonia inhibition in anaerobic digestion: A review. Process. Biochem. 2013, 48, 901–911. [Google Scholar] [CrossRef]
- Shanmugam, P.; Horan, N. Optimising the biogas production from leather fleshing waste by co-digestion with MSW. Bioresour. Technol. 2009, 100, 4117–4120. [Google Scholar] [CrossRef] [PubMed]
- Yen, H.-W.; Brune, D.E. Anaerobic co-digestion of algal sludge and waste paper to produce methane. Bioresour. Technol. 2007, 98, 130–134. [Google Scholar] [CrossRef] [PubMed]
- Schwede, S.; Kowalczyk, A.; Gerber, M.; Span, R. Anaerobic co-digestion of the marine microalga Nannochloropsis salina with energy crops. Bioresour. Technol. 2013, 148, 428–435. [Google Scholar] [CrossRef] [PubMed]
- Olsson, J.; Feng, X.M.; Ascue, J.; Gentili, F.G.; Shabiimam, M.A.; Nehrenheim, E.; Thorin, E. Co-digestion of cultivated microalgae and sewage sludge from municipal waste water treatment. Bioresour. Technol. 2014, 171, 203–210. [Google Scholar] [CrossRef] [PubMed]
- González-Fernández, C.; Molinuevo-Salces, B.; García-González, M.C. Evaluation of anaerobic codigestion of microalgal biomass and swine manure via response surface methodology. Appl. Energy 2011, 88, 3448–3453. [Google Scholar] [CrossRef]
- Prajapati, S.K.; Malik, A.; Vijay, V.K. Comparative evaluation of biomass production and bioenergy generation potential of Chlorella spp. Through anaerobic digestion. Appl. Energy 2014, 114, 790–797. [Google Scholar] [CrossRef]
- Klassen, V.; Blifernez-Klassen, O.; Wibberg, D.; Winkler, A.; Kalinowski, J.; Posten, C.; Kruse, O. Highly efficient methane generation from untreated microalgae biomass. Biotechnol. Biofuels 2017, 10, 186. [Google Scholar] [CrossRef] [PubMed]
- Mussgnug, J.H.; Klassen, V.; Schlüter, A.; Kruse, O. Microalgae as substrates for fermentative biogas production in a combined biorefinery concept. J. Biotechnol. 2010, 150, 51–56. [Google Scholar] [CrossRef] [PubMed]
- Passos, F.; Solé, M.; García, J.; Ferrer, I. Biogas production from microalgae grown in wastewater: Effect of microwave pretreatment. Appl. Energy 2013, 108, 168–175. [Google Scholar] [CrossRef]
- Passos, F.; Uggetti, E.; Carrère, H.; Ferrer, I. Pretreatment of microalgae to improve biogas production: A review. Bioresour. Technol. 2014, 172, 403–412. [Google Scholar] [CrossRef] [PubMed]
- Hamouda, R.A.; Sherif, S.A.; Ghareeb, M.M. Bioethanol production by various hydrolysis and fermentation processes with micro and macro green algae. Waste Biomass Valoriz. 2017. [Google Scholar] [CrossRef]
- Boonprab, K.; Matsui, K.; Kataoka, N. Preliminary study on bioethanol from fresh water algae, Cladophora glomerata (sarai kai) by the fungus, Monascus sp. Np1. J. Appl. Phycol. 2017. [Google Scholar] [CrossRef]
- Shokrkar, H.; Ebrahimi, S.; Zamani, M. Bioethanol production from acidic and enzymatic hydrolysates of mixed microalgae culture. Fuel 2017, 200, 380–386. [Google Scholar] [CrossRef]
- Ho, S.-H.; Chen, C.-Y.; Chang, J.-S. Effect of light intensity and nitrogen starvation on CO2 fixation and lipid/carbohydrate production of an indigenous microalga scenedesmus obliquus cnw-n. Bioresour. Technol. 2012, 113, 244–252. [Google Scholar] [CrossRef] [PubMed]
- Razzak, S.A.; Ali, S.A.M.; Hossain, M.M. Biological CO2 fixation with production of microalgae in wastewater—A review. Renew. Sust. Energy Rev. 2017, 76, 379–390. [Google Scholar] [CrossRef]
- Jin, C.; Yao, M.; Liu, H.; Chia-fon, F.L.; Ji, J. Progress in the production and application of n-butanol as a biofuel. Renew. Sust. Energy Rev. 2011, 15, 4080–4106. [Google Scholar] [CrossRef]
- Wang, Y.; Ho, S.-H.; Yen, H.-W.; Nagarajan, D.; Ren, N.-Q.; Li, S.; Hu, Z.; Lee, D.-J.; Kondo, A.; Chang, J.-S. Current advances on fermentative biobutanol production using third generation feedstock. Biotechnol. Adv. 2017, 35, 1049–1059. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Ho, S.-H.; Cheng, C.-L.; Nagarajan, D.; Guo, W.-Q.; Lin, C.; Li, S.; Ren, N.; Chang, J.-S. Nutrients and COD removal of swine wastewater with an isolated microalgal strain Neochloris aquatica cl-m1 accumulating high carbohydrate content used for biobutanol production. Bioresour. Technol. 2017, 242, 7–14. [Google Scholar] [CrossRef] [PubMed]
- Gao, K.; Orr, V.; Rehmann, L. Butanol fermentation from microalgae-derived carbohydrates after ionic liquid extraction. Bioresour. Technol. 2016, 206, 77–85. [Google Scholar] [CrossRef] [PubMed]
- Batista, A.P.; Ambrosano, L.; Graça, S.; Sousa, C.; Marques, P.A.; Ribeiro, B.; Botrel, E.P.; Neto, P.C.; Gouveia, L. Combining urban wastewater treatment with biohydrogen production—An integrated microalgae-based approach. Bioresour. Technol. 2015, 184, 230–235. [Google Scholar] [CrossRef] [PubMed]
- Batista, A.P.; Moura, P.; Marques, P.A.; Ortigueira, J.; Alves, L.; Gouveia, L. Scenedesmus obliquus as feedstock for biohydrogen production by Enterobacter aerogenes and Clostridium butyricum. Fuel 2014, 117, 537–543. [Google Scholar] [CrossRef]
- Shobana, S.; Kumar, G.; Bakonyi, P.; Saratale, G.D.; Al-Muhtaseb, A.a.H.; Nemestóthy, N.; Bélafi-Bakó, K.; Xia, A.; Chang, J.-S. A review on the biomass pretreatment and inhibitor removal methods as key-steps towards efficient macroalgae-based biohydrogen production. Bioresour. Technol. 2017, 244, 1341–1348. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.-H.; Chang, C.-Y.; Liao, Q.; Zhu, X.; Liao, C.-F.; Chang, J.-S. Biohydrogen production by a novel integration of dark fermentation and mixotrophic microalgae cultivation. Int. J. Hydrog. Energy 2013, 38, 15807–15814. [Google Scholar] [CrossRef]
- Phanduang, O.; Lunprom, S.; Salakkam, A.; Reungsang, A. Anaerobic solid-state fermentation of bio-hydrogen from microalgal Chlorella sp. Biomass. Int. J. Hydrog. Energy 2017, 42, 9650–9659. [Google Scholar] [CrossRef]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lage, S.; Gojkovic, Z.; Funk, C.; Gentili, F.G. Algal Biomass from Wastewater and Flue Gases as a Source of Bioenergy. Energies 2018, 11, 664. https://doi.org/10.3390/en11030664
Lage S, Gojkovic Z, Funk C, Gentili FG. Algal Biomass from Wastewater and Flue Gases as a Source of Bioenergy. Energies. 2018; 11(3):664. https://doi.org/10.3390/en11030664
Chicago/Turabian StyleLage, Sandra, Zivan Gojkovic, Christiane Funk, and Francesco G. Gentili. 2018. "Algal Biomass from Wastewater and Flue Gases as a Source of Bioenergy" Energies 11, no. 3: 664. https://doi.org/10.3390/en11030664
APA StyleLage, S., Gojkovic, Z., Funk, C., & Gentili, F. G. (2018). Algal Biomass from Wastewater and Flue Gases as a Source of Bioenergy. Energies, 11(3), 664. https://doi.org/10.3390/en11030664




