Conversion of Levulinic Acid from Various Herbaceous Biomass Species Using Hydrochloric Acid and Effects of Particle Size and Delignification
Abstract
1. Introduction
2. Results and Discussion
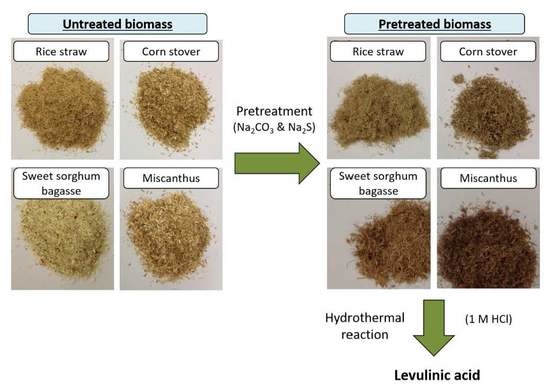
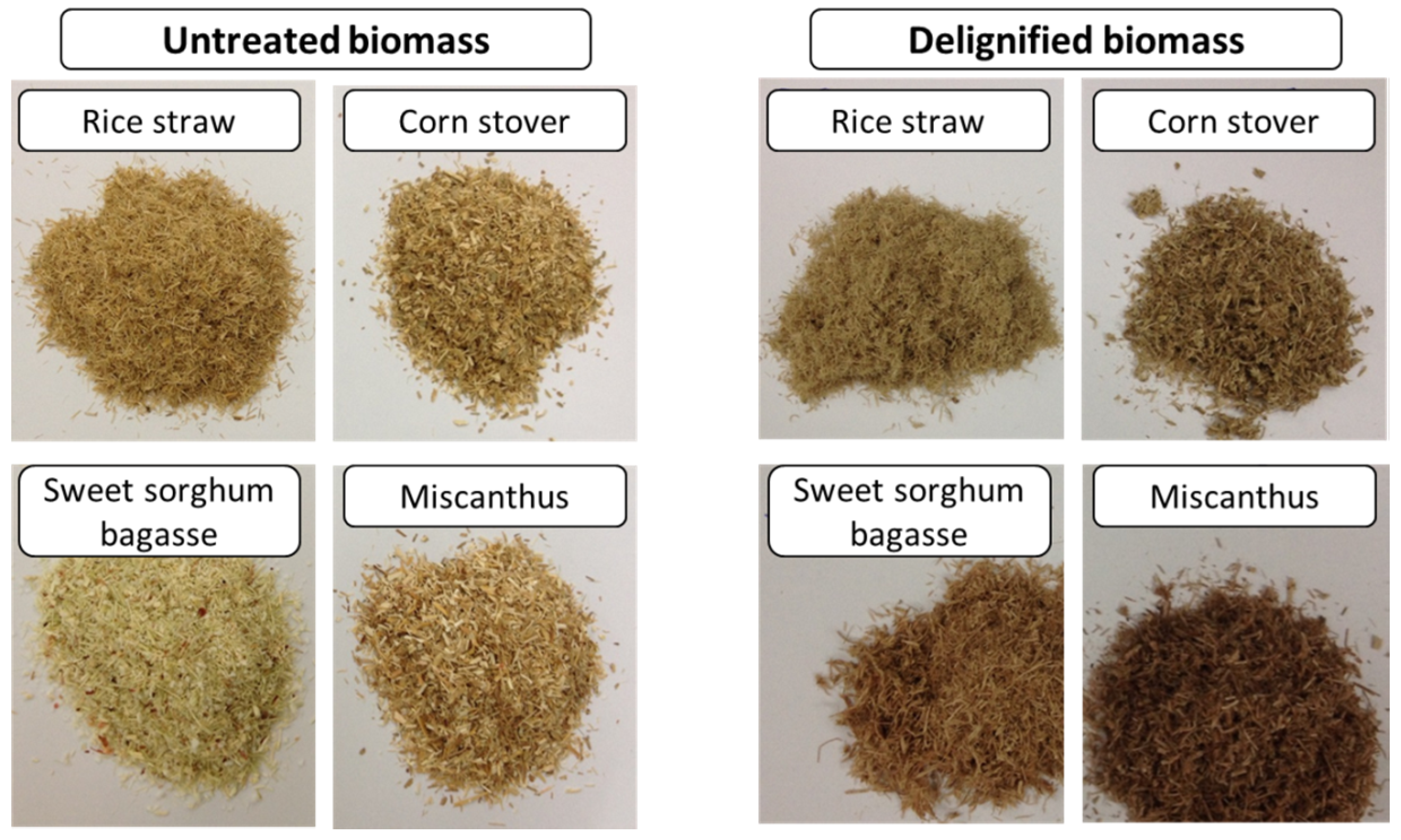
2.1. Delignification of RS, CS, SSB and MS Using SGL (Simulated Green Liquor) Pretreatment
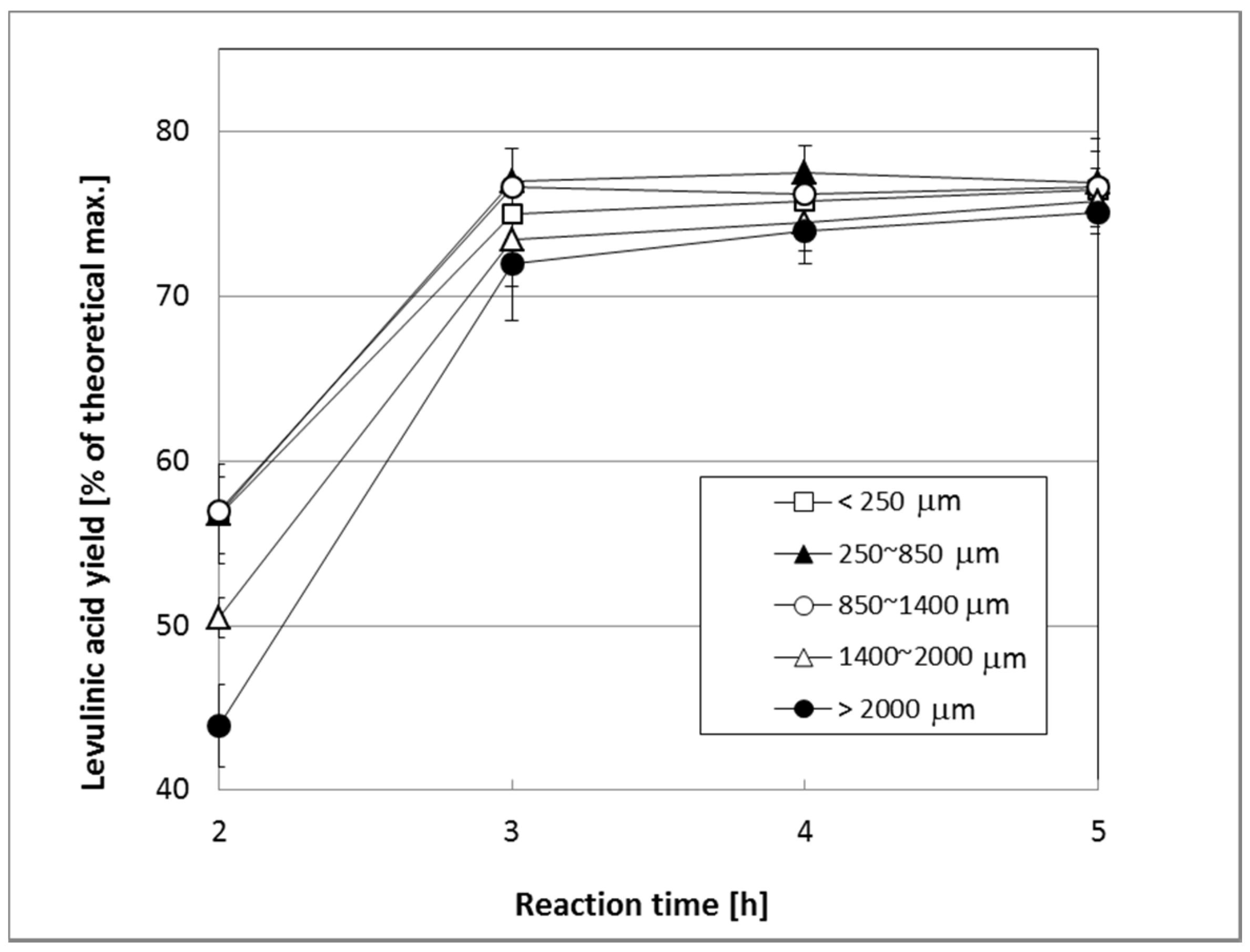
2.2. Effect of Particle Size of Biomass on the Yield of Levulinic Acid, LA
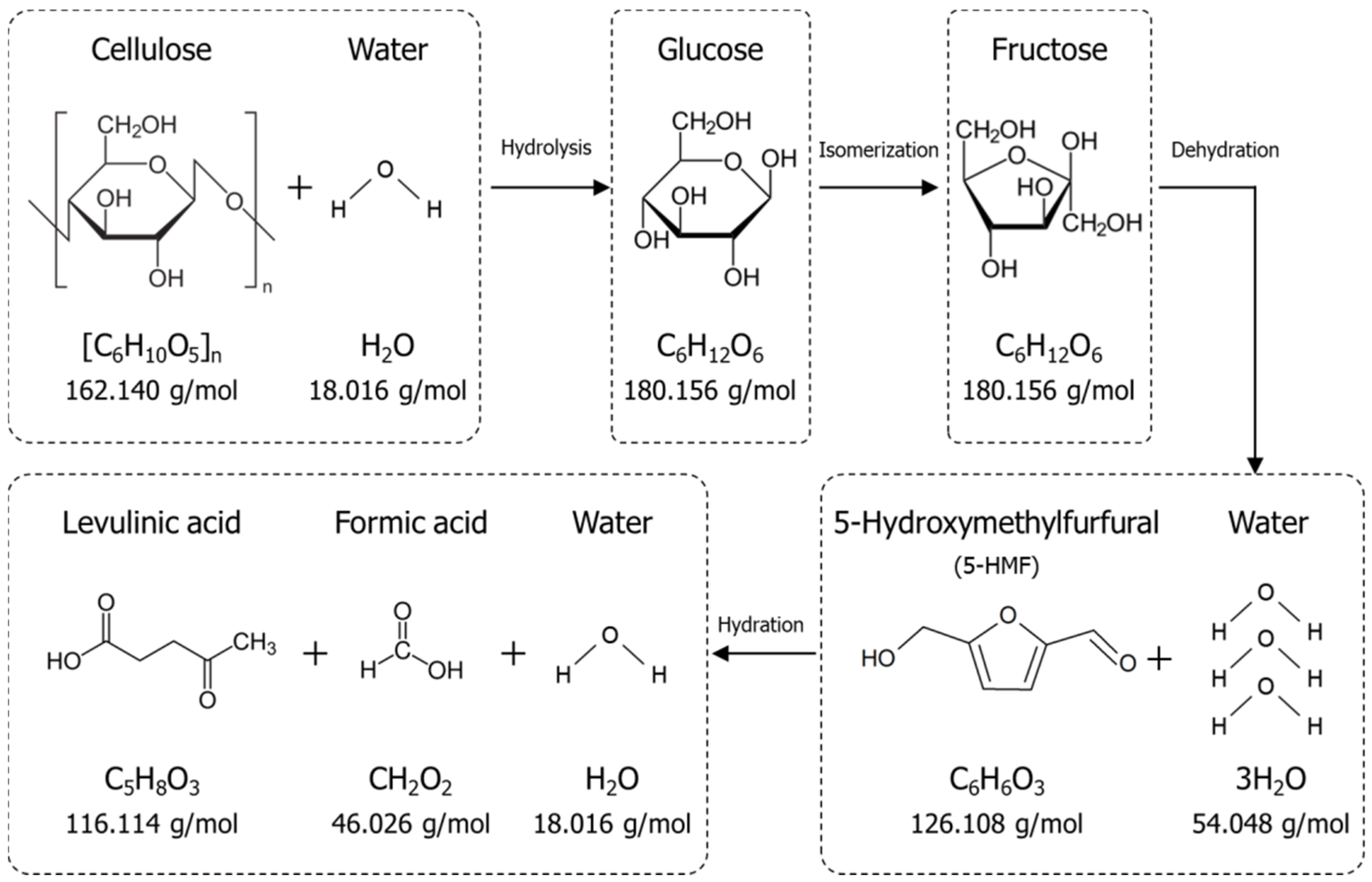
2.3. Conversion of Biomass to LA Using Hydrochloric Acid
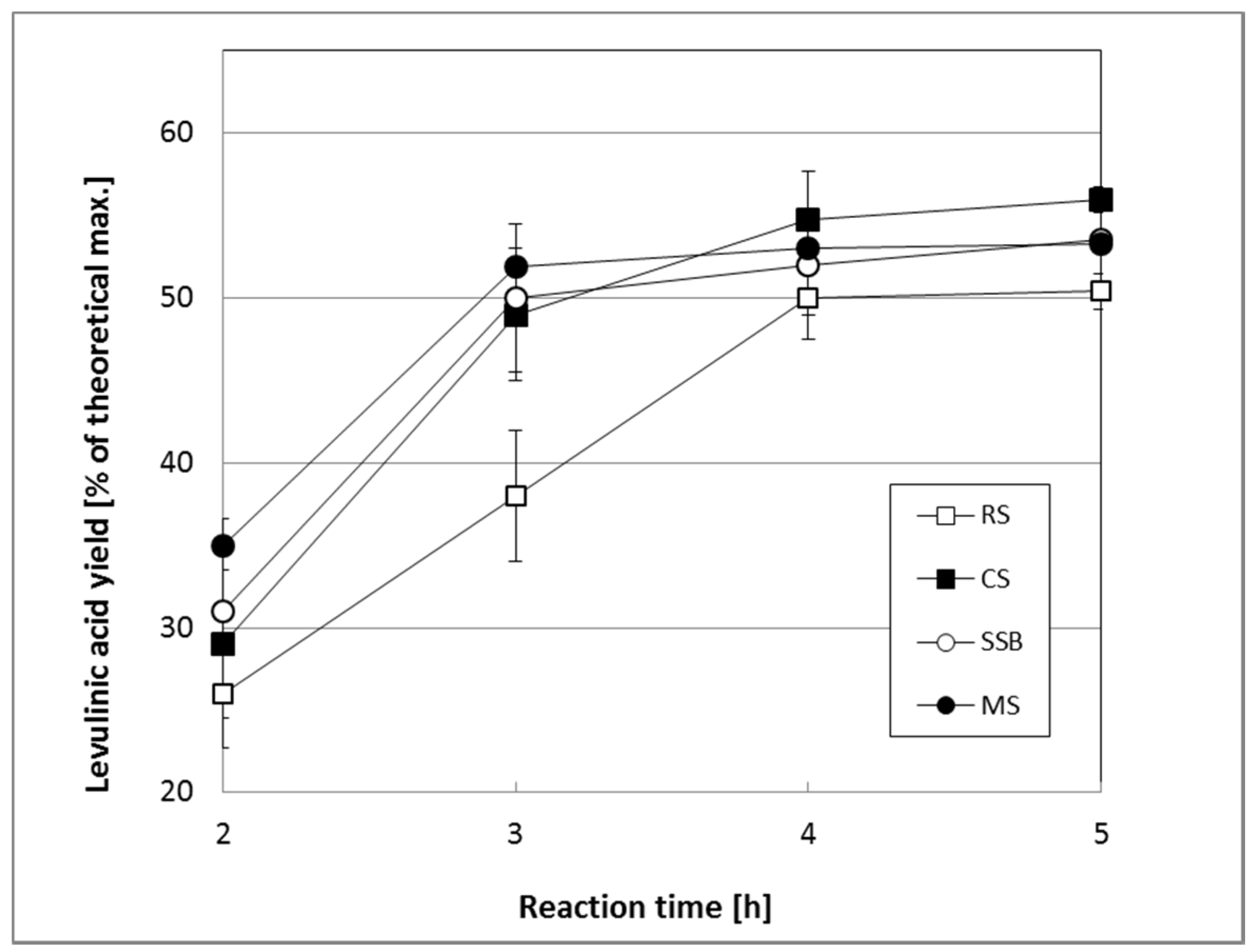
2.3.1. Production of LA Using Untreated Biomass
2.3.2. Production of LA Using Delignified Biomass
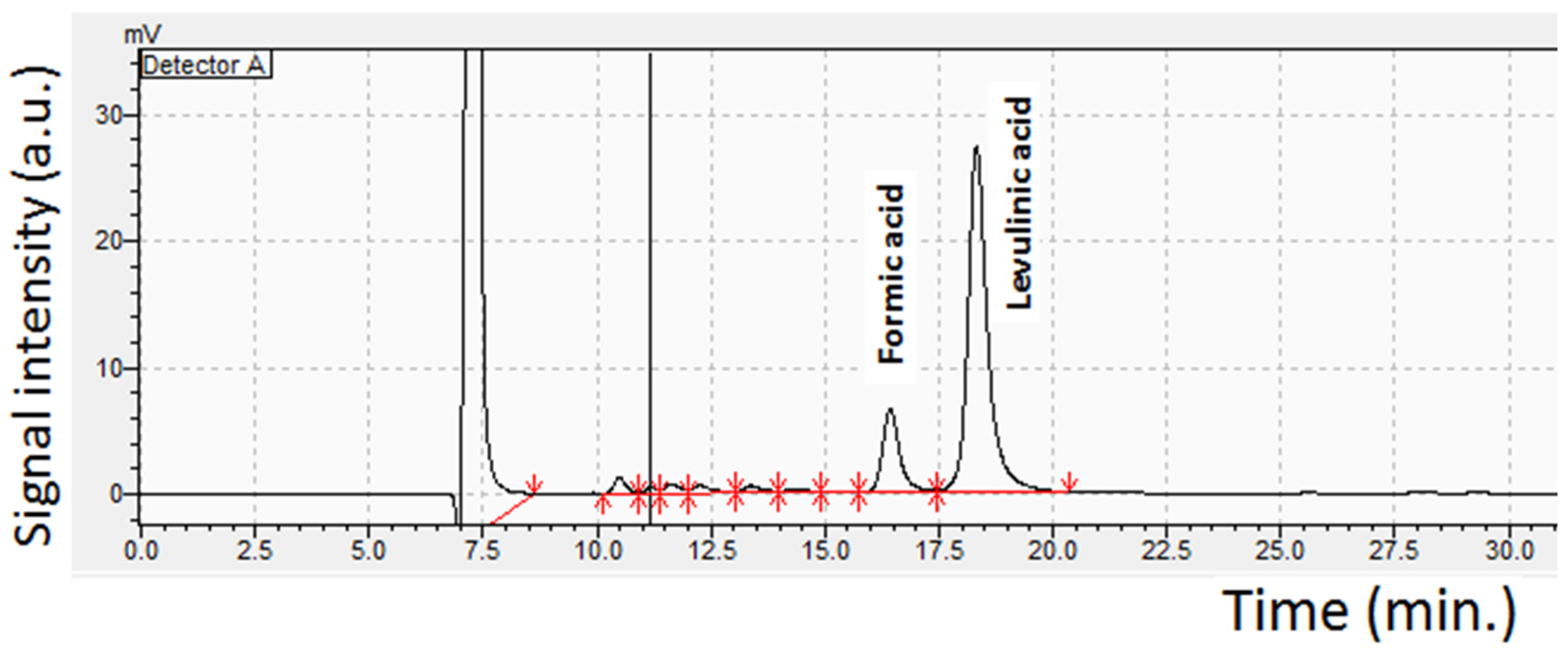
2.3.3. Byproduct Formation during LA Conversion
2.4. Other Notes
3. Materials and Methods
3.1. Feedstock
3.2. Delignification of Biomass using SGL (Na2CO3 + Na2S)
3.3. Conversion of Biomass using Hydrochloric Acid
3.4. Analytical Methods
Acknowledgments
Author Contributions
Conflicts of Interest
Appendix A

References
- Fan, M.; Yan, L. Conversion of pretreated biomass into levulinic acid via continuous extraction at atmosphere pressure. Chin. J. Chem. Phys. 2014, 27, 92–98. [Google Scholar] [CrossRef][Green Version]
- Wang, A.; Lu, Y.; Yi, Z.; Ejaz, A.; Hu, K.; Zhang, L.; Yan, K. Selective production of γ-valerolactone and valeric acid in one-pot bifunctional metal catalysts. ChemistrySelect 2018, 3, 1097–1101. [Google Scholar] [CrossRef]
- Yan, K.; Liu, Y.; Lu, Y.; Chai, J.; Sun, L. Catalytic application of layered double hydroxide-derived catalysts for the conversion of biomass-derived molecules. Catal. Sci. Technol. 2017, 7, 1622–1645. [Google Scholar] [CrossRef]
- Werpy, T.; Petersen, G. Top Value Added Chemicals from Biomass Volume I—Results of Screening for Potential Candidates from Sugars and Synthesis Gas; National Renewable Energy Laboratory (NREL): Golden City, CO, USA, 2004.
- Kang, S.; Yu, J. An intensified reaction technology for high levulinic acid concentration from lignocellulosic biomass. Biomass Bioenergy 2016, 95, 214–220. [Google Scholar] [CrossRef]
- Sultana, A.; Fujitani, T. Conversion of levulinic acid to BTX over different zeolite catalysts. Catal. Commun. 2017, 88, 26–29. [Google Scholar] [CrossRef]
- Enumula, S.S.; Gurram, V.R.B.; Chada, R.R.; Burri, D.R.; Kamaraju, S.R.R. Clean synthesis of alkyl levulinates from levulinic acid over one pot synthesized WO3-SBA-16 catalyst. J. Mol. Catal. A Chem. 2017, 426, 30–38. [Google Scholar] [CrossRef]
- Sun, D.; Takahashi, Y.; Yamada, Y.; Sato, S. Efficient formation of angelica lactones in a vapor-phase conversion of levulinic acid. Appl. Catal. A Gen. 2016, 526, 62–69. [Google Scholar] [CrossRef]
- Heredia-Olea, E.; Pérez-Carrillo, E.; Montoya-Chiw, M.; Serna-Saldívar, S.O. Effects of extrusion pretreatment parameters on sweet sorghum bagasse enzymatic hydrolysis and its subsequent conversion into bioethanol. BioMed Res. Int. 2015, 2015, 325905. [Google Scholar] [CrossRef] [PubMed]
- Jönsson, L.J.; Martín, C. Pretreatment of lignocellulose: Formation of inhibitory by-products and strategies for minimizing their effects. Bioresour. Technol. 2016, 199, 103–112. [Google Scholar] [CrossRef] [PubMed]
- Yoo, C.G.; Nghiem, N.P.; Hicks, K.B.; Kim, T.H. Pretreatment of corn stover using low-moisture anhydrous ammonia (LMAA) process. Bioresour. Technol. 2011, 102, 10028–10034. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.H.; Kim, J.S.; Sunwoo, C.S.; Lee, Y.Y. Pretreatment of corn stover by aqueous ammonia. Bioresour. Technol. 2003, 90, 39–47. [Google Scholar] [CrossRef]
- Jin, Y.; Chang, H.M.; Jameel, H.; Philips, R. Green liquor pretreatment of mixed hardwood for ethanol production in a repurposed kraft pulp mill. J. Wood Chem. Technol. 2010, 30, 86–104. [Google Scholar] [CrossRef]
- Wu, S.F.; Chang, H.M.; Jameel, H.; Philips, R. Novel green liquor pretreatment of loblolly pine chips to facilitate enzymatic hydrolysis into fermentable sugars for ethanol production. J. Wood Chem. Technol. 2010, 30, 205–218. [Google Scholar] [CrossRef]
- Zhou, Z.; Xue, W.; Lei, F.; Cheng, Y.; Jiang, J.; Sun, D. Kraft GL-ethanol pretreatment on sugarcane bagasse for effective enzymatic hydrolysis. Ind. Crops Prod. 2016, 90, 100–109. [Google Scholar] [CrossRef]
- Gu, F.; Yang, L.; Jin, Y.; Han, Q.; Chang, H.M.; Jameel, H.; Phillips, R. Green liquor pretreatment for improving enzymatic hydrolysis of corn stover. Bioresour. Technol. 2012, 24, 299–305. [Google Scholar] [CrossRef] [PubMed]
- Victor, A.; Pulidindi, I.N.; Gedanken, A. Levulinic acid production from Cicer arietinum, Cotton, Pinus radiata and Sugar cane bagasse. RSC Adv. 2014, 4, 44706–44711. [Google Scholar] [CrossRef]
- Yan, D.; Jarvis, C.; Gu, J.; Yan, Y. Production and catalytic transformation of levulinic acid: A platform for speciality chemicals and fuels. Renew. Sustain. Energy Rev. 2015, 51, 986–997. [Google Scholar] [CrossRef]
- Hayes, D.J.; Fitzpatrick, S.; Hayes, M.H.B.; Ross, J.H. The biofine process production of levulinic acid, furfural, and formic acid from lignocellulosic feedstocks. In Biorefineries-Industrial Processes and Products: Status Quo and Future Directions; Kamm, B., Gruber, P.R., Kamm, M., Eds.; Wiley-VCH Verlag, GmbH: Weinheim, Germany, 2008; pp. 139–147. ISBN 978-3-527-31027-2. [Google Scholar]
- Dussan, K.; Girisuta, B.; Haverty, D.; Leahy, J.J.; Hayes, M.H.B. Kinetics of levulinic acid and furfural production from Miscanthus giganteus. Bioresour. Technol. 2013, 149, 216–224. [Google Scholar] [CrossRef] [PubMed]
- Kang, K.E.; Han, M.; Moon, S.K.; Kang, H.W.; Kim, Y.; Cha, Y.L.; Choi, G.W. Optimization of alkali-extrusion pretreatment with twin-screw for bioethanol production from Miscanthus. Fuel 2013, 109, 520–526. [Google Scholar] [CrossRef]
- Gu, F.; Wang, W.; Jing, L.; Jin, Y. Effects of green liquor pretreatment on the chemical composition and enzymatic digestibility of rice straw. Bioresour. Technol. 2013, 149, 375–382. [Google Scholar] [CrossRef] [PubMed]
- Um, B.H.; van Walsum, G.P. Acid hydrolysis of hemicellulose in green liquor pre-pulping extract of mixed northern hardwoods. Appl. Biochem. Biotechnol. 2009, 153, 127–138. [Google Scholar] [CrossRef] [PubMed]
- Jeong, H.; Jang, S.K.; Hong, C.Y.; Kim, S.H.; Lee, S.Y.; Lee, S.M.; Choi, J.W.; Choi, I.G. Levulinic acid production by two-step acid-catalyzed treatment of Quercus mongolica using dilute sulfuric acid. Bioresour. Technol. 2017, 225, 183–190. [Google Scholar] [CrossRef] [PubMed]
- Runge, T.; Zhang, C. Two-stage acid-catalyzed conversion of carbohydrates into levulinic acid. Ind. Eng. Chem. Res. 2012, 51, 3265–3270. [Google Scholar] [CrossRef]
- Chen, H.; Yu, B.; Jin, S. Production of levulinic acid from steam exploded rice straw via solid superacid, /ZrO2-SiO2-Sm2O3. Bioresour. Technol. 2011, 102, 3568–3570. [Google Scholar] [CrossRef] [PubMed]
- Yan, L.; Yang, N.; Pang, H.; Liao, B. Production of levulinic acid from bagasse and paddy straw by liquefaction in the presence of hydrochloride acid. Clean 2008, 36, 158–163. [Google Scholar] [CrossRef]
- Francavilla, M.; Intini, S.; Luchetti, L.; Luque, R. Tunable microwave-assisted aqueous conversion of seaweed-derived agarose for the selective production of 5-hydroxymethyl furfural/levulinic acid. Green Chem. 2016, 18, 5971–5977. [Google Scholar] [CrossRef]
- Bevilaqua, D.B.; Rambo, M.K.D.; Rizzetti, T.M.; Cardoso, A.L.; Martins, A.F.J. Cleaner production: Levulinic acid from rice husks. J. Clean. Prod. 2013, 47, 96–101. [Google Scholar] [CrossRef]
- Ramli, N.A.S.; Amin, N.A.S. Optimization of renewable levulinic acid production from glucose conversion catalyzed by Fe/HY zeolite catalyst in aqueous medium. Energy Convers. Manag. 2015, 95, 10–19. [Google Scholar] [CrossRef]
- Li, S.; Qian, W.E.J. Direct saccharification of rice straw using a solid acid catalyst. Jpn. Inst. Energy 2011, 90, 1065–1071. [Google Scholar] [CrossRef][Green Version]
- Yaaini, N.; Amin, N.A.S.; Asmadi, M. Optimization of levulinic acid from lignocellulosic biomass using a new hybrid catalyst. Bioresour. Technol. 2012, 116, 58–65. [Google Scholar] [CrossRef] [PubMed]
- Victor, A.; Pulidindi, I.N.; Kim, T.H.; Gedanken, A. Design of active solid acid catalyst for the optimization of glucose production from Oryza Sativa straw. RSC Adv. 2016, 6, 31–38. [Google Scholar] [CrossRef]
- Sluiter, A.; Hames, B.; Ruiz, R.; Scarlata, C.; Sluiter, J.; Templeton, D.; Crocker, D. Determination of Structural Carbohydrates and Lignin in Biomass Laboratory Analytical Procedure (LAP); USDOE-NREL (National Renewable Energy Laboratory): Golden, CO, USA, 2012.

| Biomass | Composition | |||||||
|---|---|---|---|---|---|---|---|---|
| S.R. | Glucan | Xylan | Arabinan | AIL | ASL | Delignification | Ash | |
| (wt %) | (wt %) | (wt %) | (wt %) | (wt %) | (wt %) | (wt %) | (wt %) | |
| RS | - | 36.3 ± 0.1 | 14.0 ± 1.0 | 3.7 ± 0.0 | 15.0 ± 0.7 | 2.1 ± 0.4 | - | 8.2 ± 0.1 |
| D-RS | 57.2 | 36.3 ± 0.5 | 10.3 ± 0.1 | 2.3 ± 0.0 | 1.3 ± 0.1 | 0.9 ± 0.0 | 87.6 ± 2.0 | 2.6 ± 0.2 |
| CS | - | 33.0 ± 0.9 | 18.4 ± 0.7 | 5.3 ± 0.1 | 15.2 ± 0.3 | 2.2 ± 0.1 | - | 1.5 ± 0.1 |
| D-CS | 52.2 | 33.0 ± 3.7 | 10.0 ± 1.2 | 2.7 ± 0.1 | 0.8 ± 0.9 | 0.7 ± 0.1 | 91.2 ± 3.2 | 0.2 ± 0.0 |
| SSB | - | 41.3 ± 0.2 | 11.7 ± 0.0 | 3.1 ± 0.1 | 12.0 ± 0.3 | 1.3 ± 0.1 | - | 1.0 ± 0.1 |
| D-SSB | 60.4 | 41.1 ± 0.3 | 11.0 ± 0.2 | 2.1 ± 0.0 | 2.7 ± 0.7 | 0.9 ± 0.0 | 73.2 ± 2.1 | 0.2 ± 0.0 |
| MS | - | 44.3 ± 0.3 | 18.4 ± 0.1 | 3.5 ± 0.0 | 18.9 ± 0.3 | 0.7 ± 0.0 | - | 2.1 ± 0.3 |
| D-MS | 71.8 | 43.3 ± 0.2 | 14.7 ± 0.1 | 2.7 ± 0.0 | 5.8 ± 0.3 | 1.1 ± 0.1 | 64.8 ± 2.3 | 0.4 ± 0.2 |
| Feedstock | Reaction Conditions | LA Yield (% of Theoretical Max) | Reference |
|---|---|---|---|
| Cicer arietinum, cotton, Pinus radiata & sugarcane bagasse | Acid catalyzed hydrothermal reaction (1 M HCl, 150 °C, 2 h) | 32.6–44.0 | [17] |
| Steam exploded rice straw | S2O28−-ZrO2-SiO2-Sm2O3 | 70.0 | [26] |
| Bagasse & paddy straw | 220 °C, 4. 5 wt % HCl; | 70.0 | [27] |
| Hybrid poplar wood chips | H2SO4 (5 wt %), 190 °C, 50 min, and L/S ratio = 10 | 60.3 | [25] |
| Agarose | 1% (v/v) H2SO4; 180 °C; 10 min. Microwave irradiation | 64.0 | [28] |
| Pretreated rice husks | Pressurized acid hydrolysis: 4.5% (v/v) HCl, 170 °C, 56 bar & 60 min; | 59.4 | [29] |
| Glucose and oil palm fronds | 173.4 °C, 3.3 h, 0.93 g of glucose & 0.89 g 10% Fe/HY zeolite. | 61.8 | [30] |
| Rice straw | Amberlyst 15 dry catalyst; 150 °C, 3 h, 10% solid loading | 30–45 | [31] |
| RS, CS, SSB, MS | 1 M HCl, 5 h, 150 °C, 50 g/L solid loading | 60.2–80.0 wt % | This study |
| D-RS, D-CS, D-SSB, D-MS | 1 M HCl, 5 h, 150 °C, 50 g/L solid loading | 50.4–56.0 | This study |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pulidindi, I.N.; Kim, T.H. Conversion of Levulinic Acid from Various Herbaceous Biomass Species Using Hydrochloric Acid and Effects of Particle Size and Delignification. Energies 2018, 11, 621. https://doi.org/10.3390/en11030621
Pulidindi IN, Kim TH. Conversion of Levulinic Acid from Various Herbaceous Biomass Species Using Hydrochloric Acid and Effects of Particle Size and Delignification. Energies. 2018; 11(3):621. https://doi.org/10.3390/en11030621
Chicago/Turabian StylePulidindi, Indra Neel, and Tae Hyun Kim. 2018. "Conversion of Levulinic Acid from Various Herbaceous Biomass Species Using Hydrochloric Acid and Effects of Particle Size and Delignification" Energies 11, no. 3: 621. https://doi.org/10.3390/en11030621
APA StylePulidindi, I. N., & Kim, T. H. (2018). Conversion of Levulinic Acid from Various Herbaceous Biomass Species Using Hydrochloric Acid and Effects of Particle Size and Delignification. Energies, 11(3), 621. https://doi.org/10.3390/en11030621