Electro-Fermentation in Aid of Bioenergy and Biopolymers
Abstract
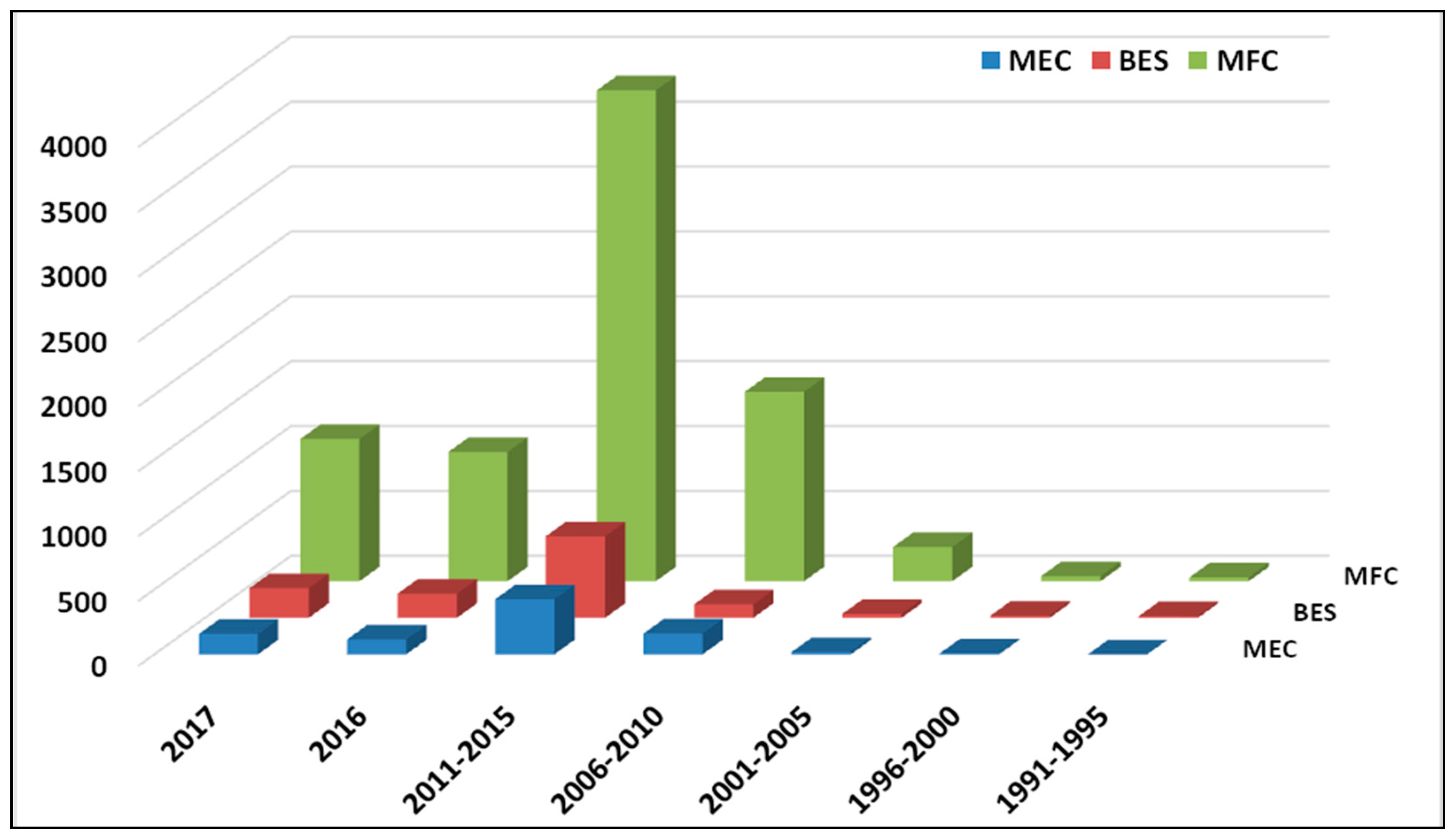
1. Introduction
2. Waste Biomass: Veiled Opportunity
2.1. Anaerobic Digestion and Its Limitations
2.2. Microbial Electro-Fermentation: An Alternative Method of Fermentation
3. Electro-Fermentation in Aid of
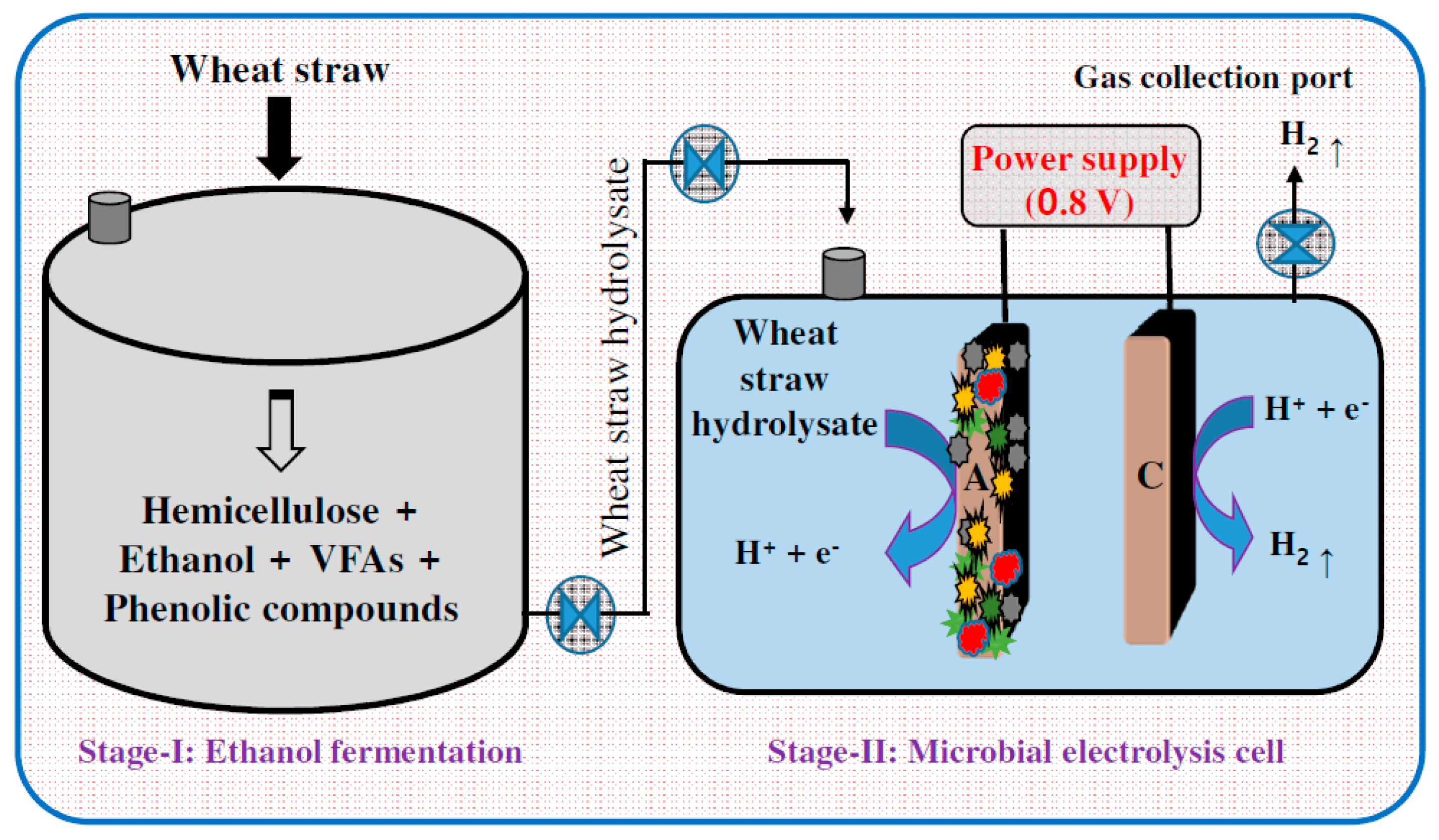
3.1. Hydrogen
3.2. Biogas
3.3. 1,3-Propanediol
3.4. Alcohols
3.5. Polyhydroxyalkanoates
3.6. Integrative Approach of Utilizing EF in a Biorefinery Prospective
4. Limitations and Challenges for Electro-Fermentations
5. Future Perspectives
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Deval, A.S.; Parikh, H.A.; Kadier, A.; Chandrasekhar, K.; Bhagwat, A.M.; Dikshit, A.K. Sequential microbial activities mediated bioelectricity production from distillery wastewater using bio-electrochemical system with simultaneous waste remediation. Int. J. Hydrogen Energy 2017, 42, 1130–1141. [Google Scholar] [CrossRef]
- Kadier, A.; Kalil, M.S.; Abdeshahian, P.; Chandrasekhar, K.; Mohamed, A.; Azman, N.F.; Logroño, W.; Simayi, Y.; Hamid, A.A. Recent advances and emerging challenges in microbial electrolysis cells (MECs) for microbial production of hydrogen and value-added chemicals. Renew. Sustain. Energy Rev. 2016, 61, 501–525. [Google Scholar] [CrossRef]
- Chandrasekhar, K.; Lee, Y.-J.; Lee, D.-W. Biohydrogen production: Strategies to improve process efficiency through microbial routes. Int. J. Mol. Sci. 2015, 16, 8266–8293. [Google Scholar] [CrossRef] [PubMed]
- Chandrasekhar, K.; Venkata Mohan, S. Induced catabolic bio-electrohydrolysis of complex food waste by regulating external resistance for enhancing acidogenic biohydrogen production. Bioresour. Technol. 2014, 165, 372–382. [Google Scholar] [CrossRef] [PubMed]
- Chandrasekhar, K.; Venkata Mohan, S. Bio-electrohydrolysis as a pretreatment strategy to catabolize complex food waste in closed circuitry: Function of electron flux to enhance acidogenic biohydrogen production. Int. J. Hydrogen Energy 2014, 39, 11411–11422. [Google Scholar] [CrossRef]
- Chandrasekhar, K.; Amulya, K.; Venkata Mohan, S. Solid phase bio-electrofermentation of food waste to harvest value-added products associated with waste remediation. Waste Manag. 2015, 45, 57–65. [Google Scholar] [CrossRef] [PubMed]
- Logan, B.E.; Rabaey, K. Conversion of wastes into bioelectricity and chemicals by using microbial electrochemical technologies. Science 2012, 337, 686–690. [Google Scholar] [CrossRef] [PubMed]
- Sustainable Wastewater Treatment Systems: Distributed Resource Recovery for Industry. Available online: www.cambrianinnovation.com (accessed on 11 January 2018).
- Patil, S.A.; Gildemyn, S.; Pant, D.; Zengler, K.; Logan, B.E.; Rabaey, K. A logical data representation framework for electricity-driven bioproduction processes. Biotechnol. Adv. 2015, 33, 736–744. [Google Scholar] [CrossRef] [PubMed]
- Plant-e Living Plants Generate Electricity. Available online: www.plant-e.com (accessed on 12 January 2018).
- Global Water, Wastewater & Reuse Solutions. Available online: www.fluencecorp.com (accessed on 12 January 2018).
- Trapero, J.R.; Horcajada, L.; Linares, J.J.; Lobato, J. Is microbial fuel cell technology ready? An economic answer towards industrial commercialization. Appl. Energy 2017, 185, 698–707. [Google Scholar] [CrossRef]
- What If Stranded Power Could Be Harnessed and Stored as Chemical Energy for Later Use? Available online: www.electrochaea.com (accessed on 11 January 2018).
- Cusick, R.D.; Logan, B.E. Phosphate recovery as struvite within a single chamber microbial electrolysis cell. Bioresour. Technol. 2012, 107, 110–115. [Google Scholar] [CrossRef] [PubMed]
- Fornero, J.J.; Rosenbaum, M.; Angenent, L.T. Electric power generation from municipal, food, and animal wastewaters using microbial fuel cells. Electroanalysis 2010, 22, 832–843. [Google Scholar] [CrossRef]
- Kadier, A.; Simayi, Y.; Chandrasekhar, K.; Ismail, M.; Kalil, M.S. Hydrogen gas production with an electroformed Ni mesh cathode catalysts in a single-chamber microbial electrolysis cell (MEC). Int. J. Hydrogen Energy 2015, 40, 14095–14103. [Google Scholar] [CrossRef]
- Kadier, A.; Simayi, Y.; Abdeshahian, P.; Azman, N.F.; Chandrasekhar, K.; Kalil, M.S. A comprehensive review of microbial electrolysis cells (MEC) reactor designs and configurations for sustainable hydrogen gas production. Alex. Eng. J. 2016, 55, 427–443. [Google Scholar] [CrossRef]
- Popp, J.; Harangi-Rákos, M.; Gabnai, Z.; Balogh, P.; Antal, G.; Bai, A. Biofuels and their co-products as livestock feed: Global economic and environmental implications. Molecules 2016, 21, 285. [Google Scholar] [CrossRef] [PubMed]
- Galanakis, C.M. Recovery of high added-value components from food wastes: Conventional, emerging technologies and commercialized applications. Trends Food Sci. Technol. 2012, 26, 68–87. [Google Scholar] [CrossRef]
- ElMekawy, A.; Srikanth, S.; Bajracharya, S.; Hegab, H.M.; Nigam, P.S.; Singh, A.; Mohan, S.V.; Pant, D. Food and agricultural wastes as substrates for bioelectrochemical system (BES): The synchronized recovery of sustainable energy and waste treatment. Food Res. Int. 2015, 73, 213–225. [Google Scholar] [CrossRef]
- Mohan, S.V.; Chandrasekhar, K. Solid phase microbial fuel cell (SMFC) for harnessing bioelectricity from composite food waste fermentation: Influence of electrode assembly and buffering capacity. Bioresour. Technol. 2011, 102, 7077–7085. [Google Scholar] [CrossRef] [PubMed]
- Reddy, M.V.; Chandrasekhar, K.; Mohan, S.V. Influence of carbohydrates and proteins concentration on fermentative hydrogen production using canteen based waste under acidophilic microenvironment. J. Biotechnol. 2011, 155, 387–395. [Google Scholar] [CrossRef] [PubMed]
- Imbert, E. Food waste valorization options: Opportunities from the bioeconomy. Open Agric. 2017, 2, 195–204. [Google Scholar] [CrossRef]
- Venkata Mohan, S.; Nikhil, G.N.; Chiranjeevi, P.; Nagendranatha Reddy, C.; Rohit, M.V.; Kumar, A.N.; Sarkar, O. Waste biorefinery models towards sustainable circular bioeconomy: Critical review and future perspectives. Bioresour. Technol. 2016, 215, 2–12. [Google Scholar] [CrossRef] [PubMed]
- Kannaiah Goud, R.; Venkata Mohan, S. Pre-fermentation of waste as a strategy to enhance the performance of single chambered microbial fuel cell (MFC). Int. J. Hydrogen Energy 2011, 36, 13753–13762. [Google Scholar] [CrossRef]
- Scopus Document Search. Available online: www.scopus.com (accessed on 12 January 2018).
- Villano, M.; Aulenta, F.; Majone, M. Perspectives of biofuels production from renewable resources with bioelectrochemical systems. Asia-Pac. J. Chem. Eng. 2012, 7, S263–S274. [Google Scholar] [CrossRef]
- Chandrasekhar, K.; Venkata Mohan, S. Bio-electrochemical remediation of real field petroleum sludge as an electron donor with simultaneous power generation facilitates biotransformation of PAH: Effect of substrate concentration. Bioresour. Technol. 2012, 110, 517–525. [Google Scholar] [CrossRef] [PubMed]
- Mohan, S.V.; Chandrasekhar, K. Self-induced bio-potential and graphite electron accepting conditions enhances petroleum sludge degradation in bio-electrochemical system with simultaneous power generation. Bioresour. Technol. 2011, 102, 9532–9541. [Google Scholar] [CrossRef] [PubMed]
- Velvizhi, G.; Venkata Mohan, S. Multi-electrode bioelectrochemical system for the treatment of high total dissolved solids bearing chemical based wastewater. Bioresour. Technol. 2017, 242, 77–86. [Google Scholar] [CrossRef] [PubMed]
- Moscoviz, R.; Toledo-Alarcón, J.; Trably, E.; Bernet, N. Electro-fermentation: How to drive fermentation using electrochemical systems. Trends Biotechnol. 2016, 34, 856–865. [Google Scholar] [CrossRef] [PubMed]
- Schievano, A.; Pepé Sciarria, T.; Vanbroekhoven, K.; De Wever, H.; Puig, S.; Andersen, S.J.; Rabaey, K.; Pant, D. Electro-Fermentation—Merging Electrochemistry with Fermentation in Industrial Applications. Trends Biotechnol. 2016, 34, 866–878. [Google Scholar] [CrossRef] [PubMed]
- Rabaey, K.; Clauwaert, P.; Aelterman, P.; Verstraete, W. Tubular microbial fuel cells for efficient electricity generation. Environ. Sci. Technol. 2005, 39, 8077–8082. [Google Scholar] [CrossRef] [PubMed]
- He, Z.; Minteer, S.D.; Angenent, L.T. Electricity generation from artificial wastewater using an upflow microbial fuel cell. Environ. Sci. Technol. 2005, 39, 5262–5267. [Google Scholar] [CrossRef] [PubMed]
- He, Z.; Wagner, N.; Minteer, S.D.; Angenent, L.T. An upflow microbial fuel cell with an interior cathode: Assessment of the internal resistance by impedance spectroscopy. Environ. Sci. Technol. 2006, 40, 5212–5217. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Zhang, J.; Yang, Q.; Feng, C.; Zhu, Y.; Ye, Z.; Ni, J. Investigation and optimization of the novel UASB–MFC integrated system for sulfate removal and bioelectricity generation using the response surface methodology (RSM). Bioresour. Technol. 2012, 124, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Lee, H.; Wang, X.; Liu, Y.; He, W. Continuous electricity generation by a graphite granule baffled air–cathode microbial fuel cell. Bioresour. Technol. 2010, 101, 632–638. [Google Scholar] [CrossRef] [PubMed]
- Choi, O.; Sang, B.-I. Extracellular electron transfer from cathode to microbes: Application for biofuel production. Biotechnol. Biofuels 2016, 9, 11. [Google Scholar] [CrossRef] [PubMed]
- Nikhil, G.N.; Suman, P.; Venkata Mohan, S.; Swamy, Y.V. Energy-positive nitrogen removal of pharmaceutical wastewater by coupling heterotrophic nitrification and electrotrophic denitrification. Chem. Eng. J. 2017, 326, 715–720. [Google Scholar] [CrossRef]
- Wang, H.; Ren, Z.J. A comprehensive review of microbial electrochemical systems as a platform technology. Biotechnol. Adv. 2013, 31, 1796–1807. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Angelidaki, I. A new method for in situ nitrate removal from groundwater using submerged microbial desalination–denitrification cell (SMDDC). Water Res. 2013, 47, 1827–1836. [Google Scholar] [CrossRef] [PubMed]
- Nikhil, G.N.; Yeruva, D.K.; Venkata Mohan, S.; Swamy, Y.V. Assessing potential cathodes for resource recovery through wastewater treatment and salinity removal using non-buffered microbial electrochemical systems. Bioresour. Technol. 2016, 215, 247–253. [Google Scholar] [CrossRef] [PubMed]
- Chandrasekhar, K.; Ahn, Y.-H. Effectiveness of piggery waste treatment using microbial fuel cells coupled with elutriated-phased acid fermentation. Bioresour. Technol. 2017, 244 Pt 1, 650–657. [Google Scholar] [CrossRef] [PubMed]
- Kiran Kumar, A.; Venkateswar Reddy, M.; Chandrasekhar, K.; Srikanth, S.; Venkata Mohan, S. Endocrine disruptive estrogens role in electron transfer: Bio-electrochemical remediation with microbial mediated electrogenesis. Bioresour. Technol. 2012, 104, 547–556. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Hu, H. Microbial Electrolysis: Novel Biotechnology for Hydrogen Production from Biomass. In Microbial Technologies in Advanced Biofuels Production; Hallenbeck, P.C., Ed.; Springer: Boston, MA, USA, 2012; pp. 93–105. [Google Scholar]
- Zhu, X.; Hatzell, M.C.; Cusick, R.D.; Logan, B.E. Microbial reverse-electrodialysis chemical-production cell for acid and alkali production. Electrochem. Commun. 2013, 31, 52–55. [Google Scholar] [CrossRef]
- Cheng, S.; Xing, D.; Call, D.F.; Logan, B.E. Direct biological conversion of electrical current into methane by electromethanogenesis. Environ. Sci. Technol. 2009, 43, 3953–3958. [Google Scholar] [CrossRef] [PubMed]
- Rabaey, K.; Bützer, S.; Brown, S.; Keller, J.; Rozendal, R.A. High current generation coupled to caustic production using a lamellar bioelectrochemical system. Environ. Sci. Technol. 2010, 44, 4315–4321. [Google Scholar] [CrossRef] [PubMed]
- Rozendal, R.A.; Leone, E.; Keller, J.; Rabaey, K. Efficient hydrogen peroxide generation from organic matter in a bioelectrochemical system. Electrochem. Commun. 2009, 11, 1752–1755. [Google Scholar] [CrossRef]
- Cao, X.; Huang, X.; Liang, P.; Xiao, K.; Zhou, Y.; Zhang, X.; Logan, B.E. A new method for water desalination using microbial desalination cells. Environ. Sci. Technol. 2009, 43, 7148–7152. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; He, Z. Integrated salinity reduction and water recovery in an osmotic microbial desalination cell. RSC Adv. 2012, 2, 3265–3269. [Google Scholar] [CrossRef]
- Forrestal, C.; Xu, P.; Jenkins, P.E.; Ren, Z. Microbial desalination cell with capacitive adsorption for ion migration control. Bioresour. Technol. 2012, 120, 332–336. [Google Scholar] [CrossRef] [PubMed]
- Morel, A.; Zuo, K.; Xia, X.; Wei, J.; Luo, X.; Liang, P.; Huang, X. Microbial desalination cells packed with ion-exchange resin to enhance water desalination rate. Bioresour. Technol. 2012, 118, 43–48. [Google Scholar] [CrossRef] [PubMed]
- Luo, H.; Jenkins, P.E.; Ren, Z. Concurrent desalination and hydrogen generation using microbial electrolysis and desalination cells. Environ. Sci. Technol. 2011, 45, 340–344. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Liu, G.; Zhang, R.; Qin, B.; Luo, Y. Development of the microbial electrolysis desalination and chemical-production cell for desalination as well as acid and alkali productions. Environ. Sci. Technol. 2012, 46, 2467–2472. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Xia, X.; Liang, P.; Cao, X.; Sun, H.; Huang, X. Stacked microbial desalination cells to enhance water desalination efficiency. Environ. Sci. Technol. 2011, 45, 2465–2470. [Google Scholar] [CrossRef] [PubMed]
- Jacobson, K.S.; Drew, D.M.; He, Z. Use of a liter-scale microbial desalination cell as a platform to study bioelectrochemical desalination with salt solution or artificial seawater. Environ. Sci. Technol. 2011, 45, 4652–4657. [Google Scholar] [CrossRef] [PubMed]
- Aelterman, P.; Rabaey, K.; Pham, H.T.; Boon, N.; Verstraete, W. Continuous electricity generation at high voltages and currents using stacked microbial fuel cells. Environ. Sci. Technol. 2006, 40, 3388–3394. [Google Scholar] [CrossRef] [PubMed]
- Cusick, R.D.; Kim, Y.; Logan, B.E. Energy capture from thermolytic solutions in microbial reverse-electrodialysis cells. Science 2012, 335, 1474–1477. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Grot, S.; Logan, B.E. Electrochemically assisted microbial production of hydrogen from acetate. Environ. Sci. Technol. 2005, 39, 4317–4320. [Google Scholar] [CrossRef] [PubMed]
- Ditzig, J.; Liu, H.; Logan, B.E. Production of hydrogen from domestic wastewater using a bioelectrochemically assisted microbial reactor (BEAMR). Int. J. Hydrogen Energy 2007, 32, 2296–2304. [Google Scholar] [CrossRef]
- Chae, K.-J.; Choi, M.-J.; Kim, K.-Y.; Ajayi, F.F.; Chang, I.-S.; Kim, I.S. A solar-powered microbial electrolysis cell with a platinum catalyst-free cathode to produce hydrogen. Environ. Sci. Technol. 2009, 43, 9525–9530. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Logan, B.E. Hydrogen production from inexhaustible supplies of fresh and salt water using microbial reverse-electrodialysis electrolysis cells. Proc. Natl. Acad. Sci. USA 2011, 108, 16176–16181. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Angelidaki, I. Innovative self-powered submersible microbial electrolysis cell (SMEC) for biohydrogen production from anaerobic reactors. Water Res. 2012, 46, 2727–2736. [Google Scholar] [CrossRef] [PubMed]
- Gong, Y.; Ebrahim, A.; Feist, A.M.; Embree, M.; Zhang, T.; Lovley, D.; Zengler, K. Sulfide-driven microbial electrosynthesis. Environ. Sci. Technol. 2013, 47, 568–573. [Google Scholar] [CrossRef] [PubMed]
- Steinbusch, K.J.J.; Hamelers, H.V.M.; Schaap, J.D.; Kampman, C.; Buisman, C.J.N. Bioelectrochemical Ethanol Production through Mediated Acetate Reduction by Mixed Cultures. Environ. Sci. Technol. 2010, 44, 513–517. [Google Scholar] [CrossRef] [PubMed]
- Nevin, K.P.; Hensley, S.A.; Franks, A.E.; Summers, Z.M.; Ou, J.; Woodard, T.L.; Snoeyenbos-West, O.L.; Lovley, D.R. Electrosynthesis of organic compounds from carbon dioxide is catalyzed by a diversity of acetogenic microorganisms. Appl. Environ. Microbiol. 2011, 77, 2882–2886. [Google Scholar] [CrossRef] [PubMed]
- Rabaey, K.; Girguis, P.; Nielsen, L.K. Metabolic and practical considerations on microbial electrosynthesis. Curr. Opin. Biotechnol. 2011, 22, 371–377. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Logan, B.E. Simultaneous removal of organic matter and salt ions from saline wastewater in bioelectrochemical systems. Desalination 2013, 308, 115–121. [Google Scholar] [CrossRef]
- Hu, H.; Fan, Y.; Liu, H. Hydrogen production using single-chamber membrane-free microbial electrolysis cells. Water Res. 2008, 42, 4172–4178. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Patel, S.K.S.; Lee, J.-K.; Kalia, V.C. Extending the limits of Bacillus for novel biotechnological applications. Biotechnol. Adv. 2013, 31, 1543–1561. [Google Scholar] [CrossRef] [PubMed]
- Call, D.; Logan, B.E. Hydrogen production in a single chamber microbial electrolysis cell lacking a membrane. Environ. Sci. Technol. 2008, 42, 3401–3406. [Google Scholar] [CrossRef] [PubMed]
- Modestra, J.A.; Babu, M.L.; Mohan, S.V. Electro-fermentation of real-field acidogenic spent wash effluents for additional biohydrogen production with simultaneous treatment in a microbial electrolysis cell. Sep. Purif. Technol. 2015, 150, 308–315. [Google Scholar] [CrossRef]
- Chandrasekhar, K.; Pandit, S.; Kadier, A.; Dasagrandhi, C.; Velpuri, J. Biohydrogen production: Integrated approaches to improve the process efficiency. In Microbial Applications Vol. 1: Bioremediation and Bioenergy; Kalia, V.C., Kumar, P., Eds.; Springer International Publishing: Cham, Switzerland, 2017; pp. 189–210. [Google Scholar]
- Villano, M.; Aulenta, F.; Ciucci, C.; Ferri, T.; Giuliano, A.; Majone, M. Bioelectrochemical reduction of CO2 to CH4 via direct and indirect extracellular electron transfer by a hydrogenophilic methanogenic culture. Bioresour. Technol. 2010, 101, 3085–3090. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Su, M.; Zhang, Y.; Zhan, G.; Tao, Y.; Li, D. Bioelectrochemical systems for simultaneously production of methane and acetate from carbon dioxide at relatively high rate. Int. J. Hydrogen Energy 2013, 38, 3497–3502. [Google Scholar] [CrossRef]
- Marshall, C.W.; Ross, D.E.; Fichot, E.B.; Norman, R.S.; May, H.D. Long-term operation of microbial electrosynthesis systems improves acetate production by autotrophic microbiomes. Environ. Sci. Technol. 2013, 47, 6023–6029. [Google Scholar] [CrossRef] [PubMed]
- Su, M.; Jiang, Y.; Li, D. Production of acetate from carbon dioxide in bioelectrochemical systems based on autotrophic mixed culture. J. Microbiol. Biotechnol. 2013, 23, 1140–1146. [Google Scholar] [CrossRef] [PubMed]
- Choi, O.; Kim, T.; Woo, H.M.; Um, Y. Electricity-driven metabolic shift through direct electron uptake by electroactive heterotroph Clostridium pasteurianum. Sci. Rep. 2014, 4, 6961. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.; Chen, J.; Freguia, S.; Rabaey, K.; Keller, J. Carbon and electron fluxes during the electricity driven 1,3-Propanediol biosynthesis from glycerol. Environ. Sci. Technol. 2013, 47, 11199–11205. [Google Scholar] [CrossRef] [PubMed]
- Xafenias, N.; Anunobi, M.O.; Mapelli, V. Electrochemical startup increases 1,3-propanediol titers in mixed-culture glycerol fermentations. Process Biochem. 2015, 50, 1499–1508. [Google Scholar] [CrossRef]
- Bajracharya, S.; Ter Heijne, A.; Dominguez Benetton, X.; Vanbroekhoven, K.; Buisman, C.J.N.; Strik, D.P.B.; Pant, D. Carbon dioxide reduction by mixed and pure cultures in microbial electrosynthesis using an assembly of graphite felt and stainless steel as a cathode. Bioresour. Technol. 2015, 195, 14–24. [Google Scholar] [CrossRef] [PubMed]
- Srikanth, S.; Venkateswar Reddy, M.; Venkata Mohan, S. Microaerophilic microenvironment at biocathode enhances electrogenesis with simultaneous synthesis of polyhydroxyalkanoates (PHA) in bioelectrochemical system (BES). Bioresour. Technol. 2012, 125, 291–299. [Google Scholar] [CrossRef] [PubMed]
- Modestra, J.A.; Mohan, S.V. Microbial electrosynthesis of carboxylic acids through CO2 reduction with selectively enriched biocatalyst: Microbial dynamics. J. CO2 Util. 2017, 20, 190–199. [Google Scholar] [CrossRef]
- Choi, K.-S.; Kondaveeti, S.; Min, B. Bioelectrochemical methane (CH4) production in anaerobic digestion at different supplemental voltages. Bioresour. Technol. 2017, 245, 826–832. [Google Scholar] [CrossRef] [PubMed]
- Clauwaert, P.; Tolêdo, R.; van der Ha, D.; Crab, R.; Verstraete, W.; Hu, H.; Udert, K.M.; Rabaey, K. Combining biocatalyzed electrolysis with anaerobic digestion. Water Sci. Technol. 2008, 57, 575–579. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Fotidis, I.A.; Angelidaki, I. Ammonia effect on hydrogenotrophic methanogens and syntrophic acetate-oxidizing bacteria. FEMS Microbiol. Ecol. 2015, 91, fiv130. [Google Scholar] [CrossRef] [PubMed]
- Michalopoulos, I.; Chatzikonstantinou, D.; Mathioudakis, D.; Vaiopoulos, I.; Tremouli, A.; Georgiopoulou, M.; Papadopoulou, K.; Lyberatos, G. Valorization of the liquid fraction of a mixture of livestock waste and cheese whey for biogas production through high-rate anaerobic co-digestion and for electricity production in a microbial fuel cell (MFC). Waste Biomass Valoriz. 2017, 8, 1759–1769. [Google Scholar] [CrossRef]
- Wang, A.; Liu, W.; Cheng, S.; Xing, D.; Zhou, J.; Logan, B.E. Source of methane and methods to control its formation in single chamber microbial electrolysis cells. Int. J. Hydrogen Energy 2009, 34, 3653–3658. [Google Scholar] [CrossRef]
- Mézes, L.; Bai, A.; Nagy, D.; Cinka, I.; Gabnai, Z. Optimization of raw material composition in an agricultural biogas plant. Trends Renew. Energy 2017, 3, 61–75. [Google Scholar] [CrossRef]
- Bai, A.; Grasselli, G.; Kormányos, S.; Szendrei, J. Economic evaluation of scaling of agricultural biogas plants. Hung. Agric. Eng. 2007, 20, 23–25. [Google Scholar]
- Kumar, P.; Singh, M.; Mehariya, S.; Patel, S.K.S.; Lee, J.-K.; Kalia, V.C. Ecobiotechnological approach for exploiting the abilities of Bacillus to produce co-polymer of polyhydroxyalkanoate. Indian J. Microbiol. 2014, 54, 151–157. [Google Scholar] [CrossRef] [PubMed]
- Pant, D.; Singh, A.; Van Bogaert, G.; Olsen, S.I.; Nigam, P.S.; Diels, L.; Vanbroekhoven, K. Bioelectrochemical systems (BES) for sustainable energy production and product recovery from organic wastes and industrial wastewaters. RSC Adv. 2012, 2, 1248–1263. [Google Scholar] [CrossRef]
- Lee, B.; Park, J.-G.; Shin, W.-B.; Tian, D.-J.; Jun, H.-B. Microbial communities change in an anaerobic digestion after application of microbial electrolysis cells. Bioresour. Technol. 2017, 234, 273–280. [Google Scholar] [CrossRef] [PubMed]
- Tartakovsky, B.; Manuel, M.F.; Neburchilov, V.; Wang, H.; Guiot, S.R. Biocatalyzed hydrogen production in a continuous flow microbial fuel cell with a gas phase cathode. J. Power Sources 2008, 182, 291–297. [Google Scholar] [CrossRef]
- Clauwaert, P.; Verstraete, W. Methanogenesis in membraneless microbial electrolysis cells. Appl. Microbiol. Biotechnol. 2009, 82, 829–836. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, K.; Sasaki, D.; Morita, M.; Hirano, S.-I.; Matsumoto, N.; Ohmura, N.; Igarashi, Y. Bioelectrochemical system stabilizes methane fermentation from garbage slurry. Bioresour. Technol. 2010, 101, 3415–3422. [Google Scholar] [CrossRef] [PubMed]
- Srikanth, S.; Kumar, M.; Singh, M.P.; Das, B.P. Bioelectro chemical systems: A sustainable and potential platform for treating waste. Procedia Environ. Sci. 2016, 35, 853–859. [Google Scholar] [CrossRef]
- Kumar, P.; Mehariya, S.; Ray, S.; Mishra, A.; Kalia, V.C. Biodiesel industry waste: A potential source of bioenergy and biopolymers. Indian J. Microbiol. 2015, 55, 1–7. [Google Scholar] [CrossRef]
- Mathew, A.S.; Wang, J.; Luo, J.; Yau, S.-T. Enhanced ethanol production via electrostatically accelerated fermentation of glucose using Saccharomyces cerevisiae. Sci. Rep. 2015, 5, 15713. [Google Scholar] [CrossRef] [PubMed]
- Shin, H.; Zeikus, J.; Jain, M. Electrically enhanced ethanol fermentation by Clostridium thermocellum and Saccharomyces cerevisiae. Appl. Microbiol. Biotechnol. 2002, 58, 476–481. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Wang, J.; Yau, S.-T. Controlled glucose consumption in yeast using a transistor-like device. Sci. Rep. 2014, 4, 5429. [Google Scholar] [CrossRef] [PubMed]
- Jeon, B.Y.; Hwang, T.S.; Park, D.H. Electrochemical and biochemical analysis of ethanol fermentation of Zymomonas mobilis KCCM11336. J. Microbiol. Biotechnol. 2009, 19, 666–674. [Google Scholar] [PubMed]
- Torella, J.P.; Gagliardi, C.J.; Chen, J.S.; Bediako, D.K.; Colón, B.; Way, J.C.; Silver, P.A.; Nocera, D.G. Efficient solar-to-fuels production from a hybrid microbial–water-splitting catalyst system. Proc. Natl. Acad. Sci. USA 2015, 112, 2337–2342. [Google Scholar] [CrossRef] [PubMed]
- Thygesen, A.; Thomsen, A.B.; Possemiers, S.; Verstraete, W. Integration of microbial electrolysis cells (MECs) in the biorefinery for production of ethanol, H2 and phenolics. Waste Biomass Valoriz. 2010, 1, 9–20. [Google Scholar] [CrossRef]
- Kumar, P.; Ray, S.; Patel, S.K.S.; Lee, J.-K.; Kalia, V.C. Bioconversion of crude glycerol to polyhydroxyalkanoate by Bacillus thuringiensis under non-limiting nitrogen conditions. Int. J. Biol. Macromol. 2015, 78, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Singh, M.; Kumar, P.; Ray, S.; Kalia, V.C. Challenges and opportunities for customizing polyhydroxyalkanoates. Indian J. Microbiol. 2015, 55, 235–249. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Jun, H.-B.; Kim, B.S. Co-production of polyhydroxyalkanoates and carotenoids through bioconversion of glycerol by Paracoccus sp. strain LL1. Int. J. Biol. Macromol. 2018, 107, 2552–2558. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Ray, S.; Kalia, V.C. Production of co-polymers of polyhydroxyalkanoates by regulating the hydrolysis of biowastes. Bioresour. Technol. 2016, 200, 413–419. [Google Scholar] [CrossRef] [PubMed]
- Pantazaki, A.A.; Papaneophytou, C.P.; Lambropoulou, D.A. Simultaneous polyhydroxyalkanoates and rhamnolipids production by Thermus thermophilus HB8. AMB Express 2011, 1, 17. [Google Scholar] [CrossRef] [PubMed]
- Redwood, M.D.; Orozco, R.L.; Majewski, A.J.; Macaskie, L.E. Electro-extractive fermentation for efficient biohydrogen production. Bioresour. Technol. 2012, 107, 166–174. [Google Scholar] [CrossRef] [PubMed]
- Redwood, M.D.; Orozco, R.L.; Majewski, A.J.; Macaskie, L.E. An integrated biohydrogen refinery: Synergy of photofermentation, extractive fermentation and hydrothermal hydrolysis of food wastes. Bioresour. Technol. 2012, 119, 384–392. [Google Scholar] [CrossRef] [PubMed]
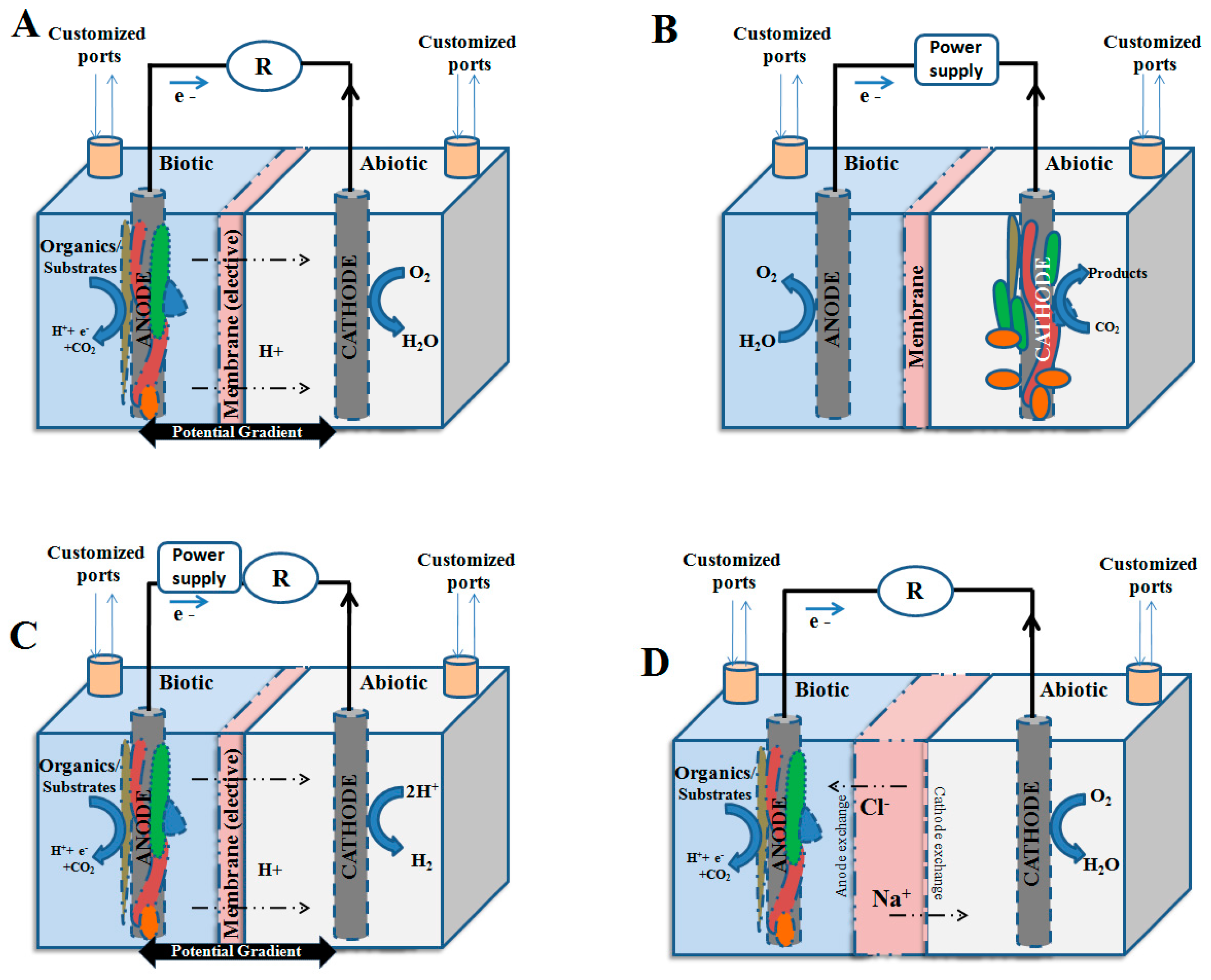
| Types | Substrates Used (Oxidation at Anode) | Reduction of Cathode | Major Output | References |
|---|---|---|---|---|
| Microbial Fuel Cells (MFC) | ||||
| Tubular MFC | Glucose, Acetate, wastewater | K3[Fe(CN)6] | Electric Current | [33] |
| Up-flow MFC (UMFC) | Sucrose | K3[Fe(CN)6], O2 | Electric Current | [34,35] |
| Up-flow anaerobic sludge blanket—MFC | Glucose | O2 | Electric Current | [36] |
| Baffled air-cathode—MFC | Glucose, corn-stover hydrolysates | O2 | Electric Current | [37] |
| Stacked—MFC | NaOAc | K3[Fe(CN)6] | Electric Current | [58] |
| Microbial reverse electrodialysis cell (MRC) | NaOAc | O2 | Electric Current | [59] |
| Microbial reverse-electrodialysis chemical-production cell (MRCC) | NaOAc | O2 | Electric Current, acid, alkali | [46] |
| Microbial Electrolysis Cells (MECs) | ||||
| MEC-based systems for chemical production Microbial electrolysis cells (MECs) | Any biodegradable material | Proton | H2, H2O2, CH4, NaOH | [47,48,49,60] |
| Bioelectro-chemically assisted microbial reactor (BEAMR) | Wastewater | Proton | H2 | [61] |
| Solar-powered microbial electrolysis fuel (solar MEC) | Acetate | Proton | H2 | [62] |
| Microbial reverse-electrodialysis electrolysis cell (MREC) | Acetate | Proton | H2 | [63] |
| Submersible microbial electrolysis cell (SMEC) | Acetate | Proton | H2 | [64] |
| Microbial electrolysis struvite-precipitation cell (MESC) | NaOAc a | Proton | H2, Struvite | [14] |
| Microbial Electrosynthesis (MES) | ||||
| MES-based systems for chemical production | Organic, H2 sulfide, H2O | Acetate or other organics, CO2 | Ethanol, acetate, 2-oxobutyrate, formate | [65,66,67,68] |
| Solid state bio-electrofermentation system (SBES) | Food wastes | O2 | Electricity, H2, Ethanol | [6] |
| Microbial Desalination Cells (MDCs) | ||||
| MDC-based systems for water desalination and beneficial reuse | Any biodegradable material | O2, K3[Fe(CN)6], organics, or other oxidants | Desalination | [50] |
| Microbial saline-wastewater electrolysis cell (MSC) | NaOAc | H2 | Treated saline wastewater, Electric Current | [69] |
| Osmotic MDC | NaOAc, xylose, wastewater | O2, K3[Fe(CN)6], proton | Water desalination, Electric Current | [51] |
| capacitive adsorption capability (cMDC) | NaOAc | K3[Fe(CN)6] | Water desalination | [52] |
| MDC packed with ion-exchange resin (R-MDC) | NaOAc | O2 | Water desalination, Electric Current | [53] |
| Electrolysis-MDC | NaOAc | Proton | H2, water desalination | [54] |
| Microbial electrolysis desalination and chemical production cell (MEDCC) | NaOAc | O2 | Water desalination, NaOH, HCl | [55] |
| Submerged MDC—denitrification cell (SMDDC) | NaOAc | Nitrate | Electric Current, N2 | [41] |
| Stacked microbial desalination cell (SMDC) | NaOAc | O2 | Water desalination, Electric Current | [56] |
| Upflow microbial desalination cell (UMDC) | NaOAc | O2 | Water desalination, Electric Current | [57] |
| Type | Inoculum | Substrate | Potential (V) | Product A | Product B | Yield of | Remarks | References | |
|---|---|---|---|---|---|---|---|---|---|
| Product A | Product B | ||||||||
| MEC | Enriched mixed culture | CO2 | −0.1 V | CH4 | - a | 4.5 L m−2 d−1 | - | 80% energy efficiency | [47] |
| MEC | Enriched mixed culture | CO2 | −0.9 V | CH4 | - | 9.2 L m−2 d−1 | - | high conversion rates (0.055 mmol d−1 mgVSS−1) | [75] |
| BES | WWTP sludge | CO2 | −1 to −1.15 V | Acetate | CH4 | 94.73 mg d−1 | 129.32 mL d−1 | 97% current capture efficiency | [76] |
| MES | Acclimatized acetogens | CO2 | −0.59 V | Acetate | H2 | 1.04 g L−1 d−1 | 0.2 g L−1 d−1 | Stabile, resilient, and improved performance over long-term operation | [77] |
| BES | Enriched electroactive mixed culture biofilm | CO2 | −1.1 V | Acetate | H2 | 2.35 mM d−1 | <0.24 mM d-1 | Cathode potential was identified as a critical factor | [78] |
| BES | Enriched electroactive mixed culture biofilm | CO2 | −1.1 V | Acetate | CH4 | 0.62 mM d−1 | 1.9 mM d−1 | Absence of methanogen inhibitor led to methane production | [78] |
| BES | Clostridium pasteurianum DSM525 | Glycerol | 0.045 V | 1,3-Propanediol | Butanol | 93 mM | 58 mM | Current consumption leads to metabolic shift | [79] |
| BES | Enriched mixed culture | Glycerol | −0.9 V | 1,3-Propanediol | H2 and CH4 | 0.8 mM L−1 h−1 | - | Direct H2 supply enhances 1,3-PDO synthesis by releasing reduced H2 during the pyruvate decarboxylation. | [80] |
| BES | Enriched mixed culture | Glycerol | −0.8 to −1.1 V | 1,3-Propanediol | - | 42 g L−1 | - | 6 times higher 1,3-PDO production compared to non-BES fermentation; no current supply leads to domination by Lactobacillus producing lactic acid | [81] |
| BES | Clostridium pasteurianum DSM525 | Glucose | 0.045 V | Butanol | Acetate/Butyrate | 1.2 mM h−1 | 3.2 mM h−1/3.7 mM h−1 | In BES, e− uptake by C. pasteurianum led to a metabolic shift where reduced nicotinamide adenine dinucleotide (NADH) consumption was quick by butanol production instead of acids. | [79] |
| MES | Clostridium ljungdahlii | CO2 | −1.1 V | Ethanol/Acetate | H2 | ~100 mg L-1/19.19 g m−2 d−1 | - | H2 production may stimulate planktonic bacteria rather than cathodic-biofilm. | [82] |
| MES | Mixed culture | CO2 | −1.1 V | Acetate | H2/CH4 | 37.89 g m−2 d−1 | - | H2 production is possibly a quick mode of transferring e− to the biocatalysts. | [82] |
| BES | Enriched mixed culture | Acetate | 0.5 V | H2O2 | - | 1.9 Kg m−3 d−1 | - | Low energy requirement of approx. 0.93 kWh/kg of H2O2 | [49] |
| MES | C. ljungdahlii DSM13528 | CO2 | −0.6 V | Acetate | Oxobutyrate | ~105 µM Acetate | - | Engineered strains of C. ljungdahlii capable of electrosynthesis could become a potential candidate for industrial biofuel production | [67] |
| MES | Moorella thermoacetica | CO2 | −0.6 V | Acetate | - | ~90 µM Acetate | - | >80% conversion efficiency by M. thermoacetica | [67] |
| BES | Mixed cultures | Glucose | - | Electricity | Polyhydroxylkanoate | 512 mV | <2% dry cell mass | Higher degradation at cathode | [83] |
| single chambered MEC | Acid-shock pretreated anaerobic consortium | Acidogenic spent wash effluents +0.6 V | 0.6 V | H2 | - | 39.35 mL at the rate of 0.057 mmol/h | - | 68% VFA utilization; Additional H2 recovery utilizing VFA rich effluents | [73] |
| Double chambered BES | Enriched culture of homoacetogenic bacteria | CO2 | −0.8 V | Acetic acid | - | 12.57 mM | - | Enrichment of biocatalysts improved the yield | [84] |
| Stacked MFC | Aerobic and anaerobic sludge mixture | sodium acetate | - | Electricity | - | 258 W m−3 | - | Coulombic efficiency 71.6% or substrate to electricity conversion of 4.7 g COD L−1 d−1 | [58] |
| MEC without membrane | Acetate-fed mixed culture | sodium acetate | 0.8 V | H2 | CH4 | 3.12 m3 H2/m3 reactor per day | 1.9% of the total gas | High H2 is possible by membrane-less MFC in a single chamber system | [72] |
| MEC | Effluent of wastewater plant | glucose | 1.0 V | CH4 | - | 408.3 mL CH4/g COD | - | 30.3% higher than in the control | [85] |
| MFC | Aerobic and anaerobic sludge mixture | Acetate | 0.6 V | CH4 | - | 0.41 mol/mol of acetate | - | lower partial pressure of H2 can improve the cathodic reduction thermodynamics | [86] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kumar, P.; Chandrasekhar, K.; Kumari, A.; Sathiyamoorthi, E.; Kim, B.S. Electro-Fermentation in Aid of Bioenergy and Biopolymers. Energies 2018, 11, 343. https://doi.org/10.3390/en11020343
Kumar P, Chandrasekhar K, Kumari A, Sathiyamoorthi E, Kim BS. Electro-Fermentation in Aid of Bioenergy and Biopolymers. Energies. 2018; 11(2):343. https://doi.org/10.3390/en11020343
Chicago/Turabian StyleKumar, Prasun, Kuppam Chandrasekhar, Archana Kumari, Ezhaveni Sathiyamoorthi, and Beom Soo Kim. 2018. "Electro-Fermentation in Aid of Bioenergy and Biopolymers" Energies 11, no. 2: 343. https://doi.org/10.3390/en11020343
APA StyleKumar, P., Chandrasekhar, K., Kumari, A., Sathiyamoorthi, E., & Kim, B. S. (2018). Electro-Fermentation in Aid of Bioenergy and Biopolymers. Energies, 11(2), 343. https://doi.org/10.3390/en11020343






