Efficient Polymer Solar Cells with Alcohol-Soluble Zirconium(IV) Isopropoxide Cathode Buffer Layer
Abstract
:1. Introduction
2. Experimental Details
2.1. Materials and Instrumentation
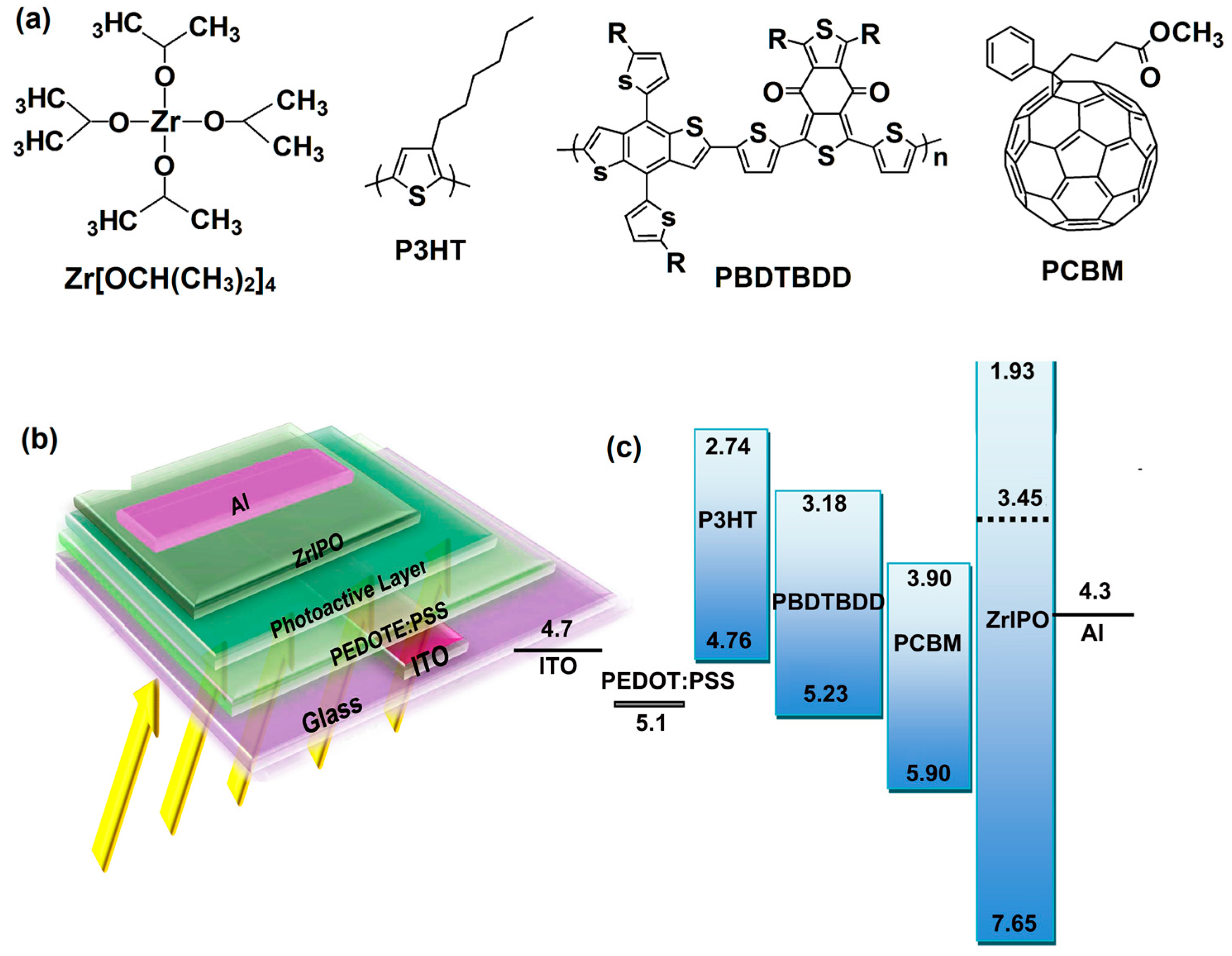
2.2. Device Fabrication and Characterization
- (A)
- ITO/PEDOT:PSS/P3HT:PCBM/Al,
- (B)
- ITO/PEDOT:PSS/P3HT:PCBM/Ca/Al,
- (C)
- ITO/PEDOT:PSS/P3HT:PCBM/ZrIPO/Al,
- (D)
- ITO/PEDOT:PSS/PBDTBDD:PCBM/Al,
- (E)
- ITO/PEDOT:PSS/PBDTBDD:PCBM/Ca/Al,
- (F)
- ITO/PEDOT:PSS/PBDTBDD:PCBM/ZrIPO/Al.
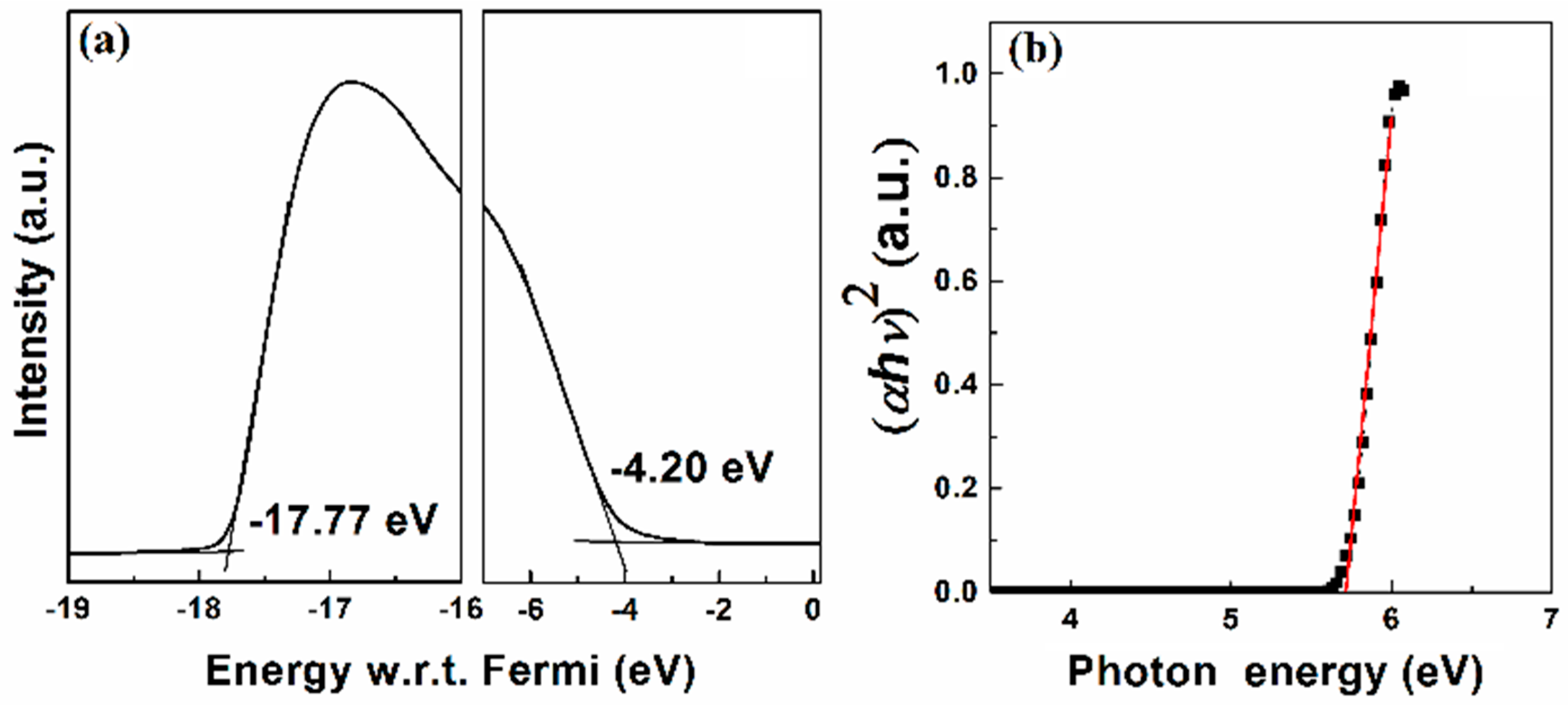
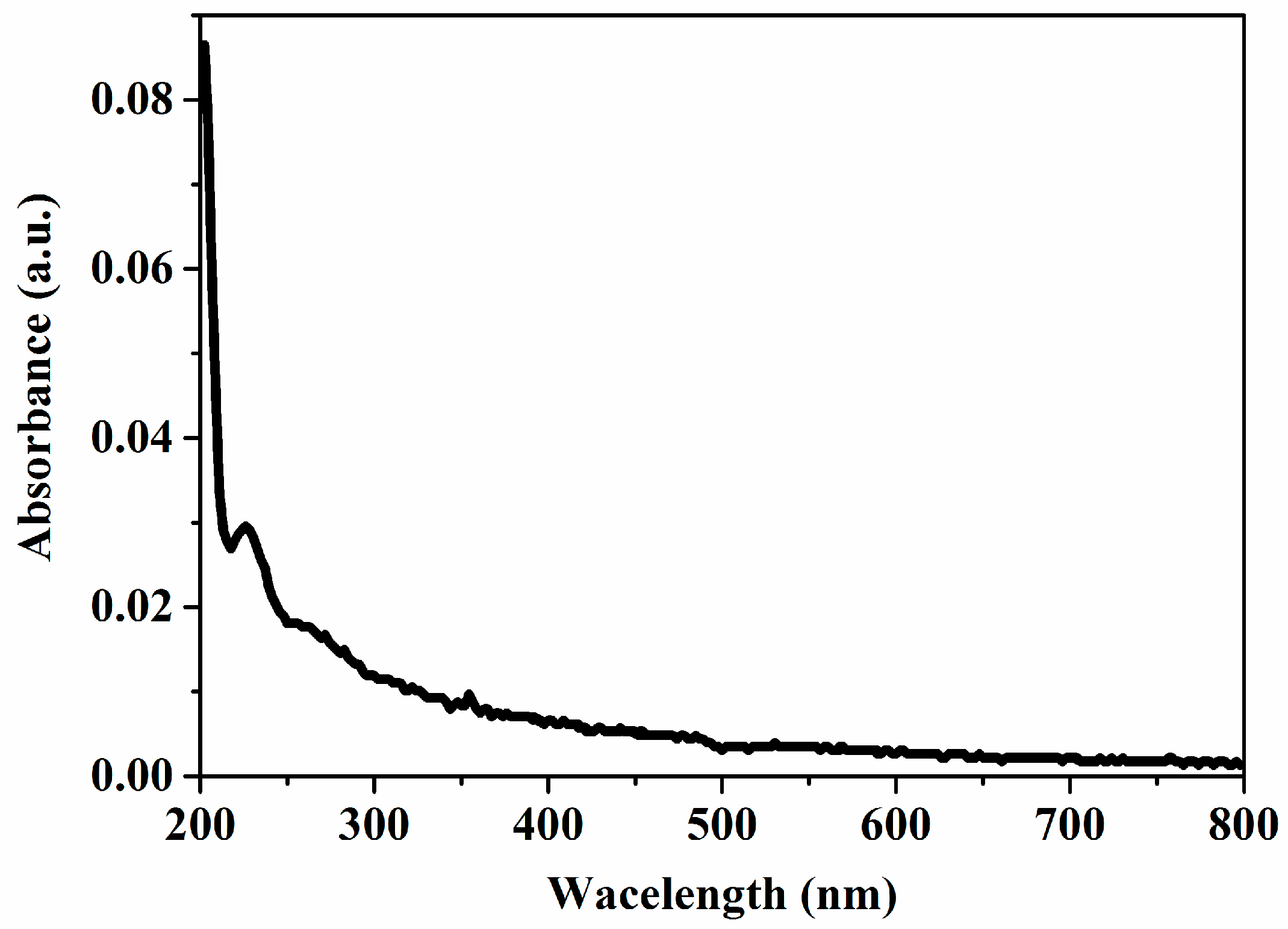
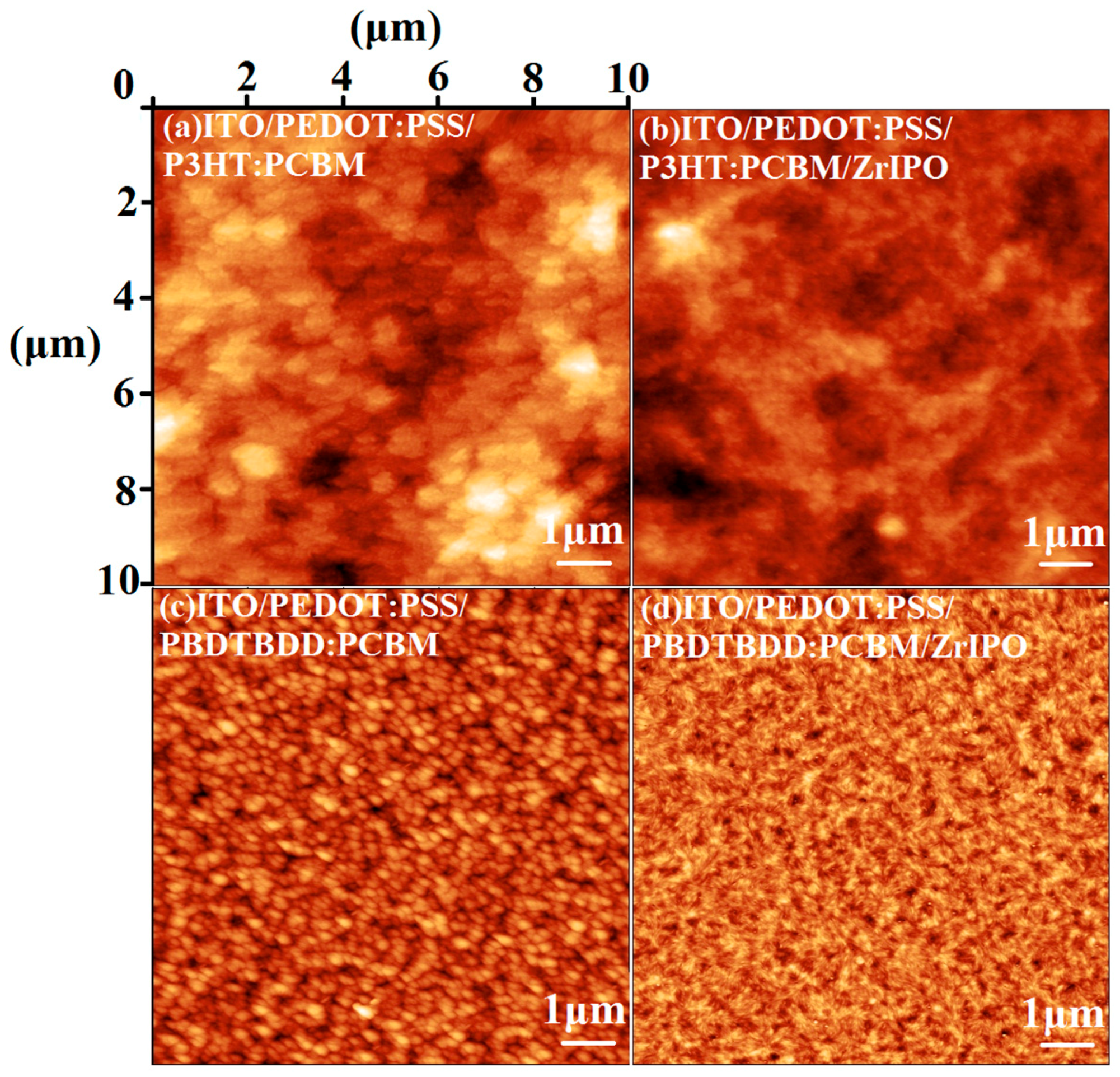
3. Results and Discussion
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Kim, J.Y.; Lee, K.; Coates, N.E.; Moses, D.; Nguyen, T.Q.; Dante, M.; Heeger, A.J. Efficient tandem polymer solar cells fabricated by all-solution processing. Science 2007, 317, 222–225. [Google Scholar] [CrossRef] [PubMed]
- Ye, L.; Xiong, Y.; Zhang, Q.Q.; Li, S.S.; Wang, C.; Jiang, Z.; Hou, J.H.; Ade, H. Surpassing 10% Efficiency Benchmark for Nonfullerene Organic Solar Cells by Scalable Coating in Air from Single Nonhalogenated Solvent. Adv. Mater. 2018. [Google Scholar] [CrossRef] [PubMed]
- Ye, L.; Zhao, W.C.; Li, S.S.; Mukherjee, S.; Carpenter, J.H.; Awartani, O.; Jiao, X.C.; Hou, J.H.; Ade, H. High-Efficiency Nonfullerene Organic Solar Cells: Critical Factors that Affect Complex Multi-Length Scale Morphology and Device Performance. Adv. Energy Mater. 2017, 7, 1602000. [Google Scholar] [CrossRef]
- Søndergaard, R.; Hösel, M.; Angmo, D.; Larsen-Olsen, T.T.; Krebs, F.C. Roll-to-roll fabrication of polymer solar cells. Mater. Today 2012, 15, 36–49. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Zhao, J.; Li, Z.; Mu, C.; Ma, W.; Hu, H.; Jiang, K.; Lin, H.; Ade, H.; Yan, H. Aggregation and morphology control enables multiple cases of high-efficiency polymer solar cells. Nat. Commun. 2014, 5, 5293. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.D. Single-junction polymer solar cells exceeding 10% power conversion efficiency. Adv. Mater. 2015, 27, 1035–1041. [Google Scholar] [CrossRef] [PubMed]
- You, J.B.; Dou, L.T.; Yoshimura, K.; Kato, T.; Ohya, K.; Moriarty, T.; Emery, K.; Chen, C.C.; Gao, J.; Li, G.; et al. A polymer tandem solar cell with 10.6% power conversion efficiency. Nat. Commun. 2013, 4, 1446. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.; Jung, J.W. High performance polymer solar cells employing a low-temperature solution-processed organic—Inorganic hybrid electron transport layer. J. Mater. Chem. A 2016, 4, 16612–16618. [Google Scholar] [CrossRef]
- Ratcliff, E.L.; Meyer, J.; Steirer, K.X.; Garcia, A.; Berry, J.J.; Ginley, D.S.; Olson, D.C.; Kahn, A.; Armstrong, N.R. Evidence for near-surface NiOOH species in solution-processed NiOx selective interlayer materials: Impact on energetics and the performance of polymer bulk heterojunction photovoltaics. Chem. Mater. 2011, 23, 4988–5000. [Google Scholar] [CrossRef]
- Zhao, W.C.; Ye, L.; Zhang, S.Q.; Yao, H.F.; Sun, M.L.; Hou, J.H. An Easily Accessible Cathode Buffer Layer for Achieving Multiple High Performance Polymer Photovoltaic Cells. J. Phys. Chem. C 2015, 119, 27322–27329. [Google Scholar] [CrossRef]
- Wang, F.Z.; Li, L.J.; Xu, Q.; Qian, D.P.; Li, S.S.; Tan, Z.A. Improved performance of polymer solar cells based on P3HT and ICBA using alcohol soluble titanium chelate as electron collection layer. Org. Electron. 2013, 14, 845–851. [Google Scholar] [CrossRef]
- Wang, F.Z.; Tan, Z.A.; Li, Y.F. Solution-processable metal oxides/chelates as electrode buffer layers for efficient and stable polymer solar cells. Energy Environ. Sci. 2015, 8, 1059–1091. [Google Scholar] [CrossRef]
- Ouyang, X.H.; Peng, R.X.; Ai, L.; Zhang, X.Y.; Ge, Z.Y. Efficient polymer solar cells employing a non-conjugated small-molecule electrolyte. Nat. Photonics 2015, 9, 520–524. [Google Scholar] [CrossRef]
- Zhang, W.F.; Wang, H.T.; Chen, B.X.; Bi, X.H.; Venkatesan, S.; Qiao, Q.Q.; Yang, S.F. Oleamide as a self-assembled cathode buffer layer for polymer solar cells: The role of the terminal group on the function of the surfactant. J. Mater. Chem. 2012, 22, 24067–24074. [Google Scholar] [CrossRef]
- Ma, W.L.; Yang, C.Y.; Gong, X.; Lee, K.; Heeger, A.J. Thermally stable efficient polymer solar cells with nanoscale control of the interpenetrating network morphology. Adv. Funct. Mater. 2005, 15, 1617–1622. [Google Scholar] [CrossRef]
- Qi, B.Y.; Zhang, Z.G.; Wang, J.Z. Uncovering the role of cathode buffer layer in organic solar cells. Sci. Rep. 2015, 5, 7803. [Google Scholar] [CrossRef] [PubMed]
- Cheng, X.; Sun, S.H.; Chen, Y.C.; Gao, Y.J.; Ai, L.; Jia, T.; Li, F.H.; Wang, Y. A water-soluble metallophthalocyanine derivative as a cathode interlayer for highly efficient polymer solar cells. J. Mater. Chem. A 2014, 2, 12484–12491. [Google Scholar] [CrossRef]
- Jung, J.W.; Jo, J.W.; Jo, W.H. Enhanced performance and air stability of polymer solar cells by formation of a self-assembled buffer layer from fullerene-endcapped poly (ethylene glycol). Adv. Mater. 2011, 23, 1782–1787. [Google Scholar] [CrossRef] [PubMed]
- Li, C.Z.; Chueh, C.C.; Yip, H.L.; O’Malley, K.M.; Chen, W.C.; Jen, A.K.Y. Effective interfacial layer to enhance efficiency of polymer solar cells via solution processed fullerene-surfactants. J. Mater. Chem. 2012, 22, 8574–8578. [Google Scholar] [CrossRef]
- Azimi, H.; Ameri, T.; Zhang, H.; Hou, Y.; Quiroz, C.O.R.; Min, J.; Hu, M.Y.; Zhang, Z.G.; Przybilla, T.; Matt, G.J.; et al. A universal interface layer based on an amine-functionalized fullerene derivative with dual functionality for efficient solution processed organic and perovskite solar cells. Adv. Energy Mater. 2015, 5, 1401692. [Google Scholar] [CrossRef]
- O’Malley, K.M.; Li, C.Z.; Yip, H.L.; Jen, A.K.Y. Enhanced open-circuit voltage in high performance polymer/fullerene bulk-heterojunction solar cells by cathode modification with a C60 surfactant. Adv. Energy Mater. 2012, 2, 82–86. [Google Scholar] [CrossRef]
- Page, Z.A.; Liu, Y.; Duzhko, V.V.; Russell, T.P.; Emrick, T. Fulleropyrrolidine interlayers: Tailoring electrodes to raise organic solar cell efficiency. Science 2014, 346, 441–444. [Google Scholar] [CrossRef] [PubMed]
- Tan, Z.A.; Zhang, W.Q.; Zhang, Z.; Qian, D.P.; Huang, Y.; Hou, J.; Li, Y.F. High-Performance Inverted Polymer Solar Cells with Solution-Processed Titanium Chelate as Electron-Collecting Layer on ITO Electrode. Adv. Mater. 2012, 24, 1476–1481. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.Q.; Wu, Q.L.; Xia, F.; Chen, X.; Liu, Y.W.; Zhang, W.F.; Zhu, J.; Dai, S.Y.; Yang, S.F. Improving the conductivity of PEDOT: PSS hole transport layer in polymer solar cells via copper (II) bromide salt doping. ACS Appl. Mater. Interfaces 2015, 7, 1439–1448. [Google Scholar] [CrossRef] [PubMed]
- Tan, Z.A.; Yang, C.H.; Zhou, E.J.; Wang, X.; Li, Y.F. Performance improvement of polymer solar cells by using a solution processible titanium chelate as cathode buffer layer. Appl. Phys. Lett. 2007, 91, 023509. [Google Scholar] [CrossRef]
- Hagfeldt, A.; Boschloo, G.; Sun, L.; Kloo, L.; Pettersson, H. Dye-Sensitized Solar Cells. Chem. Rev. 2010, 110, 6595–6663. [Google Scholar] [CrossRef] [PubMed]
- Park, M.H.; Li, J.H.; Kumar, A.; Li, G.; Yang, Y. Doping of the Metal Oxide Nanostructure and its Influence in Organic Electronics. Adv. Funct. Mater. 2009, 19, 1241–1246. [Google Scholar] [CrossRef]
- Ha, Y.E.; Jo, M.Y.; Park, J.; Kang, Y.C.; Moon, S.J.; Kim, J.H. An X-ray photoelectron spectroscopy investigation into the interface formed between poly (2-methoxy-5-(2′-ethyl-hexyloxyl)-p-phenylene vinylene) and indium tin oxide. Synth. Met. 2014, 187, 113–117. [Google Scholar] [CrossRef]
- Yoo, S.I.; Do, T.T.; Ha, Y.E.; Jo, M.Y.; Park, J.; Kang, Y.C.; Kim, J.H.; Kor, B. Effect of Self-Assembled Monolayer Treated ZnO on the Photovoltaic Properties of Inverted Polymer Solar Cells. Chem. Soc. 2014, 35, 569–574. [Google Scholar] [CrossRef]
- Steim, R.; Kogler, F.R.; Brabec, C.J. Interface materials for organic solar cells. J. Mater. Chem. A 2010, 20, 2499–2512. [Google Scholar] [CrossRef]
- Yusoff, A.R.M.; Kima, H.P.; Jang, J. High performance organic photovoltaics with zinc oxide and graphene oxide buffer layers. Nanoscale 2014, 6, 1537–1544. [Google Scholar] [CrossRef] [PubMed]
- Tan, Z.A.; Li, S.S.; Wang, F.Z.; Qian, D.P.; Lin, J.; Hou, J.H.; Li, Y.F. High performance polymer solar cells with as-prepared zirconium acetylacetonate film as cathode buffer layer. Sci. Rep. 2014, 4, 4691. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.Z.; Xu, Q.; Tan, Z.A.; Li, L.J.; Li, S.S.; Sun, G.; Tu, X.H.; Hou, J.H.; Li, Y.F. Efficient polymer solar cells with a solution-processed and thermal annealing-free RuO2 anode buffer layer. J. Mater. Chem. A 2014, 2, 1318–1324. [Google Scholar] [CrossRef]
- Wang, F.Z.; Xu, Q.; Tan, Z.A.; Qian, D.P.; Ding, Y.Q.; Li, L.J.; Li, S.S.; Li, Y.F. Alcohol soluble titanium(IV) oxide bis(2,4-pentanedionate) as electron collection layer for efficient inverted polymer solar cells. Org. Electron. 2012, 13, 2429–2435. [Google Scholar] [CrossRef]
- Wang, F.Z.; Sun, G.; Li, C.; Liu, J.Y.; Hu, S.Q.; Zheng, H.; Tan, Z.A.; Li, Y.F. Finding the Lost Open-Circuit Voltage in Polymer Solar Cells by UV-Ozone Treatment of the Nickel Acetate Anode Buffer Layer. ACS Appl. Mater. Interfaces 2014, 6, 9458–9465. [Google Scholar] [CrossRef] [PubMed]
- Ruderer, M.A.; Müller-Buschbaum, P. Morphology of polymer-based bulk heterojunction films for organic photovoltaics. Soft Matter 2011, 7, 5482–5493. [Google Scholar] [CrossRef]
- Chai, L.; White, R.T.; Greiner, M.T.; Lu, Z.H. Experimental demonstration of the universal energy level alignment rule at oxide/organic semiconductor interfaces. Phys. Rev. B 2014, 89, 035202. [Google Scholar] [CrossRef]
- Tengstedt, C.; Osikowicz, W.; Salaneck, W.R.; Parker, I.D.; Hsu, C.H.; Fahlman, M. Fermi-level pinning at conjugated polymer interfaces. Appl. Phys. Lett. 2006, 88, 053502. [Google Scholar] [CrossRef]
- Crispin, A.; Crispin, X.; Fahlman, M.; Berggren, M.; Salaneck, W.R. Transition between energy level alignment regimes at a low band gap polymer-electrode interfaces. Appl. Phys. Lett. 2006, 89, 213503. [Google Scholar] [CrossRef]
- Osikowicz, W.; de Jong, M.P.; Salaneck, W.R. Formation of the Interfacial Dipole at Organic-Organic Interfaces: C60/Polymer Interfaces. Adv. Mater. 2007, 19, 4213–4217. [Google Scholar] [CrossRef]
- Fukagawa, H.; Kera, S.; Kataoka, T.; Hosoumi, S.; Watanabe, Y.; Kudo, K.; Ueno, N. The Role of the Ionization Potential in Vacuum-Level Alignment at Organic Semiconductor Interfaces. Adv. Mater. 2007, 19, 665–668. [Google Scholar] [CrossRef]
- Greiner, M.T.; Helander, M.G.; Tang, W.M.; Wang, Z.B.; Qiu, J.; Lu, Z.H. Universal energy-level alignment of molecules on metal oxides. Nat. Mater. 2012, 11, 76–81. [Google Scholar] [CrossRef] [PubMed]
- Belmonte, G.G.; Boix, P.P.; Bisquert, J.; Lenes, M.; Bolink, H.J.; Rosa, A.L.; Filippone, S.; Martín, N. Influence of the Intermediate Density-of-States Occupancy on Open-Circuit Voltage of Bulk Heterojunction Solar Cells with Different Fullerene Acceptors. J. Phys. Chem. Lett. 2010, 1, 2566–2571. [Google Scholar] [CrossRef]
- Yip, H.L.; Hau, S.K.; Baek, N.S.; Ma, H.; Jen, A.K.Y. Polymer Solar Cells That Use Self-Assembled-Monolayer-Modified ZnO/Metals as Cathodes. Adv. Mater. 2008, 20, 2376–2382. [Google Scholar] [CrossRef]
- Vandewal, K.; Tvingstedt, K.; Gadisa, A.; Inganas, O.; Manca, J.V. On the origin of the open-circuit voltage of polymer-fullerene solar cells. Nat. Mater. 2009, 8, 904–909. [Google Scholar] [CrossRef] [PubMed]
- Murase, S.; Yang, Y. Solution Processed MoO3 Interfacial Layer for Organic Photovoltaics Prepared by a Facile Synthesis Method. Adv. Mater. 2012, 24, 2459–2462. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.Z.; Zhang, B.; Li, Q.X.; Shi, Z.Z.; Yu, L.; Liu, H.; Wang, Y.P.; Dai, S.Y.; Tan, Z.A.; Li, Y.F. Management of the light distribution within the photoactive layer for high performance conventional and inverted polymer solar cells. J. Mater. Chem. A 2016, 4, 1915–1922. [Google Scholar] [CrossRef]
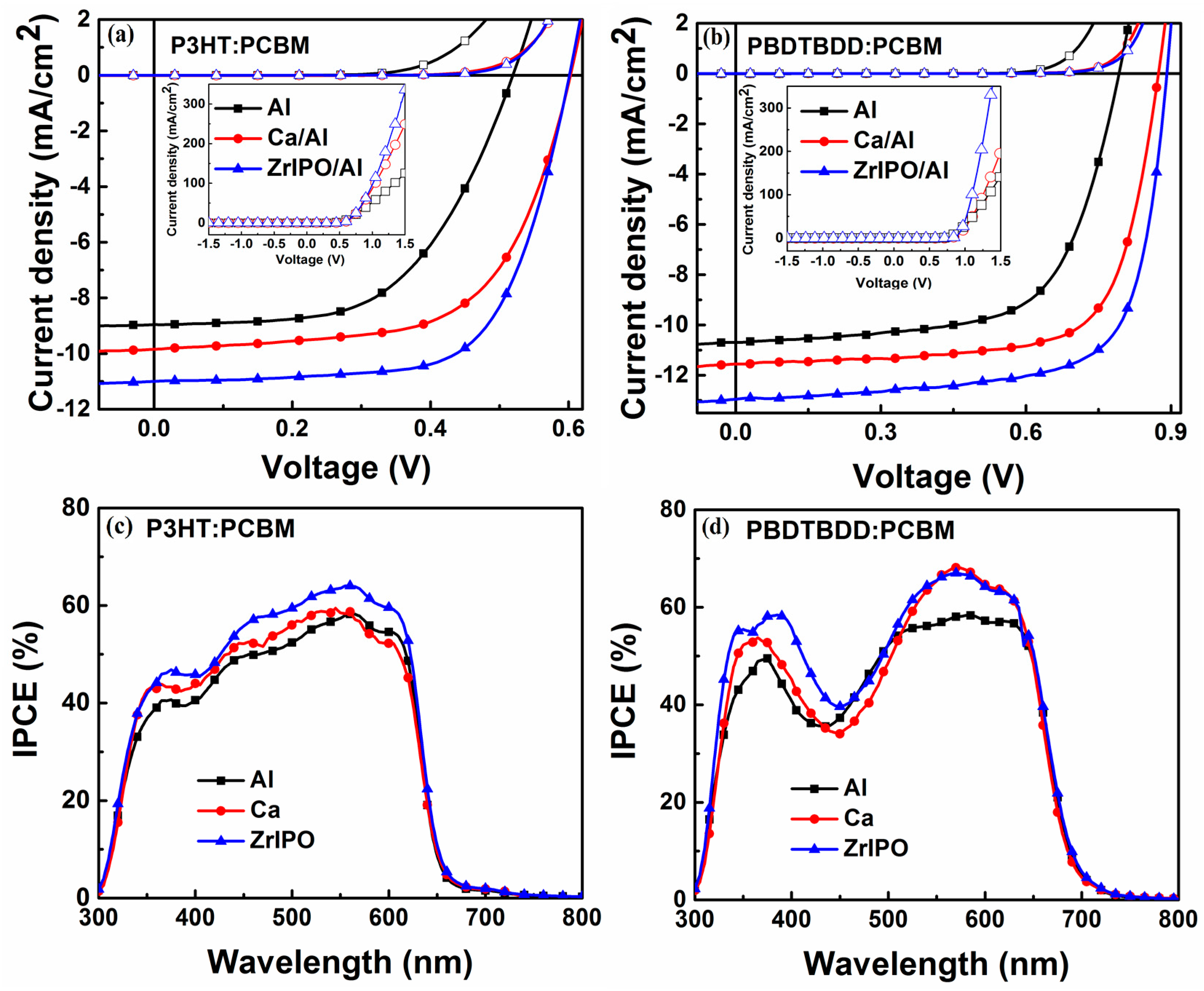

| Device | Active Layer | Cathode | Voc (V) | Jsc (mA/cm2) | FF (%) | PCE (%) | Rs a (Ω cm2) |
|---|---|---|---|---|---|---|---|
| A | P3HT:PCBM | Al | 0.52 (±0.01) | 8.96 (±0.02) | 55.9 (±0.01) | 2.60 (±0.05) | 7.6 (±0.04) |
| B | Ca/Al | 0.60 (±0.01) | 9.85 (±0.03) | 62.4 (±0.02) | 3.69 (±0.03) | 3.5 (±0.03) | |
| C | ZrIPO/Al | 0.60 (±0.01) | 11.0 (±0.01) | 67.7 (±0.01) | 4.47 (±0.02) | 2.8 (±0.02) | |
| D | PBDTBDD:PCBM | Al | 0.79 (±0.01) | 10.7 (±0.02) | 64.8 (±0.02) | 5.48 (±0.04) | 12.5 (±0.05) |
| E | Ca/Al | 0.87 (±0.01) | 11.56 (±0.01) | 71.2 (±0.02) | 7.16 (±0.03) | 4.6 (±0.03) | |
| F | ZrIPO/Al | 0.89 (±0.01) | 12.95 (±0.01) | 70.0 (±0.01) | 8.07 (±0.03) | 1.2 (±0.02) |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luo, Z.; Yang, B.; Bai, Y.; Hayat, T.; Alsaedi, A.; Tan, Z. Efficient Polymer Solar Cells with Alcohol-Soluble Zirconium(IV) Isopropoxide Cathode Buffer Layer. Energies 2018, 11, 328. https://doi.org/10.3390/en11020328
Luo Z, Yang B, Bai Y, Hayat T, Alsaedi A, Tan Z. Efficient Polymer Solar Cells with Alcohol-Soluble Zirconium(IV) Isopropoxide Cathode Buffer Layer. Energies. 2018; 11(2):328. https://doi.org/10.3390/en11020328
Chicago/Turabian StyleLuo, Zhen, Bo Yang, Yiming Bai, Tasawar Hayat, Ahmed Alsaedi, and Zhan’ao Tan. 2018. "Efficient Polymer Solar Cells with Alcohol-Soluble Zirconium(IV) Isopropoxide Cathode Buffer Layer" Energies 11, no. 2: 328. https://doi.org/10.3390/en11020328
APA StyleLuo, Z., Yang, B., Bai, Y., Hayat, T., Alsaedi, A., & Tan, Z. (2018). Efficient Polymer Solar Cells with Alcohol-Soluble Zirconium(IV) Isopropoxide Cathode Buffer Layer. Energies, 11(2), 328. https://doi.org/10.3390/en11020328






