Experimental Investigations of Innovative Biomass Energy Harnessing Solutions
Abstract
:1. Introduction
2. Experimental Investigations
2.1. Energy Valorization of Animal Fat Waste from the Leather Industry
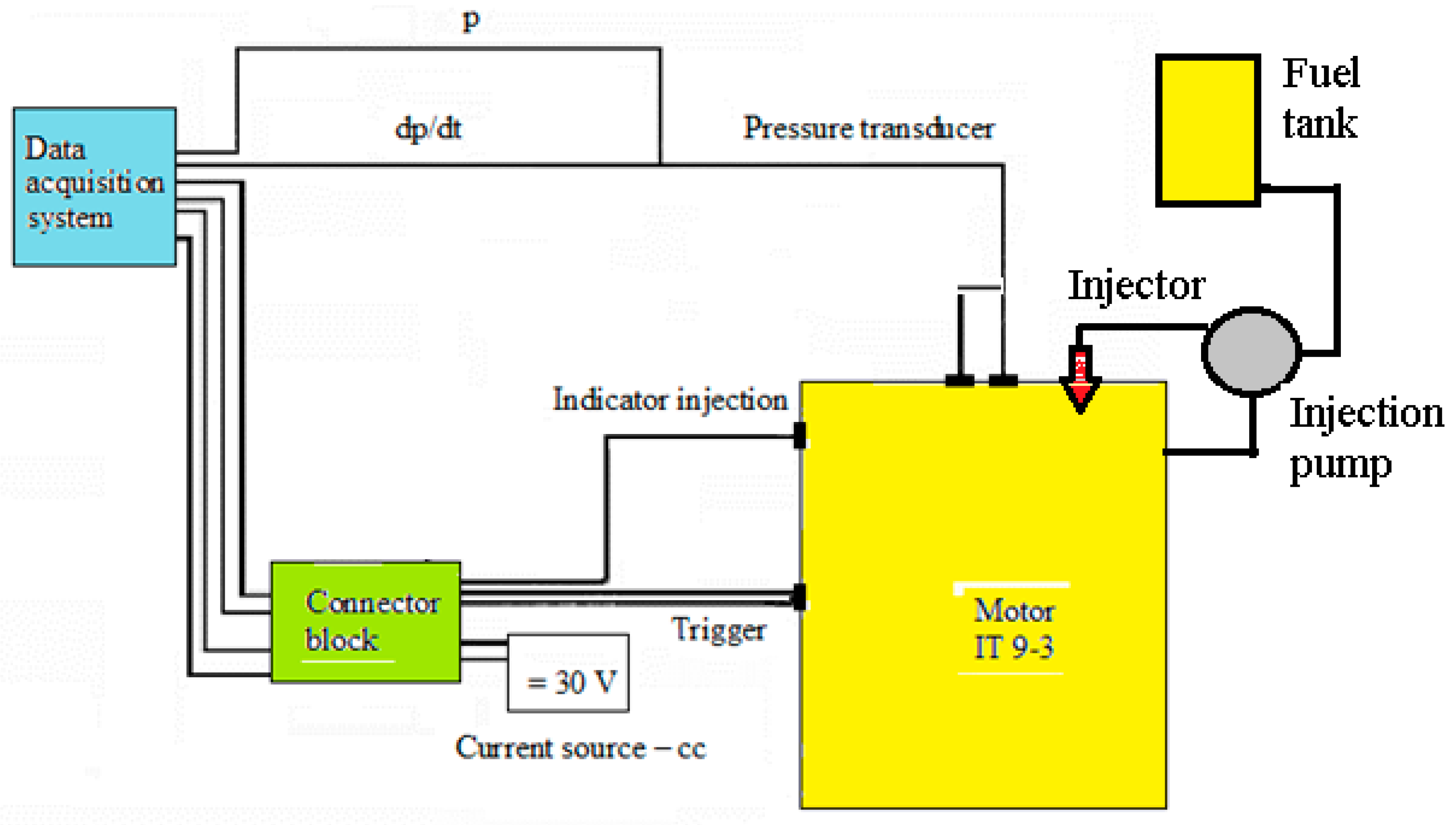
2.1.1. Diesel Engines Fueled with Animal Fats
- In-cylinder pressure transducer line—Anstalt für Verbrennungskraftmaschinen List Engineering Company (AVL), with an accuracy of ±0.05% for 0–250 bar measuring domain;
- Angle encoder—Kubler, with a resolution of 0.05 °CA (crank angle) and accuracy of ±0.1 °CA;
- Real-time data acquisition system—AVL for recording and processing of the in-cylinder data (256 RAM for Indimodul 621);
- Gas analyzer—AVL (with 1 ppm for NOx accuracy);
- Smoke meter for diesel engines—AVL (with 0.1% accuracy).
- Initially, components of the installation were calibrated.
- For the animal fat-diesel fuel mixture preparation both fuels required preheating at temperatures over 40 °C. At this temperature animal fats are in a liquid state and become soluble in diesel fuel, and a finally homogenous mixture results.
- The engine was firstly fueled exclusively with diesel fuel, to set up the reference regime, defined by xc = 0. Secondly, different diesel fuel-animal fat blends were used, which were prepared as per the previously described procedure.
- For each experiment, several parameters were monitored: the auto-ignition delay; injection timing; engine compression ratio; in-cylinder pressure variation; and temperatures of the exhaust gases, cooling liquid, lubricant oil and inlet air. The concentration of pollutants emissions was also recorded.
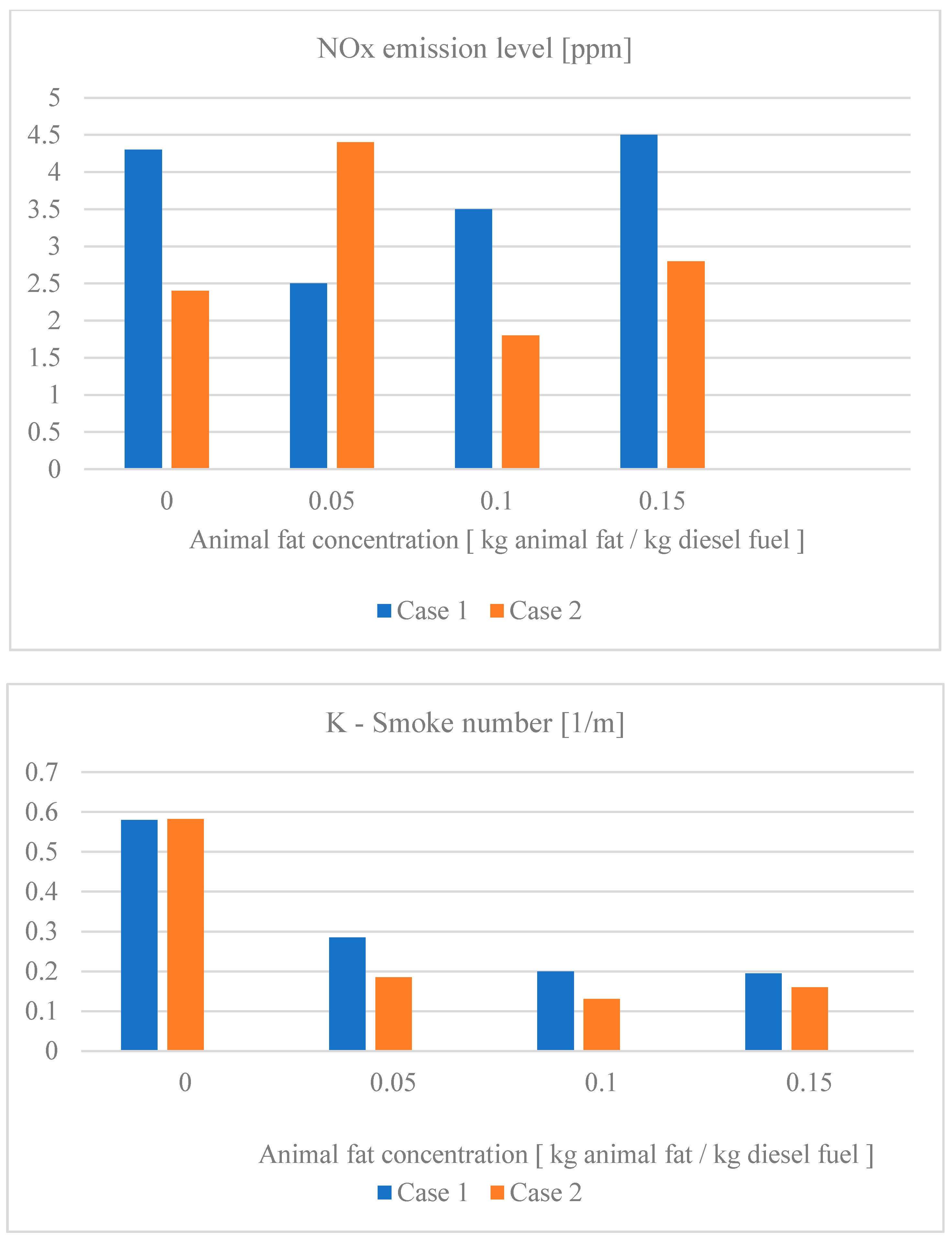
- Case I—constant engine parameters, namely: injection timing (β = 13 °CA, crank angle), compression ratio (ε = 13.80), and the values corresponding to a diesel fuel auto-ignition delay equal to the injection timing.
- Case II—variable engine parameters; adjustment of the injection timing from the standard value of 13 °CA to 15.8 °CA, for the same compression ratio, in order to maintain the start of the combustion process at TDC (top dead center).
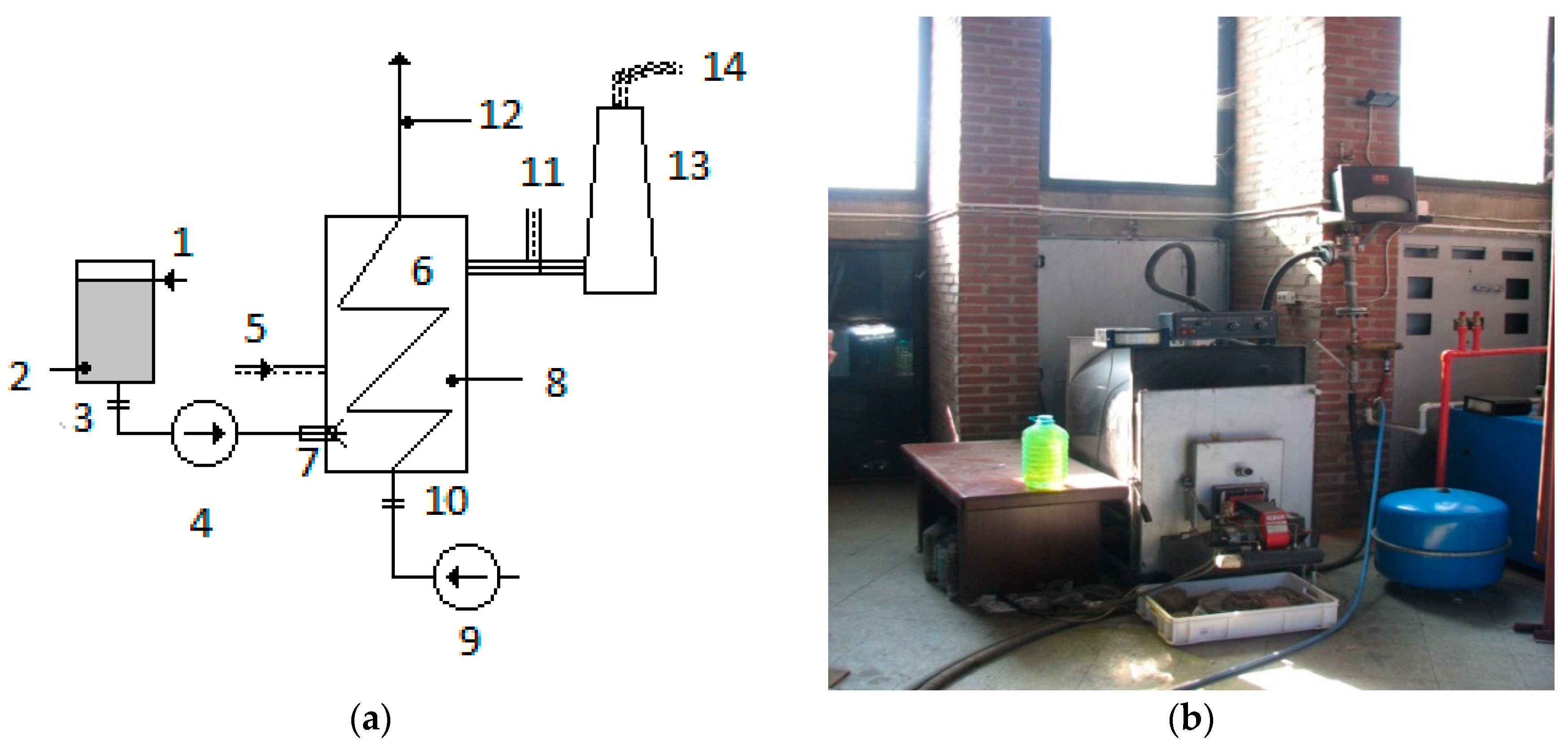
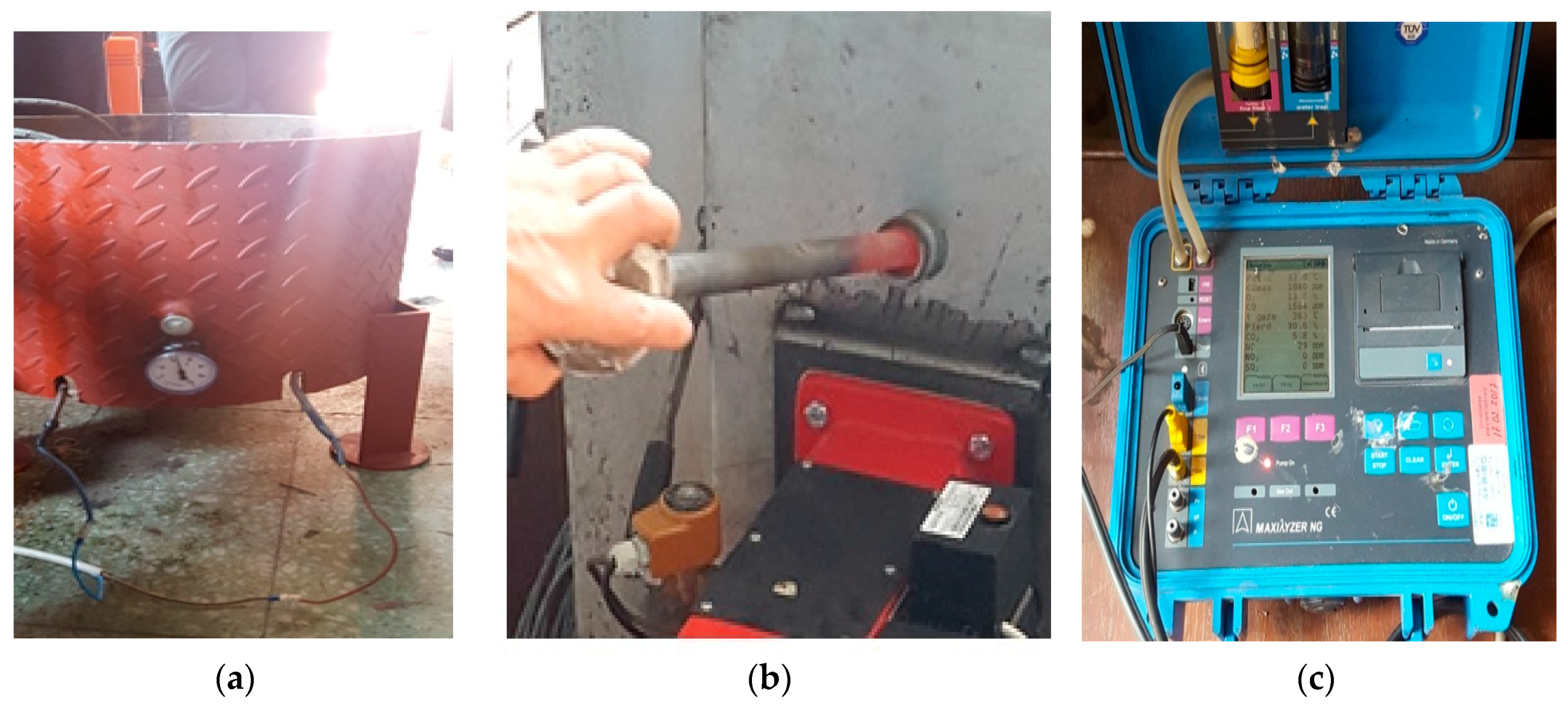
2.1.2. Pilot Boiler Fed with Animal Fat
2.2. Production of Biogas from Protein Waste in the Leather Industry
- Methane (CH4), 42–64%;
- Carbon dioxide (CO2), 55–30%;
- Hydrogen (H2), 0.95–1.15%;
- Hydrogen sulfide (H2S), 0.01–0.8%;
- Nitrogen (N2), 2.04–4.05%.
2.3. Energy Use of Raw Vegetable Oils
- Mix 1: 20% vegetable oil and the rest LLF;
- Mix 2: 50% vegetable oil and the rest LLF;
- Mix 3: 20% vegetable oil and the rest diesel;
- Mix 4: 50% vegetable oil and the rest diesel.
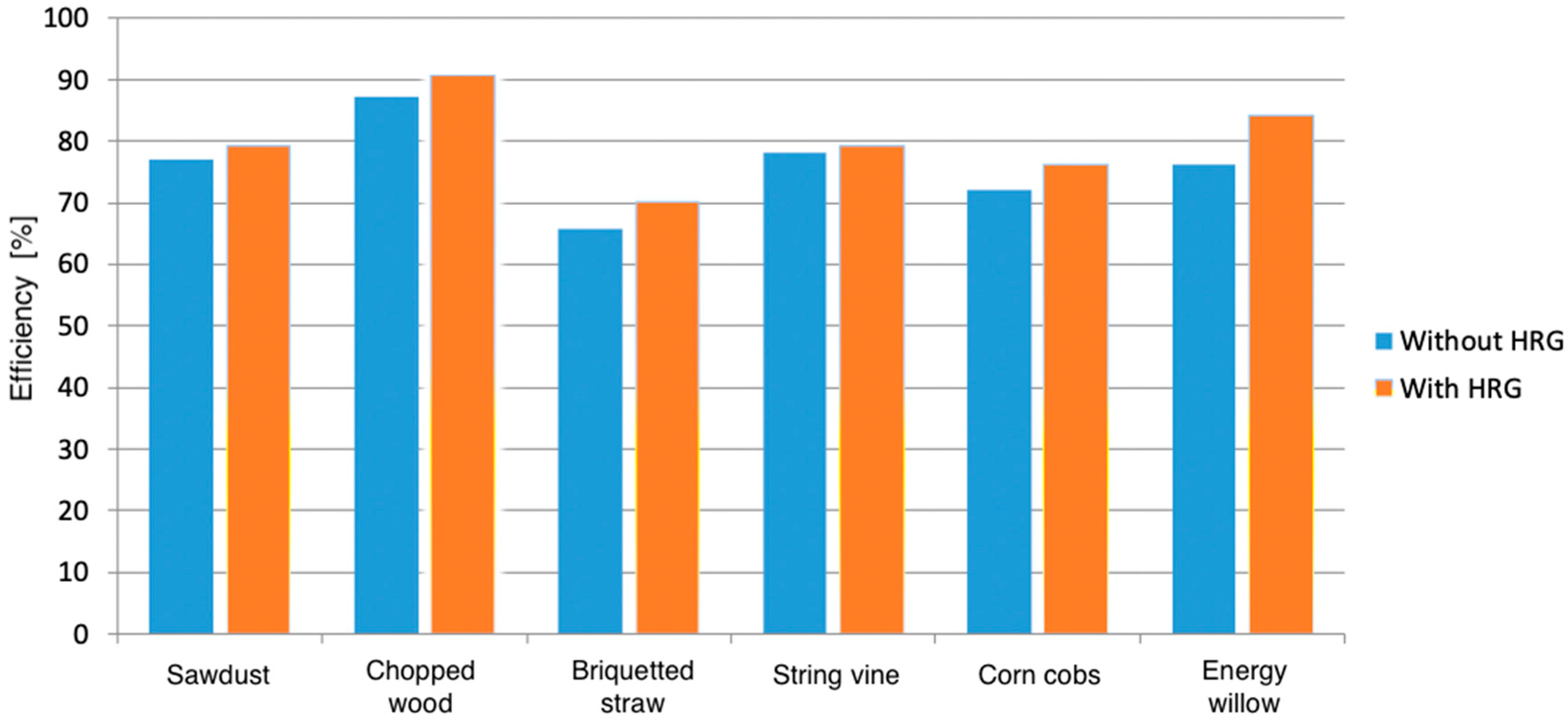
2.4. Innovative Energy Vector: Clean Hydrogen-Solid Biomass
3. Results
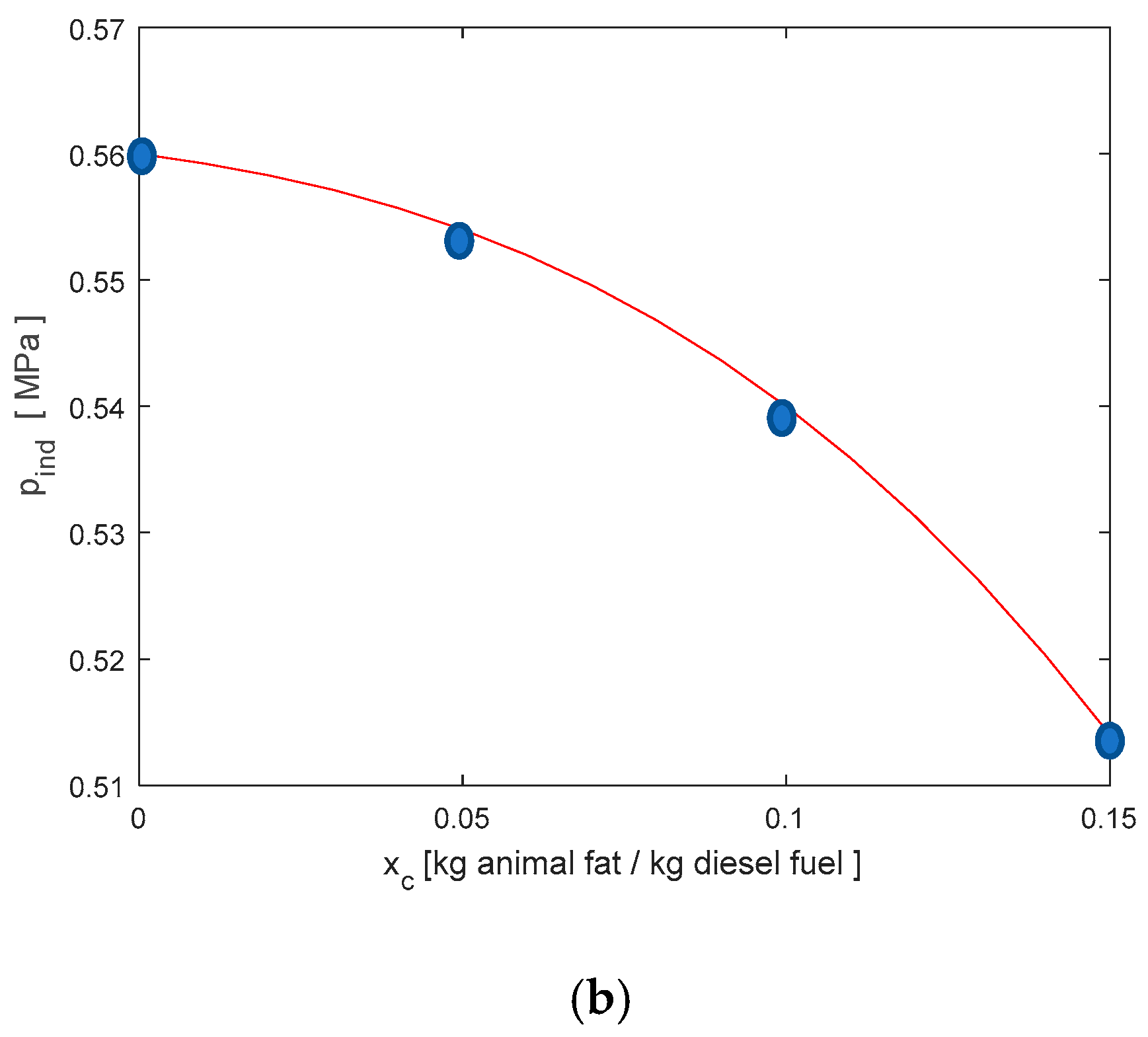
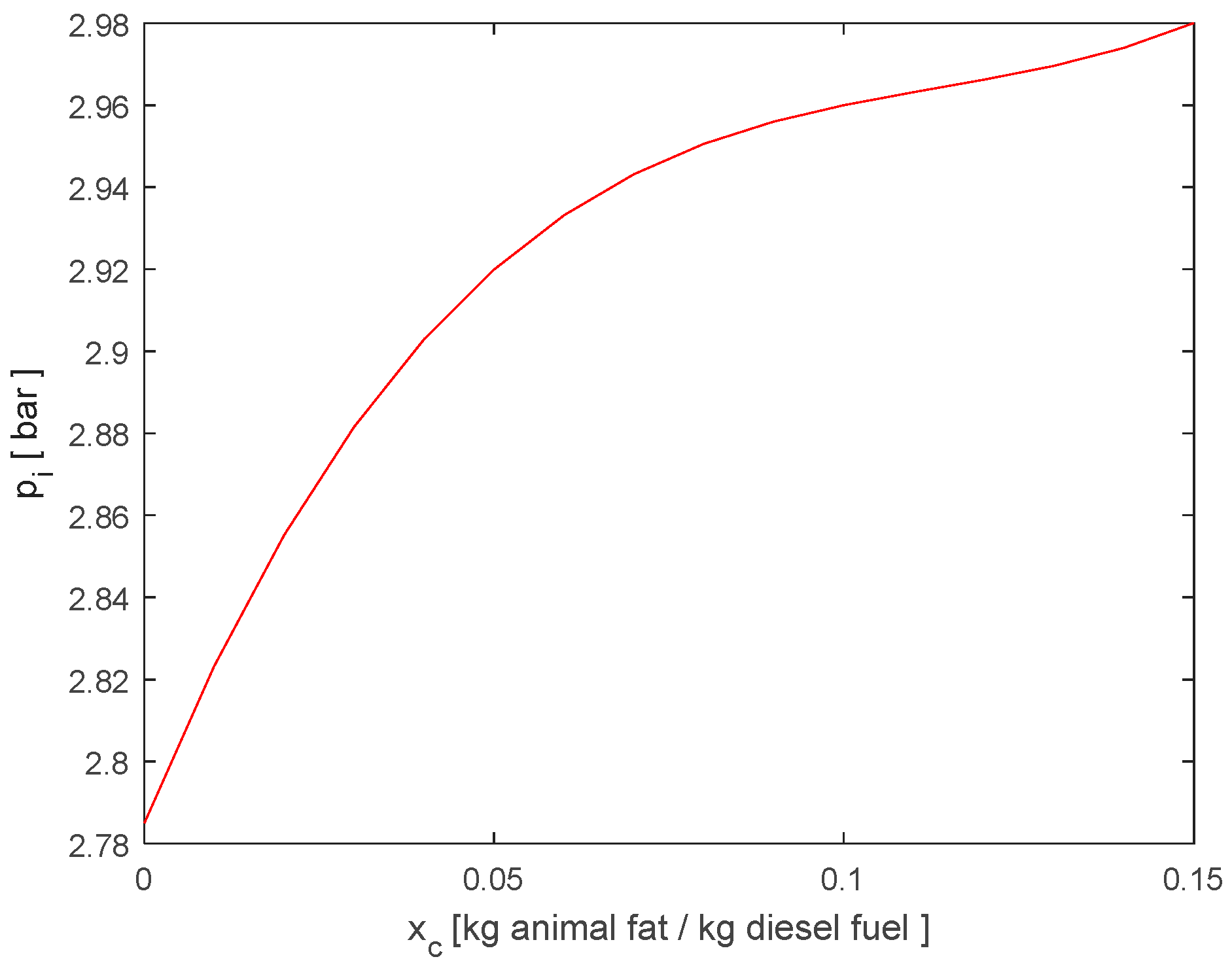
3.1. Animal Fat
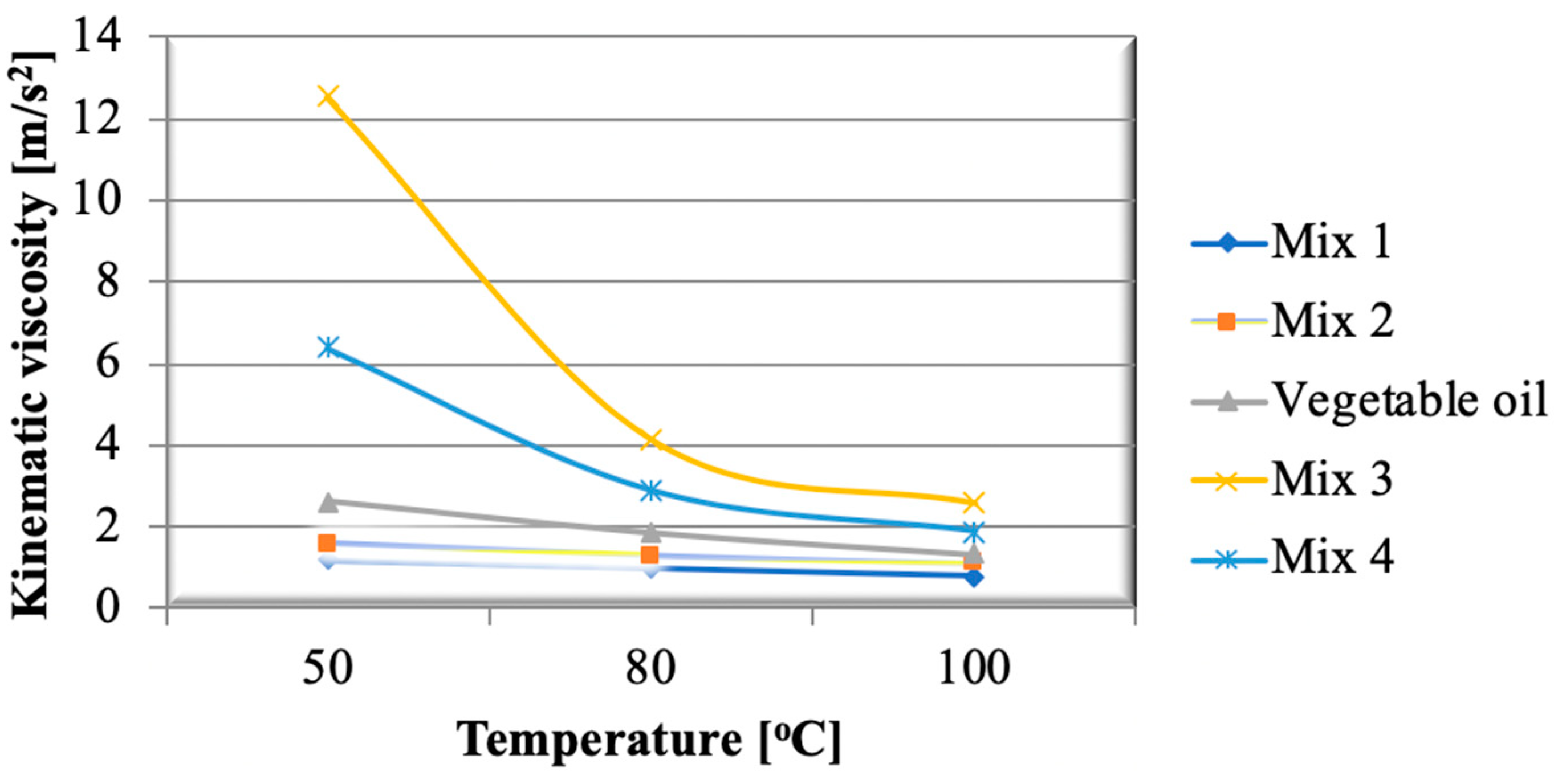
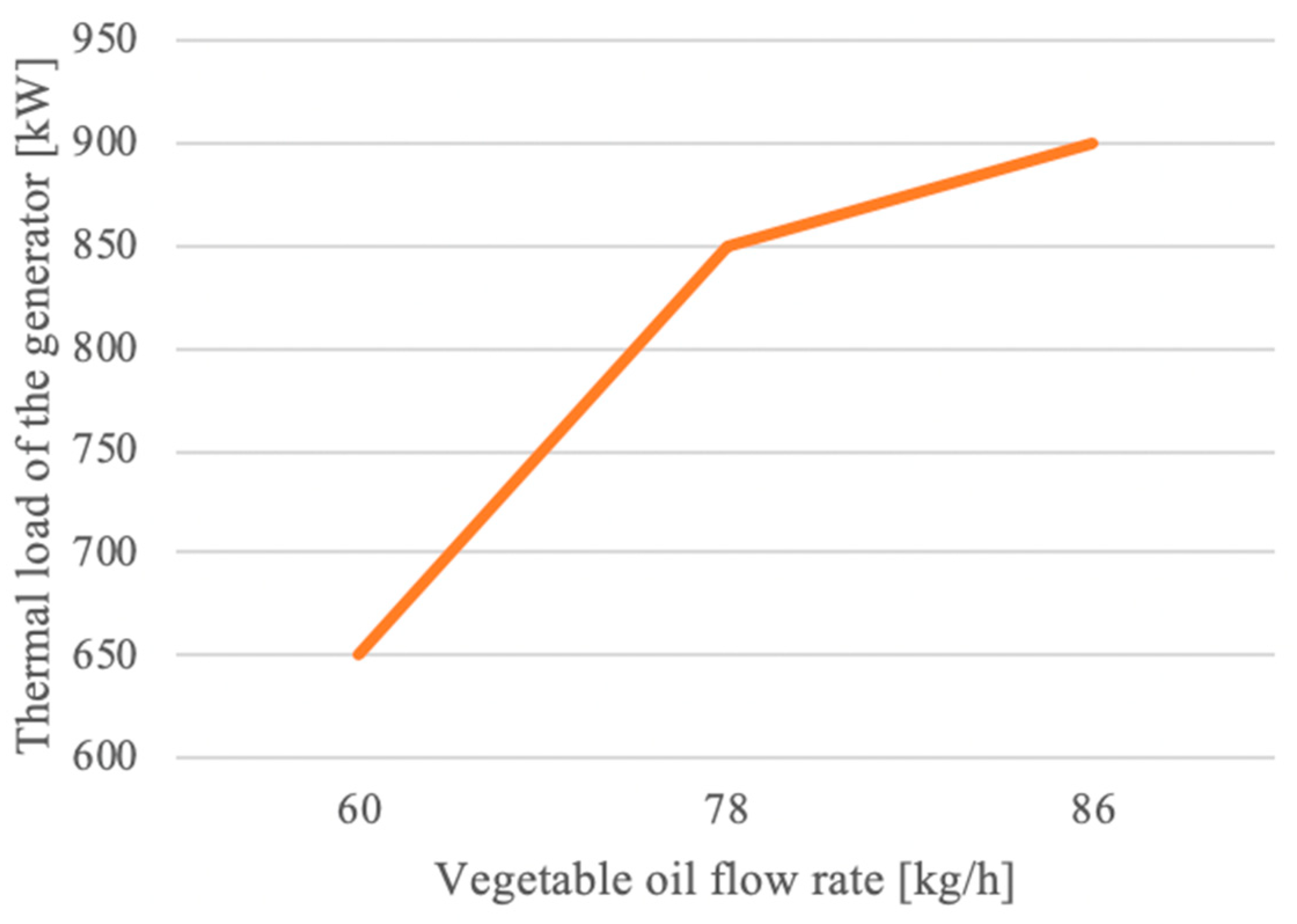
3.2. Crude Vegetable Oils
- The air excess decreased with the thermal loading of the generator, stabilizing at 1.6 close to the nominal load;
- Exhaust gas temperatures were only slightly influenced by variation in both thermal loading of the generator and vegetable oil flow rate;
- Pollutant emissions were also only slightly influenced by the thermal loading of the generator and vegetable oil flow rate, with the following limits: 240–250 mg/m3 carbon oxide, 140–150 mg/m3 nitrogen oxides. The sulfur dioxide emission was constant, at 6 mg/m3. These low recorded values indicate that the burning of vegetable oils has a reduced impact on the environment;
- Analysis of the composition of the combustion gases showed that the percentage of carbon dioxide did not exceed 11.5%.
3.3. Hydrogen-Solid Biomass
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Future Transport Fuels. Report of the European Expert Group on Future Transport Fuels; ALBA-Fachverlag: Dusseldorf, Germany, 2011. [Google Scholar]
- United Nations Industrial Development Organization. Wastes generated in the leather products industry. In Proceedings of the Fourteenth Session of the Leather and Leather Products Industry Panel, Zlin, Czech Republic, 13–15 December 2000. [Google Scholar]
- Lazaroiu, G.; Pana, C.; Mihaescu, L.; Cernat, A.; Negurescu, N.; Mocanu, R.; Negreanu, G. Solutions for energy recovery of animal waste from leather industry. Energy Convers. Manag. 2017, 149, 1085–1095. [Google Scholar] [CrossRef]
- Kumar, A.; Kerihuel, J.; Bellettre, J.; Tazerout, M.; Senthyl, M. The use of the preheated animal fat as fuel in a compression ignition engine. Renew. Energy 2005, 30, 1443–1456. [Google Scholar] [CrossRef]
- Kumar, M.S.; Kerihuel, J.; Bellettre, J.; Tazerout, M. A comparative study of a different methods of using animal fat as a fuel in a compression ignition engine. J. Eng. Gas Turbines Power 2006, 128, 907–914. [Google Scholar] [CrossRef]
- Pham, P.X.; Bodisco, T.A.; Stevanovic, S.; Rahman, M.D.; Wang, H.; Ristovski, Z.D.; Brown, R.J.; Masri, A.R. Engine performance characteristics for biodiesels of different degrees of saturation and carbon chain lengths. SAE Int. J. Fuel Lubr. 2013, 6, 188–198. [Google Scholar] [CrossRef]
- Stengel, B.; Vium, J.H. Synthesis, Characterization and Use of Hydro-Treated Oils and Fats for Engine Operation. In A Report from IEA Advanced Motor Fuels Implementing Agreement; Alberta Innovates Technology Futures-Fuels and Lubricants: Edmonton, AB, Canada, 2015; pp. 1–51. [Google Scholar]
- Lazaroiu, G.; Pop, E.; Negreanu, G.; Pisa, I.; Mihaescu, L.; Bondrea, A.; Berbece, V. Biomass combustion with hydrogen injection for energy applications. Energy 2017, 127, 351–357. [Google Scholar] [CrossRef]
- Pisa, I.; Lazaroiu, G.; Mihaescu, L.; Prisecaru, T.; Negreanu, G.P. Mathematical model and experimental tests of hydrogen diffusion in the porous system of biomass. Int. J. Green Energy 2016, 13, 774–780. [Google Scholar] [CrossRef]
- Pisa, I.; Lazaroiu, G.; Prisecaru, T. Influence of hydrogen enriched gas injection upon polluting emissions from pulverized coal combustion. Int. J. Hydrog. Energy 2014, 39, 17702–17709. [Google Scholar] [CrossRef]
- Neri, E.; Cespi, D.; Setti, L.; Gombi, E.; Bernardi, E.; Vassura, I.; Passarini, F. Biomass residues to renewable energy: a life cycle perspective applied at a local scale. Energies 2016, 9, 922. [Google Scholar] [CrossRef]
- Maj, G. Emission factors and energy properties of Agro and forest biomass in aspect of sustainability of energy sector. Energies 2018, 11, 1516. [Google Scholar] [CrossRef]
- Adam, I.K.; Abdul Aziz, A.R.; Heikal, M.R.; Yusup, S.; Firmansyah; Ahmad, A.S.; Zainal Abidin, E.Z. Performance and emission analysis of rubber seed, palm, and their combined blend in a multi-cylinder diesel engine. Energies 2018, 11, 1522. [Google Scholar] [CrossRef]
- Prochazka, P.; Honig, V. Economic Analysis of diesel-fuel replacement by crude palm oil in indonesian power plants. Energies 2018, 11, 504. [Google Scholar] [CrossRef]
- Bartocci, P.; Zampilli, M.; Bidini, G.; Fantozzi, F. Hydrogen rich gas production through gasification of charcoal pellet. Appl. Therm. Eng. 2018, 132, 817–823. [Google Scholar] [CrossRef]
- Molino, A.; Larocca, V.; Chianese, S.; Musmarra, D. Biofuels production by biomass gasification: A review. Energies 2018, 11, 811. [Google Scholar] [CrossRef]
- Cancela, A.; Pérez, L.; Febrero, A.; Sánchez, A.; Salgueiro, J.L.; Ortiz, L. Exploitation of Nannochloropsis gaditana biomass for biodiesel and pellet production. Renew. Energy 2019, 133, 725–730. [Google Scholar] [CrossRef]
- Bartels, J.R.; Pate, M.B.; Norman, K.; Olson, N.K. An economic survey of hydrogen production from conventional and alternative energy sources. Int. J. Hydrog. Energy 2010, 35, 8371–8384. [Google Scholar] [CrossRef]
- Ge, J.G.; Kim, M.S.; Yoon, S.K.; Choi, N.J. Effects of the pilot injection timing and egr on combustion, performance and exhaust emissions in a common rail diesel engine fueled with canola oil biodiesel-diesel blend. Energies 2015, 8, 7312–7325. [Google Scholar] [CrossRef]









| Properties | Diesel Fuel | MEGA | EEGA | Fats of Animal Origin | |
|---|---|---|---|---|---|
| Composition [wt %] | C | 86 | 76 | 76 | 72–74 |
| H2 | 13 | 13 | 12 | 12–13 | |
| O2 | 0.4 | 11 | 12 | 12–13 | |
| Ash | 0.001 | 0.001 | |||
| Heating Value Hi [MJ/kg] | 43 | 37 | 38 | 38–39 | |
| Cetane Number CN [−] | 79 | 73 | 72 | 40–45 | |
| Density [kg/m3] | 850 | 880 | 870 | 920–940 | |
| Ignition Point [°C] | 75 | 160 | 185 | 95–100 | |
| Biomass Type | Parameter | ||
|---|---|---|---|
| LHV [kJ/kg] | Wt [%] | Ai [%] | |
| sawdust | 15,500–16,500 | 14.0–14.2 | 2.4–2.5 |
| chopped wood | 16,900–17,500 | 10.5–11.2 | 0.4–0.5 |
| briquetted straw | 14,500–15,000 | 10.3–10.5 | 4.6–4.8 |
| string vine | 13,300–13,800 | 15.8–16.1 | 4.8–4.9 |
| corn cobs | 13,000–13,500 | 7.9–8.0 | 4.9–5.0 |
| energy willow | 14,000–14,500 | 17.7–18.1 | 2.3–2.4 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lazaroiu, G.; Mihaescu, L.; Negreanu, G.; Pana, C.; Pisa, I.; Cernat, A.; Ciupageanu, D.-A. Experimental Investigations of Innovative Biomass Energy Harnessing Solutions. Energies 2018, 11, 3469. https://doi.org/10.3390/en11123469
Lazaroiu G, Mihaescu L, Negreanu G, Pana C, Pisa I, Cernat A, Ciupageanu D-A. Experimental Investigations of Innovative Biomass Energy Harnessing Solutions. Energies. 2018; 11(12):3469. https://doi.org/10.3390/en11123469
Chicago/Turabian StyleLazaroiu, Gheorghe, Lucian Mihaescu, Gabriel Negreanu, Constantin Pana, Ionel Pisa, Alexandru Cernat, and Dana-Alexandra Ciupageanu. 2018. "Experimental Investigations of Innovative Biomass Energy Harnessing Solutions" Energies 11, no. 12: 3469. https://doi.org/10.3390/en11123469
APA StyleLazaroiu, G., Mihaescu, L., Negreanu, G., Pana, C., Pisa, I., Cernat, A., & Ciupageanu, D.-A. (2018). Experimental Investigations of Innovative Biomass Energy Harnessing Solutions. Energies, 11(12), 3469. https://doi.org/10.3390/en11123469