Derating Guidelines for Lithium-Ion Batteries
Abstract
1. Introduction
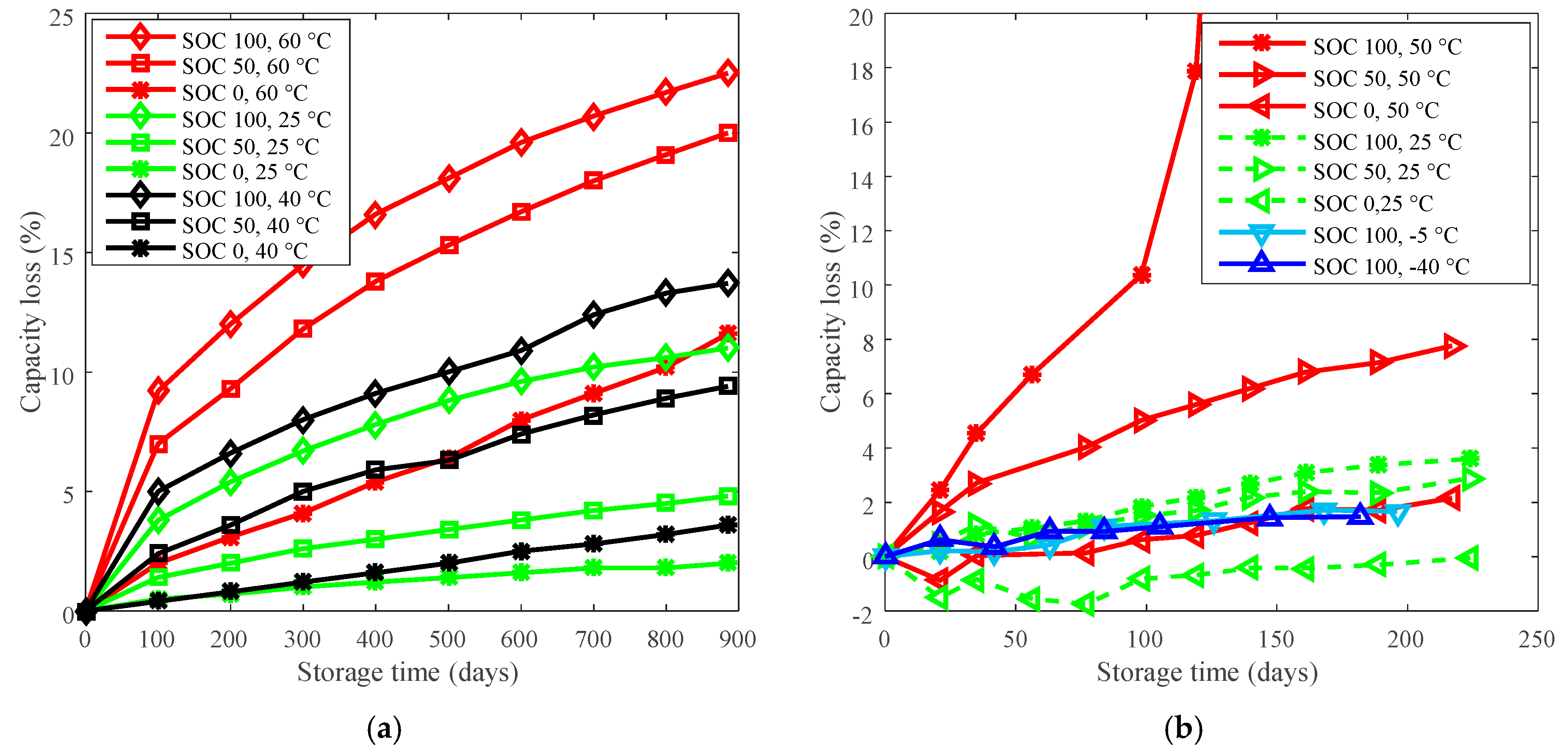
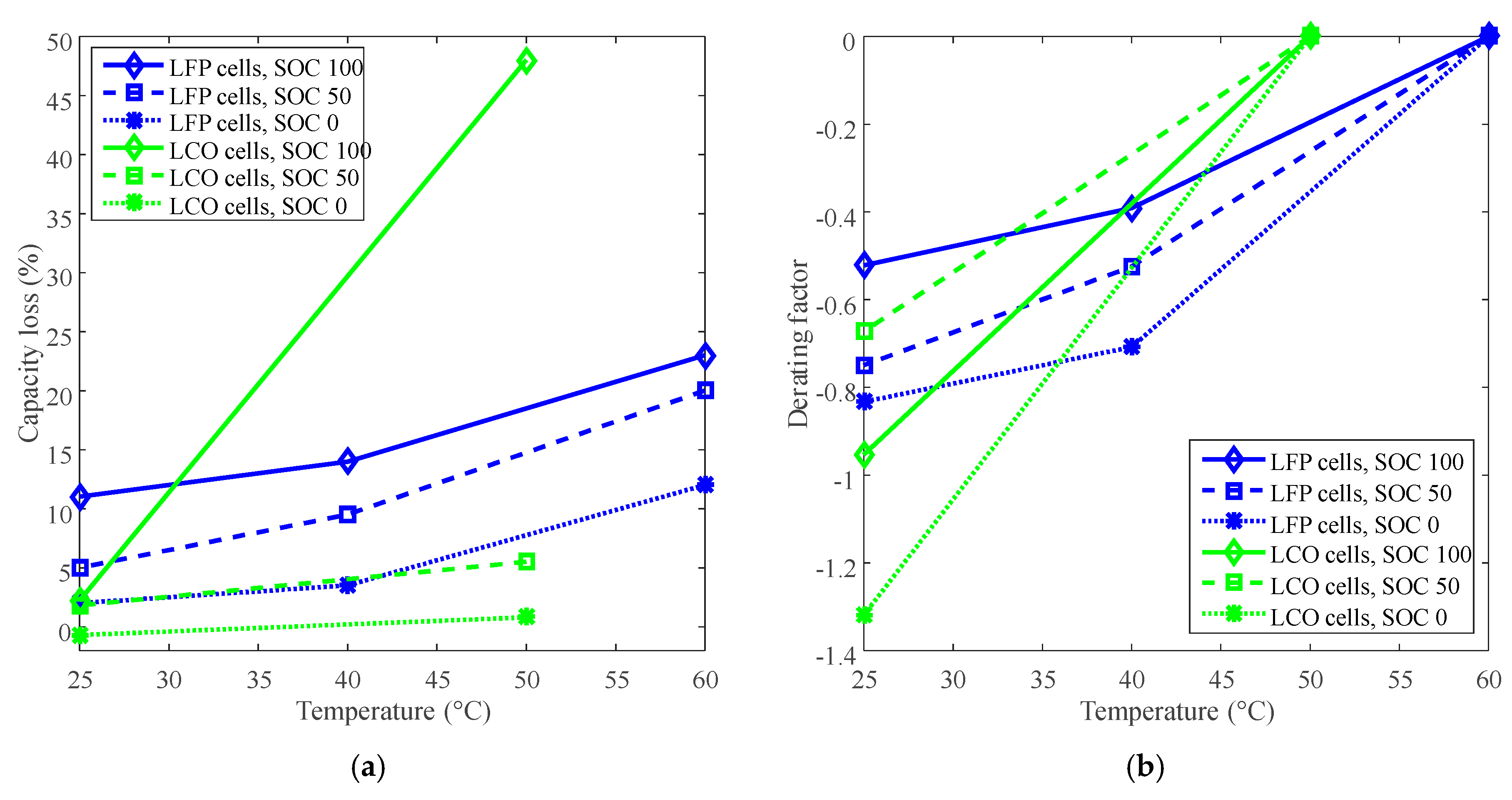
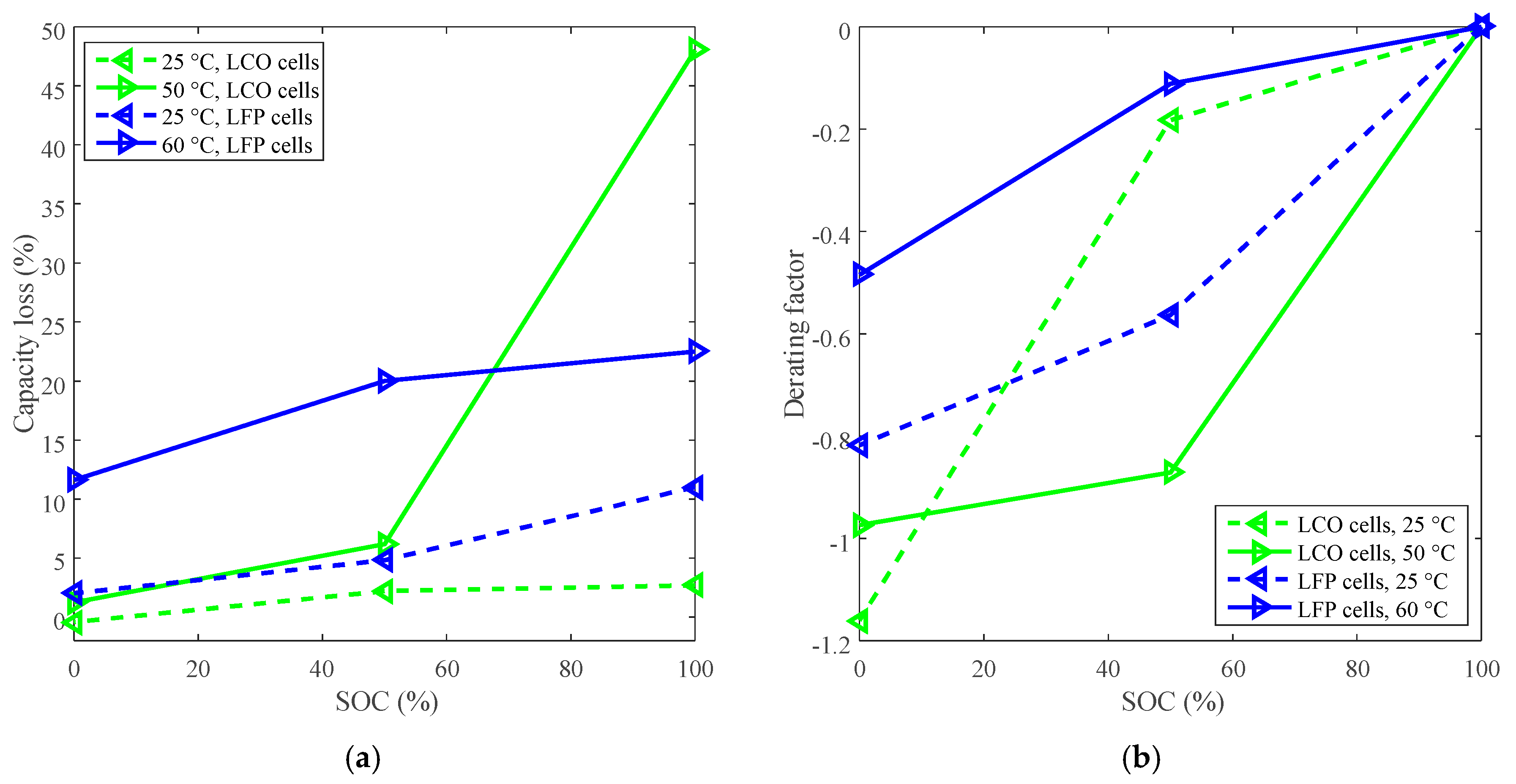
2. Derating for Calendar Life Improvement
3. Derating for Cycle Life Improvement
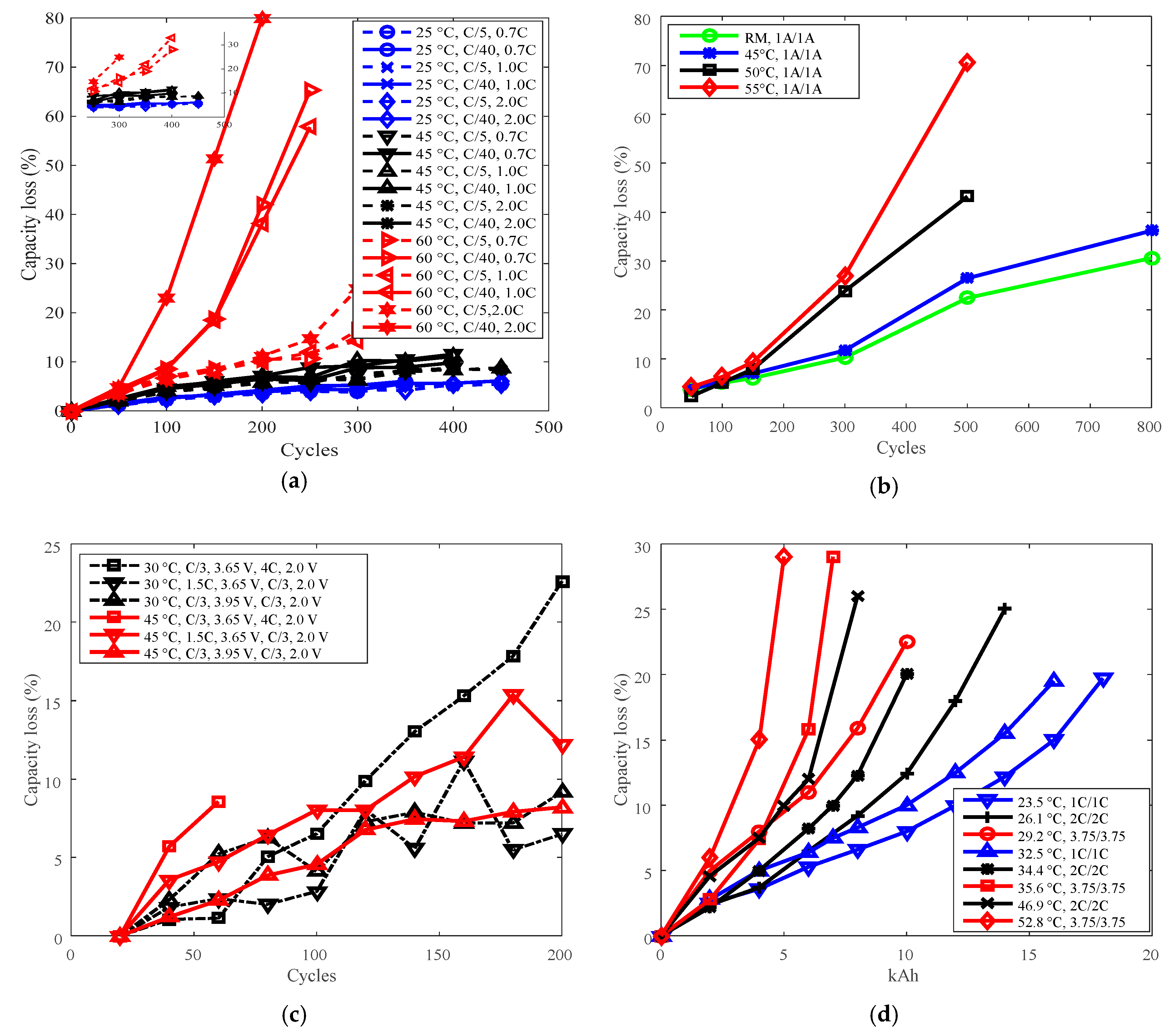
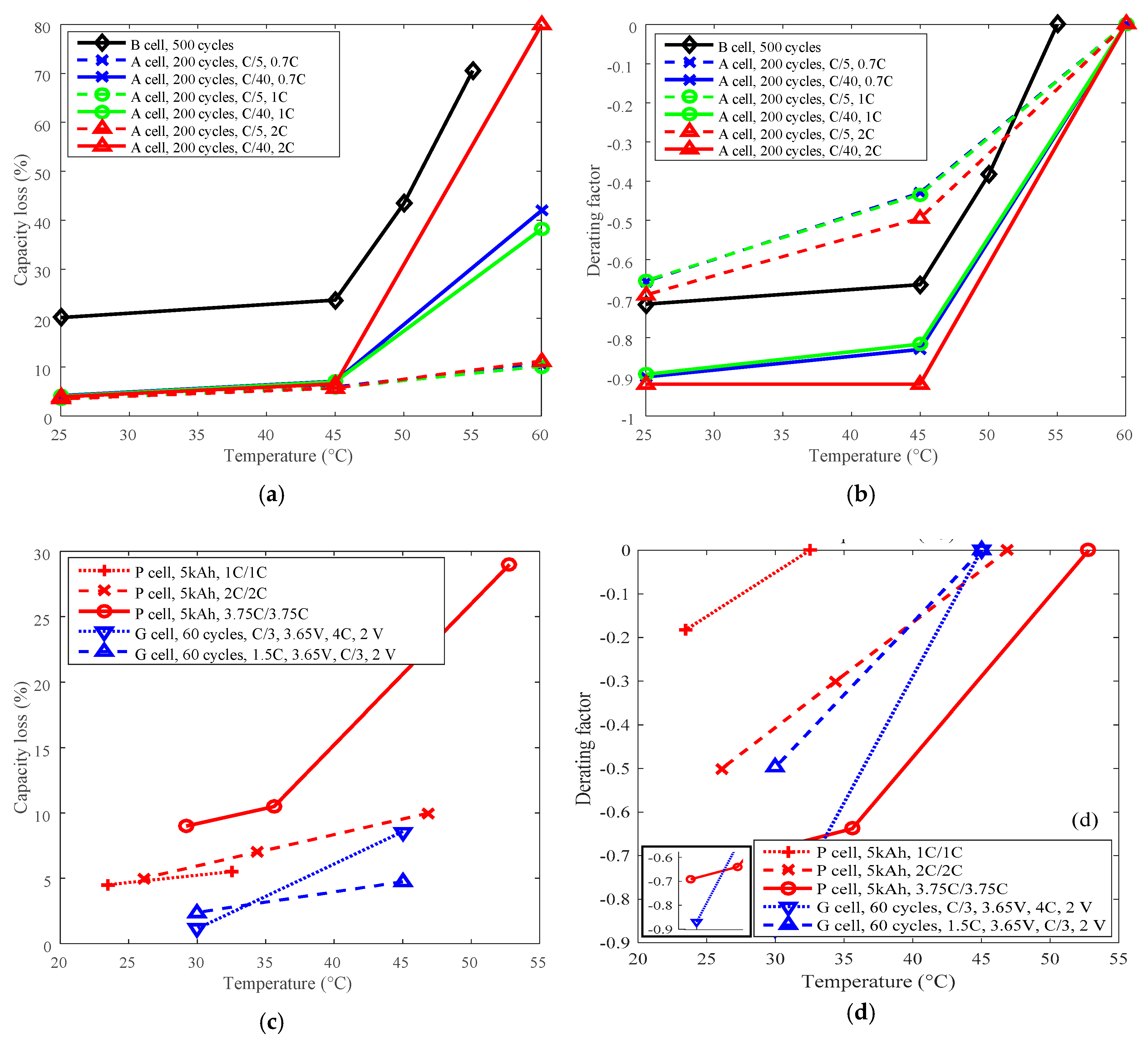
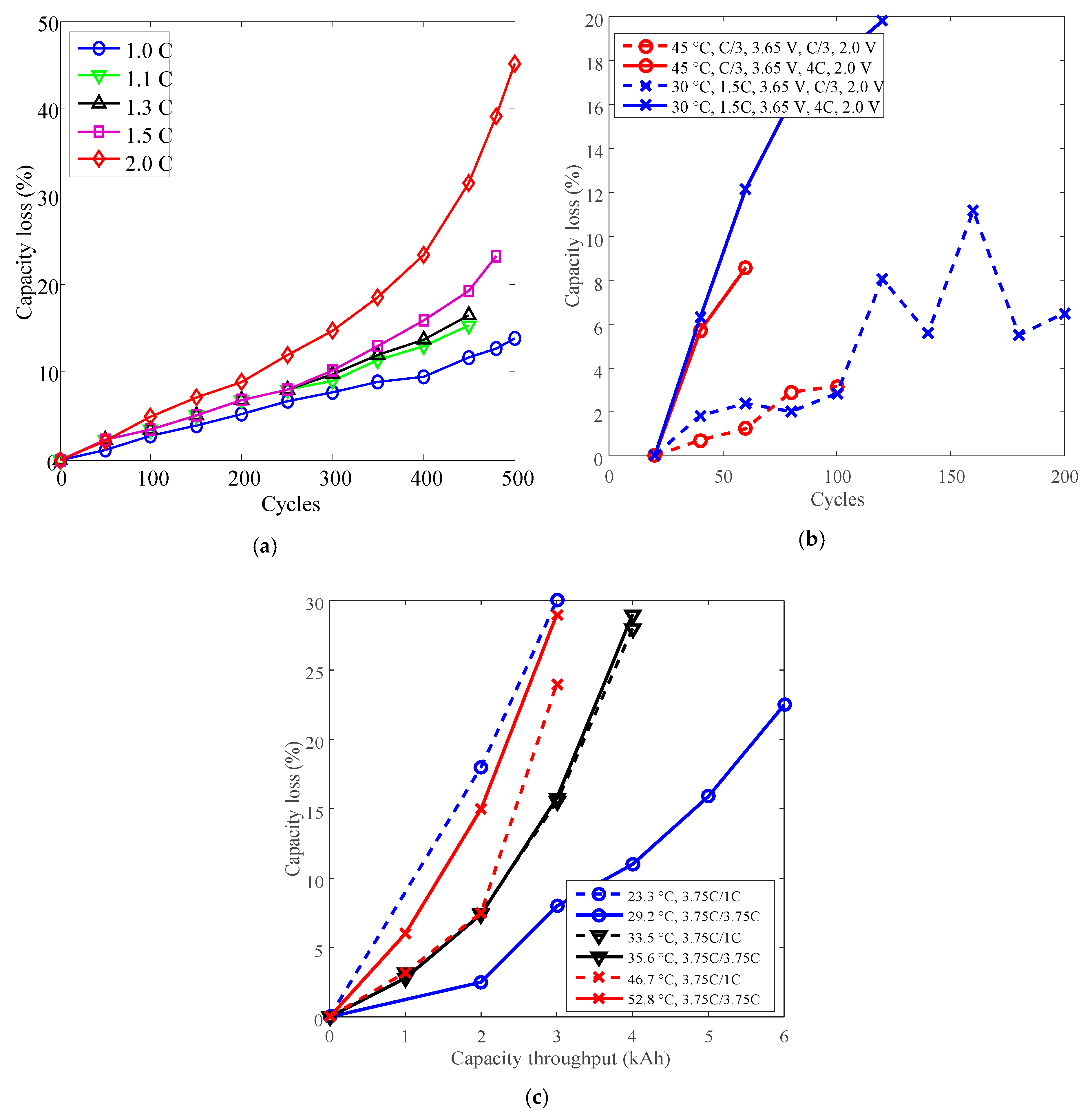
3.1. Cycle Life Improvement by Temperature Derating
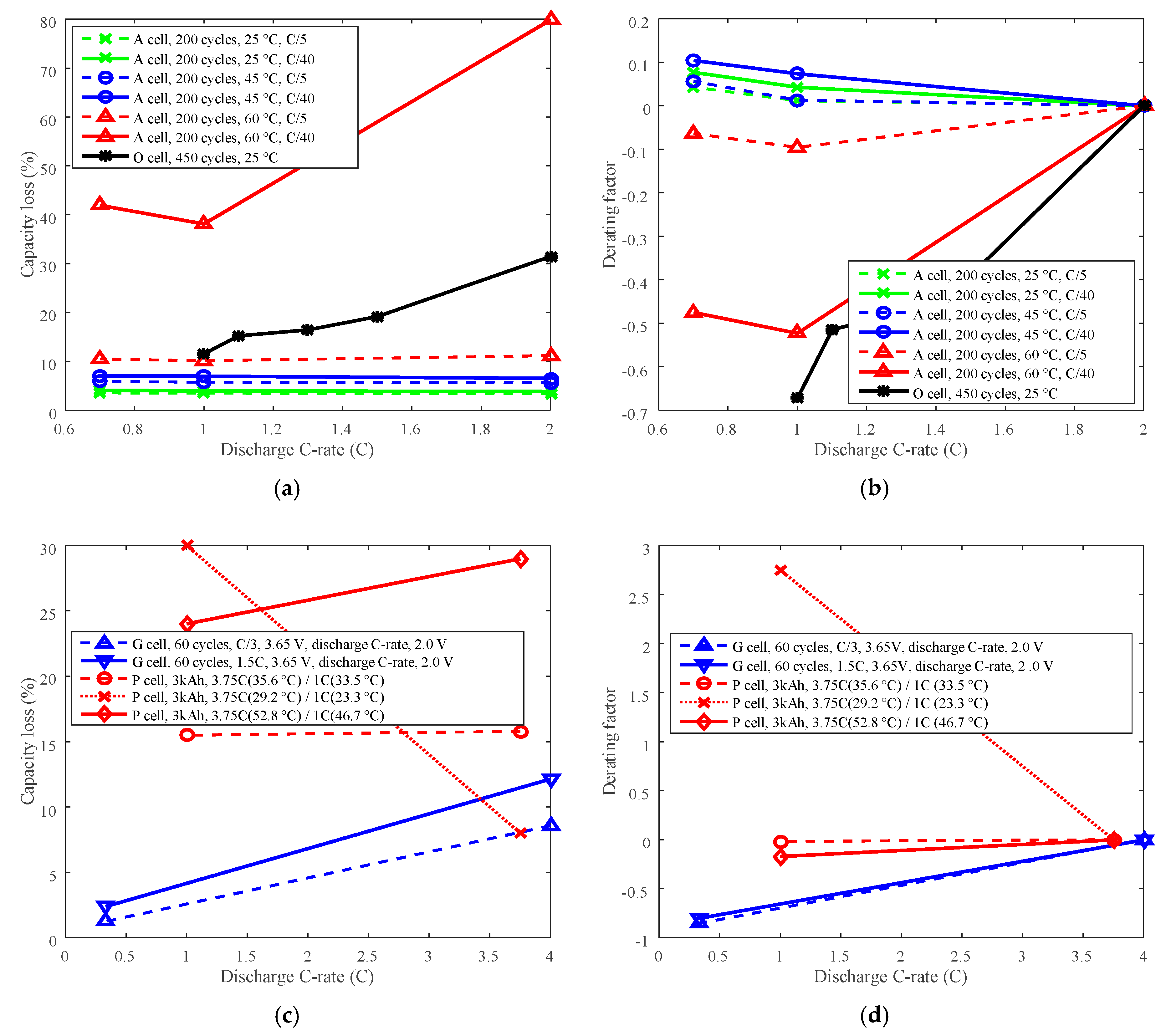
3.2. Cycle Life Improvement by Discharge C-Rate Derating
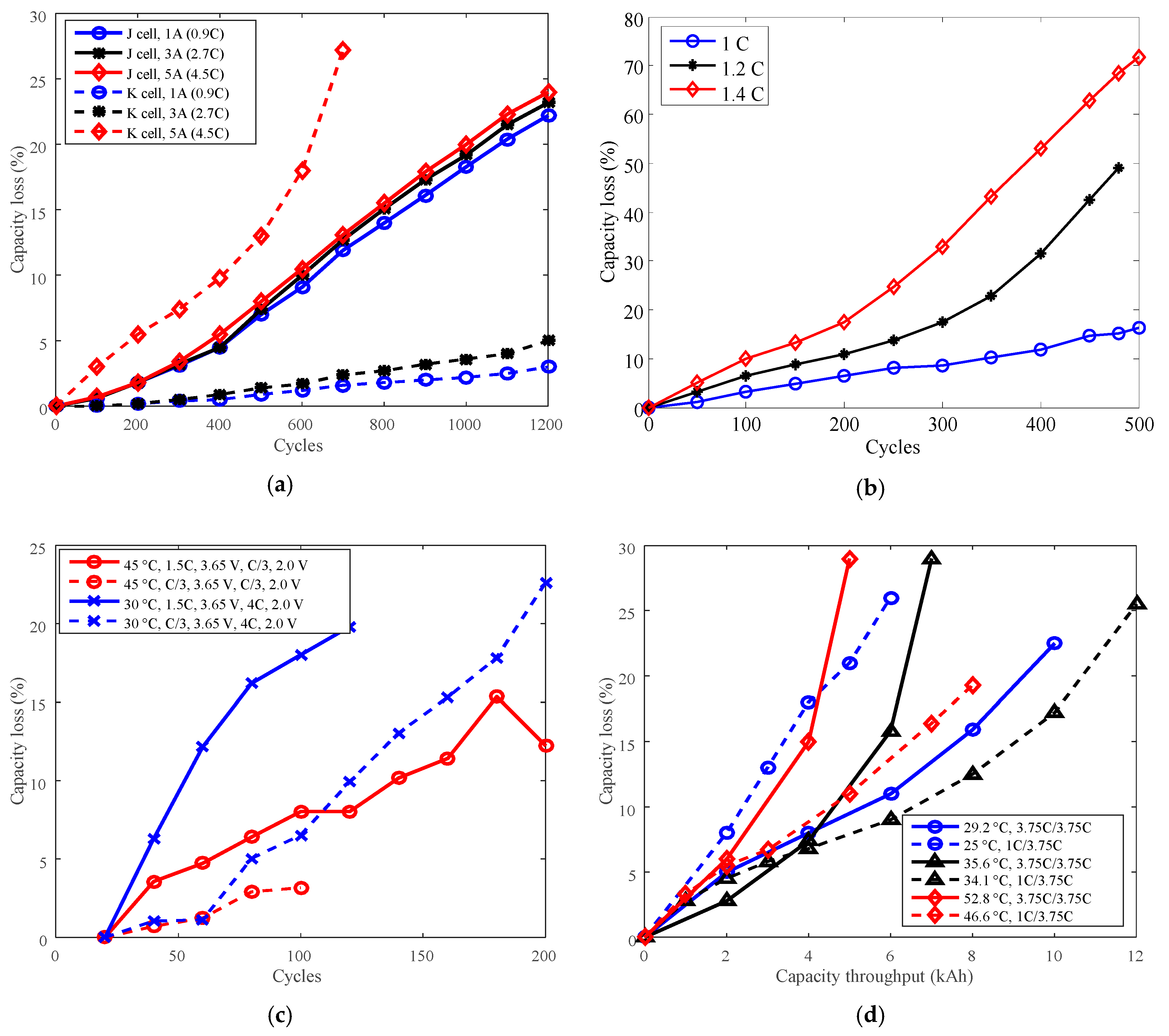
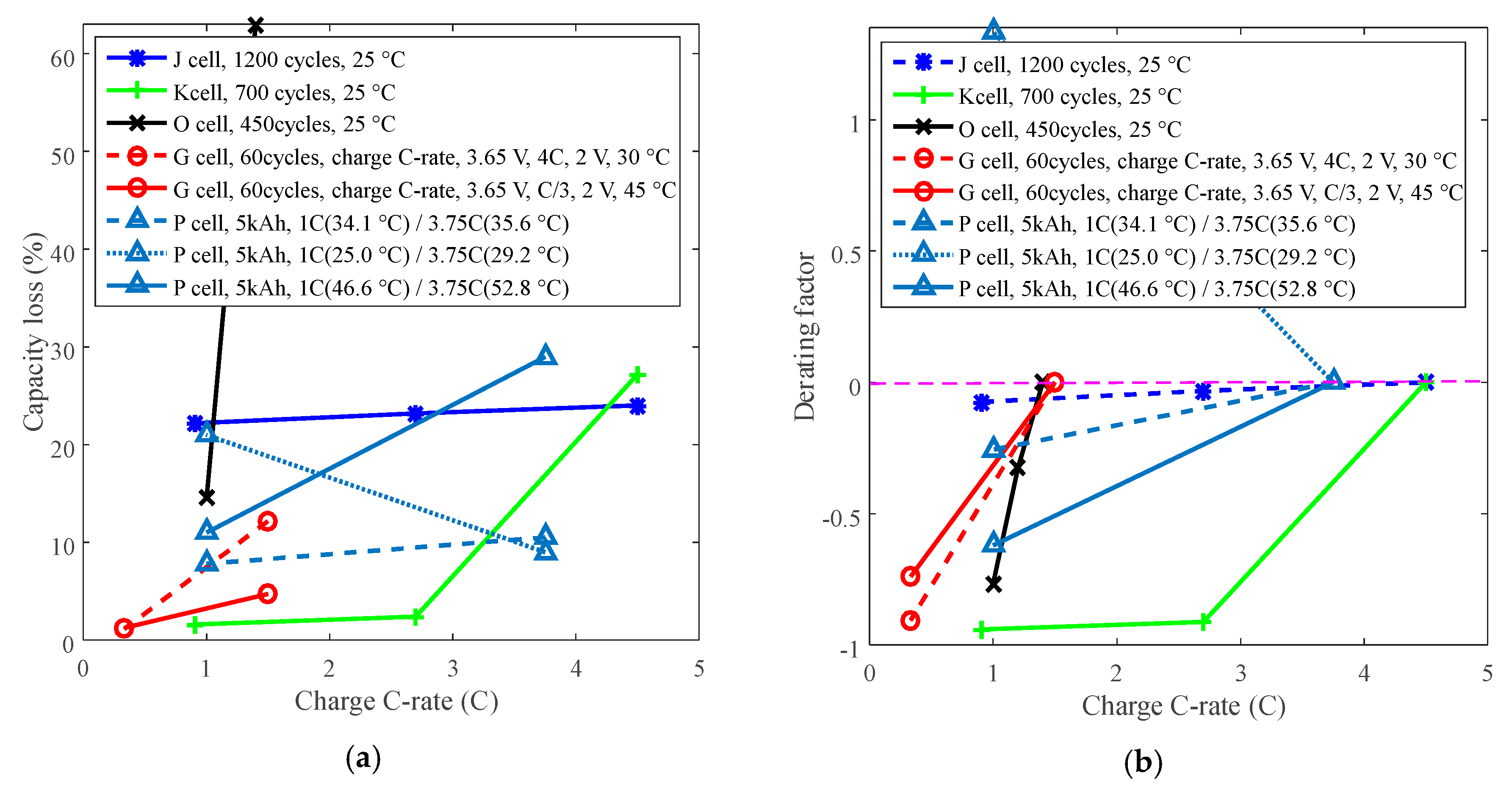
3.3. Cycle Life Improvement by Charge C-Rate Derating
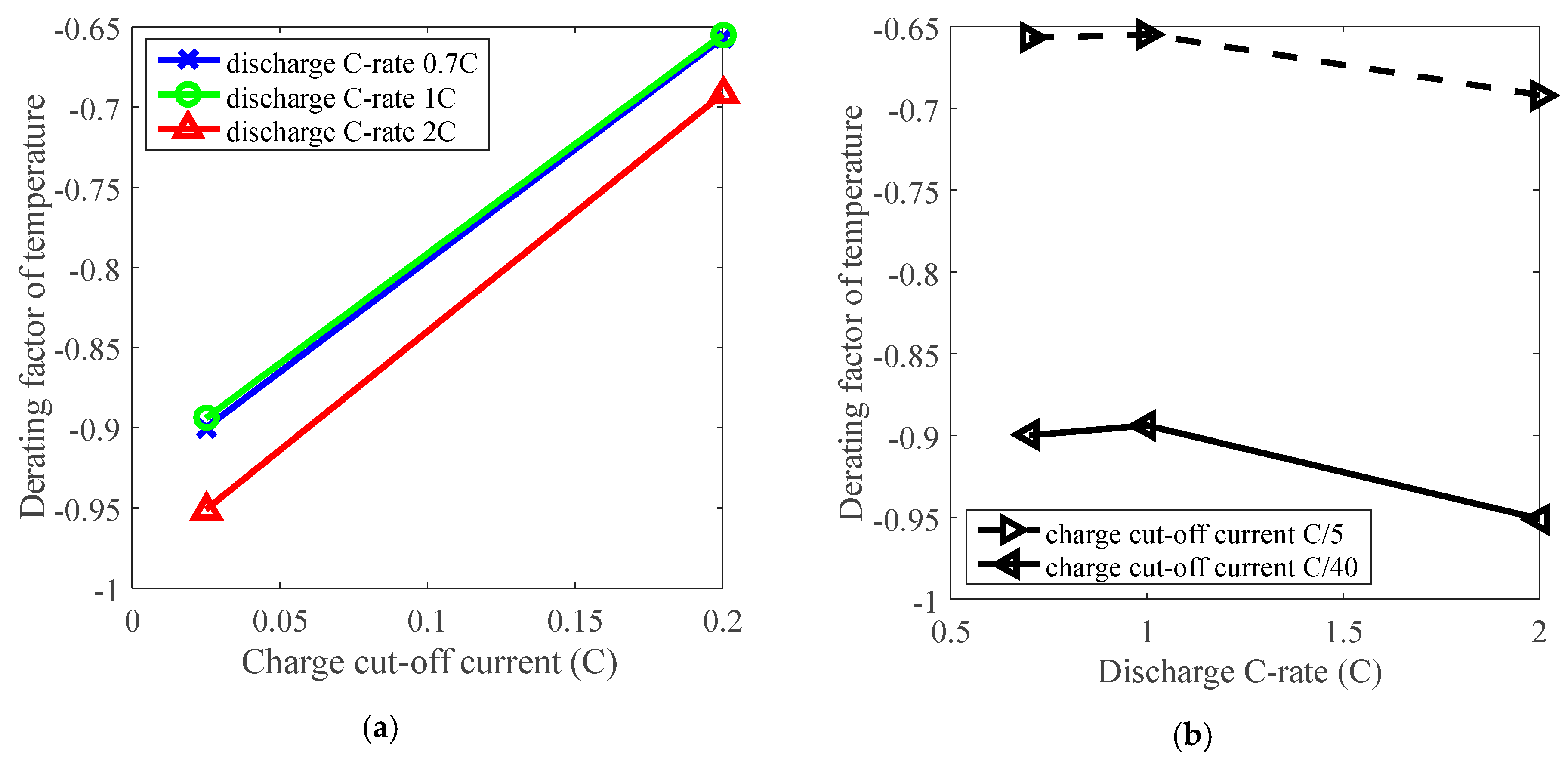
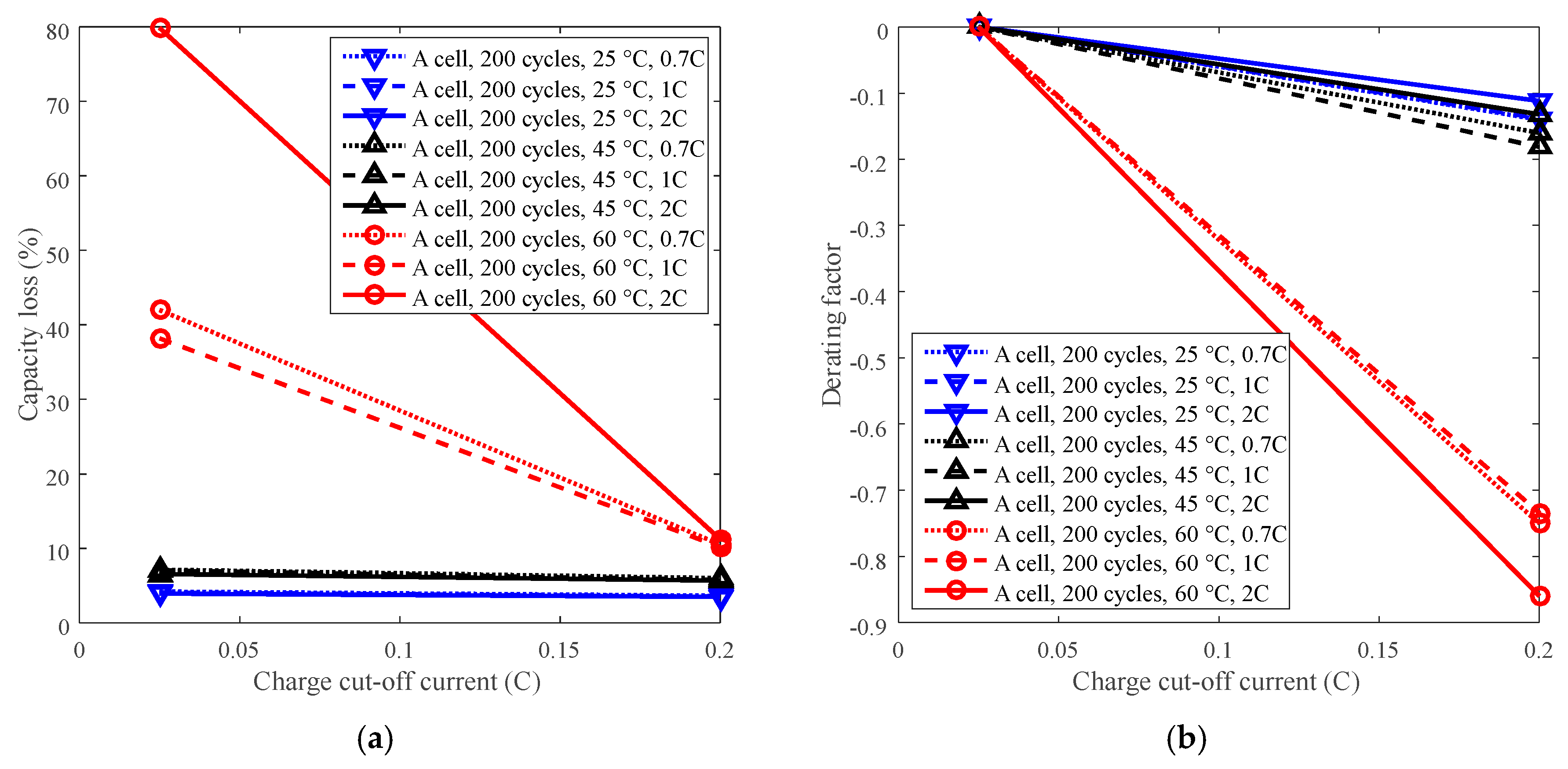
3.4. Cycle Life Improvement by Charge Cut-Off Current Derating
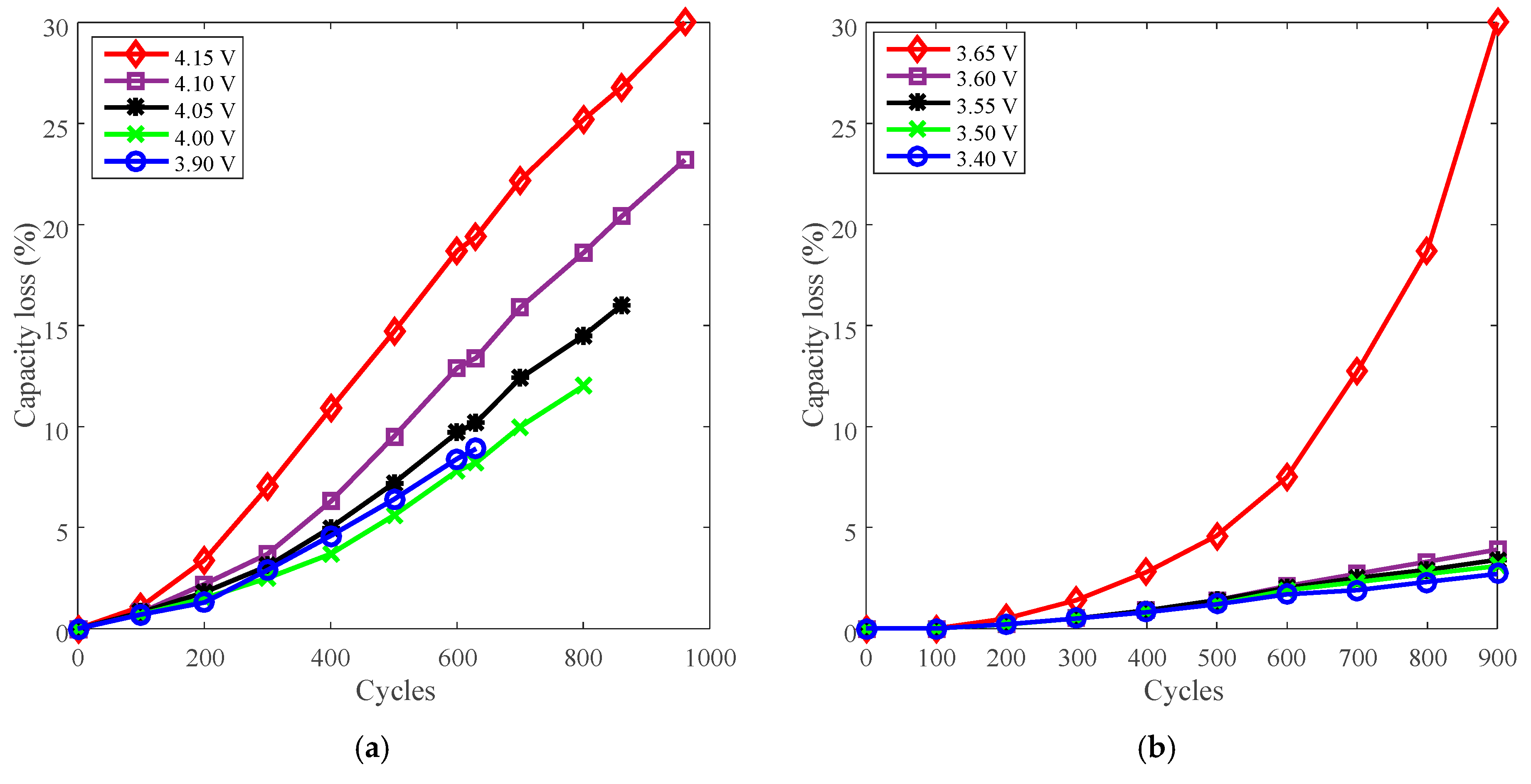
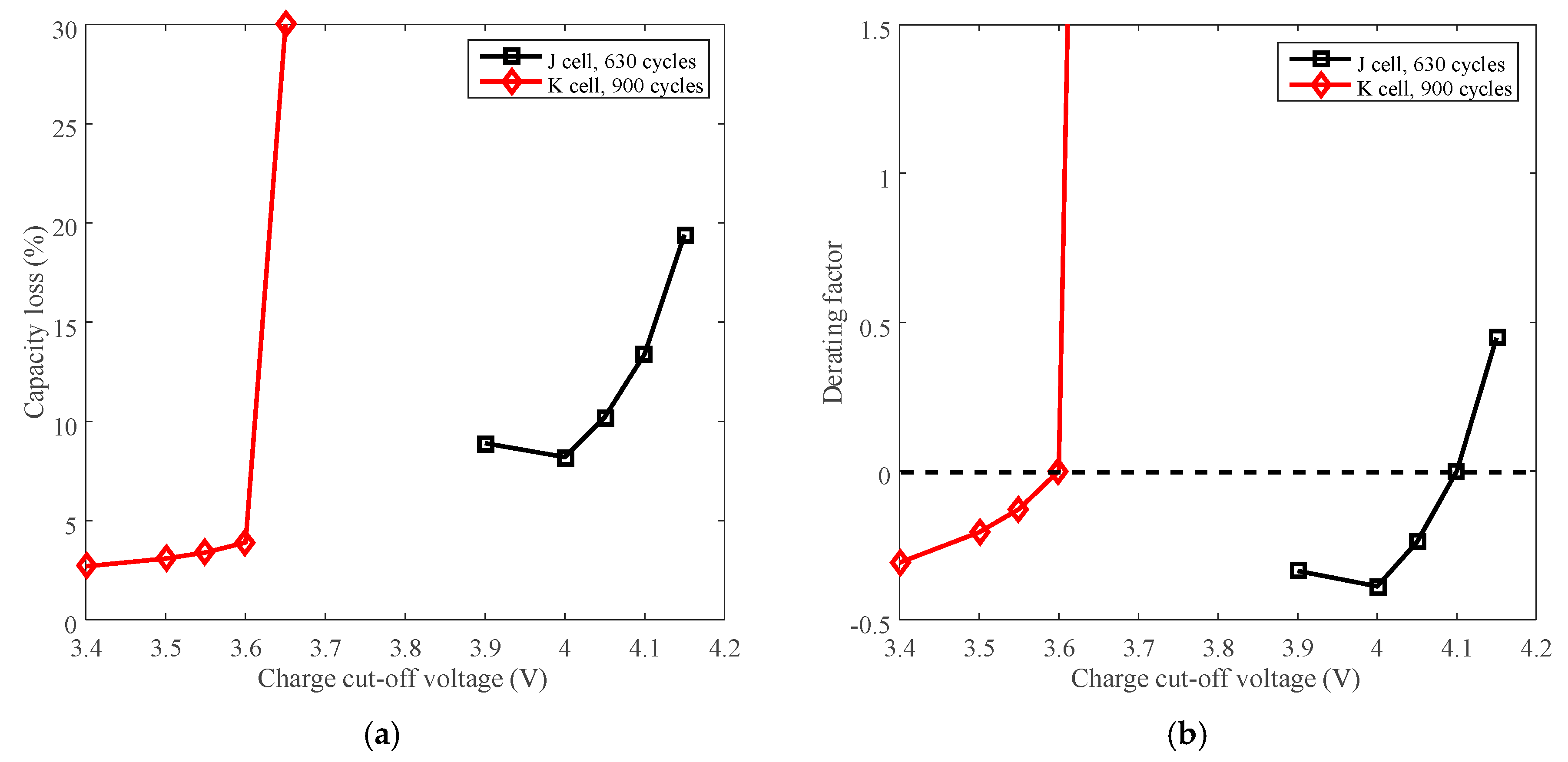
3.5. Cycle Life Improvement by Charge Cut-Off Voltage Derating
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Du, J.; Zhang, X.; Wang, T.; Song, Z.; Yang, X.; Wang, H.; Wu, X. Battery degradation minimization oriented energy management strategy for plug-in hybrid electric bus with multi-energy storage system. Energy 2018, 165, 153–163. [Google Scholar] [CrossRef]
- Instructions for EEE Parts Selection, Screening, Qualification and Derating (EEE-INST-002). Available online: https://nepp.nasa.gov/docuploads/FFB52B88-36AE-4378-A05B2C084B5EE2CC/EEE-INST-002_add1.pdf (accessed on 22 November 2018).
- Recommended Practice for Maintenance, Testing, and Replacement of Valve-Regulated Lead-Acid Batteries for Stationary Applications (IEEE 1188). Available online: https://standards.ieee.org/project/1188.html (accessed on 20 November 2018).
- Freescale Semiconductor. MPC7447AECS01AD Specification. 7 November 2018. Available online: https://www.nxp.com/docs/en/data-sheet/MPC7447AECS01AD.pdf (accessed on 7 November 2018).
- Hitachi. Instructions for Use of Hitachi High-Voltage Monolithic ICs. 7 November 2018. Available online: http://www.hitachi-power-semiconductor-device.co.jp/en/products/ic/pdf/Instructions_for_Use_EN.pdf (accessed on 7 November 2018).
- Yang, F.; Wang, D.; Zhao, Y.; Tsui, K.L.; Bae, S.J. A study of the relationship between coulombic efficiency and capacity degradation of commercial lithium-ion batteries. Energy 2018, 145, 486–495. [Google Scholar] [CrossRef]
- Guan, T.; Zuo, P.; Sun, S.; Du, C.; Zhang, L.; Cui, Y.; Wang, F. Degradation mechanism of LCO/mesocarbon microbeads battery based on accelerated aging tests. J. Power Sources 2014, 268, 816–823. [Google Scholar] [CrossRef]
- Choi, S.S.; Lim, H.S. Factors that affect cycle-life and possible degradation mechanisms of a Li-ion cell based on LiCoO2. J. Power Sources 2002, 111, 130–136. [Google Scholar] [CrossRef]
- Ning, G.; Haran, B.; Popov, B.N. Capacity fade study of lithium-ion batteries cycled at high discharge rates. J. Power Sources 2003, 117, 160–169. [Google Scholar] [CrossRef]
- Wang, J.; Liu, P.; Hicks-Garner, J.; Sherman, E.; Soukiazian, S.; Verbrugge, M.; Finamore, P. Cycle-life model for graphite-LFP cells. J. Power Sources 2011, 196, 3942–3948. [Google Scholar] [CrossRef]
- Ecker, M.; Nieto, N.; Käbitz, S.; Schmalstieg, J.; Blanke, H.; Warnecke, A.; Sauer, D.U. Calendar and cycle life study of Li (NiMnCo) O2-based 18650 lithium-ion batteries. J. Power Sources 2014, 248, 839–851. [Google Scholar] [CrossRef]
- Saxena, S.; Hendricks, C.; Pecht, M. Cycle life testing and modeling of graphite/LiCoO2 cells under different state of charge ranges. J. Power Sources 2016, 327, 394–400. [Google Scholar] [CrossRef]
- Wikner, E.; Thiringer, T. Extending Battery Lifetime by Avoiding High SOC. Appl. Sci. 2018, 8, 1825. [Google Scholar] [CrossRef]
- IEEE Standard Procedure for the Determination of the Ampacity Derating Factor for Fire-Protected Cable Systems. (IEEE Std 848-2015). Available online: https://ieeexplore.ieee.org/stamp/stamp.jsp?tp=&arnumber=7111195 (accessed on 20 November 2018).
- Peng, W.; Li, Y.F.; Yang, Y.J.; Mi, J.; Huang, H.Z. Bayesian degradation analysis with inverse Gaussian process models under time-varying degradation rates. IEEE Trans. Reliab. 2017, 66, 84–96. [Google Scholar] [CrossRef]
- Naumann, M.; Schimpe, M.; Keil, P.; Hesse, H.C.; Jossen, A. Analysis and modeling of calendar aging of a commercial LiFePO 4/graphite cell. J. Energy Storage 2018, 17, 153–169. [Google Scholar] [CrossRef]
- Rodrigues, M.T.F.; Sayed, F.N.; Gullapalli, H.; Ajayan, P.M. High-temperature solid electrolyte interphases (SEI) in graphite electrodes. J. Power Sources 2018, 381, 107–115. [Google Scholar] [CrossRef]
- Ploehn, H.J.; Ramadass, P.; White, R.E. Solvent diffusion model for aging of lithium-ion battery cells. J. Electrochem. Soc. 2004, 151, A456–A462. [Google Scholar] [CrossRef]
- Ramadass, P.; Haran, B.; White, R.; Popov, B.N. Capacity fade of Sony 18650 cells cycled at elevated temperatures: Part I. Cycling performance. J. Power Sources 2002, 112, 606–613. [Google Scholar] [CrossRef]
- Li, Z.; Lu, L.; Ouyang, M.; Xiao, Y. Modeling the capacity degradation of LiFePO4/graphite batteries based on stress coupling analysis. J. Power Sources 2011, 196, 9757–9766. [Google Scholar] [CrossRef]
- Keil, P.; Jossen, A. Charging protocols for lithium-ion batteries and their impact on cycle life—An experimental study with different 18650 high-power cells. J. Energy Storage 2016, 6, 125–141. [Google Scholar] [CrossRef]
- Groot, J.; Swierczynski, M.; Stan, A.I.; Kær, S.K. On the complex ageing characteristics of high-power LiFePO4/graphite battery cells cycled with high charge and discharge currents. J. Power Sources 2015, 286, 475–487. [Google Scholar] [CrossRef]
- Liu, G.; Lu, W. A Model of Concurrent Lithium Dendrite Growth, SEI Growth, SEI Penetration and Regrowth. J. Electrochem. Soc. 2017, 164, A1826–A1833. [Google Scholar] [CrossRef]
- Deshpande, R.; Verbrugge, M.; Cheng, Y.T.; Wang, J.; Liu, P. Battery cycle life prediction with coupled chemical degradation and fatigue mechanics. J. Electrochem. Soc. 2012, 159, A1730–A1738. [Google Scholar] [CrossRef]
- Guan, T.; Sun, S.; Yu, F.; Gao, Y.; Fan, P.; Zuo, P.; Yin, G. The degradation of LiCoO2/graphite batteries at different rates. Electrochim. Acta 2018, 279, 204–212. [Google Scholar] [CrossRef]
- Birkl, C.R.; McTurk, E.; Roberts, M.R.; Bruce, P.G.; Howey, D.A. A parametric open circuit voltage model for lithium ion batteries. J. Electrochem. Soc. 2015, 162, A2271–A2280. [Google Scholar] [CrossRef]
| Chemistry | Nominal Capacity (Ah) | Charge Cut-Off Voltage (V) | Discharge Cut-Off Voltage (V) |
|---|---|---|---|
| Graphite/LFP [16] | 3.0 | 3.6 | 2.0 |
| Graphite/LCO | 1.5 | 4.2 | 2.75 |
| Derated Stresses | Chemistry | Battery | Levels of Derated Stresses |
|---|---|---|---|
| Temperature | LCO | A | 10 °C, 25 °C, 45 °C, 60 °C |
| B | 25 °C, 45 °C, 50 °C, 55 °C | ||
| LFP | G | 45 °C, 30 °C | |
| P | 29.2 °C, 35.6 °C, 52.8 °C | ||
| Discharge C-rate | LCO | A | 0.7C, 1.0C, 2.0C |
| O | 1C, 1.1C, 1.3C, 1.5C, 2.0C | ||
| LFP | G | C/3, 4C | |
| P | 1C, 3.75C | ||
| Charge C-rate | LCO | J | 1A (0.91C), 3A, 5A (4.5C) |
| O | 1C, 1.2C, 1.4C | ||
| LFP | K | 1A (0.91C), 3A, 5A (4.5C) | |
| G | C/3, 1.5C | ||
| P | 1C, 3.75C | ||
| Charge cut-off current | LCO | A | C/5, C/40 |
| O | 0 min, 28 min, 53 min, 100 min | ||
| Charge cut-off voltage | LCO | J | 4.15 V, 4.1 V, 4.05 V, 4.0 V, 3.9 V |
| LFP | K | 3.65 V, 3.6 V, 3.55 V, 3.5 V, 3.4 V |
| Battery | Battery Specifications | Stress | Testing Procedures and Conditions |
|---|---|---|---|
| A | Normal capacity: 3.36 Ah Cut-off voltage: 4.4 V/3.0 V Positive electrode: LCO Negative electrode: graphite | Temperature: 25 °C, 45 °C, 60 °C | Charge: CC 1.5C to 4.2 V, CV to 1C, then CC 1C to 4.4 V, CV to Ccut-off Discharge: CC C discharge to 3.0 V Charge cut-off current (Ccut-off): C/5, C/40 Discharge constant current (Cdischarge): 0.7C, 1.0C, 2.0C |
| Charge cut-off current: C/5, C/40 | |||
| Discharge constant current: 0.7C, 1.0C, 2.0C | |||
| B [19] | Normal capacity:1.8 Ah Cut-off voltage: 4.2 V/2.0 V Positive electrode: LCO Negative electrode: carbon | Temperature: 25 °C, 45 °C, 50 °C, 55 °C | Charge: CC 1 A to 4.2 V, CV to 50 mA; discharge: CC 1 A to 2.0 V under 25 °C, 45 °C, 50 °C, and 55 °C. |
| G [20] | Nominal capacity: 11 Ah Cut-off voltage: 3.65 V/2.0 V Positive electrode: LFP Negative electrode: graphite | Temperature: 45 °C, 30 °C | (1) Charge CC C/3 to 3.65 V, then CV to C/10; discharge CC C/3 to 2.0 V under 45 °C. (2) Charge CC C/3 to 3.65 V, then CV to C/10; discharge CC 4C to 2.0 V under 30 °C. (3) Charge CC 1.5C to 3.65 V, then CV to C/10; discharge CC C/3 to 2.0 V under 30 °C. (4) Charge CC C/3 to 3.65 V, then CV to C/10; discharge CC 4C to 2.0 V under 45 °C. (5) Charge CC 1.5C to 3.65 V, then CV to C/10; discharge CC C/3 to 2.0 V under 45 °C. (6) Charge, CC 1.5C to 3.65 V, then CV to C/10; discharge CC 4C to 2.0 V under 30 °C. |
| Charge C-rate: 1.5C, C/3 | |||
| Discharge C-rate: 4C, C/3 | |||
| J [20] | Nominal capacity: 1.1 Ah Cut-off voltage: 4.1 V/2.5 V Positive electrode: LiNiCoMnO2 + LCO Negative electrode: graphite | Charge C-rate: 1 A, 3 A, 5 A | Charge CC 1/3/5 A to 4.1 V, then CV to 100 mA; discharge CC 3 A to 2.5 V under 25 °C. |
| Charge cut-off voltage: 4.15 V, 4.1 V, 4.05 V, 4.0 V, 3.9 V | Charge CC 3 A to CV, then CV to 100 mA; discharge CC 3 A to 2.5 V under 25 °C. | ||
| K [21] | Nominal capacity: 1.1 Ah Cut-off voltage: 3.6 V/2.0 V Positive electrode: LFP Negative electrode: graphite | Charge C-rate: 1 A, 3 A, 5 A | Charge CC 1/3/5 A to 3.6 V, then CV to 100 mA; discharge: CC 3 A to 2.0 V under 25 °C. |
| Charge cut-off voltage: 3.65 V, 3.6 V, 3.55 V, 3.5 V, 3.4 V | Charge CC 3 A to CV, then CV to 100 mA; discharge CC 3 A to 2.0 V under 25 °C. | ||
| O [8] | Rated capacity: 900 mAh Cut-off voltage: 4.2 V/2.75 V Positive electrode: LCO Negative electrode: graphite | CV charge period: 0 min, 28 min, 53 min, 100 min | Charge CC 1C to 4.2V, CV hold thp; discharge CC 1C to 2.75 V under 25 °C. |
| Charge C-rate: 1C, 1.2C, 1.4C | Charge CC to 4.2 V, CV hold 2.5 h; discharge CC 1C to 2.75 V under 25 °C. | ||
| Discharge C-rate: 1C, 1.1C, 1.3C, 1.5C, 2.0C | Charge CC 1C to 4.2 V, CV to 90 mA; discharge CC to 2.75 V under 25 °C. | ||
| P [22] | Rated capacity: 2.3 Ah Manufacturer: A123 Systems Positive electrode: LFP Negative electrode: graphite | Temperature: | (1) Charge CC 1C to SOC 100%; discharge CC 1C to SOC 0% under 23.5 °C. (2) Charge CC 1C to SOC 100%; discharge CC 1C to SOC 0% under 32.5 °C. (3) Charge CC 2C to SOC 100%; discharge: CC 2C to SOC 0% under 26.1 °C. (4) Charge CC 2C to SOC 100%; discharge: CC 2C to SOC 0% under 34.4 °C. (5) Charge CC 2C to SOC 100%; discharge: CC 2C to SOC 0% under 46.9 °C. (6) Charge CC 3.75C to SOC 100%; discharge: CC 3.75C to SOC 0%, under 29.2 °C. (7) Charge CC 3.75C to SOC 100%; discharge: CC 3.75C to SOC 0%, under 35.6 °C. (8) Charge CC 3.75C to SOC 100%; discharge: CC 3.75C to SOC 0%, under 52.8 °C. (9) Charge CC 1C to SOC 100%; discharge CC 3.75C to SOC 0% under 34.1 °C. (10) Charge CC 1C to SOC 100%; discharge CC 3.75C to SOC 0% under 25.0 °C. (11) Charge CC 1C to SOC 100%; discharge: CC 3.75C to SOC 0% under 46.6 °C. (12) Charge CC 3.75C to SOC 100%; discharge: CC 1C to SOC 0% under 33.5 °C. (13) Charge CC 3.75C to SOC 100%; discharge: CC to SOC 0% under 23.3 °C. (14) Charge CC 3.75C to SOC 100%; discharge: CC to SOC 0% under 46.7 °C. |
| Charge C-rate: 1C, 3.75C | |||
| Discharge C-rate: 1C. 3.75C |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, Y.; Saxena, S.; Pecht, M. Derating Guidelines for Lithium-Ion Batteries. Energies 2018, 11, 3295. https://doi.org/10.3390/en11123295
Sun Y, Saxena S, Pecht M. Derating Guidelines for Lithium-Ion Batteries. Energies. 2018; 11(12):3295. https://doi.org/10.3390/en11123295
Chicago/Turabian StyleSun, Yongquan, Saurabh Saxena, and Michael Pecht. 2018. "Derating Guidelines for Lithium-Ion Batteries" Energies 11, no. 12: 3295. https://doi.org/10.3390/en11123295
APA StyleSun, Y., Saxena, S., & Pecht, M. (2018). Derating Guidelines for Lithium-Ion Batteries. Energies, 11(12), 3295. https://doi.org/10.3390/en11123295







