1. Introduction
With the increasing production of electricity from intermittent renewable energy sources (e.g., wind and solar), the need for an effective energy storage is becoming imperative. It is therefore necessary to accumulate energy at the time it is not requested, and use it later when renewable energy is lacking, and energy is still demanded.
The European Union (EU-28) has seen an increased renewable energy production over the years and it is aiming to reach 20% of the gross final energy consumption by 2020. It is estimated that between 2006 and 2016 there was an increase in renewable energy production by two-thirds [
1].
Among the different options for energy storage, PEM electrolysis has recently attracted attention because it utilizes the same technology as PEM fuel cells, which has been developed for a long time and proven successful. The electrolyzer is able to produce hydrogen from electricity by an electrochemical reaction for later use in a fuel cell, moreover hydrogen can be used to produce other carbon-neutral fuels such as syngas and alcohols (e.g., methanol) which despite containing carbon, can be produced by renewable sources [
2,
3].
Early electrolyzer models and simulations in Matlab/Simulink
® were developed among others by [
4,
5]. Such a dynamic modelling software platform is well suited for energy case scenarios where input and output are continuously changing over the time. In particular in [
4], the authors describe a model with all the components from renewable energy generation including the wind turbines, electrolyzer, fuel cell and power conditioning. System transient responses to different case scenarios are also presented.
One of the first Simulink mathematical models of the gas porous diffusion electrode and ion exchange membranes of a PEM electrolyzer can be attributed to Görgün et al. [
6]. This model is in fact a steady state model, as it does not consider thermal and electrical capacitance dynamic effects. Awasthi et al. [
7] followed a similar approach.
Marangio et al. [
8] presented a validated PEM electrolyzer semi-empirical model including overvoltages and resistances along the electrodes, flow plates and electrolyte. Abdin et al. [
9] included Knudsen diffusion and molecular diffusion to characterize cathode and anode porous media. Choi et al. [
10] developed an electrolyzer model with the Butler-Volmer kinetics including the effect of cell temperature on the exchange current density. More recently, Yigit et al. [
11] developed a dynamic model of a high pressure PEM electrolyzer system, the model is only partially validated. This study gives detailed information of the energy losses in the system at different current density of the electrolyzer showing that above 1 A/cm
2 efficiency become significantly low.
In the aforementioned models, authors implemented the differential equations describing the physical phenomena directly in the software platform. Other authors have found other ways to approach this complex dynamic system modelling effort. For instance, Olivier et al. [
12] developed a model based on the “bond graph” method. The model includes stack and BoP and simulates the behavior under intermittent condition. This graphical approach is helpful to simplify the representation of complex dynamic system behavior and convert the system in a state-space mode. The model was then implemented in Matlab/Simulink
®, showing good agreement between experimental and model data.
Zhou et al. [
13] developed a control oriented electrolyzer model and tested it in real time with a hardware-in-the-loop emulator of the electrolyzer and wind energy system. The emulator is able to test different electrolyzer specifications given by the manufacturers. In [
14], Ruuskanen et al. followed a similar approach where only the power conditioning was experimentally tested and the rest of the system was implemented in a power-hardware-in-loop.
This paper provide a validated PEM electrolyzer model that includes the physical principles introduced in previous papers [
4,
5,
8,
9]. In addition, in this study we estimate cell efficiency and heat dissipation. Besides differently from [
8,
9], water gas pressure was calculated using the water saturation pressure. The model is able to predict cell performance at a large range of different temperatures. Exchange current densities parameters and membrane conductivity was chosen as closely depending on temperature.
This study is divided in two main parts. In the first part, the experimental test is described where an electrolyzer cell is characterized and performance are measured. In the second part, the model is detailed described and the experimental results are used to validate the model.
2. Experimental
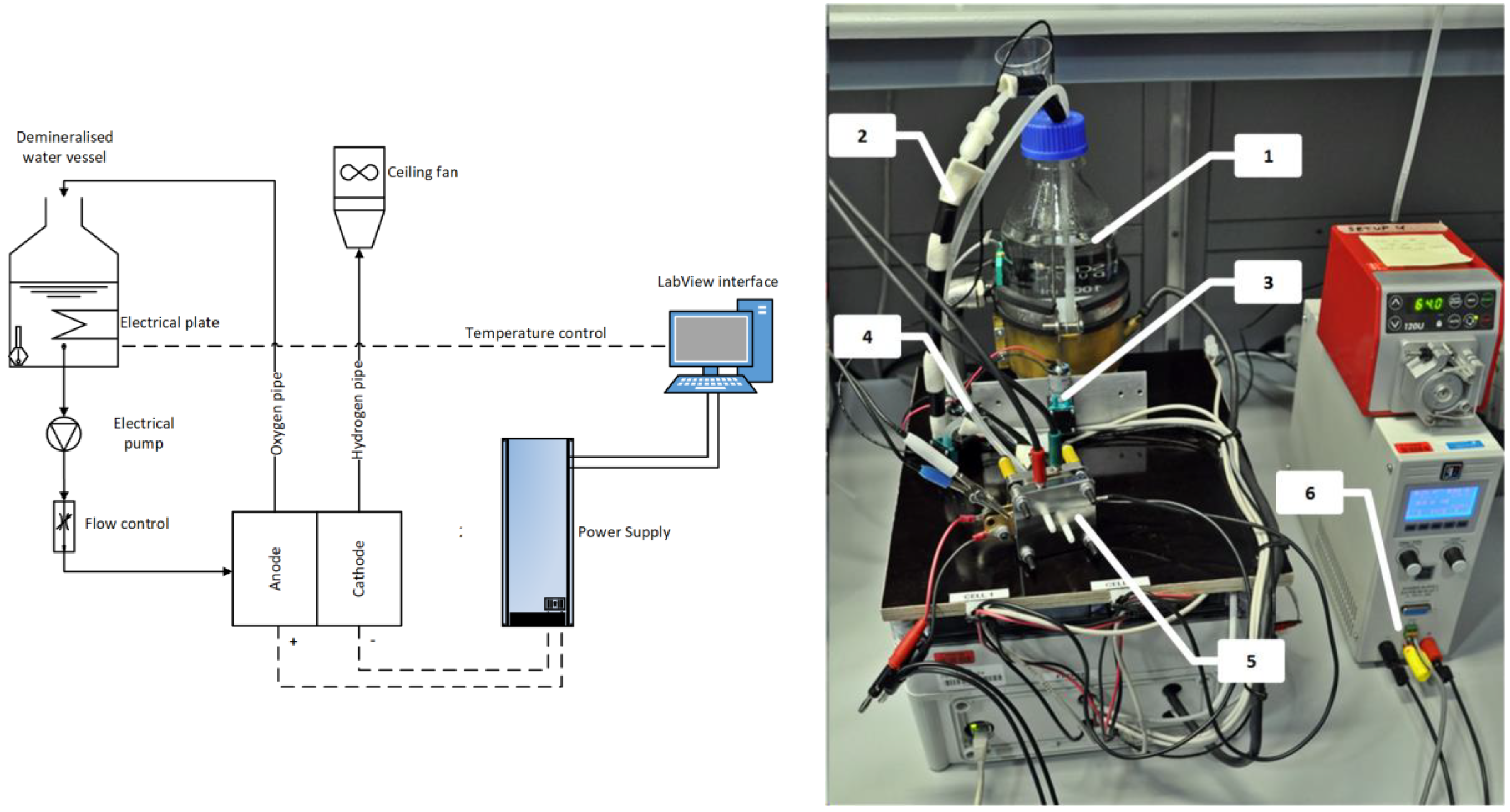
The experimental setup is shown in
Figure 1. The core of test is the electrolyzer cell assembly that is supplied by electrical power and the required reactants. The polarization curve was registered at two fixed operating temperatures, i.e., 60 °C and 80 °C. A start-up phase initiated each test in which the cell gradually reached the set temperature.
The cell has an active area of 2.89 cm2. The cathode has a catalyst loading of 0.5 mg/cm2 Pt/C, carbon cloth (C-cloth PTL) with parallel flow field. The anode has a catalyst loading of 0.3 mg/cm2 IrO2, 2.7 mg/cm2 Ir, porous Ti PTL, with an interdigitated flow field. The Nafion polymer membrane is type N117.
Since the first part of the cell polarization curve has a greater slope, measurement were more frequent at lower current densities than at high current density. At each step, the voltage was measured as average of 3 min measurements. Up to 0.289 A, steps were every 0.01 A cm2; between 0.289 A and 0.578 A, steps were every 0.1 A cm2. Finally, steps were every 0.2 A cm2, from 0.578 A up to the maximum voltage which was fixed at 2.2 A.
3. Modelling
A simplified mathematical model was developed in Matlab/Simulink
®. The approach follows the same modelling structure initiated by Abdin et al. [
9] and Marangio et al. [
8]. The model is divided into four sub-sections: Anode and Cathode chambers, Membrane and Voltage.
The model was fitted to experimental electrolyzer polarization curve operating at 60 °C and 80 °C. The electrodes reference exchange current densities at the anode and cathode, electrodes charge transfer coefficients and the membrane hydrogen ion diffusivity were estimated from the cell curve performance as they closely relate to the cell performance at different temperature.
As
Figure 2 depicts at the anode side, water is introduced and then split into hydrogen ions and oxygen gas. Hydrogen positive ions cross the membrane and recombine at the cathode side forming hydrogen gas. At the same time electrons travel through the external circuit, which is connected to the power supply that provide the electromotive force for the electrochemical reaction to happen. The basic reactions taking place to the electrolyte/electrode interface are given below:
The model follows similar approach to the one used by Görgün in [
6] and later by [
6,
9]. The model is divided in four Simulink blocks in which mass flow rate of different species are computed (i.e., Anode chamber, Cathode chamber, Membrane, Voltage). Main assumption of this model is to consider steady-state electrochemical mechanism for the electrolyzer model and therefore there is an instantaneous response to input changes with no time delays. This approach is justified by the fact that transient response is very fast in PEM electrolyzer as shown in experimental work by [
6,
9].
3.1. Anode Chamber
In the anode chamber, four moles of oxygen are generated for each electron. According to the “Faraday’s law” we can define the molar flow rate of generated oxygen as:
Similarly, two moles of water are consumed for each electron.
is the current which is function of the current density, , and the cell area, , i.e., .
The accumulation of oxygen gas in the anode chamber is calculated as the difference between the oxygen gas at the chamber inlet and outlet plus the oxygen generated by the electrochemical reaction [
9]:
Similarly the accumulation of water takes into account the water consumed by the electrochemical reaction and the net water flow through the membrane which is the combination of multiple processes as described in section “Membrane” [
9].
The partial pressure of the species in the channel can be calculated using the “Dalton law” in which oxygen, water and hydrogen are considered in the gas phase. Such an approach was used, among others, in [
8,
9]. As in the anode chamber water is in liquid phase, we calculate the oxygen gas partial as a difference between the anode total pressure and the water saturation pressure. In this way, the water gas phase is accounted equal to its saturation pressure.
In the present study, the anode pressure was considered atmospheric, i.e.,
The water saturation pressure can be calculated using the “Antoine equation” which is function of temperature and other parameters shown in
Table 1:
Similar approach for the gas species partial pressures calculation was used for the cathode.
3.2. Cathode Chamber
At the cathode side, hydrogen is generated by the electrochemical reaction. The molar balance and the gas partial pressure can be calculated similarly to the anode side.
The hydrogen gas accumulation is calculated as a difference between the hydrogen molar flow rate at the inlet and outlet plus the product hydrogen:
Product hydrogen is calculated using “Faraday’s law” considering that for two moles of electrons one mole of hydrogen is generated:
The hydrogen partial pressure is calculated as a difference between the cathode pressure,
, and the water saturation pressure:
3.3. Membrane
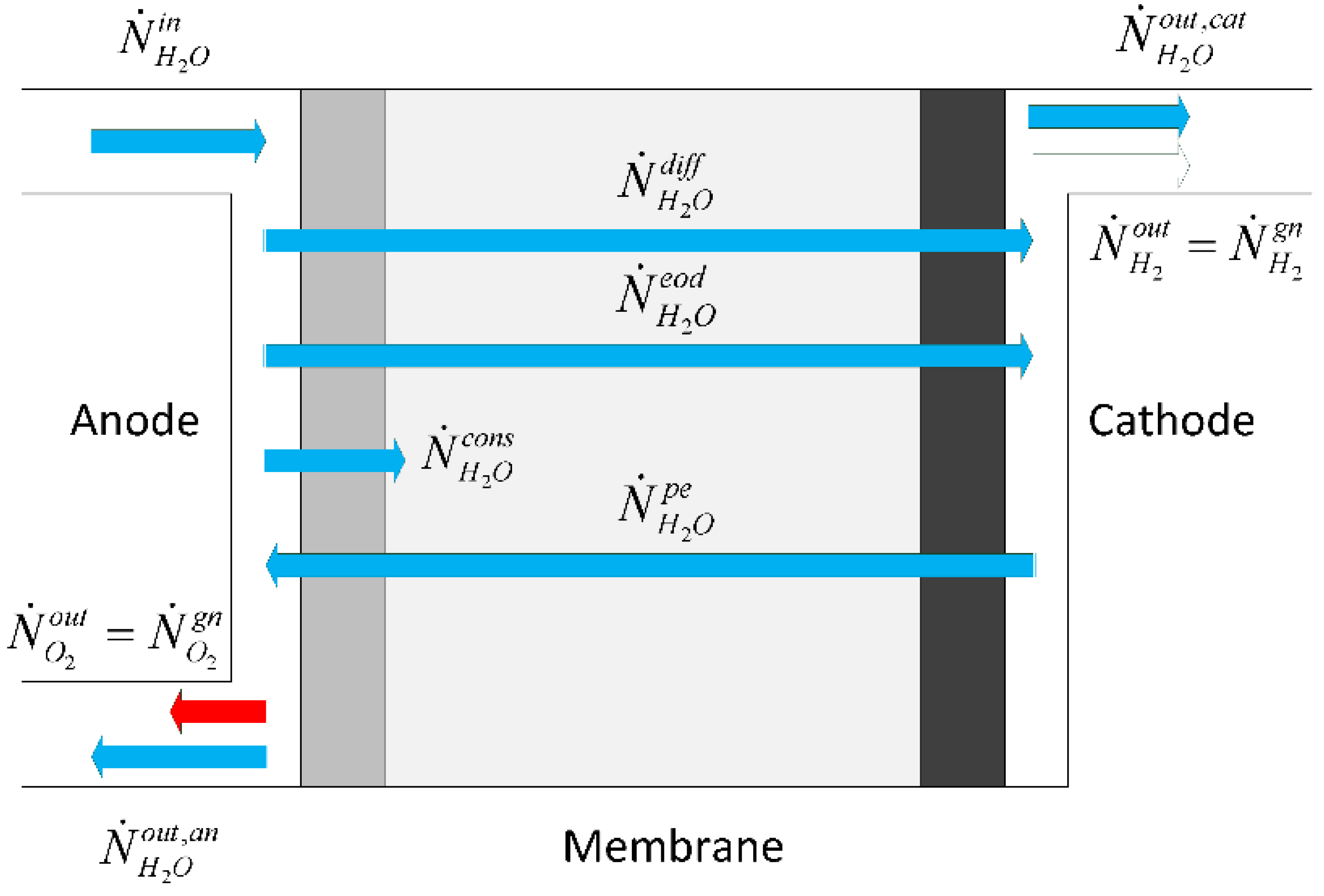
Abdin et al. [
9] identify three main relevant phenomena for water transport, namely diffusion, electro osmotic drag and hydraulic pressure, which combined provide the membrane net water flow:
Diffusion mechanism refers to the transport phenomena due to concentration gradients across the membrane, whereas electro-osmotic drag refers to water which is dragged by hydrogen protons in the membrane, and hydraulic pressure refers to pressure asymmetries between the anode and cathode that cause water transport. We describe these three water transport processes in the next three sections.
3.4. Water Diffusion
Diffusion of water refers to the transport of water from high to low concentration regions prevalently from anode to cathode as water is formed at anode side. Fick’s law is used to calculate the water transport by integrating water concentration across the membrane between the two electrodes [
16]:
Water diffusion is function of active area of the membrane
, the water diffusion coefficient,
, and the water concentration in the membrane,
. In
Figure 3, the concentration of the species at the membrane interface and inside the membrane is illustrated. We can assume that the water concentration at the electrode/membrane interface is approximated with the water concentration in the electrode channel.
Assuming a linear water concentration gradient, we can simply calculate the concentration gradients across the membrane as a difference instead of using integral function [
8,
9,
11,
17]. With this assumption in mind, we calculate the water molar flow rate due to diffusion as:
where
is the membrane water diffusion coefficient,
is the thickness of the membrane and
,
are the water concentration at the electrolyte/electrode interfaces.
The diffusion process of a multi-component gas mixture across the electrode porous media is accounted using the Stefan-Maxwell approach, which considers an effective binary diffusion coefficient. Such a coefficient is used to estimate the diffusivity as a function of temperature, pressure and other geometric parameters [
18]. Similarly to the approach described in [
3,
8], we can calculate the water concentration using the Fick’s law of diffusion in the electrolyte as shown in the equations below:
where
is the anode effective binary diffusion coefficient for
,
is the cathode effective binary diffusion coefficient for the gas pair
,
and
are the thicknesses of the electrodes and
and
are the water gas molar concentration.
The anode and cathode electrodes effective binary diffusion coefficient of transport,
, is calibrated by applying the porosity correction [
7,
8]:
is the porosity correction,
is the percolation threshold and
is an experimental factor. The binary diffusion coefficient is a proportionality factor that depends on temperature and pressure of generic two gas species A and B:
In the equation,
is the electrode pressure,
and
are coefficients that depends on the gas type,
is the molar mass of species A and B. The water concentration in liquid form at anode and cathode can be expressed as:
The water density in this equation is calculated as [
19]:
The empirical parameters, A, B, C and D are provided in
Table 2.
3.5. Electro Osmotic Drag
The water transport due to electro osmotic drag
represents the number of moles of water molecules which are dragged by each mole of hydrogen ions through the membrane and it is proportional to the osmotic drag coefficient and the hydrogen ions i.e.,
.
The osmotic drag coefficient,
, represents the number of water molecules carried by each hydrogen ions and it has been measured experimentally by different authors and resulted values have shown large variance. Awasthi et al. considered a value
which is in line with the relationship below that is function of temperature and pressure [
17]:
In this work we consider the experimental relationship by Onda et al. [
20] which applies to a fully hydrated membrane:
3.6. Hydraulic Pressure
Water transport due to pressure asymmetry,
, between anode and cathode depends on permeability of the membrane and can be calculated using the Darcy’s Law. Similar approach was followed by [
7,
8,
9,
17]. The relationship is function of the membrane permeability to water,
, the viscosity,
, and the molar mass of water,
:
5. Efficiency
A simplified system input-output thermodynamic analysis to determine the electrolyzer efficiency is provided in [
23]. For a PEM electrolyzer we consider as input the electric work, the cooling and water for the electrochemical reaction. The system output will be the hydrogen and oxygen gas formed by the electrochemical reaction. This steady-state approach disregards, among other things, losses due mechanical work provided by ancillary equipment and the mass accumulation due to the dynamic performance.
The electrolyzer first law efficiency considers as input the electric work
provided by the power supply and as output the enthalpy change in standard condition of the electrochemical reaction to obtain hydrogen gas
.
We assume that the remaining part of the electrical work, which is not converted in hydrogen gas, is the rejected heat,
. We notice that
has a negative sign for a mere convention as in fact we provide cooling to the electrolyzer stack. We can write the cooling as a function of the electrical work and first law efficiency as:
The electrolyzer second law efficiency considers as input the electric work
provided by the power supply and as output the Gibbs free energy change in standard condition of the electrochemical reaction to obtain hydrogen gas,
:
The Gibbs free energy at standard condition,
, is calculated by subtracting from
, the reversible heat:
The electrical work is function of the cell voltage,
and the faradaic efficiency:
In [
20] the faradaic efficiency,
, is function of hydrogen and oxygen membrane crossover and is close to unity, however at low current densities it can be significant. Gas crossover occurs generally due to solution-diffusion mechanism. The relationship is function of the equivalent current of hydrogen and oxygen crossover:
The equivalent current of hydrogen crossover,
, is defined as [
20]:
The hydrogen permeability in Nafion is defined as [
20]:
The equivalent current of oxygen crossover,
, is calculated similarly to that of hydrogen:
The membrane permeability of oxygen is approximated as one-half of the hydrogen permeability as mentioned in [
15,
27]:
6. Results and Discussion
In order to fit the model to the experimental data, five empirical parameters were calibrated i.e.,
,
,
,
and
. Other fixed parameters were from experimental measurements and various sources as shown in
Table 3.
The fitting results are given in the
Table 4. Exchange current densities have high impact on the activation overpotential [
9]. Among others, Espinoza et al. [
28] and Biaku et al. [
29] found similar values of
and
. Choi et al. [
10] suggested values of
and
in the same range as the ones found in this model validation. Regarding the diffusivity of hydrogen protons in water,
, the value obtained in this study is consistent with the ones found, among others, in [
8,
30]. It is worth mentioning that as suggested in [
30], this coefficient is strongly correlated to the cell temperature and in particular, hydrogen diffusivity increases with temperature. In [
30], in the temperature range similar to the one in this study it was found a limear increase in 20% of the
. For this reason, we assumed two different values for
at the temperature of 60 °C and 80 °C as shown in the
Table 4.
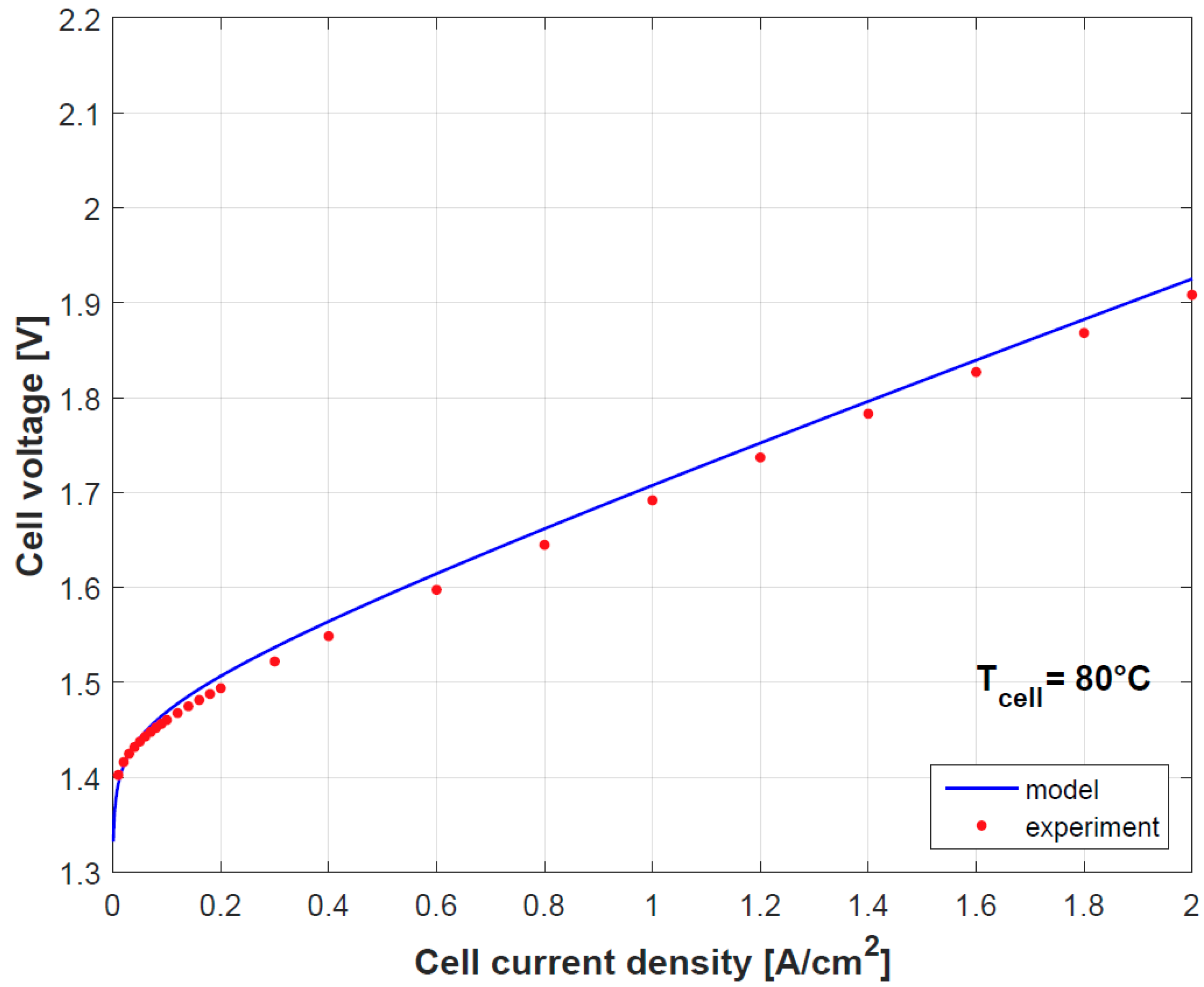
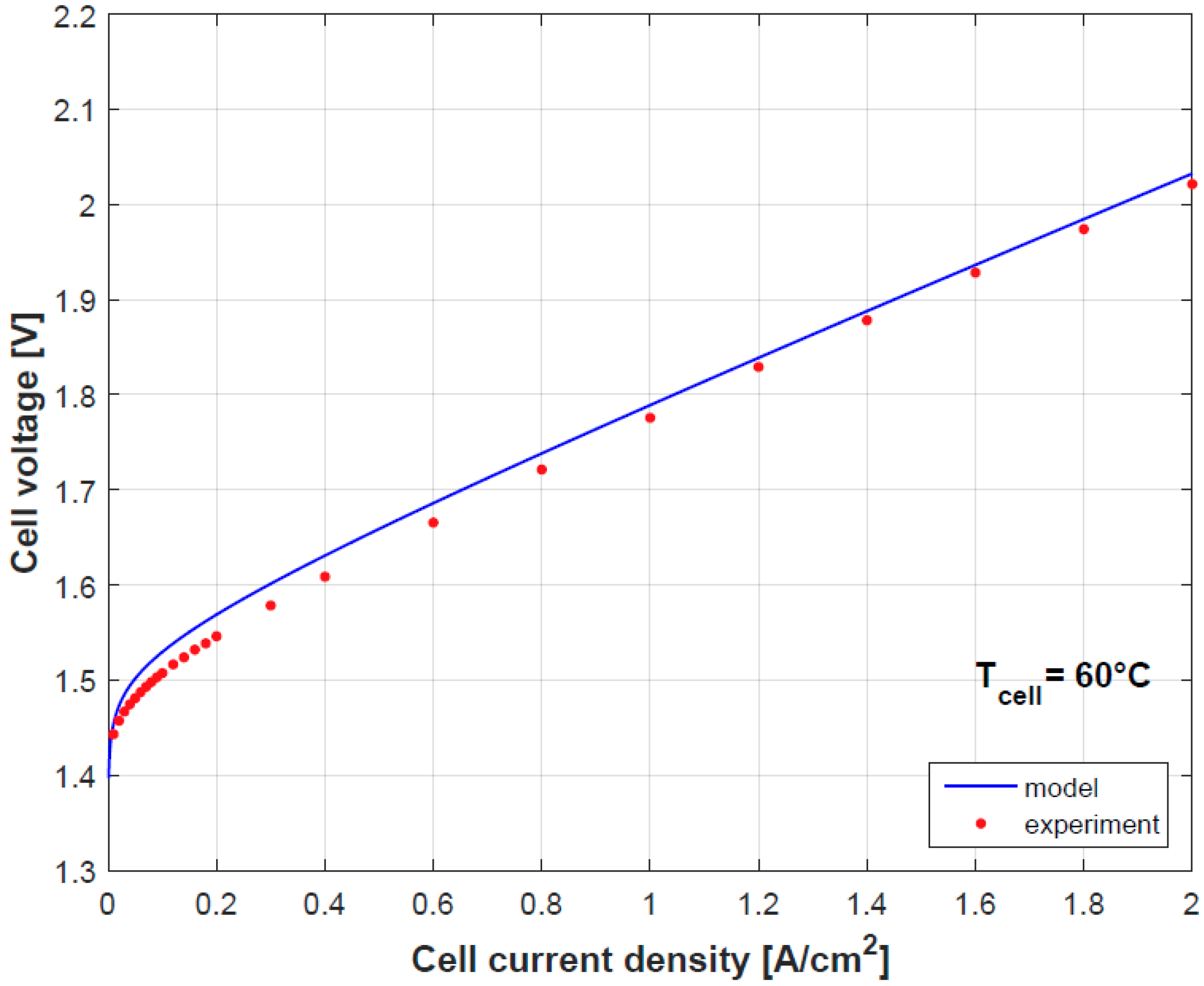
Figure 4 and
Figure 5 provide a comparison of the results obtained by both model and experimental tests using the empirically fitted parameters in
Table 4. A small discrepancy in the polarization curve is seen due to the model assumption of negligible Ohmic overpotentials in the electrodes and plates, which indeed contribute in limited proportion [
9]. Besides as mention before, the temperature dependence of
gives a good prediction for the cell performance.
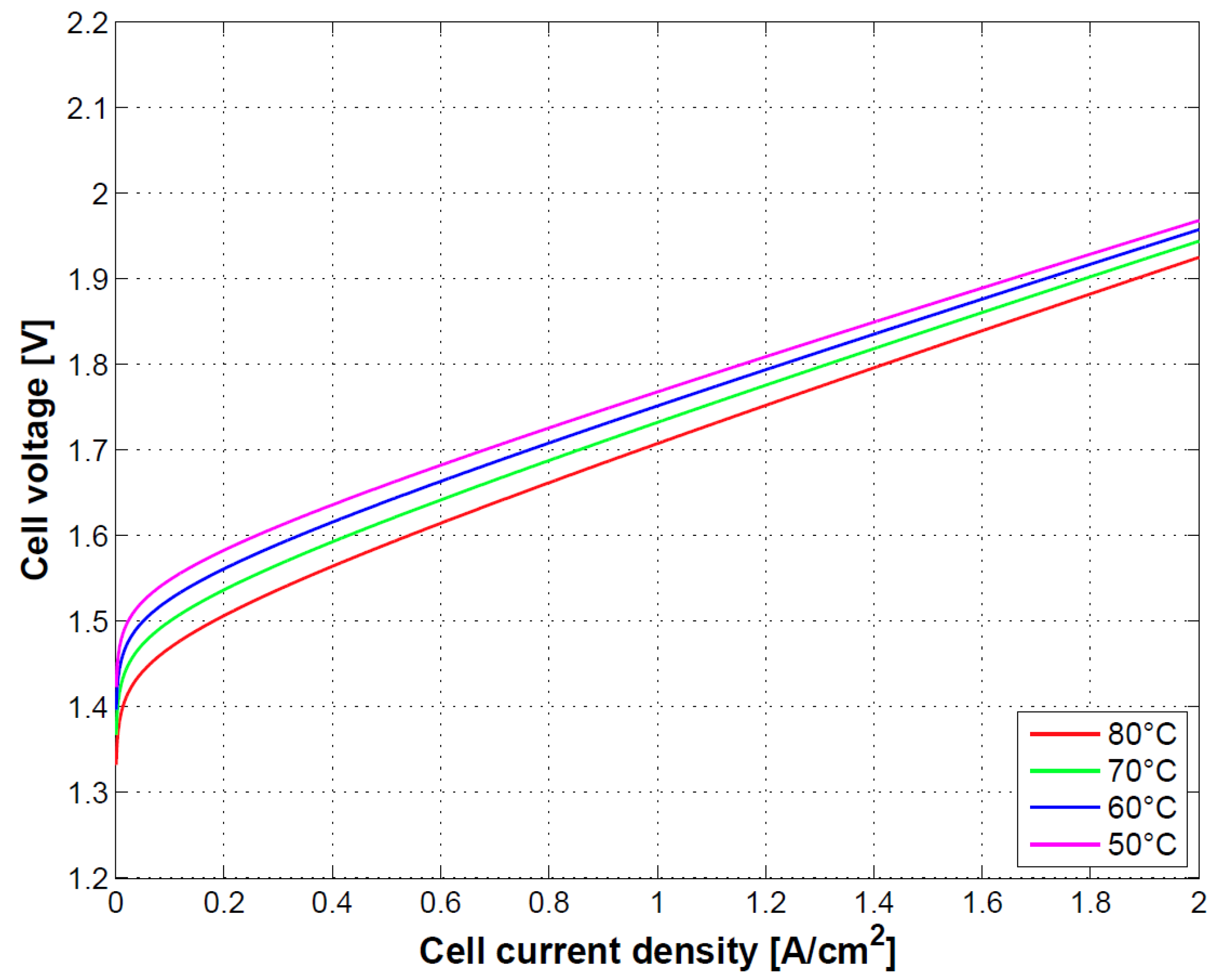
In
Figure 6, the effect of temperature on cell performance is depicted. Increasing temperature of operation will reduce the Gibbs free energy of the electrochemical reaction thereby increasing the cell performance and energy conversion. This is in agreement with results provided in [
9].
The Ohmic overpotential depends on temperature through the conductivity relationship. This is reflected on a slight increase in the slope of the curves at mid-high current densities.
Figure 7 shows the relatively higher contribution of the anode activation overpotential to the overall activation over potential. The kinetics of the oxygen evolution reaction at the anode side is slower than the hydrogen evolution reaction at cathode side resulting in higher overpotentials at the anode. Nevertheless, the reaction kinetics also depends on physical properties of the electrode material e.g., roughness factor. The charge transfer coefficients at anode and cathode,
and
, gave values similar to those in [
9].
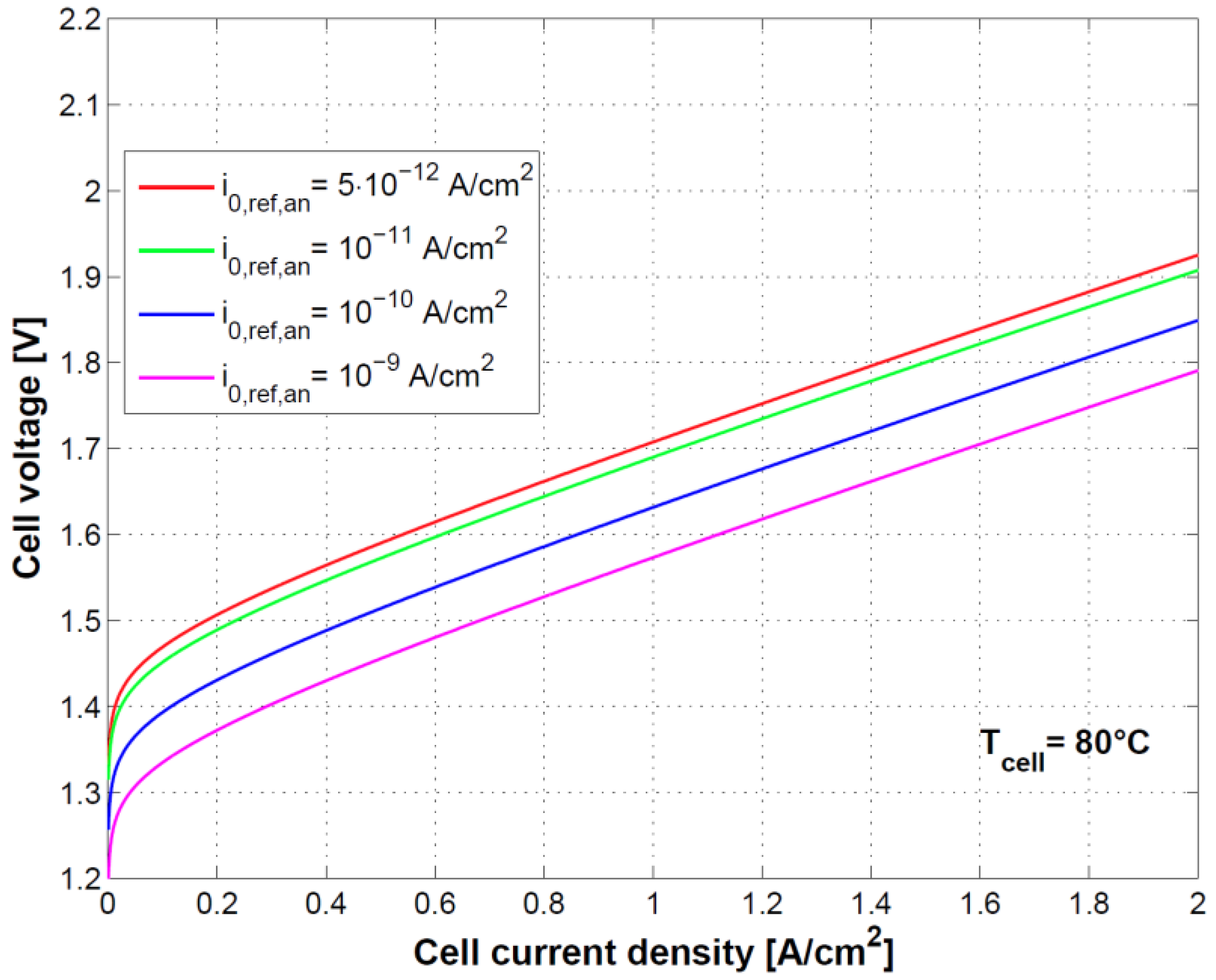
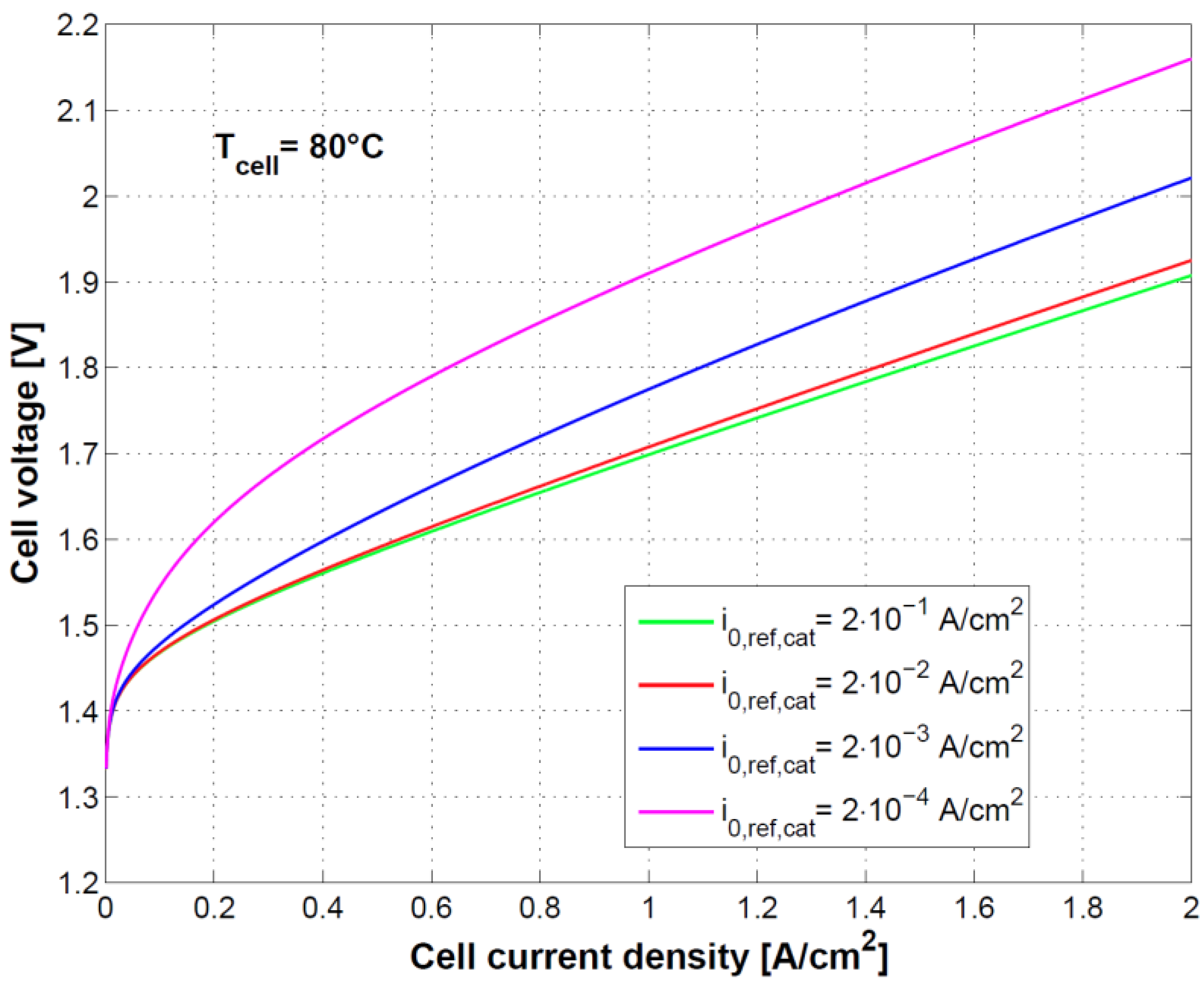
Figure 8 and
Figure 9 show the effect of using different exchange current densities at anode and cathode. This parameter mainly affects activation overvoltage as a consequence of different kinetics of charge transfer reactions.
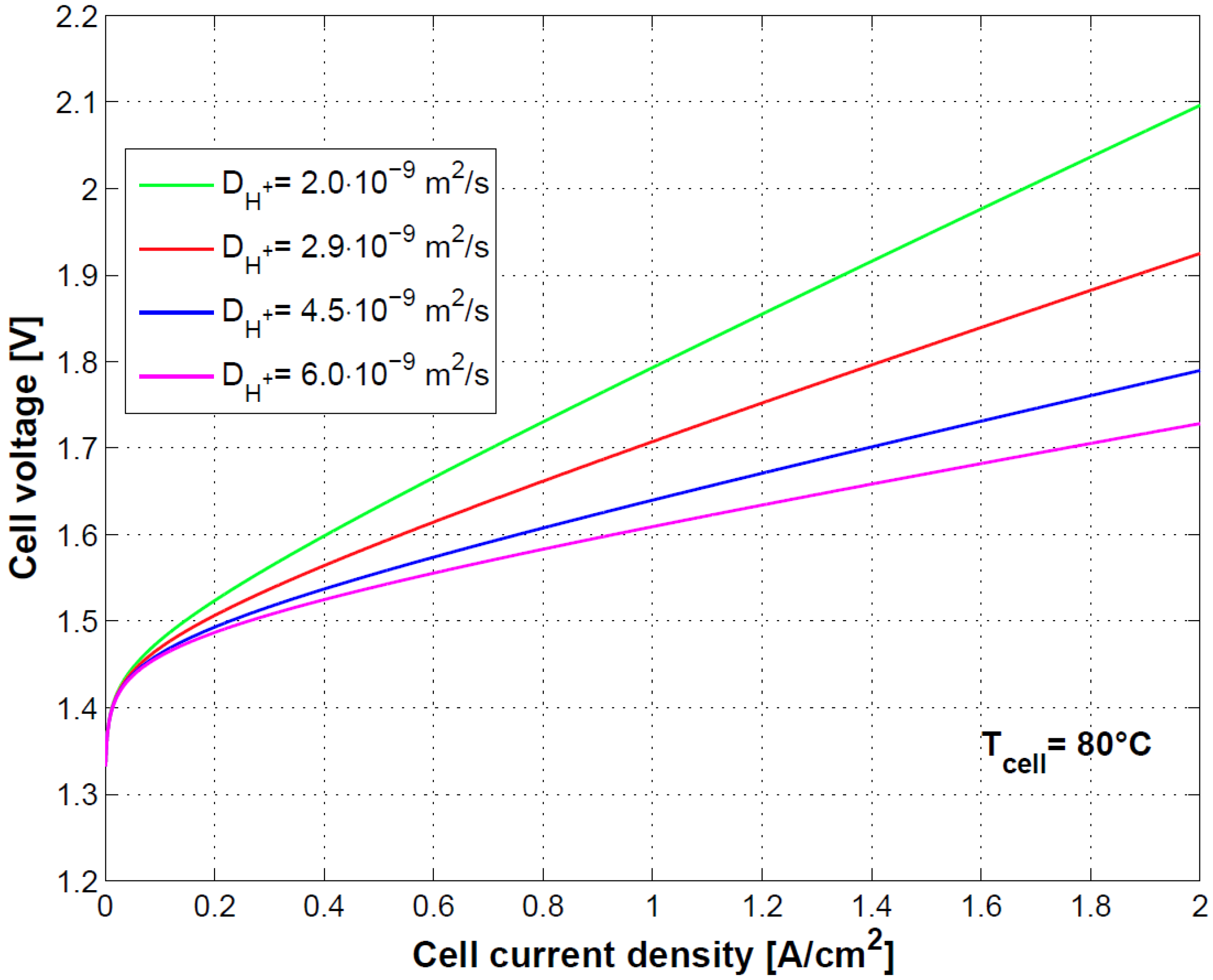
The sensitivity on the cell model to the diffusivity of H
+ ions is shown in
Figure 10. The link of diffusivity of H
+ ions to membrane conductivity and therefore to ohmic losses is evident as previously shown in Equation (34) and [
8].
In
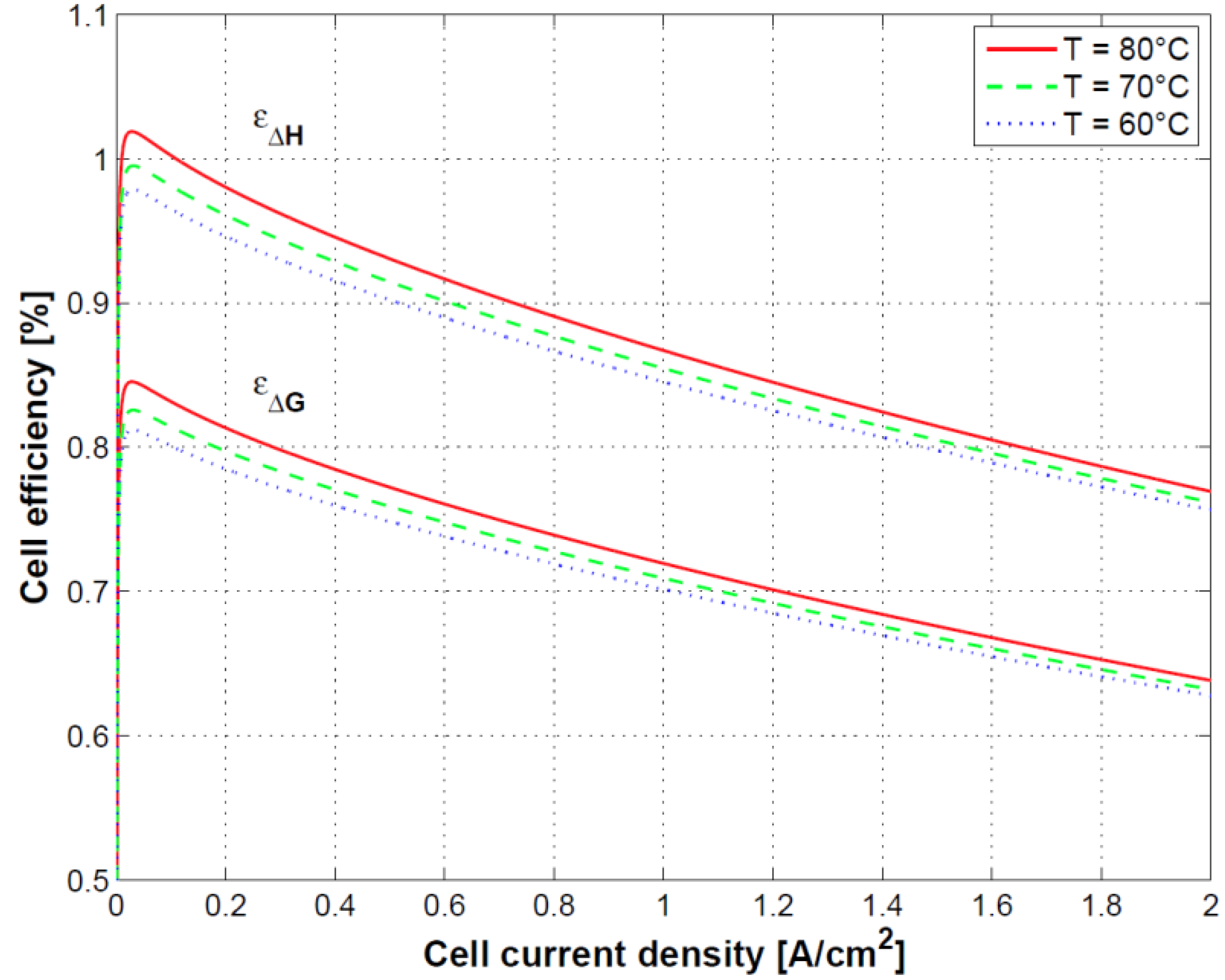
Figure 11, the impact of the temperature on the first and second law efficiencies defined in Equations (37) and (39) is shown. First law efficiency can reach values higher than 100% at low current density due to cell heat absorption and strongly reduces with the increase of current density by around 30% over the current density range of operation.
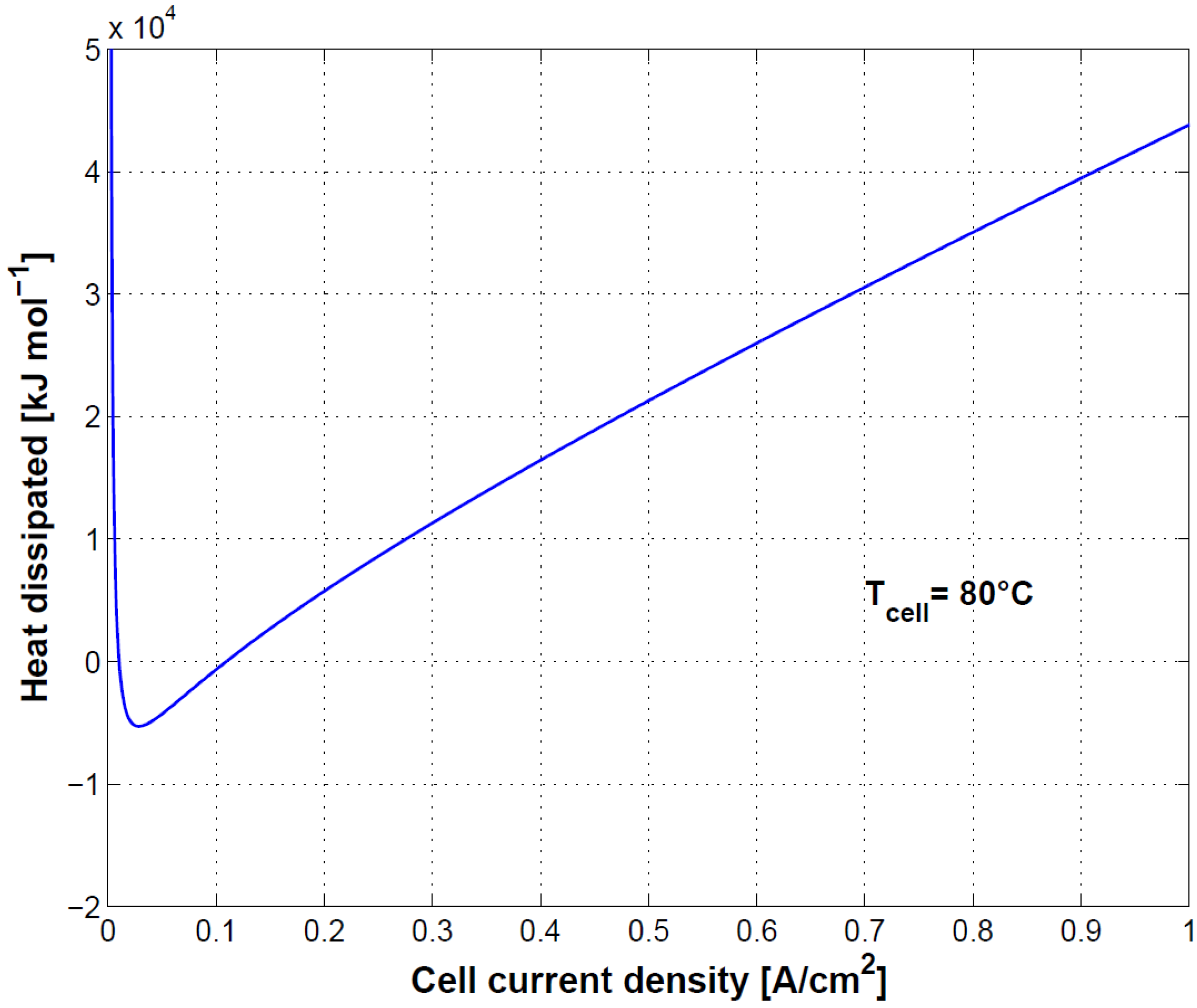
In
Figure 12, cell heat loss is shown. At low current density, heat dissipation is negative, meaning that the cell absorbs heat from the surroundings. When hydrogen production is low, the enthalpy change of the electrochemical reaction is higher than the electrical work [
23]. At high current density, a high increase in heat dissipation is to be expected.
7. Conclusions
A model of a PEM electrolyzer cell was developed including electrochemical mechanism at the anode, cathode and in the membrane. Cell performance were analyses by defining an efficiency relationship. The model was able to reasonably fit the experimental data at two different temperature values i.e., 60 °C and 80 °C.
In previous studies it has been shown that activation and ohmic over voltages are closely related to temperature. For this reason, key parameters in the overvoltages relationship were chosen for the performance fitting. The electrodes reference exchange current density and electrodes change transfer coefficients showed a sensitivity to temperature; these parameters are related to the activation overvoltage. The hydrogen ion diffusivity is closely related to temperature in the ohmic overvoltage relationship.
Finally, the connection between cell polarization curve, efficiency and heat dissipation was shown. Specifically, because of the heat absorption at low current densities, first principle efficiency can reach values higher than 100%. At high current density efficiency decreases as a result of the reduced performance and related heat dissipation. Oxygen and hydrogen crossover played a less relevant role in this case as the test was conducted at ambient pressure.